Abstract
Polynitroxylated pegylated hemoglobin (PNPH) as a multifunctional therapeutic takes advantage of the oxygen transport ability of hemoglobin, the anti-oxidative stress activities from the redox coupling of nitroxide and heme iron, and the hyper-colloid properties of pegylation. The published pre-clinical data demonstrating PNPH acts as a neurovascular-protective multifunctional therapeutic in an animal model simulating pre-hospital resuscitation of traumatic brain injury (TBI) with hemorrhagic shock (HS) is reviewed. Preliminary results on the potential utility of PNPH for neurovascular protection in thrombolytic stroke therapy and for correction of vascular dysfunction through transfusion in sickle cell disease (SCD) are also discussed. We hypothesize that with PNPH hemoglobin has more than been tamed – it has become a therapeutic and not just a non-toxic extracellular oxygen carrier - and that successful PNPH development as a multifunctional therapeutic that protects the neurovasculature and reduces oxidative stress may represent a paradigm shift in transfusion and critical care medicine, which may meet a number of un-met medical needs resulting from oxidative stress and inadequate blood flow, such as HS, TBI, SCD and stroke.
Keywords: Nitroxide, Hemoglobin, poly (ethylene) glycol, oxidative stress, HBOC
Current Generation HBOCs
A blood substitute that eliminates the need for refrigeration and crossmatching, can be manufactured in quantity, has a long shelf life, and reduces the risk of iatrogenic infection is a worthwhile goal. However, hemoglobin-based oxygen carriers (HBOCs) developed during the past half century and tested in humans have done more harm than good by increasing risk of death and myocardial infarction (1). This abysmal history, and the resulting negative views of HBOCs held by the medical, regulatory, and investment communities, means that a paradigm shift is required. The outcome of the 2008 FDA/NIH co-sponsored workshop on HBOCs was the view that in order to be successful, an HBOC must demonstrate a therapeutic index with evidence of both safety and efficacy (2). This is based primarily on the association of increased risk of heart attack and death but also in part on the lack of therapeutic efficacy of current generation HBOCs.
The conventional wisdom concerning the causes of the adverse effects of experimental HBOCs is that the primary mechanism of toxicity is nitric oxide (NO) scavenging at the endothelium, causing vasoconstriction and, paradoxically for a product intended to deliver oxygen, compromising tissue perfusion and oxygenation (3). After unsuccessful phase II clinical trials of diaspirin cross-linked hemoglobin by Baxter (4), a number of HBOC developers sought to tame hemoglobin (Hb) by creating Hb derivatives, which either reduced NO binding or compensated for NO binding. These strategies included increasing the molecular weight of modified Hb to reduce Hb extravasation into the endothelium and binding of NO by 1) glutaraldehyde polymerization of human (5) and bovine Hb (6) by former Northfield Laboratories and Biopure Corp., respectively, and 2) O-raffinose cross-linking human Hb with residual unpolymerized tetramers by former Hemosol Inc. (7). An alternative strategy included genetically modifying Hb to reduce NO binding as was done by Baxter (8). These products were tested in advanced clinical trials but the results were negative (1)
Additionally, it appears that cell-free Hb derivatives tested to date also bring another set of toxicities in addition to NO scavenging. These toxicities are related to pro-oxidant activity, and are not addressed by the above approaches. They are the result of the combination of oxygen and heme iron in the absence of the controlling influence of the antioxidant enzymes of the red cell. The result can be generation of toxic molecules like superoxide, hydrogen peroxide, hydroxyl radical, oxoferryl porphyrin, etc. (3,4). Thus the reduction in oxygen delivery resulting from Hb’s NO scavenging and vasoconstriction is further exacerbated by oxidative stress through a burst of superoxide generation from heme iron auto oxidation in the transfused HBOCs (9). In this view, cell-free Hb without appropriate regulation would actually add to the inflammatory insult of ischemia and reperfusion, adding to the underlying pathology in the very clinical situations where a blood substitute would be most useful. Polynitroxylation may well provide the controlling influence to tame the pro-oxidant activity of Hb (10).
In addition to lipid encapsulated or “cellular” hemoglobin as done by Waseda University and Terumo Corporation, the most recent strategies to develop cell-free hemoglobins that address both vasoconstriction and the pro-oxidant activity of cell-free Hb include:
Polynitroxylating the HBOC to introduce SOD- and catalase-mimetic activities by Hsia et. al. (10,17, 21).
