Abstract
The breast cancer resistance protein, also known as ABCG2, is one of the most studied ATP-binding cassette (ABC) transporters, due to its ability to confer multidrug resistance1,2. The lack of information on the physiological roles of ABCG2 in humans severely limits cancer chemotherapeutic approaches targeting this transporter. We report here that ABCG2 comprises the molecular basis of a new blood group system (Junior, Jr), and that individuals of the Jr(a−) blood type have inherited two null alleles of ABCG2. We thus identified 5 frameshift and 3 nonsense mutations in ABCG2. Furthermore, we show that the prevalence of the Jr(a−) blood type in the Japanese and European Gypsy populations is related to the mutations p.Q126X and p.R236X, respectively. The identification of ABCG2−/− (Jr(a−)) individuals, who appear phenotypically normal, is an essential step towards targeting ABCG2 in cancer, but also understanding the physiological and pharmacological roles of this promiscuous transporter in humans.
In an accompanying paper (NG-LE30664, Helias et al.), we have determined the genetic basis of the Lan(−) blood type, and thus identified individuals homozygous for null mutations in ABCB6, which encodes another ABC transporter family member. While this has direct implications in transfusion, it allowed us to investigate the physiological role of this ABC transporter, and highlighted the need of a comprehensive understanding of blood types to fully realize the goals of personalized medicine.
As part of an effort to identify the genes encoding the few remaining high-frequency blood group antigens with an unknown molecular basis, we focused our research on Junior antigen a (hereafter abbreviated as Jra). The corresponding alloantibody, anti-Jra, may appear on immunization of the individuals who do not express the Jra antigen, i.e. who have the Jr(a−) blood type. Anti-Jra may be responsible for acute hemolytic transfusion reactions3 but is of particular concern in obstetrics, as it can cause fatal hemolytic disease of the fetus and newborn (HDFN)4. In fact, anti-Jra is usually detected during pregnancy of Jr(a−) mothers, whose Jr(a−) blood type remains ignored until they develop an anti-Jra induced by the Jr(a+) cells of their fetus. While the Jr(a−) blood type is rare worldwide, many cases have been found in the Japanese population5,6 and in European Gypsy communities4,7. Transfusion support of Jr(a−) patients is highly difficult to manage because of the extreme rarity of Jr(a−) blood donors. A monoclonal antibody specific for the Jra antigen, HMR0921 (Fig. 1a), has been available since 19946 but has not yet allowed elucidation of the genetic basis of the Jra antigen. In particular, we were unable to immunoprecipitate the Jra antigen with HMR0921 from human red blood cells (RBCs), either due to the low abundance of the Jra antigen, the low affinity of HMR0921, or other biophysical properties.
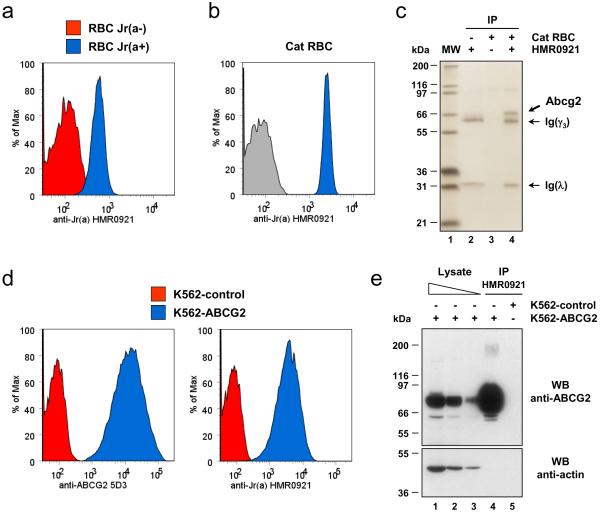
Figure 1.
The transporter ABCG2 is the carrier of the Jra blood group antigen.
(a) The monoclonal antibody HMR0921 shows a strict Jra specificity with human RBCs. RBCs from a Jr(a+) subject (blue profile) or a Jr(a−) subject (red profile) were labeled with HMR0921. Of note, the histogram profiles of Jr(a−) RBCs labeled with or without HMR0921 were superposable.
(b) Cat RBCs are highly reactive with anti-Jra HMR0921. Cat RBCs were labeled with HMR0921 (blue profiles) or without (grey profiles).
