Abstract
Dopamine (DA) is known to play essential roles in neural function and behavior. Accordingly, DA neurons have been the focus of intense experimental investigation that has led to many important advances in our understanding of how DA influences these processes. However, it is becoming increasingly appreciated that delineating the precise contributions of DA neurons to cellular, circuit, and systems-level phenomena will require more sophisticated control over their patterns of activity than conventional techniques can provide. Specifically, the roles played by DA neurons are likely to depend on their afferent and efferent connectivity, the timing and length of their neural activation, and the nature of the behavior under investigation. Recently developed optogenetic tools hold great promise for disentangling these complex issues. Here we discuss the use of light-sensitive microbial opsins in the context of outstanding questions in DA research. A major technical advance offered by these proteins is the ability to bidirectionally modulate DA neuron activity in in vitro and in vivo preparations on a time scale that more closely approximates those of neural, perceptual and behavioral events. In addition, continued advances in rodent genetics and viral-mediated gene delivery have contributed to the ability to selectively target DA neurons or their individual afferent and efferent connections. Further, these tools are suitable for use in experimental subjects engaged in complex behaviors. After reviewing the strengths and limitations of optogenetic methodologies, we conclude by describing early efforts in the application of this valuable new approach that demonstrate its potential to improve our understanding of the neural and behavioral functions of DA.
Keywords: dopamine, optogenetics, channelrhodopsin, halorhodopsin, archaerhodopsin, reward, accumbens, striatum, ventral tegmental area, substantia nigra
1. Introduction
The neural and behavioral functions of dopamine have been studied using a variety of experimental approaches, including lesioning of DA neurons, application of DA receptor agonists and antagonists, neurochemical measurements of DA concentrations and DA release, and electrophysiological measurements of DA neuronal activity. These and other techniques have been utilized alone and in combination to great effect. But, like all techniques, these methods have some limitations. For example, observance of a behavioral effect after injection of a DA receptor antagonist indicates that endogenous DA actions at DA receptors contribute to that behavioral process. However, the relatively long time course of drug action makes it difficult to relate temporally-specific patterns of DA neuronal activity and release to behavior emitted on short time scales. On the other hand, the precision afforded by electrophysiological recordings from the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc), where the predominant populations of DA neurons reside, allows for tight temporal correlation of these neurons’ activity with perceptual events and behavior. However, the ability to demonstrate causality, and neurochemical specificity, has remained limited. Similarly, with in vitro slice recording, we can learn much about DA effects upon synapses and circuits, but the afferents that are stimulated are often mixed, limiting conclusions. Here we review the use of optogenetic tools to activate or inhibit the activity of midbrain DA neurons as well as their afferents and efferents in neural slices and in behaving animals, and discuss the advantages provided by these tools for studies that seek to link DA neuronal activity, circuit function, and behavior. To provide context for the discussion of optogenetic approaches, we first briefly review ideas of the role of DA in behavior. We next discuss outstanding questions where further experimentation is needed to elucidate causal roles for dopamine neurons in neural circuit function and behavior. We then discuss the merits and limitations of using optogenetics to address these questions. Finally, we close with a review of exciting recent findings using optogenetic approaches that have successfully begun to address the critical questions raised in the initial sections of this review.
2. Dopamine and behavior
Dopamine neurons in the SNc and the VTA send long-range projections to many sites in the diencephalon and telencephalon, with the SNc DA neurons projecting primarily to the dorsal striatum (i.e., the caudate/putamen) and the VTA projecting primarily to the ventral striatum (the nucleus accumbens (NAc) and olfactory tubercle) as well as prefrontal cortex, the amygdala and the hippocampus (Beckstead et al., 1979; Swanson, 1982). Given this broad pattern of efferents, it is not surprising that the literature implicating DA in the acquisition and expression of a number of cognitive and behavioral processes is quite extensive. Because the densest DA projections are to the striatum, most of the findings have focused on DA function within midbrain-striatal circuits. However as discussed below, DA influences behavior via its actions at multiple projection targets, and the nature of behavioral changes observed after manipulations of DAergic transmission can vary depending on which terminal field is affected. Here we briefly touch upon some of the primary conceptions regarding the role of DA neuron activity with an eye towards understanding how application of optogenetic tools can lead to testable experiments that address these roles.
2.1. Historical and current conceptions of DA and behavior
Initial interest in DA neurons was largely motivated by the striking behavioral consequences of naturally-occurring or experimentally-induced decrements in DA transmission. The early discovery that a hallmark symptom of Parkinson’s disease, a disease characterized by tremor, bradykinesia and rigidity, is the degeneration of DA neurons focused research on the role of DA in motor function. Depletion of striatal DA via the neurotoxin 6-hydroxy-dopamine in rats produces aphagia, adipsia, and akinesia, recapitulating some aspects of Parkinson’s. Further, DA agonists can ameliorate some difficulties in motor initiation and performance in patients and experimental animals, supporting a role for DA in motor function (Carlsson, 1964; Bernheimer et al., 1973; Nisenbaum et al., 1986; Zetterström et al., 1986; Carey, 1990). The specific means by which nigostriatal DA contributes to motor function have since been refined such that DA projections from the SNc to the dorsal striatum are considered to influence motor action selection in response to environmental stimuli, as well as to mediate learning of the stimulus-action association itself (Hikosaka, 1998; Costa, 2007; Wickens et al., 2007; Redgrave et al., 2011).
Other early studies suggested that the mesolimbic DA pathway from the VTA to the NAc was also critical for mounting a behavioral response to sensory stimuli (Koob et al., 1978), similar to ideas for nigrostriatal DA. In fact, many contemporary ideas fit within the larger context of facilitation of behavioral responding to salient stimuli but the details of these conceptions vary. A broad hypothesis, described by Robbins and Everitt (2007) but similar to other descriptions (Ikemoto and Panksepp, 1999; Wise, 2006; Costa, 2007), is that DA neuronal activity is triggered by a range of behaviorally-important stimuli and behavioral states (hunger, stress) and that this DA system activation leads to the activation, or enhancement, of behavior made to obtain a particular adaptively-relevant goal (such as food). Further, the specifics of the behaviors that are activated are dependent upon which distinct neural circuits are affected, such that DA release at one set of terminals may facilitate different behaviors than DA release at other sets of terminals (Robbins and Everitt, 1992; Amalric and Koob, 1993; Nicola, 2007; Robbins and Everitt, 2007; Nicola, 2010). For example, in reaction time tasks in which rats are well-trained to respond rapidly to a stimulus, DAergic depletions of the doral striatum decrease the rapidity of the response (Brown and Robbins, 1991), while depletions of the NAc have no effect (Amalric and Koob, 1987; Brown and Robbins, 1991). In contrast, NAc DA depletions or local DA antagonist infusion into the NAc impair approach behavior in response to food and drug rewards particularly for Pavlovian conditioned responses (Parkinson et al., 2002; Saunders and Robinson, 2012).
A number of ideas regarding DA function specifically in the NAc have been put forth (Salamone et al., 1997; Berridge and Robinson, 1998; Nicola, 2007; 2010) for example, an influential theory by Berridge and Robinson (1998) proposes that NAc DA is required for the ability of incentive stimuli, such as reward-paired cues, to motivate behavioral responding towards that cue, and to allow the cue to enhance behavior directed toward obtaining the reward. An additional conception, by Salamone, proposes that DA allows for the activation of outcome-motivated actions under conditions requiring effort, whether they are triggered by cue presentation or not (Salamone et al., 1997).
DA release in other brain regions, such as the prefrontal cortex and the amygdala, has also been studied. DA in the prefrontal cortex appears to affect working memory and impulsivity (Goldman-Rakic, 1990; Dagher and Robbins, 2009; Cools and D’Esposito, 2011; Dalley et al., 2011) while in the amygdala it may be critical for the modulation of emotional learning (Lamont and Kokkinidis, 1998; Rosenkranz and Grace, 2002; Fadok et al., 2010; Phillips et al., 2010; Tye et al., 2010). Taken together, the effects of manipulation of the DA system clearly depend upon which DAergic projection is affected.
2.2. Insights from electrophysiology
The ideas and findings just discussed have emerged primarily from studies using pharmacological agents injected systemically or directly into DA terminal regions to modulate endogenous DA transmission, or using DA neurotoxins that ablate the DA system. They are complemented by ideas derived from the recording of phasic neural activity in the VTA and SNc during behavior, and of extracellular DA transients measured by voltammetry in DA terminal regions. These studies, along with pharmacological studies, suggest a role for DA neural activity in reinforcement learning (White and Milner, 1992; Wickens et al., 2003; Wise, 2006; Costa, 2007). Classic studies from Schultz and colleagues demonstrated that a substantial proportion of putative DA neurons recorded from the primate SNc and VTA respond to the presence of unpredicted reward, such as food or juice, with a burst of phasic activity, i.e., a brief series of action potentials (Ljungberg et al., 1992; Schultz et al., 1993). Phasic bursts are one of the characteristic firing modes of DA neurons and will be described in more detail in Section 3.1. After repeated pairing of a visual stimulus prior to the delivery of the reward, these neurons cease to respond to reward delivery and instead respond to the onset of the cue with phasic increases in activity (Ljungberg et al., 1992; Schultz et al., 1993; Houk and Wise, 1995; Kimura, 1995; Reynolds and Wickens, 2002; Wickens et al., 2003). In time, a specific accounting of DA’s role in learning emerged when it was realized that the DA neuron response to unpredicted, predicted, and omitted reward could signal for errors in reward prediction; this type of error signal is a central component of reinforcement learning theory (Schultz et al., 1997; Schultz and Dickinson, 2000; Glimcher, 2011). In this conception, increases in phasic DA firing in response to unpredicted reward are proposed to be necessary for at least some types of reward learning; the conditions under which these bursts occur, and how they contribute to an organism’s ability to predict the value of an upcoming event (and, hence affect subsequent behavior), can be quantified using temporal difference learning models (Schultz et al., 1997; Glimcher, 2011). These ideas have stimulated extensive interest into the neural and behavioral effects of DA. DA neuron firing has also been linked with encoding of relative reward risk and uncertainty (Fiorillo et al., 2003; Schultz, 2010).