Co-polymerizing the HBOC with the antioxidant enzymes SOD and catalase to neutralize the natural pro-oxidant activities of heme iron and to enhance the anti-oxidant and anti-inflammatory activities by D’Angillo and Chang (11).
Pharmacological cross-linking the HBOC with ATP, adenosine and reduced glutathione to diminish intrinsic toxic effect of cell-free Hb by Simoni et al. (12).
Limiting the excessive oxygen–delivery capacity of the HBOC by modifying the Hb in such a way that oxygen-binding affinity is increased (P50 is decreased) by Winslow (13).
S-Nitrosylating and PEGylating the HBOC to avoid NO scavenging by heme by Nakai. (14).
Adjunct therapies of nitric oxide inhalation by Zapol et. al. (15) or sodium nitrite therapy by Petal and Kerby et. al. (16) to attenuate the hypertensive effect of HBOC.
The ideal HBOC would not only expand circulatory volume and deliver oxygen, but also do so in a manner that treats the oxidative insult of ischemia and reperfusion. It should perform at least as well as blood if not better. In fact, the ideal HBOC could conceivably perform better than stored red cells, given recent evidence that the safety of packed red cells drops off as they are stored for more than a week or two (18). We propose that PNPH with its added catalytic antioxidant activity of nitroxides may hold the key to meeting this challenge.
A New Generation Hemoglobin Based Therapeutic - PNPH - Structure and Development
In this review we describe further refinement of the last approach through the creation of polynitroxylated pegylated Hb (PNPH). A schematic of PNPH is shown in Figure 1 and the legend gives structural details on the molecule. The PNPH has five structural and functional components contributing to its unique therapeutic activities: 1) Hb as the protein center provides oxygen carrier capability but is caboxylated to provide thermo stability and added anti-inflammatory activity, 2) the hydrated polyethylene glycol moieties of PNPH provide hyper-colloid properties important to stabilizing hemodynamics during hypotension and hypovolemia, 3) the nitroxide moieties of PNPH not only improve the safety of cell-free Hb but also provide anti-oxidant/anti-inflammatory and neuroprotective activities, 4) the desirable redox coupling of heme iron with the nitroxide is promoted in the stoichiometry, and 5) further redox coupling of the nitroxide/heme iron complex with endogenous plasma anti-oxidants such as ascorbate provides additional anti-oxidant activities (19). These added intra-vascular and extra-vascular coupled redox reactions that attenuate vasoconstriction and the vascular inflammatory effects of ischemia and reperfusion are the new anti-oxidative stress therapeutic activities of this unique PNPH. Thus this approach represents a paradigm shift in blood substitute development, away from the traditional focus on oxygen delivery and NO supplementation and toward a new focus on safely correcting inadequate blood flow and oxidative stress.
Figure 1.
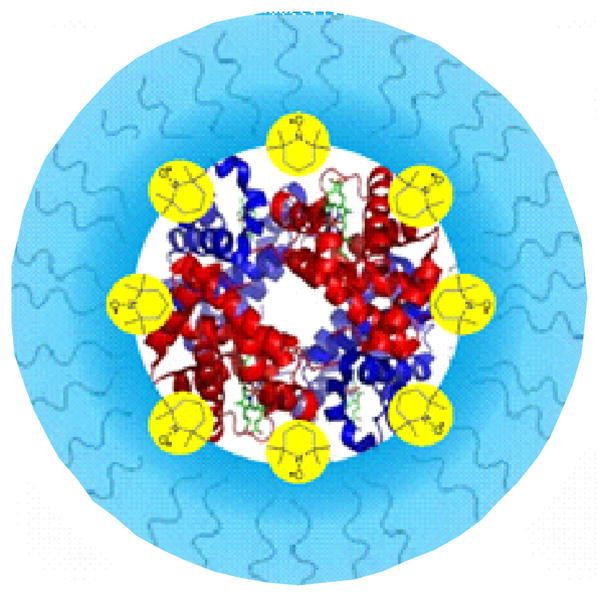
Schematic of PNPH: 8–10 5kD Peg chains (blue) protrude to form an outer shell, which shields the 12–14 nitroxides (yellow) and the hemoglobin (Hb) in the center. PNPH has a P50 of 10–12mmHg and has a molecular radius of approximately 6.8 nm and a hydrodynamic volume corresponding to that of an oligomerized Hb of 256kD.