(c) The ortholog of human ABCG2 is immunoprecipitated by anti-Jra HMR0921 from cat RBCs. Lysates were prepared from membranes of cat RBCs labeled with or without HMR0921, and corresponding immune complexes (lanes 3 and 4) were analyzed by polyacrylamide gel electrophoresis under reducing conditions with heat denaturation, and silver staining. The identity of the different bands present in HMR0921 immune complex was determined by mass spectrometry. Molecular weight markers are shown in lane 1 and pure HMR0921 in lane 2.
(d) Exogenous expression of human ABCG2 in K-562 cells results in cell surface expression of the Jra antigen. Live K-562 cells stably transfected with an episomal expression construct of ABCG2 cDNA (blue profiles) or the corresponding empty vector (red profiles) were analyzed by flow cytometry with anti-Jra HMR0921 (right panel) or anti-ABCG2 5D3 (left panel).
(e) Anti-Jra HMR0921 is able to immunoprecipitate human ABCG2. Lysates (lane 1: 1/500th, lane 2: 1/1,500th and lane 3: 1/4,500th) were prepared from ABCG2-expressing or control K-562 cells labeled with HMR0921, and corresponding immune complexes (lanes 4 and 5) were analyzed by polyacrylamide gel electrophoresis under reducing conditions with heat denaturation, and western blot with anti-ABCG2 BXP21 (top panel) or anti-actin C4 (bottom panel).
While exploring the existence of the Jra antigen in different mammalian species by analyzing their RBCs by flow cytometry with HMR0921, we observed that the RBCs from most species showed no reactivity with HMR0921 (Supplementary Fig. 1) but cat RBCs exhibited a much stronger reactivity with HMR0921 than human RBCs (Fig. 1a–b). We therefore decided to identify the antigen recognized by HMR0921 on cat RBCs, assuming it would eventually lead to the identification of the human Jra blood group antigen. A protein of approximately 70 kDa was efficiently immunoprecipitated by HMR0921 from cat RBCs (Fig. 1c) and was identified by mass spectrometry as being Abcg2 (Supplementary Fig. 2), the cat ortholog of the human transporter ABCG2 (see next paragraph for details). In order to check whether human ABCG2 similarly carries the Jra antigen, we transfected an ABCG2 expression construct in K-562 erythroleukemia cells, which do not express this transporter8, and first analyzed them by flow cytometry. As shown in Figure 1d, we observed a strong expression of ABCG2 as well as the Jra antigen at the surface of ABCG2-transfected K-562 cells. We then used these cells to immunoprecipitate ABCG2 with HMR0921 (Fig. 1e), ultimately demonstrating that the Jra antigen is carried by ABCG2. These data established that ABCG2 (4q22) defines a novel blood group system.
Initially discovered for being highly expressed in placenta9 as well as conferring anthracyclin or mitoxantrone resistance in MCF-7 breast cancer cells10,11, the plasma membrane transporter ABCG2 has since been shown to confer multidrug resistance in several cancer cells by actively exporting a wide variety of drugs across the plasma membrane (see1,2). Under normal conditions, ABCG2 is thought to play an important role in protecting the organism against various toxic substances, by restricting their absorption or facilitating their elimination, as demonstrated with Abcg2−/− mice (see12). Consistently, ABCG2 is extensively expressed in barrier tissues such as intestine and placenta, as well as the blood-brain barrier13. ABCG2 is also highly expressed on the canalicular membrane of hepatocytes13 and the brush border membrane of renal proximal tubular cells14, in addition to its well-known expression on hematopoietic stem cells8 and mature RBCs15. Due to the potential impact of altered ABCG2 function on the bioavailability or pharmacokinetics of its numerous drug substrates, ABCG2 has been the subject of intense pharmacogenetic studies (see16). Hence, several hundred ABCG2 polymorphisms are currently reported in NCBI dbSNP (Build 132).