Recent studies have demonstrated that a subset of putative DA neurons in primate and rodent SNc and VTA are activated by noxious stimuli (Brischoux et al., 2009; Matsumoto and Hikosaka, 2009; Zweifel et al., 2011), and further investigations revealed that genetic modulation of DAergic transmission can impact fear learning (Fadok et al., 2009; Zweifel et al., 2011). These studies are challenging to integrate into the simple reward prediction error framework described above, and helped lead to the idea that DA signals contribute to motivation to respond to stimuli based on their behavioral salience, in addition to valence (Bromberg-Martin et al., 2010).
2.3. Insights from neurochemical measurement
Measurements of DA release over brief (milliseconds to seconds) time spans can be accomplished using in vivo voltammetry; these experiments have supplemented findings from in vivo electrophysiological recordings. These measurements made in DA terminal regions of rodents also reveal increases in DA release after rewards and reward-predictive cues, and in some cases, as with neural recording, the DAergic response to reward disappears when fully predicted by a preceding stimulus (Roitman, 2004; Day et al., 2007). While rewarding tastes increase DA release, aversive tastes decrease DA release in the NAc (Roitman et al., 2008; Wheeler et al., 2011). Voltammetric studies have additionally revealed that transient DA signals can precede the initiation of internally-generated behavioral responding (Phillips et al., 2003; Wassum et al., 2012) indicating that phasic DA activity may be important for both internally-generated and cue-evoked behavior. Interestingly, DA release has also been observed in striatal “reward” regions in response to stress-inducing or painful stimuli (Anstrom et al., 2009, Budygin et al., 2012), in line with observed electrophysiological responses.
2.4. Summary
In summary, DA neuron activity and subsequent release in terminal regions is generally proposed to activate or modulate ongoing behavior, especially in response to external sensory stimuli, and to promote changes in postsynaptic targets that may increase the likelihood of the repetition of the behavior that was associated with preceding and/or concomitant DA neuronal activity. There is also evidence that DA release over longer time scales (seconds to minutes; termed ‘tonic’) is required for ongoing production of behavior, and these tonic levels may also play a role in neural changes that underlie learning of multiple behaviors (Grace, 1991; Robbins and Everitt, 2007; Schultz, 2007). Notably, the nature of the behavior that is affected by phasic or tonic DA release is considered to depend upon the specific functions of the circuits postsynaptic to the DA terminals.
The rich literature briefly described here supports the idea that DA neurons contribute substantially to many essential behaviors, although the nature of this contribution is still a matter of debate (for a more extensive review of these issues, see Salamone et al., 1997; Berridge and Robinson, 1998; Schultz and Dickinson, 2000; Robbins and Everitt, 2007; Bromberg-Martin et al., 2010; Nicola, 2010). In resolving these behavioral roles of DA, it is important to remember that behavior is a function of neural circuits. Parsing the many roles ascribed to DA will require an understanding of the circuits in which DA neurons participate, and the temporal windows during which DA influences activity in those circuits. With this in mind, several outstanding questions regarding DA neuronal function are addressed in the next section.
3. Outstanding physiological questions
Although decades of pharmacological, behavioral and electrophysiological studies have greatly increased our understanding of how DA influences neural activity, and, hence, behavior, many outstanding questions remain. Some of these questions are discussed below.
3.1. What are the functional consequences of temporal coding by DA neurons?
Studies that employ electrophysiological techniques to record the activity of putative DA neurons have revealed that they fire in distinct temporal modes, either phasic or tonic. Within these classes, sustained activations as well as brief pauses in activity have also been observed. Phasic activity has received the most experimental attention. As previously introduced (Section 2), recordings from behaving primates and rodents consistently show that putative DA neurons in VTA and SNc are briefly activated by primary rewards, reward-predictive cues, salient stimuli, and in some cases, aversive stimuli (Strecker and Jacobs, 1985; Mirenowicz and Schultz, 1994; Tobler et al., 2005; Roesch et al., 2007; Brischoux et al., 2009; Matsumoto and Hikosaka, 2009). These responses have an onset latency of 60-100 ms and typically last for less than 200 ms; they consist of a single action potential or a burst of spikes with an average frequency of 20-100 Hz (Schultz, 2007).
Rewards and reward-predictive cues also elicit phasic DA release in efferent targets (Roitman, 2004; Flagel et al., 2010; McCutcheon et al., 2011), although the relationship between DA neuron activation and DA release can be complex, and a one-to-one correspondence between DA neuron activation and DA release should not be assumed. For example, Garris and colleagues showed that electrical stimulation of the VTA evokes either robust or very little DA release, depending on whether the stimulation was delivered by the experimenter or contingent on an instrumental response made by a well-trained subject (Garris et al., 1999). Similarly, Montague and colleagues demonstrated that the magnitude of an electrical stimulation-evoked DA transient depended strongly on the neuron’s recent firing history (Montague, 2004). Phasic burst firing has been proposed to depend on glutamatergic and cholinergic excitatory synaptic drive onto DA neurons from a number of areas, including the pedunculopontine tegmental nucleus, laterodorsal tegmental nucleus, prefrontal cortex and subthalamic nucleus (Smith and Grace, 1992; Tong et al., 1996; Floresco et al., 2003; Lodge and Grace, 2006). In addition to stimulus-evoked bursts, most putative DA neurons also exhibit spontaneous bursts of comparable intensity and duration (Grace and Bunney, 1984a; Hyland et al., 2002). These bursts may be the source of spontaneous dopamine transients observed in target structures such as the NAc (Sombers et al., 2009).
A subset of putative DA neurons has been reported to exhibit sustained activity prior to reward if reward delivery is uncertain (i.e., rewards given with p < 1). Because the magnitude of sustained activation is greatest when reward delivery is least certain (at p = 0.5), this signal has been suggested to code for reward uncertainty. This signal lasts at least 5-10 times longer than the phasic activations seen to rewards and cues but only represents a modest increase (up to 40%) from baseline activity levels, much lower than the peak rate during phasic activations (Fiorillo et al., 2003; 2005; Schultz, 2010).
In the absence of specific stimuli, putative DA neurons mostly show low-level tonic activity that is driven by pacemaker-like membrane currents (Grace and Bunney, 1984b; Grace and Onn, 1989; Hyland et al., 2002). Tonic activity has been shown to be regulated by afferent structures such as the ventral pallidum (Floresco et al., 2001; 2003). Tonic firing of DA neurons is thought to generate low-level DA release that constitutes a basal DA “tone” (Goto and Grace, 2005; Schultz, 2007), although spontaneous bursts have recently been suggested to make a substantial contribution (Owesson-White et al., 2012). Noteably, phasic firing is substantially more effective than tonic firing at evoking DA release (Gonon, 1988; Tsai et al., 2009).
Under some conditions, such as when a predicted reward is unexpectedly omitted, putative DA neurons briefly stop firing. The resulting pause begins approximately 100 ms after reward would have been delivered and lasts approximately 400 ms (Hollerman et al. 1998) creating a break in tonic activity. Because this pause in firing is a disruption in the normally continuous DA signal, it may represent another form of temporal coding by DA neurons. In fact, several studies suggest that these pauses in DA neuron activity are uniquely poised to impact synaptic transmission in downstream structures (Goto and Grace, 2005; Matsuda, 2006).
After a DA neuron fires an action potential, the DA signal is still subject to modifications that can affect its temporal profile. DA release can be modulated at the level of the axon terminal; striatal afferents of varied neurotransmitter content can alter DA release by activating presynaptic receptors on DA neuron terminals, and this influence is independent of DA neuron activity (Chesselet, 1984; Zhang and Sulzer, 2012). Activation of presynaptic DA “autoreceptors” can suppress further DA release, either at the level of the axon terminal or via local DA release in VTA/SN at somatic autoreceptors (Chesselet, 1984; Fields et al., 2007). Other types of presynaptic receptors, including metabotropic glutamate receptors, opiod receptors, acetylcholine receptors and GABAB receptors have also been shown to modulate DA release in the striatum, in some cases elevating DA tone but in most cases suppressing it (Zhang and Sulzer, 2012). Further, DA is actively cleared from the extracellular space by dopamine transporters located presynaptically near synaptic contacts, restricting the amount of time in which released DA can act upon its receptors (Giros et al., 1991; Nirenberg et al., 1996; Schultz, 2007). Importantly, the kinetics of DA release and reuptake vary substantially by projection target, with relatively slow clearance in the basolateral amygdala and prefrontal cortex compared to striatal regions (Garris and Wightman, 1994).