To support this paradigm shift, we review published and preliminary data on a PNPH formulation that is efficacious in oxidative stress models of traumatic brain injury (TBI) compounded by hemorrhagic shock (HS), stroke, and sickle cell disease (SCD). The data presented below support the following therapeutic functions:
PNPH normalizes blood flow, which is at least as important as or even more important than its ability to deliver oxygen. The new molecular design of PNPH greatly decreases the natural tendency of heme iron to auto oxidize and generate superoxide, thus preventing vasoconstriction and correcting inadequate blood flow.
PNPH has the ability to catalytically reduce oxidative stress through the redox coupling of the nitroxide moieties with the heme iron resulting in increased SOD- and catalase-mimetic activities, instead of the natural pro-oxidant activities of the heme iron, and thus converts PNPH to a potent neurovascular protectant.
PNPH has the ability to further redox couple with plasma ascorbate resulting in augmentation of SOD- and catalase-mimetic activities in the form of a novel peroxidase activity (19).
The PNPH structure of multiple (up to 14) nitroxides covalently labeled onto the core Hb which is shielded by a hydrated polyethylene glycol shell is what uniquely allows it to function as neurovascular protective multi-functional therapeutic in the vascular space.
Recent studies with controlled inhalation of CO and infusion of carboxy pegylated Hbs (PegHbs) have indicated that CO can have beneficial anti-inflammatory activity (20). In fact, both Sangart Inc. and Prolong Pharmaceuticals Inc. are now developing their respective PegHbs as CO formulations. Both companies are targeting approvals as orphan drugs for SCD therapy. This confirms the benefits of the CO formulation of polynitroxylated dextran conjugated Hb used in an earlier report (21).
Experimental Evaluation of PNPH
The multifunctional therapeutic effects of PNPH prepared from bovine PegHb is indicated by its efficacy in three indications: 1) TBI+HS, 2) stroke and 3) SCD.
1) PNPH for TBI+HS
A full paper has been published (22) which describes the study of PNPH as an efficacious, novel, anti-oxidative hyper-colloid neuroprotective small volume resuscitative fluid for TBI+HS when compared to standard therapy in a model simulating the pre-hospital setting. In this paper we present data, which demonstrate that the pathology and neurological deficits from TBI can be minimized with PNPH as a pre-hospital treatment and the exacerbation of edema caused by the large volume resuscitation of current standard care can be avoided. PNPH requires the least volume to restore and maintain mean arterial blood pressure when compared to Lactated Ringer’s (LR), standard civilian therapy, or Hextend (HEX), standard military therapy, and confers neuroprotection in a relevant mouse model of TBI+HS. Mice resuscitated with PNPH had fewer Fluoro-Jade C+ identified dying neurons in CA1 vs. HEX and LR. PNPH was also shown not only to be non-toxic but also to be neuroprotective against injury (glutamate/glycine exposure and neuronal stretch) in rat primary cortical neuron cultures (p< 0.01). PNPH also maintained cerebral oxygenation better that LR and HEX as measured by implanted direct oxygen electrodes. In addition, recent data suggest a substantive attenuation of the development of intracranial hypertension during resuscitation compared to conventional therapy in TBI plus severe HS in mice. PNPH as a therapeutic for TBI represents a major improvement over current HBOCs suggesting that the improvement is due to the combined refinements of polynitroxylation and pegylation technologies.
In a separate publication studying TBI with HS, in vivo measurement of hemodynamic response, cerebral perfusion and intracranial pressure (ICP) demonstrated a beneficial synergistic effect of breathing 100% oxygen and PNPH resuscitation, which included reduced ICP (23).
The covalently labeled nitroxides on PNPH also appear to also have potent anti-oxidative stress activities in the reduced state. Recently published results show that plasma ascorbate participates in the redox cycling of the covalently labeled nitroxides on PNPH through an unusual redox-catalytic mechanism whereby reduction of H2O2 is achieved at the expense of reducing equivalents of ascorbate converted into reduced nitroxides (19).