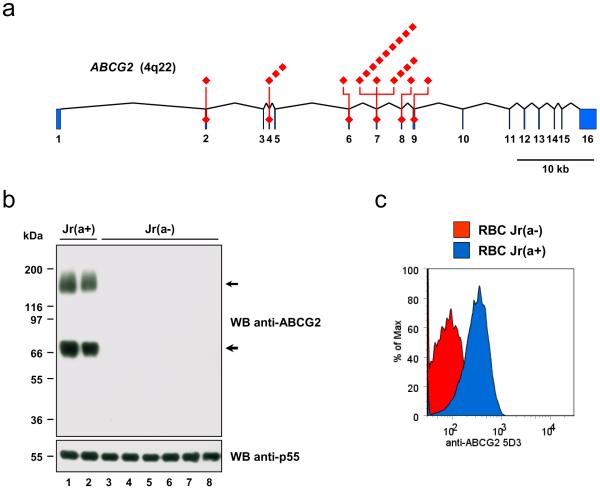
To determine which ABCG2 polymorphisms are responsible for the Jr(a−) blood type, we sequenced ABCG2 in a cohort of 18 unrelated Jr(a−) subjects (see Methods for details). We found nonsense and frameshift mutations in ABCG2 in all analyzed Jr(a−) subjects, and identified 8 different ABCG2 mutations altogether (Table 1, Fig. 2a and Supplementary Fig. 3a–h). Most of these Jr(a−) subjects were homozygous for a single ABCG2 mutation while the others were double heterozygous (Supplementary Table 1), which strongly suggested that the Jr(a−) blood type results from recessive inheritance of ABCG2 null mutations. We first confirmed the recessive inheritance by analyzing pedigrees of Jr(a−) probands (Supplementary Fig. 4). We then confirmed the absence of ABCG2 transporter in Jr(a−) individuals by western blot analysis of RBC membrane lysates (Fig. 2b) and flow cytometry analysis of native RBCs (Fig. 2c). On the basis of these data, we concluded that the ABCG2 mutations identified in the Jr(a−) subjects correspond to null alleles of ABCG2 and are responsible for their Jr(a−) blood type. Despite the large number of reported polymorphisms within ABCG2, the 8 null mutations identified were not present in NCBI dbSNP (Build 132), except the nonsense mutation c.376C>T (p.Q126X). This mutation was identified at the heterozygous state by Imai and colleagues17 while searching for non-synonymous SNP of ABCG2 in the Japanese population, and later found in the Korean population18, which is consistent with our results (Supplementary Table 1, subjects YAN, KAN and LEV). Furthermore, with an estimated frequency of 1.6–2.4% in the Japanese population17,19, the mutation c.376C>T may thus alone account for the frequency of the Jr(a−) blood type in this population (0.026–0.066%)5,6.
Table 1.
ABCG2 null mutations causing the Jr(a−) blood type
| Nucleotide changea | Predicted amino acid changeb | Location |
|---|---|---|
| 1 c.187_197delATATTATCGAA | p.I63YfsX54 | exon 2 |
| 2 c.376C>T | p.Q126X | exon4 |
| 3 c.542_543insA | p.F182VfsX14 | exon 6 |
| 4 c.706C>T | p.R236X | exon 7 |
| 5 c.730C>T | p.Q244X | exon 7 |
| 6 c.791_792delTT | p.L264HfsX14 | exon 7 |
| 7 c.875_878dupACTT | p.F293LfsX8 | exon 8 |
| 8 c.1111_1112delAC | p.T371LfsX20 | exon 9 |
Reference sequence NM_004827.1
Reference sequence NP_004818.1
Figure 2.
Null alleles of ABCG2 are responsible for the Jr(a−) blood type.
(a) Diagram showing the positions of ABCG2 null mutations identified in this study (see Table 1 for details). Blue boxes represent exons and broken lines introns. Red diamonds at the top represent the number of unrelated Jr(a−) subjects found with each mutation.
(b) Western blot analysis of ABCG2 expression in the membrane of RBCs from Jr(a+) and Jr(a−) individuals. Membranes were prepared from RBCs of 2 Jr(a+) (lanes 1 and 2) and 6 Jr(a−) (lanes 3 to 8) individuals and analyzed by polyacrylamide gel electrophoresis under reducing conditions without heat denaturation, and western blot with anti-ABCG2 BXP21 (top panel); similar results were obtained with rat anti-ABCG2 BXP34 (data not shown). The membranes were reprobed with a rabbit antiserum against p55, a membrane palmitoylated scaffolding protein of the RBC membrane (bottom panel). Of note, the different electrophoretic mobilities detected for ABCG2 here and in Figure 1e depends on the heat denaturation of lysates prior to electrophoresis.