After release, DA can bind to receptors located either pre- or postsynaptically. Interestingly, nearly half of the principal cell types in the dorsal striatum, the medium spiny neurons (MSN) exclusively express D1Rs, while an equivalent number exclusively express D2Rs. In the dorsal striatum, the D1R-expressing neurons express substance P and dynorphin, project directly to the SNr and internal segment of the globus pallidus, and are commonly referred to as the “direct” pathway. The D2R-expressing neurons express enkephalin, project to the external segment of the globus pallidus, and are referred to as the “indirect” pathway (Gerfen and Surmeier, 2011; Lerner and Kreitzer, 2011). The direct and indirect pathways are proposed to have opposing effects on behavioral output, with activation of the direct pathway facilitating movement, and activation of the indirect pathway inhibiting movement (Gerfen and Surmeier, 2011). A recent study employed optogenetic techniques to test this hypothesis directly (Kravitz et al., 2010); the results will be described in Section 6.4. A similar dichotomy between D1R- and D2R-expressing MSNs may be present within the ventral striatum (Lobo et al., 2010). Notably, a minority of MSNs that express both D1Rs and D2Rs have been described (Surmeier et al., 1996), and these neurons may be more numerous in ventral striatum than in dorsal striatum (Perreault et al., 2011).
Relevant to the discussion of temporal factors, the firing mode of the DA-releasing neuron is proposed to impact which type of striatal DA receptor, and hence neuronal pathway, is likely to be more strongly activated after release. D1Rs are reported to be less efficacious at binding DA than D2Rs as measured by quantitative autoradiography in rat striatum (Richfield et al., 1989). Thus, when DA concentrations are low, such as during tonic firing, D2Rs may be preferentially activated. In contrast, when DA concentrations are high, such as during phasic firing, D1Rs may also be engaged (Gonon, 1997; Schultz, 2007; Dreyer et al., 2010), although limited experimental evidence exists to support (or refute) this claim. Thus, in the striatum, it is possible that phasic and tonic DA neuron activation may initiate differential downstream effects by acting on different receptors, and, by virtue of the divergent anatomical connectivity of the cells containing these receptors, engage different neural circuits.
A major outstanding question is whether the rich temporal information contained in DA signals (along with the distinct changes in activity and plasticity these patterns can elicit) has meaningful effects on behavior. Studies that used genetic strategies to preferentially alter tonic or phasic DA neuron activity suggest that the answer to this question is yes. Long-term increases in tonic DA signaling (accomplished via knockdown of the DA transporter) or reductions in phasic DA signaling (accomplished via a selective knockout of NMDA receptors required for burst firing in DA neurons) induce specific and divergent behavioral changes (Cagniard et al., 2006; Zweifel et al., 2008; 2009; 2011; Wang et al., 2011). Although elegant, these studies utilize chronic interventions and thus lack the temporal precision to manipulate phasic or tonic signaling during discreet behavioral events (for example, decreasing phasic DA activation during predictive cues, or increasing tonic activity during delay periods in working-memory tasks). Because optogenetic approaches can affect DA neuron function with precise temporal resolution, they have the potential to provide new avenues for exploring the causal relationship between temporally patterned DA signals and subsequent effects on DA release and receptor actions, as well as on behavior, as we will see in Section 6.
3.2. Can DA and non-DA neurons be reliably identified in electrophysiological recordings?
One of the main objectives of electrophysiology experiments is to observe the physiological properties of specific neural population(s) in order to understand how these population(s) integrate into larger neural circuits. This approach is only valid to the extent that the neural populations of interest can be reliably identified.
A large number of recording studies (both in vitro and in vivo) have used electrophysiological and pharmacological criteria to identify DA neurons. These criteria include: (1) long duration action potentials and a slow spontaneous firing rate (2-10 Hz) with some bursting, (2) presence of a hyperpolarization-activated inward current, referred to as an Ih, (3) DA or D2R agonist-mediated inhibition, and (4) lack of opioid receptor agonist-induced inhibition (Ungless and Grace, 2012). These criteria were based on early recordings made primarily in the SNc (Bunney et al., 1973; Grace and Bunney, 1980; 1983; Lacey et al., 1987; Grace and Onn, 1989; Lacey et al., 1989) where recorded neurons were cytochemically identified in vitro and in vivo; neurons that were later confirmed to be DAergic had these electrophysiological and pharmacological properties.
The SNc is approximately 90% DAergic (Margolis et al., 2006b), and in this structure DA neurons can be reliably identified using these characteristics (Brown et al., 2009). However these criteria have been called into question when used to identify DA neurons in the VTA, where only 60% of the neurons are DAergic (Swanson, 1982; Margolis et al., 2006b; Nair-Roberts et al., 2008). Intriguingly, numerous exceptions to the “characteristic” electrophysiological and pharmacological properties of DA neurons can be found in this area. First, when considered as a population, cytochemically identified DA and non-DA neurons have overlapping firing rates and action potential widths in vitro and in vivo (Ungless et al., 2004; Margolis et al., 2006a; 2006b; 2012 but see Ungless and Grace, 2012). Second, some identified non-DA neurons exhibit an Ih (Cameron et al., 1997; Jones and Kauer, 1999; Margolis et al., 2003; 2006a; 2006b; 2012 but see Wanat et al., 2008; Mao et al., 2011) and some identified DA neurons do not have an Ih (Jones and Kauer, 1999; Lammel et al., 2008 but see Margolis et al., 2006a; 2006b; 2012). Third, some identified non-DA neurons are inhibited by DA agonists (Johnson and North, 1992; Cameron et al., 1997; Margolis et al., 2006b; 2008) and some identified DA neurons are not inhibited by DA agonists (Margolis et al., 2006b; Lammel et al., 2008; Margolis et al., 2008). In fact, up to 40% of cells that were presumed to be non-DA neurons based on their electrophysiological properties were inhibited by DA in vivo (Yim and Mogenson, 1980). Fourth, some DA neurons are actually inhibited by opioid agonists (Cameron et al., 1997; Margolis et al., 2003). Fifth, a significant proportion of rodent VTA, especially in the medial regions, consists of glutamatergic neurons, whose physiological properties have not been reported (Yamaguchi et al., 2007; Gorelova et al., 2012).
These findings indicate that judgments regarding the neurotransmitter content of recorded VTA neurons solely on the basis their electrophysiological and pharmacological properties are problematic (note this issue has been recently reviewed in Margolis et al., 2006b; Ungless and Grace, 2012). Existing solutions to this problem are juxtacellular or intracellular labeling of recorded cells with post-hoc cytochemical verification of neurotransmitter content; alternatively transgenic animals with genetically labeled DA or non-DA neurons can be used. However, labeling or visualizing recorded cells is not practical for gathering large data sets from in vivo preparations.
Undoubtedly, some (perhaps even most) of the neurons identified with existing criteria are indeed DAergic. However any misattribution of response properties will make the task of understanding a complex and multi-functional circuitry more difficult; this issue will be particularly true when sample sizes are small or responses are heterogeneous (Ungless and Grace, 2012). Therefore, optogenetic approaches that allow one to unequivocally identify and manipulate DA and non-DA neural populations in the midbrain are well-suited to further reveal the physiological properties of DA and non-DA neurons with exceptional accuracy.
3.3. How are afferent inputs integrated to generate DA signals?
Anatomical tracing studies reveal that DA neurons receive inputs from many sources (Bunney and Aghajanian, 1976; Phillipson, 1979; Geisler and Zahm, 2005; Geisler et al., 2007; Zahm et al., 2011; Watabe-Uchida et al., 2012). Both VTA and SN receive inputs from neurons in the striatum, pallidum, hypothalamus, amygdala, cortex, thalamus, hindbrain and other midbrain regions although in most cases distinct groups of neurons within these structures innervate the VTA and SN (Watabe-Uchida et al., 2012). In addition, DA neurons receive input from local GABA, glutamate and DA neurons (Fields et al., 2007). An outstanding question of great interest is how these diverse inputs are integrated by DA neurons to generate observed neuronal firing patterns.
Inroads have already been made in identifying afferent structures that selectively control burst or tonic firing, as described in Section 3.1 (Floresco et al., 2003; Lodge and Grace, 2006). Additionally, using a combination of transsynaptic retrograde tracing, pharmacology and in vivo electrophysiology, Luo and colleagues (2011) elucidated a disynaptic circuit from hippocampal CA3 neurons to VTA neurons via the lateral septum. Activating this circuit at theta frequencies inhibited non-DA neurons and excited DA neurons whose neurochemical content was identified by juxtacellular labeling. Furthermore, interfering with neural transmission in this circuit via a pharmacological disconnection procedure blocked expression of a context-dependent memory, demonstrating the functional importance of this afferent pathway (Luo et al., 2011).
Hikosaka and colleagues have elegantly demonstrated that lateral habenula (LHb) neurons are excited and inhibited by stimuli that inhibit or excite putative DA neurons, respectively, indicating that these structures have opposing response patterns (Matsumoto and Hikosaka, 2007). Importantly, the excitatory responses of LHb neurons recorded in awake primates occurs with shorter latency than the inhibitory response of putative DA neurons (although the reverse is not true), suggesting that the LHb may be responsible for inhibitions observed in DA neurons. Furthermore, electrical stimulation of LHb neurons inhibits putative DA neurons at short latency (~10ms), strongly implicating the LHb in shaping the reward-related signals of DA neurons (Matsumoto and Hikosaka, 2007). However, anatomical evidence is not consistent with direct inhibitory actions of LHb neurons onto DA neurons (Omelchenko et al., 2009) and new studies point to the involvement of an intermediate GABAergic structure, the rostromedial tegmental nucleus (RMTg), in conveying LHb information to DA neurons (Balcita-Pedicino et al., 2011; Hong et al., 2011).