2) PNPH for stroke
PNPH was studied as a first treatment for neuroprotection in ischemic stroke. PNPH reduced infarct volume by 58%, similar to that published for PegHb at 55% (24, 25). Further studies are planned to exploit the neurovascular protective activities of PNPH to extend the therapeutic window of tPA treatment.
Several factors contribute to the rationale for the use of PNPH in stroke. First, PNPH has the ability to deliver O2 to ischemic tissue through collateral arteries and thereby prolong viability until full reperfusion can be achieved. By increasing the amount of O2 carried in the plasma, PNPH with a P50 of 10 mmHg (intermediate between red blood cell (RBC) Hb and ischemic tissue PO2) will facilitate O2 delivery to the endothelial wall. The plasma-based O2 carrier will also deliver O2 to capillaries that are narrowed or partially obstructed and poorly perfused by RBCs during ischemia. Second, PNPH possesses antioxidant properties by virtue of its SOD, catalase and peroxidase mimetic activities. These properties are likely to stabilize the vasculature during ischemia and reduce hemorrhagic transformation arising from delayed reperfusion. Third, PNPH is synthesized and stored with the heme in the carboxy state. When transfused, the carbon monoxide (CO) is released within a matter of minutes and over 97% of the Hb in the RBC and the plasma is capable of carrying O2 soon after a 10 ml/kg transfusion. Thus, PNPH also acts as a CO donor that is rapidly converted into an O2 carrier in the circulation. Because CO donors are known to possess vasodilatory, anti-inflammatory and anti-apoptotic properties, PNPH may limit reperfusion injury through multiple mechanisms. With decreases in NO production in the endothelium during middle cerebral artery occlusion (MCAo), collateral blood flow is not maximized and the platelet inhibitory role of endothelial-derived NO is lost. CO derived from PNPH can act to increase cGMP and supplant the loss of NO. Fourth, PNPH has been observed to directly protect cultured neurons from NMDA. Since activation of NMDA receptors is well known to contribute to ischemic injury and to injury from tPA, PNPH may also act to directly protect neurons from ischemia and tPA administration in addition to Hb in the case of hemorrhagic stroke.
These therapeutic mechanisms strongly suggest that besides neuroprotection when there is compromised blood brain barrier as in TBI and hemorrhagic stroke, PNPH enhances the blood flow in the penumbra of the ischemic brain and serves as an antioxidant to ameliorate reperfusion injury, thereby leading to infarct reduction.
3) PNPH for SCD
Again, several factors contribute to the rationale for the use of PNPH in SCD. The chronic hemolytic anemia of SCD produces NO scavenging by the cell-free Hb causing NO-dependent vascular dysfunction (26), which can be ameliorated by the anti-oxidant and NO-sparing effects of PNPH. The cell-free Hb is also naturally a pro-oxidant and leads to overproduction of superoxide which can also be ameliorated by the multiple anti-oxidant properties of PNPH including its SOD, catalase, and peroxidase mimetic activities. PNPH, as a therapeutic fluid to not only augment oxygen delivery but also correct inadequate blood flow while conferring antioxidant and NO-sparing effects, could also represent a paradigm change in the treatment of vaso-occlusive crisis and acute chest syndrome in SCD. In preliminary collaborative PNPH studies, PNPH was shown to correct NO-dependent vascular dysfunctions induced by NO depletion and overproduction of superoxide in a transgenic sickle mouse model (27). Inhibition of sickling and acute hemolysis by PNPH coupled with its enhancement of NO bioavailability may represent another paradigm shift in transfusion therapy in SCD patients.
Conclusions
A growing body of preclinical evidence suggests that PNPH represents a paradigm shift in HBOC development toward a new focus on correcting inadequate blood flow due to oxidative stress and attenuating ischemia/reperfusion/inflammation injury. Also, if this holds up in clinical trials, PNPH appears likely to meet the FDA mandate for an HBOC with a meaningful increase in therapeutic index. The biology of PNPH suggests that it will be of therapeutic value in a wide range of clinical indications; current thinking is that TBI, stroke, and SCD are a reasonable first set of indications to prove the benefits of PNPH. With renewed interest and funding by NIH and Dept. of Defense for development of therapeutic Hb, PNPH (and other related high therapeutic index products) could 1) be further studied to better define the contribution to favorable outcome of each of the rich array of potential mechanistic effects of this multifunctional therapeutic and 2) be developed to commercialization and given the opportunity to address major healthcare concerns and unmet medical needs in transfusion and critical care medicine.