(c) Flow cytometry analysis of ABCG2 expression at the surface of native RBCs from Jr(a+) and Jr(a−) individuals. RBCs from a Jr(a+) subject (blue profile) or a Jr(a−) subject (red profile) were labeled with mouse anti-ABCG2 5D3 (see Supplementary Figure 5 for a comparative study of 5D3 and HMR0921 antibodies); the histogram profiles of Jr(a−) RBCs, labeled with and without 5D3, were perfectly superposable. Similar results were obtained with mouse anti-ABCG2 F9123 (data not shown).
Six out of the seven unrelated Jr(a−) subjects homozygous for the nonsense mutation c.706C>T (p.R236X) (Supplementary Table 1, subjects GIM, PAT, BENO, REI, REN and KAR) belonged to Gypsy communities of Southwestern Europe. This suggested that the mutation c.706C>T is the genetic basis of the Jr(a−) blood type in this ethnic group. As shown in Supplementary Table 1, these six Jr(a−) subjects shared the same ABCG2 haplotype, consistent with the hypothesis of a founder mutation in an ancestral allele. However, the mutation c.706C>T was also found on another haplotype in two other Jr(a−) subjects (Supplementary Table 1, subjects BOU and BER) suggesting that this mutation has arisen twice independently.
The discovery of ABCG2−/− (Jr(a−)) individuals is an essential step toward understanding the physiological roles of ABCG2 in humans. Toward this goal, we first chose to evaluate the plasma levels of urate in Jr(a−) individuals, since genome-wide association studies (GWAS) of large cohorts have recently identified the minor allele of rs2231142 in ABCG2 (encoding the defective variant p.Q141K17,20) as a risk factor for hyperuricemia and gout21,22, and revealed the capacity of ABCG2 to transport urate23. We used cryopreserved plasma samples from pregnant Jr(a−) women (see Methods for details) and observed that the levels of urate were not significantly increased in the absence of ABCG2 (220 μmol/L ± 50 (n=9) for ABCG2−/− vs. 214 μmol/L ± 76 (n=9) for controls). This indicated that urate homeostasis is not markedly impaired in pregnant ABCG2−/− women, and suggested that other mechanisms may compensate for the absence of ABCG2. Alternatively, this unexpected result may reflect important differences in hormonal controls. Indeed, Matsuo and colleagues24 have reported that the ABCG2 null mutation c.376C>T (p.Q126X) is strongly associated with the risk of hyperuricemia and gout in Japanese men, and they suggested that men homozygous for this mutation may be at higher risk, due to their complete deficiency in ABCG2. Future studies to validate this hypothesis should be greatly facilitated since we show here that men homozygous for the mutation c.376C>T have the Jr(a−) blood type and represent a significant fraction of the Japanese population5,6.
As knockout mouse studies indicated an essential role of ABCG2 in maintaining porphyrin homeostasis in RBCs15,25, we then sought to examine the porphyrin levels in the blood of Jr(a-) individuals. We procured fresh Jr(a-) blood samples during the monitoring of Jr(a-) pregnancies. Consistent with the suspected role of ABCG2 in exporting porphyrin excess from RBCs15,25, the plasma levels of porphyrin were unusually low in these Jr(a-) blood samples (undetectable, i.e. < 5.0 nmol/L (n=3); 6.5 < normal range < 20.0), while RBC levels of porphyrin were higher than the normal range (3.3 μmol/L ± 1.7 (n=3); 0.1 < normal range < 1.9) but not 10-fold higher as observed in Abcg2−/− mice25. These data should be confirmed outside of pregnancy and in larger cohorts, but suggest that while ABCG2−/− individuals exhibit no symptoms of porphyria26, aberrancies in porphyrin transport do exist and may place them at risk under certain conditions. Of note, the transporter ABCB6 also contributes to porphyrin export from RBCs (see accompanying paper (NG-LE30664, Helias et al.)) and may compensate, albeit not fully, for the absence of ABCG2 in humans.
In addition to the immediate impact on transfusion medicine and obstetrics, elucidating the genetic basis of the Jr(a-) blood type has uncovered a significant number of individuals who lack the promiscuous transporter ABCG2. These ABCG2−/− (Jr(a-)) individuals are expected to be hypersensitive to the drugs transported by ABCG2, and our ability to identify them by blood typing is of major clinical interest, in order to predict drug responses and to establish optimal and more personalized drug dosages.