Although these studies represent major advances in our understanding of how DA firing patterns are generated, methodological drawbacks limit what can be gleaned from these experiments. For example, pharmacological approaches cause activity changes in afferent structures for minutes or hours, making it difficult to correlate particular patterns of activity in afferent structures with potential changes in DA neuron firing. Electrical stimulation provides greatly enhanced temporal resolution, but is likely to indiscriminately activate cells or fibers of passage in the vicinity of the stimulating electrode (in addition to the targeted neurons) making the identity and spatial distribution of cells affected by this technique difficult to predict (Histed et al., 2009).
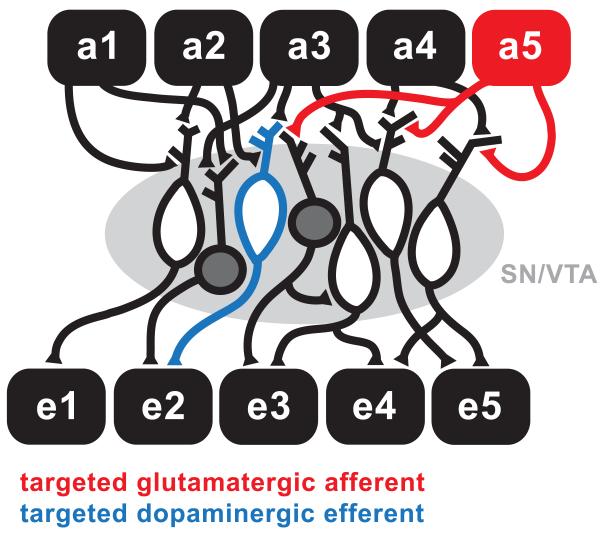
Importantly, both of these approaches will impact all of the efferent targets of the structure under investigation, of which VTA neurons are undoubtedly one of many. Furthermore, manipulations of specific afferent inputs can only be achieved in certain in vitro brain slice preparations where both the projecting neurons and their targets are preserved. More commonly, electrical stimulation is applied in close proximity to recorded cells, activating a heterogeneous and unidentified afferent (and perhaps efferent) population. Thus, it is not easy to confirm that changes in DA neural activity elicited by these types of manipulations are mediated by direct or specific actions of the afferent in question. As we shall see, techniques such as optogenetics that allow single afferents to be manipulated in isolation are extremely useful in overcoming these limitations (Figure 1).
Figure 1. Establishing causal roles for individual components of dopaminergic circuits.
The substantia nigra (SN) and ventral tegmental area (VTA) contain both dopaminergic (white) and non-dopaminergic neurons (dark grey). Both types of neurons receive mixed input from multiple afferent structures (a1 – a5), and send axonal projections to one or more efferent targets (e1-e5). Selectively manipulating individual afferent (red) or efferent (blue) projections in experimental preparations will facilitate an understanding of the complex circuitry in which dopamine neurons are embedded.
3.4. How do specific efferent DA projections impact neural activity and behavior?
Just as VTA and SNc neurons receive diverse inputs, these neuronal populations also send efferent projections to many parts of the brain including the striatum, globus pallidus/ventral pallidum, subthalamic nucleus, thalamus, LHb, amygdala, bed nucleus of the stria terminalis, hippocampus, lateral septum, lateral hypothalamus, prefrontal and entorhinal cortex, raphe nuclei, parabrachial nucleus, locus coeruleus, and other midbrain regions including the periaqueductal grey (Beckstead et al., 1979; Swanson, 1982; Fields et al., 2007). Many of these areas also send a reciprocal projection. VTA and SN projection neurons target largely non-overlapping neural populations in these structures, especially in striatum where VTA projects primarily to ventral striatum while SN projects primarily to dorsal striatum (Beckstead et al., 1979). As described in Section 3.2, there are a significant number of non-DA (primarily GABAergic, with some glutamatergic) neurons in the VTA, and to a lesser extent in the SNc. These non-DA neurons send projections both within and outside of the VTA and SNc (Swanson, 1982; Margolis et al., 2006; Fields et al., 2007; Dobi et al., 2010; Yamaguchi et al., 2011). In the VTA, the non-DA content for efferent projections ranges from 20% (NAc) to >99% (lateral habenula and locus coeruleus) (Swanson, 1982).
As previously mentioned in Section 3.1, DA produces effects in its target regions upon binding to D1 type or D2 type receptors, whose activation serves to modulate intrinsic cell excitability and excitatory and inhibitory synaptic input. DA-mediated plasticity has been extensively studied in the dorsal striatum. DA modulates the strength and direction of synaptic plasticity, transitions between up and down states, and the ion channels that control spiking in dorsal striatal MSNs (Gerfen and Surmeier, 2011; Lerner and Kreitzer, 2011). Importantly, the features of DA modulation depend on which receptor subtype is being stimulated. DA acting on D1R-expressing direct-pathway neurons is required for LTP and suppresses LTD in these cells (Pawlak and Kerr, 2008; Shen et al., 2008). In contrast, DA acting on D2R-expressing indirect-pathway neurons is required for LTD, and suppresses LTP (Shen et al., 2008). Additionally, DA modulates local inhibition in the striatum by affecting GABA release by MSNs at recurrent axon collaterals; D1R activation potentiates local inhibition while D2R activation suppresses it (Guzmán et al., 2003). This type of lateral inhibition has been suggested to be a potential mechanism whereby ensembles of striatal neurons can compete for control over behavior (Groves, 1983).
Clearly, the effects of DA release upon striatal network function, both for short- and long-term plasticity, are complicated. The ability to manipulate DAergic efferents in the slice preparation and in vivo using optogenetics, rather than pharmacological agents, may be useful for understanding these effects (Figure 1). Another critical advance would be the ability to control neural activity of specific glutamatergic afferents that impinge on neurons during tests of DA-dependent plasticity, as the post-synaptic changes induced by DA may depend on the properties of incoming glutamatergic signals. For example, does DA modify all inputs received by a given striatal MSNs equally? In addition, the distinct effects of DA on direct and indirect pathway MSNs suggest that the ability to manipulate these pathways independently, ideally in vivo, would greatly enhance our understanding of their role in behavior.
A multitude of studies that employ targeted intracranial infusions of DA agonists and antagonists in specific brain areas or bath application of these drugs in brain slices provide a substantial framework for understanding how DA neurons impact neural activity and behavior in downstream targets. However, the limited temporal precision of these approaches leaves many questions open. Additionally, using pharmacology to parse the actions of non-DA projection neurons of the VTA or SN in downstream areas can be problematic because GABAergic and glutamatergic transmission is so ubiquitous. Some studies have used microstimulation in DAergic regions to assess the effects of temporally-specific patterns of DA neuron activity on plasticity in downstream areas such as auditory cortex (Bao et al., 2001). However, these studies are subject to the same limitations that make understanding the roles of specific afferent pathways complicated: observed effects are difficult to ascribe to direct and specific actions of DA because electrical stimulation affects both targeted and non-targeted neurons (Histed et al., 2009). Optogenetics offers solutions to many of the technical limitations described in this section.
4. Suitability of optogenetics to address outstanding questions
The recent development of genetically-encoded light-sensitive proteins capable of directly modifying neural activity (opsins) promises to overcome many of the technical problems described in the preceding sections. Here, we discuss some of the advantages that make optogenetics uniquely able to address many outstanding questions regarding DA function, in concert with conventional (but still valuable) behavioral, electrophysiological and pharmacological techniques.
4.1. Temporal control
A primary advantage of optogenetic tools such as channelrhodopsin2 (ChR2) is the ability to control neural activity in mammalian cells with millisecond precision (Nagel et al., 2003; Boyden et al., 2005) without off-target actions on fibers of passage. This has immediate implications for understanding the functional effects of temporal coding by DA neurons, especially during discreet behavioral events. For example, as discussed, compelling electrophysiological evidence suggests that DA neurons signal reward prediction errors. Optogenetics should make it possible to determine if these firing patterns are indeed causal for the learned appetitive behaviors during which they are typically observed by selectively inhibiting or inducing phasic DA neuron activity. It would also be interesting to determine if phasic DA neuron activation has different effects when emitted immediately prior to, during, or immediately following reward-predictive cues or operant responses. Additionally, targeted mutations led to the development of an opsin that induces stable depolarization over longer timescales (up to 30 minutes) with the possibility of fast reversibility induced by a second light pulse. The most recent versions of these opsins are referred to as stable step function opsins (SSFOs) (Yizhar et al., 2011b). SSFOs may be useful for studying the effects of increased tonic activity in DA neurons during defined time periods.
4.2. Bidirectional action
Another significant advantage of optogenetics is the ability to bidirectionally modulate membrane potential, thus making possible temporally-specific inhibition of neural activity (Zhang et al., 2007b). Two types of opsins have been developed that provide fast optical inhibition. Halorhodopsin (NpHR) is a light-activated chloride pump; the inward flow of negative chloride ions through this pump hyperpolarizes neurons (Zhang et al., 2007b). In addition, archaerhodopsin (Arch) is a light-activated proton pump that hyperpolarizes neurons by removing positive ions from the intracellular space (Chow et al., 2010). While existing electrical microstimulation techniques allowed for temporally precise activation of DA neurons (even if these neurons were not selectively activated), no technique prior to the development of optogenetics allowed for inhibition of DA neurons on physiologically and behaviorally relevant timescales (i.e., milliseconds to seconds). This functionality is crucial for establishing the necessity of specific patterns of DA neuron activity for plasticity and behavior. For example, suppression of DA neurons limited to specific perceptual events, such as cue or reward presentation, and behavioral events, such as initiation of a movement, may lead to expansion of our understanding of the role of DA in behavior and refinement of the ideas touched upon in Section 2. Conversely, inhibitory opsins could be used to mimic naturally-occurring pauses in DA neuron firing (such as those observed when expected rewards are omitted) and establish the sufficiency of pauses to mediate neural or behavioral changes.