Acknowledgments
The work in TBI+HS in collaboration with Safar Center at UPMC was supported in part by grant PR054755 W81XWH-06-1-0247 from the US Army. The work in stroke and SCD at Johns Hopkins University Medical Center was funded via a Collaborative Agreement between SynZyme Technologies LLC and Prolong Pharmaceuticals Inc.
Footnotes
Disclosure: The authors are shareholders of SynZyme Technologies LLC.
References
- 1.Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 2008;299:2304–12. doi: 10.1001/jama.299.19.jrv80007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FDA/NIH Workshop. HEMOGLOBIN BASED OXYGEN CARRIERS: CURRENT STATUS AND FUTURE DIRECTIONS; Bethesda, Maryland. Wednesday, April 29–30, 2008; http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/WorkshopsMeetingsConferences/TranscriptsMinutes/UCM051545. [Google Scholar]
- 3.Alayash AI. Hemoglobin-based blood substitutes: oxygen carriers, pressor agents, or oxidants? Nat Biotechnol. 1999;17:545–49. doi: 10.1038/9849. [DOI] [PubMed] [Google Scholar]
- 4.Sloan EP. The clinical trials of diaspirin cross-linked hemoglobin (DCLHb) in severe traumatic hemorrhagic shock: the tale of two continents. Intensive Care Med. 2003 Mar;29:347–9. doi: 10.1007/s00134-003-1637-y. [DOI] [PubMed] [Google Scholar]
- 5.Gould SA, Moore EE, Hoyt DB, Ness PM, Norris EJ, Carson JL, Hides GA, Freeman IH, DeWoskin R, Moss GS. The life-sustaining capacity of human polymerized hemoglobin when red cells might be unavailable. J Am Coll Surg. 2002;195:445–52. doi: 10.1016/s1072-7515(02)01335-2. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs E. Clinical update: Hemopure(r) - a room temperature stable hemoglobin oxygen carrier. Anasthesiol Intensivmed Notfallmed Schmerzther. 2001;36 (Suppl 2):S121–2. doi: 10.1055/s-2001-18203. [DOI] [PubMed] [Google Scholar]
- 7.Hsia JC, Song DL, Er SS, Wong LT, Keipert PE, Gomez CL, Gonzales A, Macdonald VW, Hess JR, Winslow RM. Pharmacokinetic studies in the rat on a o-raffinose polymerized human hemoglobin. Biomater Artif Cells Immobilization Biotechnol. 1992;20(2–4):587–95. doi: 10.3109/10731199209119687. [DOI] [PubMed] [Google Scholar]
- 8.Burhop K. The Development of a Second-Generation, Designer, Recombinant Hemoglobin. Artificial Oxygen Carrier. 2005;12:127–34. [Google Scholar]
- 9.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nature Reviews Drug Discovery. 2004;3:152–59. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 10.Ma L, Hsia CJC. Taming the Hemoglobin: Therapeutic and imaging Applications of Redox Coupled Nitroxide and Hemoglobin. Xth International Symposium on Blood Substitutes; Providence, RI, USA. June 12–15, 2005. [Google Scholar]
- 11.D’Agnillo F, Chang TM. Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotechnol. 1998;16:667–71. doi: 10.1038/nbt0798-667. [DOI] [PubMed] [Google Scholar]
- 12.Simoni J, Simoni G, Martinez-Zaguilan R, Wesson DE, Lox CD, Prien SD, Kumar RV. Improved blood substitute: evaluation of its effects on human endothelial cells. ASAIO J. 1998;44(5):M356–67. [PubMed] [Google Scholar]
- 13.Winslow RM. The role of hemoglobin oxygen affinity in oxygen transport at high altitude. Respir Physiol Neurobiol. 2007;158:121–127. doi: 10.1016/j.resp.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi AT, Nakai K, Fukumoto D, Yamano M, Haida M, Tsukada H. S-Nitrosylated Pegylated Hemoglobin Reduces the Size of cerebral Infarction in Rats. Artificial Organs. 2009;33(2):183–188. doi: 10.1111/j.1525-1594.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- 15.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation. 