METHODS
Subjects
The cohort of 18 Jr(a-) subjects analyzed in this study corresponded to all the Jr(a-) probands who had been investigated at the National Reference Laboratory for Blood Groups (Paris, France), and from whom a frozen blood sample was still available; these blood samples are primarily used for serologic investigations when an antibody against a high-frequency blood group antigen has been detected. Of note, all 18 subjects were women whose Jr(a-) blood type had been identified during pregnancy, after they had developed an anti-Jra. Fresh blood samples were obtained after informed consent, and the study was conducted according to the ethical standards of the National Institute for Blood Transfusion (Paris, France).
Animal blood samples
Animal blood samples were taken according to the ethical standards of the Ecole Nationale Vétérinaire d'Alfort (Maisons-Alfort, France).
Monoclonal antibody HMR0921
The production and characterization of monoclonal anti-Jra HMR0921 has been described in detail previously6. Briefly, peripheral blood lymphocytes from a healthy Japanese woman whose serum contained an anti-Jra (titer 128) were transformed with Epstein-Barr virus (EBV) then fused with the mouse myeloma cell line P3X63Ag8.653 using polyethylene glycol (PEG), subjected to hypoxanthine-aminopterin-thymidine (HAT) - ouabain selection, and finally cloned by repeated limiting dilution. Anti-Jra-secreting hybridoma were identified by antiglobulin tests with Jr(a+) and Jr(a-) RBCs. Clone HMR0921 was expanded for antibody production. The reactivity titer of produced HMR0921 was 512 with native or trypsin-, alpha-chymotrypsin-, papain- or DTT-treated Jr(a+) RBCs as determined by antiglobulin test.
Sequencing
The primers used to amplify and sequence ABCG2 have been described previously18,27,28 and are listed in Supplementary Table 2. Detailed PCR conditions are available upon request. PCR products were sequenced with ABI BigDye terminator chemistry after ExoSAP treatment (GATC Biotech). Sequence analysis was performed using DNA Workbench software (CLC bio). Mutations were screened by unidirectional sequencing and confirmed by bidirectional sequencing.
Flow cytometry analysis
Animal RBCs were from fresh blood samples taken on EDTA, extensively washed in Dulbecco's phosphate-buffered saline solution (DPBS, Gibco), while human RBCs were from frozen blood samples, thawed, resuspended in stabilization solution (ID-CellStab, DiaMed) and washed in DPBS. RBCs were resuspended either in low-ionic strength solution (LISS, Formule 735, B. Braun Medical) supplemented with 0.15 % bovine serum albumin (BSA) and incubated with anti-Jra HMR09216 (1:50; hybridoma supernatant), or in DPBS supplemented with 0.15 % BSA and incubated with anti-ABCG2 5D3 (1:20; Santa Cruz Biotechnology). K-562 cells were washed and resuspended in DPBS supplemented with 0.15 % BSA and then incubated with anti-Jra HMR0921 (1:50) or anti-ABCG2 5D3 (1:20). Labeling with anti-Jra HMR0921 and anti-ABCG2 5D3 were revealed with goat F(ab')2 anti-human IgG(H+L)-PE (1:100; Beckman Coulter) and goat F(ab')2 anti-mouse IgG(H+L)-PE (1:100; Beckman Coulter), respectively, and immediately analyzed with a FACSCanto II flow cytometer (BD Bioscience) equipped with FACSDiva software (v. 6.1.2) (BD Bioscience). Ten thousand RBCs or viable K-562 cells, gated on forward scatter (FSC) vs. side scatter (SSC), were collected for each sample. Data were analyzed with FlowJo software (v. 7.2.5) (TreeStar).