4.3. Genetic specificity
Unlike conventional electrical stimulation techniques, the machinery for optically-induced activity manipulation is a single protein that can be genetically targeted to desired cell populations. This represents a long sought-after experimental goal of selectively manipulating one cell population without affecting others (Zhang et al., 2007a). Transgenic lines for both mouse (Lindeberg et al., 2004; Bäckman et al., 2006) and rat (Witten et al., 2011) exist that allow for selective targeting of DA neurons using the robust Cre-dependent opsin expressing viral constructs now commonly employed (Atasoy et al., 2008; Tsai et al., 2009). This approach will be particularly advantageous when applied to the study of DA neurons in the VTA, as the cellular heterogeneity of the VTA makes it difficult to manipulate DA neurons without causing concurrent changes in local GABAergic or glutamatergic neurons. Genetic specificity will also prove to be valuable if genetically distinct populations of non-DA neurons (for example VTA GABA neurons, or an afferent projection such as orexin-expressing neurons of the lateral hypothalamus) are the subjects of study.
4.4. Anatomical specificity
As described in Section 3, many questions remain regarding the functional effects of specific afferents onto DA neurons and actions of DA (or local non-DA neurons) on specific efferent targets. Several studies have demonstrated the feasibility of activating the axon terminals of opsin-expressing neurons to achieve selective control over specific neural projections (e.g., Petreanu et al., 2007; Gradinaru et al., 2009; Tye et al., 2011). Additionally, neural populations can be labeled based on their projection patterns using retrograde or anterograde viruses that have been specially engineered for monosynaptic transsynaptic transport (Wickersham et al., 2007, Beier et al., 2011). If the virus utilized expresses an opsin, neurons can be activated or inhibited based on their afferent or efferent connectivity (Lima et al., 2009). Further, this can be accomplished while targeting light delivery to cell bodies, which has proven to be a particularly robust method for optically manipulating neural activity. Using either of these strategies to activate or inhibit specific DA afferents or efferents will undoubtedly be an enormously informative technique for understanding the functions of individual components of the circuits in which DA neurons are embedded (Figure 1), especially in in vitro brain slice preparations where wide-field light delivery is easily achieved.
4.5. Independent control of multiple neural populations
The activation maxima of excitatory and inhibitory opsins are approximately 100 nm apart, allowing for independent control using blue and yellow light. These opsins can be targeted to the same neuron for bidirectional control of neural activity, or they can be targeted to genetically or anatomically distinct neural populations and the effects of combinatorial or sequential activation and inhibition can be examined. In addition, additional excitatory opsins were identified with sufficiently separated activation maxima from ChR2 to make possible independent excitation of two neural populations using red-shifted cation channels (Zhang et al., 2008; Yizhar et al., 2011b) or independent inhibition of two neural populations using blue-shifted outward proton pumps (Chow et al., 2010). Thus activity in two neural populations can be manipulated concurrently and independently. Additionally, partial overlap of the spectral sensitivities of some optogenetic tools (for example, ChR2 and VChR1) also make possible progressive recruitment of two populations of neurons (Zhang et al., 2007a).
Combinatorial control may be useful to simultaneously and selectively manipulate multiple DA afferents or efferents (for example, to compare properties of striatal and frontal cortical inputs to DA neurons in slice preparations). Also, it may be useful to independently manipulate multiple elements of local circuitry (e.g. GABA and DA neurons in the VTA).
4.6. Optimized integration with electrophysiological recording
Conventional electrical stimulation techniques produce large electrical artifacts at the time of stimulation, obscuring concurrent neural responses. These stimulation artifacts are virtually nonexistent with optical excitation and inhibition (Zhang et al., 2007a; Yizhar et al., 2011a), making true dual recording and activity manipulation possible (e.g., Petreanu et al., 2007; Anikeeva et al., 2011). This will be particularly useful for in vitro and in vivo electrophysiological studies of DA function.
In summary, the advantages in temporal, neurochemical, anatomical, and combinatorial control make optogenetics an ideal tool to answer many outstanding questions in DA research. Importantly, many efforts have already been made to optimize these tools for use in neuroscience experiments (Bernstein and Boyden, 2011; Sparta et al., 2011; Yizhar et al., 2011a; Tye and Deisseroth, 2012).
5. Caveats for optogenetic approaches
Although optogenetics offers numerous and compelling advantages, there are limitations to this technique that may impact the interpretation or feasibility of some experiments. Some of these caveats are discussed below.
5.1. Interpretability of projection-specific manipulations may be limited by anatomical properties
While activation of terminal axon fields to identify selective roles for afferent or efferent projections is a powerful and specific approach, it can suffer from multiple off-target effects that depend on the anatomy of the projection being targeted. First, opsin-expressing axons that pass through the targeted illuminated region but terminate elsewhere will also be affected. Second, changes in membrane voltage may backpropagate from the illuminated axons to their cell bodies, triggering somatic depolarization or hyperpolarization. This second issue is a serious concern for studies that aim to perform efferent-specific manipulations of SN neurons, as most of these neurons have branching axons that make connections in multiple target regions (Fallon, 1981; Prensa and Parent, 2001); it is a less significant issue for VTA neurons, which primarily innervate only one target (Fallon, 1981; Swanson, 1982; Margolis et al., 2006a; 2006b; Lammel et al., 2008).
Another related anatomical consideration is that somatic depolarization of either VTA or SNc DA neurons can also impact local neural activity via somatodendritic DA release (Rice et al., 1997). Locally-released DA is then available to bind to receptors expressed on DA and non-DA neurons within these structures. Collectively, these off-target actions could compromise the intended specificity of the optogenetic manipulation. Strategies that employ transsynaptic viruses to express opsins in anatomically defined neural populations will likely avoid the first issue, but cannot bypass the others.
5.2. Activity of DA neurons is not equivalent to actions of dopamine
Although DA neurons are commonly referred to as just that – DA neurons – many of these cells also co-release peptides and other neurotransmitters. Thus, when DA neurons are activated or inhibited, release of these molecules may also be modulated. Some DA neurons co-release other neurotransmitters such as glutamate (Stuber et al., 2010; Tecuapetla et al., 2010), or GABA (Tritsch et al., 2012) and contain several peptides which also have the potential to potently modulate neural activity in downstream structures. These include cholecystokinin (Seroogy et al., 1989; Hamilton et al., 2000) and neurotensin (Seroogy et al., 1988). Activity-dependent release of neurotrophic factors, such as neurotrophin3 (Seroogy and Gall, 1993), and brain-derived neurotrophic factor (Seroogy and Gall, 1993) could also occur following optogenetic activation of DA neurons. Under some conditions, DA neurons have been reported to co-release serotonin (Zhou et al., 2005). It should be noted that this confound is not specific to optogenetic techniques, as many manipulations (such as electrical stimulation or pharmacological inhibition) that act to enhance or suppress DA neuron activity could cause corresponding changes in the release of other signaling molecules present in DA neurons. When possible, attempts should be made to block/rescue optogenetically-induced effects with DA antagonists or agonists to provide further evidence that they are specifically caused by actions of DA.
Additionally, as mentioned previously in Section 3.1, DA neuron activation and DA release are separate processes and the relationship between the two can be complex (Garris et al., 1999; Montague, 2004). Voltammetry can be employed to directly measure changes in DA release caused by optical manipulations.
5.3. Opsin expression may affect the normal function of DA neurons
While changes in neural responsivity and gross cell death have not been widely reported in optogenetic studies published to date, some as yet undetected changes may occur that affect cell function or health. Findings from a recent study highlight this issue. This study demonstrated that in hippocampal neurons, inhibition via NpHR, but not Arch, can lead to changes in cell excitability for a period of time following illumination. The altered responsivity was the result of changes in the GABAA receptor reversal potential induced by entry of chloride ions through the opsin (Raimondo et al., 2012). This important finding makes clear the necessity of validating the efficacy of optogenetic tools under conditions that resemble experimental situations as closely as possible. Additionally, care should be taken to include appropriate controls for exogenous gene expression and heating during light delivery (see Yizhar et al., 2011 for review).
It is also worth noting that ChR2, the most commonly used opsin, is Ca2+ permeable (Nagel et al., 2003). In fact, increasing Ca2+ permeability in a ChR2 variant was shown to improve the kinetics and light sensitivity of the channel (Kleinlogel et al., 2011). Ca2+ entry via opsins may impact Ca2+ dependent cellular processes; this may be a feature or a bug depending on the experimental question of interest.
5.4. Light delivery to large structures or diffuse projections may be challenging
While the soma of DA neurons are located in relatively small and tightly packed clusters suitable for broad illumination with small-diameter optical fibers, the afferent or efferent structures that target or are targeted by DA neurons are often much larger and achieving light delivery that encompasses the entirety of such areas may be challenging. For example, DA projections from the SNc to dorsal striatum would be very difficult to target in their entirety as the dorsal striatum is large even in mice. Modifying fiber tips to increase light spread (Kravitz and Kreitzer, 2011), using fibers with wide numerical apertures (Yizhar et al., 2011), or using multiple fibers (Bernstein and Boyden, 2011) may mitigate this problem in some cases. Properties of optical fiber implants, which are readily available with diameters from tens to hundreds of m and divergent beam angles of 8-42°, should be chosen with the goals and constraints of the specific application in mind.
Conversely, the ability to target substructures (such as dorsomedial and dorsolateral striatum, or the core and shell subregions of the nucleus accumbens) is likely to be excellent, particularly in larger rodents and primates.