2008;117:1982–1990. doi: 10.1161/CIRCULATIONAHA.107.729137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez C, Vitturi DA, He J, Vandromme M, Brandon A, Hutchings A, Rue L, Kerby JD, Patel RP. Sodium nitrite therapy attenuates hypertensive effects of HBOC-201 via nitrite reduction. Biochem J. 2009;422(3):423–32. doi: 10.1042/BJ20090735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishna MC, Ma L, Samuni A, Trimble CE, Mitchell JB, Hsia CJC. Superoxide Dismutase- and Catalase-Mimetic Activity of a Polynitroxyl Hemoglobin (PNH) Artif Cells, Blood Sub lmmob Biotech. 1996;24:369. [Google Scholar]
- 18.Spinella PC, Carroll CL, Staff I, Gross R, Mc Quay J, Keibel L, Wade CE, Holcomb JB. Duration of red blood cell storage is associated with increased incidence of deep vein thrombosis and in hospital mortality in patients with traumatic injuries. Crit Care. 2009;13:R151. doi: 10.1186/cc8050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoyanovsky DA, Kapralov A, Huang Z, Maeda A, Osipov A, Hsia CJ, Ma L, Kochanek PM, Bayr H, Kagan VE. Unusual peroxidase activity of polynitroxylated pegylated hemoglobin: Elimination of H(2)O(2) coupled with intramolecular oxidation of nitroxides. Biochem Biophys Res Commun. 2010;399(2):139–43. doi: 10.1016/j.bbrc.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Beckman JD, Belcher JD, Vineyard JV, Chen C, Nguyen J, Nwaneri MO, O’Sullivan MG, Gulbahce E, Hebbel RP, Vercellotti GM. Inhaled carbon monoxide reduces leukocytosis in a murine model of sickle cell disease. Am J Physiol Heart Circ Physiol. 2009;297(4):H1243–53. doi: 10.1152/ajpheart.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buehler PW, Haney CR, Gulati A, Ma L, Hsia CJ. Polynitroxyl hemoglobin: a pharmacokinetic study of covalently bound nitroxides to hemoglobin platforms. Free Radic Biol Med. 2004;37:124–35. doi: 10.1016/j.freeradbiomed.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Shellington DK, Du L, Wu X, Exo J, Vagni V, Ma L, Janesko-Feldman K, Clark RSB, Bayır H, Dixon CE, Jenkins LW, Hsia CJC, Kochanek PM. Polynitroxylated Pegylated Hemoglobin: A Novel Neuroprotective Hemoglobin for Acute Volume-Limited Fluid Resuscitation after Combined Traumatic Brain Injury and Hemorrhagic Hypotension in Mice. Crit Care Med. 2011;39(3):494–505. doi: 10.1097/CCM.0b013e318206b1fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blasiole B, Bayir H, Vagni V, Feldman K, Ma L, Hsia C, Kochanek P. Resuscitation of experimental traumatic brain injury and hemorrhagic shock in mice: Acute effects of polynitroxylated pegylated hemoglobin and 100% oxygen. Crit Care Med. 2010;38(12 Suppl):Abstract 145. [Google Scholar]
- 24.Koehler RC, Cao S, Zhanf J, Kwansa H. Effect of cell-free hemoglobin on the cerebral circulation. XIIth ISBS meeting in Parma; Italy. August 25–27, 2009. [Google Scholar]
- 25.Klaus JA, Kibler KK, Abuchowski A, Koehler RC. Early treatment of Transient Focal Cerebral Ischemia with Bovine PEGylated Carboxy Hemoglobin Transfusion. Artif Cells Blood Substit Immobil Biotechnol. 2010;38(5):223–9. doi: 10.3109/10731199.2010.488635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu LL, Champion HC, Campbell-Lee SA, Bivalacqua TJ, Manci EA, Diwan BA, Schimel DM, Cochard AE, Wang X, Schechter AN, Noguchi CT, Gladwin MT. Hemolysis in sickle cell mice causes pulmonary hypertension due to global impairment in nitric oxide bioavailability. Blood. 2007;109:3088–9. doi: 10.1182/blood-2006-08-039438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsia CJC, Ma L. PNPH - A therapeutic for inadequate blood flow and superoxide, nitric oxide dependent vascular dysfunctions. XIIth ISBS meeting in Parma; Italy. August 25–27, 2009. [Google Scholar]