Mass spectrometry analysis
Polyacrylamide gels were stained with the SilverQuest Silver Staining kit (Invitrogen). Excised bands were diced into small pieces, washed with water and destained with the Silver D-Stain kit (G-Biosciences). Gel pieces were subjected to two rounds of the following: washing with water for 5 min at room temperature and then incubation with 50 % acetonitrile, 50 mM ammonium bicarbonate for 30 min at 37°C. Gel pieces were then completely dehydrated by adding 100 % acetonitrile. After removal of acetonitrile, gel pieces were dried in a speed vacuum centrifuge then placed on ice and allowed to swell with 12.5 ng/μl Sequencing Grade Modified Trypsin (Promega) in 50 mM ammonium bicarbonate for 30 min on ice. An equal volume of 50 mM ammonium bicarbonate was then added and the samples were incubated overnight at 37°C. Peptides were collected and gel pieces were further extracted once with 50 % acetronitrile, 2.5 % formic acid then once with 100 % acetonitrile. Pooled peptide extractions were dried in a speed vacuum centrifuge, and peptides were resuspended in 2.5 % acetonitrile, 2.5 % formic acid, and loaded for nanoscale liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis in an LTQ Orbitrap mass spectrometer (ThermoElectron) as previously described29. Tandem mass spectra were searched against a concatenated forward and reverse human IPI database30 using SEQUEST software and requiring fully tryptic peptides, allowing for a precursor mass tolerance of 30 PPM, and dynamic modification of methionine (15.9949 for oxidation) and cysteine (71.0371 for acrylamidation). Requiring 3 unique peptide identifications, precursor measurements within 8 PPM of the theoretical mass, and XCorr values of 1.8, 2.2, 2.5 and 2.8 for 1+, 2+, 3+ and 4+ charge states respectively yielded only one protein hit and no reverse hits. The identified protein was not found in the control sample.
Western blot analysis
Lysates of RBC membranes were prepared from frozen blood samples, thawed, resuspended in stabilization solution (ID-CellStab, DiaMed) and washed in 0.9 % NaCl (B. Braun Medical). RBC membranes were prepared at 0–4 °C by hypotonic lysis with 5P8 buffer (5 mM Na2HPO4 pH8.0 and 350 μM EDTA pH8.0) supplemented with 1 mM AEBSF, stripped by incubation with 10 mM NaOH and finally solubilized with an equal volume of 4X LDS Sample Buffer (Invitrogen). Equal amounts of RBC membrane lysates were reduced with 100 mM DTT without boiling, resolved by Tris-Glycine 8 % SDS-PAGE and transferred to PolyScreen PVDF Transfer Membrane (Perkin Elmer) by submarine transfer. The Mark12 Unstained Standard (Invitrogen) was used as reference of molecular weights. Membranes were blocked in 1X Blocking Buffer (Sigma) overnight at 4 °C and then incubated with mouse monoclonal anti-ABCG2 BXP21 (1:1,000; Santa Cruz Biotechnology) for 90 min at 21 °C. Anti-ABCG2 labeling was revealed with an anti-mouse IgG(H+L) horseradish-peroxidase-linked goat antibody (1:1,000; P.A.R.I.S Biotech) for 45 min at 21 °C, the Amersham ECL Plus Western Blotting Detection System (GE Healthcare) and Kodak BioMax MR films (Eastman Kodak Company). Membranes were similarly reprobed with a rabbit serum anti-p5531 (1:100,000).
Plasmid construction
The coding sequence of human ABCG2 cDNA was amplified from a Human Fetal Liver Marathon-Ready cDNA library (Clontech), cloned into pCR4Blunt-TOPO vector (Invitrogen), sequence-verified (identical to NM_004827.2) and subcloned as an NotI/SbfI fragment into the pCEP5 episomal vector, a pCEP4 vector (Invitrogen) with a modified polylinker. Complete sequence of pCEP5-ABCG2 plasmid is available upon request.
Cell culture and transfection
Human K-562 cells were grown in Iscove's modified Dulbecco's medium (IMDM + GlutaMAXI, Gibco) supplemented with 10 % fetal bovine serum (Pan-Biotech) and 0.5 × antibiotic-antimycotic solution (Gibco) at 37 °C under a humidified atmosphere containing 5 % CO2. Cells were periodically tested for mycoplasma contamination using a home-made PCR assay. To obtain K-562 transfectants, 106 K-562 cells were first transfected with 2 μg of pCEP5-ABCG2 plasmid by nucleofection using the Nucleofector II device and the Cell Line Nucleofector Kit V (Amaxa) according to the manufacturer's protocol (program T-016). Stable K-562 transfectants were obtained after 13–15 days of selection with hygromycin B (0.5 mg/ml, Invitrogen). Plasmid DNA used for transfection was purified with NucleoBond Xtra Midi Plus (Macherey-Nagel) and verified by restriction analysis before transfection.