5.5. Common equipment configurations for optogenetic experiments require tethering
In many studies that use optogenetics in behaving animals, light is delivered via a lightweight and flexible optical cable that must be attached to the experimental animal. These cables enable use of high-powered light sources such as lasers. While behavior is virtually unrestricted in many cases, tethering may present a challenge for some in vivo applications (for example, when combined with intravenous drug self-administration or electrophysiological studies). Solutions exist to circumvent these problems such as the use of combined optical/fluid or optical/electrophysiological commutators, or the utilization of head-mounted LED light sources that preclude tethering (Bernstein and Boyden, 2011; Yizhar et al., 2011).
6. Early optogenetic applications to DA research
The promise of optogenetics to establish causal roles for DA neurons in neural activity and behavior is quickly becoming a reality. A number of recent studies that employ optogenetic techniques have already made significant contributions to our understanding of how DA neurons promote behavior, what they encode, and how their signals are generated and utilized by afferent and efferent circuitry. Lastly, these studies have been extended to shed light on causes of and treatments for DA-related diseases.
6.1. Sufficiency of phasic DA signals to promote behavior
As described in Section 2, phasic signaling by DA neurons is implicated in promoting a variety of behavioral functions. Causal tests of the sufficiency of phasic DA neuron activation to promote specific behaviors have been the focus of several early studies. These studies reveal that phasic activation of DA neurons is sufficient to support conditioned place preference and positive reinforcement in the absence of any natural reward (Tsai et al., 2009; Witten et al., 2011; Kim et al., 2012). Interestingly, in one of these studies, the ability to mimic phasic or tonic DA neuron firing with optogenetics was used by Tsai and colleagues to demonstrate that only phasic firing modes are sufficient to induce a conditioned place preference (Tsai et al., 2009).
In a second of these studies, Witten and colleagues demonstrated that selective optical stimulation of VTA DA neurons supports robust intracranial self-stimulation behavior (ICSS) that is sensitive to changes in the magnitude of DA neuron activation, or alterations in behavioral contingencies such as extinction or degradation of the temporal relationship between response and reinforcement. This resolved a historical controversy regarding the identity of cells that support electrical ICSS (Figure 2). First demonstrated in a landmark study by Olds and Milner (Olds and Milner, 1954), ICSS is perhaps the simplest behavioral paradigm for studying the minimal neural elements that mediate positive reinforcement. Furthermore, this study utilized a novel transgenic rat line expressing Cre recombinase in catecholaminergic neurons to target ChR2 selectively to DA neurons of the VTA and activate these neurons in behaving rats. Since many DA-dependent behaviors have been optimized for rats, and their size makes them the preferred rodent model for in vivo electrophysiology and voltammetry, the ability to utilize selective optogenetic tools in this species represents a valuable advance.
Figure 2. Comparison of response patterns from individual rats performing operant behaviors to obtain electrical stimulation of the septum or optogenetic stimulation of dopamine neurons.
(A) Cumulative bar press responses from a high-performing rat implanted with a stimulating electrode in the septum. Each bar press was reinforced with current delivered to the tip of the implanted electrode; background color indicates the voltage of current applied. The rate of responding increased with voltage, and ceased when current was not applied. Note that behavioral data from multiple daily sessions were collapsed to produce the figure depicted. Adapted with permission from Olds and Milner (1954). (B) Cumulative nosepoke responses from a high-performing rat receiving optogenetic stimulation of ventral tegmental area dopamine neurons. Each nosepoke was reinforced with 1 second of 20 Hz optical stimulation. The color of the line indicates behavior across 4 consecutive daily sessions. (C) Response rate from the same animal shown in B during a behavioral session where the length of optical stimulation delivered with each response was systematically varied. Each stimulation length was tested multiple times; error bars represent SEM. Response rate increased with stimulation length. (D) Cumulative responses for the same animal during a within-session extinction test. Responding ceased when no longer reinforced, and resumed when the laser was turn back on. Stimulation length was 1 sec as in B. Panels b-d were adapted with permission from Witten et al. (2011). Note the close correspondence of the pattern of responding in A with the patterns observed in B-D.
Phasic activation of DA neurons can also alter behaviors directed at obtaining natural rewards. Phasic DA can alter preference for nutritive vs. non-nutritive sweeteners, and this effect can be modulated by metabolic state (Domingos et al., 2011). Additionally, when given a choice between two operant responses that result in the same food reward, mice prefer to perform the response that also results in DA neuron activation (Adamantidis et al., 2011). These studies demonstrate how useful temporal control of DA neurons is for understanding their behavioral effects.
6.2. Unambiguous identification of DA and GABA neurons in vivo
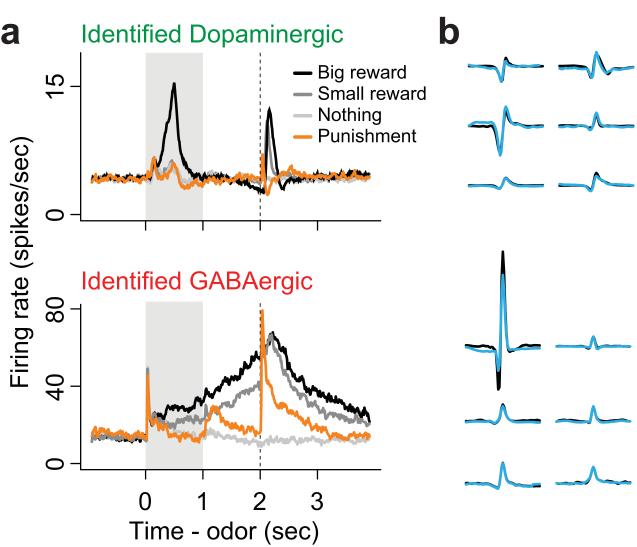
A recently-published study elegantly illustrates the benefits of integrating optogenetics with in vivo electrophysiology. Cohen and colleagues used ChR2 to identify DAergic and GABAergic neurons in the VTA of behaving mice, circumventing the difficulties associated with identifying DA neurons using electrophysiological and pharmacological criteria (Cohen et al., 2012). In this study, neurons had to meet several rigorous standards to be identified as DAergic or non-DAergic. Identified neurons showed close correspondence between optically-evoked and spontaneous waveform shapes (Figure 3b), short latency and low temporal jitter of optically-evoked spikes, and the ability to follow high-frequency (50 hz) trains of optical stimulation with high fidelity. Notably, no relationship was observed in identified neurons between neurotransmitter content and electrophysiological properties such as spike duration or spontaneous firing rate, criteria that are commonly used to identify putative DA neurons (see Section 3.2).
Figure 3. Electrophysiological properties of optogenetically identified dopamine and GABA neurons recorded in vivo.
Electrophysiological recordings were performed in vivo in two strains of transgenic mice expressing channelrhodopsin2 in either dopamine or GABA neurons. During recording sessions, subjects were engaged in an odor-outcome association task with positive, negative and neutral outcomes. (A) Average responses of identified dopamine neurons (top; n=26) and identified GABA neurons (bottom; n=17) to odor cues (0-1 seconds, grey bar) and associated outcomes (delivered at 2 seconds, dashed line). Note that GABA neurons increase their firing rate during reward-predictive cues and this activity is sustained until the expected time of reward delivery. (B) Spontaneous (black) and optically evoked (blue) waveforms from 6 individual neurons recorded in mice expressing channelrhodopsin2 in dopamine (top) or GABA neurons (bottom). Close correspondence between spontaneously occurring and optically evoked waveform shapes was one of the criteria used to identify the neurotransmitter content of recorded neurons. Adapted with permission from Cohen et al. (2012).
The authors proceeded to correlate neurotransmitter content with electrophysiological responses during an odor-outcome association task. Because of the unique ability – conferred by optogenetics -- to unambiguously identify the neurotransmitter content of recorded cells, the authors were able to show that GABA neurons are excited by reward-predictive cues and may encode reward expectation, revealing a previously unappreciated capability of this neural population (Figure 3a). This observation reshapes conventional views of how VTA neurons collectively encode motivationally-significant conditioned and unconditioned stimuli.
6.3. Delineating connectivity and function of specific afferent projections
Optogenetic tools have already proven useful in understanding the detailed anatomy and functional impact of afferent projections to DAergic regions. Two recent in vitro studies used selective optical stimulation of afferents from dorsal and ventral striatum to determine that striatal MSNs exclusively target GABAergic neurons in the SNr and VTA (Chuhma et al., 2011; Xia et al., 2011; but see Watabe-Uchida et al., 2012).
VTA GABA neurons were themselves the focus of two studies from independent labs (Tan et al., 2012; van Zessen et al., 2012). In each of these studies, the authors used ChR2 to directly activate GABA neurons in the VTA and assess the effects of this component of VTA circuitry on the activity of DA neurons. They also went on to explore how activation of this circuit element influenced motivated behaviors. Tan and colleagues found that optogenetically-activated GABA neurons inhibit putative DA neurons via actions at GABAA receptors, and that putative GABA neurons recorded in vivo increase their firing in response to an aversive stimulus while putative DA neurons decrease their firing (Tan et al., 2012). In this study, identification of the neurotransmitter content of recorded cells was made using the pharmacological and electrophysiological criteria previously described. Furthermore, the authors found that optogenetically-increasing GABA neuron activity or decreasing DA neuron activity is sufficient to cause conditioned place aversion. This study establishes a role for VTA GABA neurons in the neural processing of aversive stimuli, although it does not preclude a role for DA neurons suggested by previous observations (Anstrom et al., 2009; Brischoux et al., 2009; Fadok et al., 2009; Matsumoto and Hikosaka, 2009; Fadok et al., 2010; Zweifel et al., 2011; Budygin et al., 2012).