Urate and porphyrin analysis
Urate levels were measured with an uricase method adapted to Dimension Clinical Chemistry System by Siemens Healthcare Diagnostics Inc., in plasma samples that were cryopreserved at the National Reference Laboratory for Blood Groups, as they contained an alloantibody of interest. Nine plasma samples from pregnant Jr(a-) women were available; four plasma samples from pregnant Yt(a-) women and five plasma samples from pregnant Lu(b-) women were used as control. Porphyrin levels in plasma and RBCs were measured on blood samples, taken on EDTA and kept less than 24 h at 4–8 °C in the dark, as previously described32.
Supplementary Material
Acknowledgments
The authors are indebted to all present and past members of the National Reference Center for Blood Groups (CNRGS) for identifying and conserving Jr(a−) blood samples, and extracting genomic DNA. We greatly appreciate Maude Le Gall for her thoughtful comments on the manuscript. This study was supported in part by the National Institute of Blood Transfusion (INTS), the National Institute for Health and Medical Research (INSERM) and Paris Diderot University (Paris 7). B.A.B was funded by the Vermont Genetics Network through NIH/NCRR grant P20 RR16462.
Footnotes
Contributions C.S. performed immunoprecipitation, genomic DNA sequencing and western blot analysis. V.H. performed flow cytometry, made expression constructs and cell culture. B.A.B. performed mass spectrometry analysis. T.P. provided immunohematological information and provided most Jr(a−) blood samples. H.P. performed porphyrin and urate analysis. T.M. provided the monoclonal antibody HMR0921. S.P. and M.V.T. provided animal blood samples. M.W. provided two Jr(a−) blood samples. P.-Y.L.P. was a former chief operating officer of CNRGS. J.-P.C. initiated the study by contacting T.M. for HMR0921, contacted H.P. for porphyrin and urate analysis, and continuously supported the study. L.A. conceived and supervised the study, performed experiments, made the figures and wrote the manuscript, which was reviewed by J.-P.C., T.P. and B.A.B. All authors approved the submitted manuscript.
Competing financial interests The authors declare no competing financial interests.
REFERENCES
- 1.Polgar O, Robey RW, Bates SE. ABCG2: structure, function and role in drug response. Expert Opin Drug Metab Toxicol. 2008;4:1–15. doi: 10.1517/17425255.4.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Noguchi K, Katayama K, Mitsuhashi J, Sugimoto Y. Functions of the breast cancer resistance protein (BCRP/ABCG2) in chemotherapy. Adv Drug Deliv Rev. 2009;61:26–33. doi: 10.1016/j.addr.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Kwon MY, Su L, Arndt PA, Garratty G, Blackall DP. Clinical significance of anti-Jra: report of two cases and review of the literature. Transfusion. 2004;44:197–201. doi: 10.1111/j.1537-2995.2004.00643.x. [DOI] [PubMed] [Google Scholar]
- 4.Peyrard T, et al. Fatal hemolytic disease of the fetus and newborn associated with anti-Jr. Transfusion. 2008;48:1906–11. doi: 10.1111/j.1537-2995.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 5.Nakajima H, Ito K. An example of anti-Jra causing hemolytic disease of the newborn and frequency of Jra antigen in the Japanese population. Vox Sang. 1978;35:265–7. doi: 10.1111/j.1423-0410.1978.tb02932.x. [DOI] [PubMed] [Google Scholar]
- 6.Miyazaki T, et al. A human monoclonal antibody to high-frequency red cell antigen Jra. Vox Sang. 1994;66:51–4. doi: 10.1111/j.1423-0410.1994.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 7.Pisacka M, et al. Six cases of anti-Jr(a) antibody detected in one year - A probable relation with Gypsy ethnic minority from central Slovakia. Proc. 26th Congr. Internat. Soc. Blood Transfusion. Vienna. 2000;78:P146. [Google Scholar]
- 8.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–12. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 9.Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M. A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res. 1998;58:5337–9. [PubMed] [Google Scholar]
- 10.Doyle LA, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A. 1998;95:15665–70. doi: 10.1073/pnas.95.26.15665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake K, et al. Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res. 1999;59:8–13. [PubMed] [Google Scholar]