In the second of these studies, Van Zessen and colleagues found that optogenetic activation of VTA GABA neurons suppressed the activity and excitability of DA neurons, as well as suppressing DA release in ventral striatum (Van Zessen et al., 2012). Optogenetic activation of GABA neurons during reward-seeking behavior disrupted reward consummatory behavior without affecting conditioned anticipatory behavior. Behavior was not disrupted when GABAergic projections to the NAc were selectively activated, indicating that intra-VTA actions, or actions via a different projection target, mediate this effect. Interestingly, the authors of this study found that VTA GABA neurons could only suppress evoked DA release when DA neurons were activated at low frequencies (10 Hz or less). This raises the intriguing possibility that VTA GABA neurons may exert greater control over nearby DA neurons when these neurons fire at low rates, such as during tonic activity.
Two other studies have helped to delineate the precise anatomical connections between LHb and VTA neurons. Stamatakis et al. (Stamatakis and Stuber, 2012) used optogenetic activation of LHb inputs to assess the functional connectivity of this projection with ventral midbrain neurons. The authors found that LHb afferents primarily targeted neurons in the posterior VTA and RMTg. Furthermore, the vast majority of VTA neurons responsive to LHb input were non-DAergic, although only 50% of sampled VTA non-DA neurons were responsive to LHb stimulation. This study provided additional compelling evidence that LHb inputs to RMTg mediate avoidance behaviors, presumably via a connection between RMTg and VTA. This analysis was complemented by a second study (Matsui and Williams, 2011) that used selective optical stimulation of GABAergic RMTg afferents to VTA to determine that this pathway evokes GABAA IPSCs in putative DA neurons. Collectively, these studies add to mounting anatomical and electrophysiological evidence pointing to a disynaptic LHb-RMTg-VTA circuit that signals negative value.
6.4. Consequences of modulating activity in efferent striatal targets
A considerable body of research has focused on DA’s actions in the striatum, in part because it receives an extremely robust DAergic projection. A number of recent studies have used optogenetics to selectively activate or inhibit D1R- and D2R-expressing MSNs in dorsal and ventral striatum and assess the effects of these manipulations on a variety of behaviors.
Using transgenic mice that allow for selective targeting of D1R and D2R neurons in dorsal striatum, Kravitz and colleagures found that activation of D1R MSNs was sufficient to induce movement and decrease freezing, consistent with the hypothesized role of direct-pathway neurons in promoting movement (Kravitz et al., 2010). Conversely, activation of D2R neurons suppressed movement and increased freezing, consistent with the hypothesized role of indirect-pathway neurons in inhibiting movement. This finding has implications for a functional dichotomy in the downstream effects of phasic and tonic DA release in dorsal striatum, which are proposed to differentially impact these two neural populations.
Additional roles for dorsal striatal D1R and D2R neurons were found in positive reinforcement and action choice. In a separate study, Kravitz et al. found that activation of D1R neurons was reinforcing, as mice would perform an operant response to receive activation of D1R neurons (Kravitz et al., 2012). In contrast, mice actually decreased their responding below baseline levels if D2R neurons were activated. Complementing this finding, Tai and colleagues found that transient, unilateral activation of dorsal striatal D1R and D2R neurons during decision-making introduces opposing biases in the distribution of choices (Tai et al. 2012). In a forced-choice task with left and right responses, activation of D1R neurons increased the probability of making a contralateral response and activation of D2R neurons increased the probability of an ipsilateral response. Notably, the effect of stimulation depended on reward history and could not be explained as a pure effect on motor behavior.
Two other studies examined the effects of activity manipulations in specific neural populations of the ventral striatum in the context of cocaine-related behaviors. In contrast to the robust conditioned place preference observed when DA neurons in the VTA are activated (Tsai et al., 2009), Lobo and colleagues found that selective activation of D1R or D2R MSNs in the NAc failed to support a place preference (Lobo et al., 2010). Similarly, Witten et al. observed that optogenetic inhibition of NAc cholinergic interneurons (which also express DA receptors and potently, though indirectly, inhibit MSNs) did not alter place preference (Witten et al., 2010). In both studies, the same manipulations were effective in shifting place preference in the presence of cocaine.
Working in vitro, two independent studies (Stuber et al., 2010; Tecuapetla et al., 2010) provided unequivocal evidence that DA neurons can co-release glutamate in the striatum and lead to postsynaptic EPSCs in MSNs, resolving a long-standing controversy. Interestingly, glutamatergic currents evoked by optical stimulation of DAergic terminals were more prominent in ventral striatum MSNs; the mean magnitude of these currents in dorsal striatum was 10-fold lower (Stuber et al., 2010). This suggests that DA neurons in some brain regions emit a short-timescale excitatory postsynaptic signal in parallel with slower neuromodulatory effects.
6.5. Applications to DA-related disease states
Many serious and pervasive diseases involve dysfunction of DAergic systems. These include addictions, Parkinson’s disease, schizophrenia, and perhaps depression and attention deficit hyperactivity disorder as well. Optogenetic tools are likely to help improve our understanding of the etiology and treatment of these diseases.
For example, Brown and colleagues found that optogenetic activation of DA neurons mimicked drug-induced AMPA receptor redistribution (Brown et al., 2010). Additionally, several studies have used optogenetics to examine cocaine-induced changes in the NAc (Lobo et al., 2010; Witten et al., 2010; Pascoli et al., 2011) and to elucidate the neural mechanisms that underlie the therapeutic efficacy of deep brain stimulation in models of Parkinson’s disease (Gradinaru et al., 2009) or that open new avenues for treatment of this debilitating condition (Kravitz et al., 2010). The application of optogenetics to selectively manipulate specific cell types and/or specific synaptic inputs has greatly enhanced the ability to draw causal conclusions about the contribution of DA and related circuitry in these preclinical disease models.
7. Summary and conclusions
DA neurons have received a great deal of experimental attention since they were first identified in the middle of the last century (Carlsson et al., 1962). They feature prominently in theories that describe how experimental animals (and presumably humans) move, eat, learn, remember, and experience natural and drug rewards. DA neurons have even been suggested to play a major role in the neural basis of consciousness (Palmiter, 2011). Despite a wealth of knowledge gained from thousands of DA studies that have been conducted to date, many significant questions remain. Many of these questions could be best answered by directly modifying the activity of DA neurons or other elements of the circuits in which they participate. Optogenetics is clearly suited to meet this need, and will be especially powerful when used in conjunction with existing behavioral, electrophysiological, pharmacological and anatomical techniques.
It is interesting to speculate that the relative homogeneity of DA neuronal population responses observed in vivo may facilitate attempts to mimic or delete their signals using optogenetic tools. For example, 75% of putative DA neurons are activated by unpredicted rewards; for comparison, only a small fraction of hippocampal pyramidal neurons are synchronously active during spatial exploration (Wilson and McNaughton, 1993; Hollerman and Schultz, 1998). Also, the comparatively slow timing of modulatory DA signals may provide more leeway when attempting to match exogenously-applied signals to endogenously-timed plasticity mechanisms which often have a narrow window of efficacy (Dan and Poo, 2004). Thus, the study of DA neurons may represent a case where the power of optogenetics can be put to particularly effective use.