- 12.Vlaming ML, Lagas JS, Schinkel AH. Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev. 2009;61:14–25. doi: 10.1016/j.addr.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Maliepaard M, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61:3458–64. [PubMed] [Google Scholar]
- 14.Huls M, et al. The breast cancer resistance protein transporter ABCG2 is expressed in the human kidney proximal tubule apical membrane. Kidney Int. 2008;73:220–5. doi: 10.1038/sj.ki.5002645. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, et al. Increased expression of the Abcg2 transporter during erythroid maturation plays a role in decreasing cellular protoporphyrin IX levels. Blood. 2005;105:2571–6. doi: 10.1182/blood-2004-04-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cusatis G, Sparreboom A. Pharmacogenomic importance of ABCG2. Pharmacogenomics. 2008;9:1005–9. doi: 10.2217/14622416.9.8.1005. [DOI] [PubMed] [Google Scholar]
- 17.Imai Y, et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol Cancer Ther. 2002;1:611–6. [PubMed] [Google Scholar]
- 18.Lee SS, et al. Identification and functional assessment of BCRP polymorphisms in a Korean population. Drug Metab Dispos. 2007;35:623–32. doi: 10.1124/dmd.106.012302. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi D, et al. Functional assessment of ABCG2 (BCRP) gene polymorphisms to protein expression in human placenta. Drug Metab Dispos. 2005;33:94–101. doi: 10.1124/dmd.104.001628. [DOI] [PubMed] [Google Scholar]
- 20.Tamura A, et al. Re-evaluation and functional classification of non-synonymous single nucleotide polymorphisms of the human ATP-binding cassette transporter ABCG2. Cancer Sci. 2007;98:231–9. doi: 10.1111/j.1349-7006.2006.00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehghan A, et al. Association of three genetic loci with uric acid concentration and risk of gout: a genome-wide association study. Lancet. 2008;372:1953–61. doi: 10.1016/S0140-6736(08)61343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kolz M, et al. Meta-analysis of 28,141 individuals identifies common variants within five new loci that influence uric acid concentrations. PLoS Genet. 2009;5:e1000504. doi: 10.1371/journal.pgen.1000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woodward OM, et al. Identification of a urate transporter, ABCG2, with a common functional polymorphism causing gout. Proc Natl Acad Sci U S A. 2009;106:10338–42. doi: 10.1073/pnas.0901249106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuo H, et al. Common defects of ABCG2, a high-capacity urate exporter, cause gout: a function-based genetic analysis in a Japanese population. Sci Transl Med. 2009;1:5–11. doi: 10.1126/scitranslmed.3000237. [DOI] [PubMed] [Google Scholar]
- 25.Jonker JW, et al. The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A. 2002;99:15649–54. doi: 10.1073/pnas.202607599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924–37. doi: 10.1016/S0140-6736(09)61925-5. [DOI] [PubMed] [Google Scholar]
- 27.Itoda M, et al. Eight novel single nucleotide polymorphisms in ABCG2/BCRP in Japanese cancer patients administered irinotacan. Drug Metab Pharmacokinet. 2003;18:212–7. doi: 10.2133/dmpk.18.212. [DOI] [PubMed] [Google Scholar]
- 28.Backstrom G, et al. Genetic variation in the ATP-binding cassette transporter gene ABCG2 (BCRP) in a Swedish population. Eur J Pharm Sci. 2003;18:359–64. doi: 10.1016/s0928-0987(03)00038-1. [DOI] [PubMed] [Google Scholar]
- 29.Ballif BA, Carey GR, Sunyaev SR, Gygi SP. Large-scale identification and evolution indexing of tyrosine phosphorylation sites from murine brain. J Proteome Res. 2008;7:311–8. doi: 10.1021/pr0701254. [DOI] [PubMed] [Google Scholar]
- 30.Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat Methods. 2007;4:207–14. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- 31.Arnaud L, et al. Identification and characterization of a novel XK splice site mutation in a patient with McLeod syndrome. Transfusion. 2009;49:479–84. doi: 10.1111/j.1537-2995.2008.02003.x. [DOI] [PubMed] [Google Scholar]
- 32.Gouya L, et al. Contribution of a common single-nucleotide polymorphism to the genetic predisposition for erythropoietic protoporphyria. Am J Hum Genet. 2006;78:2–14. doi: 10.1086/498620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.