Acknowledgements
Supported by DA015096, AA014925, AA018025 and funds from the State of California through UCSF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamantidis AR, Tsai H-C, Boutrel B, Zhang F, Stuber GD, Budygin EA, Touriño C, Bonci A, Deisseroth K, de Lecea L. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J. Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Depletion of dopamine in the caudate nucleus but not in nucleus accumbens impairs reaction-time performance in rats. J. Neurosci. 1987;7:2129–2134. doi: 10.1523/JNEUROSCI.07-07-02129.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M, Koob GF. Functionally selective neurochemical afferents and efferents of the mesocorticolimbic and nigrostriatal dopamine system. Prog. Brain Res. 1993;99:209–226. doi: 10.1016/s0079-6123(08)61348-5. [DOI] [PubMed] [Google Scholar]
- Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nature Neurosci. 2011;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neurosci. 2009;161:3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcita-Pedicino JJ, Omelchenko N, Bell R, Sesack SR. The inhibitory influence of the lateral habenula on midbrain dopamine cells: Ultrastructural evidence for indirect mediation via the rostromedial mesopontine tegmental nucleus. J. Comp. Neurol. 2011;519:1143–1164. doi: 10.1002/cne.22561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- Bäckman CM, Malik N, Zhang Y, Shan L, Grinberg A, Hoffer BJ, Westphal H, Tomac AC. Characterization of a mouse strain expressing Cre recombinase from the 3 untranslated region of the dopamine transporter locus. Genesis. 2006;44:383–390. doi: 10.1002/dvg.20228. [DOI] [PubMed] [Google Scholar]
- Beckstead RM, Domesick VB, Nauta WJ. Efferent connections of the substantia nigra and ventral tegmental area in the rat. Brain Res. 1979;175:191–217. doi: 10.1016/0006-8993(79)91001-1. [DOI] [PubMed] [Google Scholar]
- Beier KT, Saunders A, Oldenburg IA, Miyamichi K, Akhtar N, Luo L, Whelan SPJ, Sabatini B, Cepko CL. Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors. Proc Natl Acad Sci. 2011;108:15414–15419. doi: 10.1073/pnas.1110854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cognitive Sci. 2011;15:592–600. doi: 10.1016/j.tics.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U.S.A. 2009;106:4894–4899. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MTC, Bellone C, Mameli M, Labouèbe G, Bocklisch C, Balland B, Dahan L, Luján R, Deisseroth K, Lüscher C. Drug-driven AMPA receptor redistribution mimicked by selective dopamine neuron stimulation. PLoS ONE. 2010;5:e15870. doi: 10.1371/journal.pone.0015870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MTC, Henny P, Bolam JP, Magill PJ. Activity of neurochemically heterogeneous dopaminergic neurons in the substantia nigra during spontaneous and driven changes in brain state. J Neurosci. 2009;29:2915–2925. doi: 10.1523/JNEUROSCI.4423-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown VJ, Robbins TW. Simple and choice reaction time performance following unilateral striatal dopamine depletion in the rat. Impaired motor readiness but preserved response preparation. Brain. 1991;114:513–525. doi: 10.1093/brain/114.1.513. [DOI] [PubMed] [Google Scholar]
- Budygin EA, Park J, Bass CE, Grinevich VP, Bonin KD, Wightman RM. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neurosci. 2012;201:331–337. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney BS, Aghajanian GK. The precise localization of nigral afferents in the rat as determined by a retrograde tracing technique. Brain Res. 1976;117:423–435. doi: 10.1016/0006-8993(76)90751-4. [DOI] [PubMed] [Google Scholar]
- Bunney BS, Walters JR, Roth RH, Aghajanian GK. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J. Pharmacol. Exp. Ther. 1973;185:560–571. [PubMed] [Google Scholar]
- Cagniard B, Beeler JA, Britt JP, McGehee DS, Marinelli M, Zhuang X. Dopamine scales performance in the absence of new learning. Neuron. 2006;51:541–547. doi: 10.1016/j.neuron.2006.07.026. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neurosci. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Carey RJ. Dopamine receptors mediate drug-induced but not Pavlovian conditioned contralateral rotation in the unilateral 6-OHDA animal model. Brain Res. 1990;515:292–298. doi: 10.1016/0006-8993(90)90609-f. [DOI] [PubMed] [Google Scholar]
- Carlsson A. Evidence for a role of dopamine in extrapyramidal functions. Acta Neuroveg (Wien) 1964;26:484–493. doi: 10.1007/BF01252144. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Falck B, Hillarp NA. Cellular localization of brain monoamines. Acta Physiol Scand Suppl. 1962;56:1–28. [PubMed] [Google Scholar]
- Chesselet MF. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neurosci. 1984;12:347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Tanaka KF, Hen R, Rayport S. Functional connectome of the striatal medium spiny neuron. J Neurosci. 2011;31:1183–1192. doi: 10.1523/JNEUROSCI.3833-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa RM. Plastic corticostriatal circuits for action learning: what’s dopamine got to do with it? Ann New York Acad Sci. 2007;1104:172–191. doi: 10.1196/annals.1390.015. [DOI] [PubMed] [Google Scholar]
- Dagher A, Robbins TW. Personality, addiction, dopamine: insights from Parkinson’s disease. Neuron. 2009;61:502–510. doi: 10.1016/j.neuron.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo M-M. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dobi A, Margolis EB, Wang H-L, Harvey BK, Morales M. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JK, Herrik KF, Berg RW, Hounsgaard JD. Influence of phasic and tonic dopamine release on receptor activation. J Neurosci. 2010;30:14273–14283. doi: 10.1523/JNEUROSCI.1894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TMK, Palmiter RD. Dopamine is necessary for cue-dependent fear conditioning. J Neurosci. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok JP, Darvas M, Dickerson TMK, Palmiter RD. Long-term memory for pavlovian fear conditioning requires dopamine in the nucleus accumbens and basolateral amygdala. PLoS ONE. 2010;5:e12751. doi: 10.1371/journal.pone.0012751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH. Collateralization of monoamine neurons: mesotelencephalic dopamine projections to caudate, septum, and frontal cortex. J. Neurosci. 1981;1:1361–1368. doi: 10.1523/JNEUROSCI.01-12-01361.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields HL, Hjelmstad GO, Margolis EB, Nicola SM. Ventral tegmental area neurons in learned appetitive behavior and positive reinforcement. Annu. Rev. Neurosci. 2007;30:289–316. doi: 10.1146/annurev.neuro.30.051606.094341. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Evidence that the delay-period activity of dopamine neurons corresponds to reward uncertainty rather than backpropagating TD errors. Behav Brain Funct. 2005;1:7. doi: 10.1186/1744-9081-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, Akers CA, Clinton SM, Phillips PEM, Akil H. A selective role for dopamine in stimulus-reward learning. Nature. 2010;469:53–57. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–973. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Garris PA, Wightman RM. Different kinetics govern dopaminergic transmission in the amygdala, prefrontal cortex, and striatum: an in vivo voltammetric study. J. Neurosci. 1994;14:442–450. doi: 10.1523/JNEUROSCI.14-01-00442.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garris PA, Kilpatrick M, Bunin MA, Michael D, Walker QD, Wightman RM. Dissociation of dopamine release in the nucleus accumbens from intracranial self-stimulation. Nature. 1999;398:67–69. doi: 10.1038/18019. [DOI] [PubMed] [Google Scholar]
- Geisler S, Zahm DS. Afferents of the ventral tegmental area in the rat-anatomical substratum for integrative functions. J. Comp. Neurol. 2005;490:270–294. doi: 10.1002/cne.20668. [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS. Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci. 2007;27:5730–5743. doi: 10.1523/JNEUROSCI.0012-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Mestikawy el, S., Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- Glimcher PW. Colloquium Paper: Understanding dopamine and reinforcement learning: The dopamine reward prediction error hypothesis. Proc Natl Acad Sci. 2011;108:15647–15654. doi: 10.1073/pnas.1014269108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular and circuit basis of working memory in prefrontal cortex of nonhuman primates. Prog. Brain Res. 1990;85:325–35. doi: 10.1016/s0079-6123(08)62688-6. discussion335–6. [DOI] [PubMed] [Google Scholar]
- Gonon F. Prolonged and extrasynaptic excitatory action of dopamine mediated by D1 receptors in the rat striatum in vivo. J. Neurosci. 1997;17:5972–5978. doi: 10.1523/JNEUROSCI.17-15-05972.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neurosci. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Gorelova N, Mulholland PJ, Chandler LJ, Seamans JK. The glutamatergic component of the mesocortical pathway emanating from different subregions of the ventral midbrain. Cerebral Cortex. 2012;22:327–336. doi: 10.1093/cercor/bhr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neurosci. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Nigral dopamine neurons: intracellular recording and identification with L-dopa injection and histofluorescence. Science. 1980;210:654–656. doi: 10.1126/science.7433992. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neurosci. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984a;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: single spike firing. J. Neurosci. 1984b;4:2866–2876. doi: 10.1523/JNEUROSCI.04-11-02866.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J. Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM. A theory of the functional organization of the neostriatum and the neostriatal control of voluntary movement. Brain Res. 1983;286:109–132. doi: 10.1016/0165-0173(83)90011-5. [DOI] [PubMed] [Google Scholar]
- Guzmán JN, Hernández A, Galarraga E, Tapia D, Laville A, Vergara R, Aceves J, Bargas J. Dopaminergic modulation of axon collaterals interconnecting spiny neurons of the rat striatum. J Neurosci. 2003;23:8931–8940. doi: 10.1523/JNEUROSCI.23-26-08931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton ME, Redondo JL, Freeman AS. Overflow of dopamine and cholecystokinin in rat nucleus accumbens in response to acute drug administration. Synapse. 2000;38:238–242. doi: 10.1002/1098-2396(20001201)38:3<238::AID-SYN2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. Neural systems for control of voluntary action--a hypothesis. Adv. Biophys. 1998;35:81–102. [PubMed] [Google Scholar]
- Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–309. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Hong S, Jhou TC, Smith M, Saleem KS, Hikosaka O. Negative reward signals from the lateral habenula to dopamine neurons are mediated by rostromedial tegmental nucleus in primates. J Neurosci. 2011;31:11457–11471. doi: 10.1523/JNEUROSCI.1384-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houk JC, Wise SP. Distributed modular architectures linking basal ganglia, cerebellum, and cerebral cortex: their role in planning and controlling action. Cereb. Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JNJ, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neurosci. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res. Brain Res. Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Kauer JA. Amphetamine depresses excitatory synaptic transmission via serotonin receptors in the ventral tegmental area. J Neurosci. 1999;19:9780–9787. doi: 10.1523/JNEUROSCI.19-22-09780.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Baratta MV, Yang A, Lee D, Boyden ES, Fiorillo CD. Optogenetic mimicry of the transient activation of dopamine neurons by natural reward is sufficient for operant reinforcement. PLoS ONE. 2012;7:e33612. doi: 10.1371/journal.pone.0033612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. Role of basal ganglia in behavioral learning. Neurosci Res. 1995;22:353–358. doi: 10.1016/0168-0102(95)00914-f. [DOI] [PubMed] [Google Scholar]
- Kleinlogel S, Feldbauer K, Dempski RE, Fotis H, Wood PG, Bamann C, Bamberg E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat Neurosci. 2011;14:513–518. doi: 10.1038/nn.2776. [DOI] [PubMed] [Google Scholar]
- Koob GF, Riley SJ, Smith SC, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi and olfactory tubercle on feeding, locomotor activity, and amphetamine anorexia in the rat. J Comp Physiol Psychol. 1978;92:917–927. doi: 10.1037/h0077542. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Kreitzer AC. Optogenetic manipulation of neural circuitry in vivo. Curr Opinion Neurobiol. 2011;21:433–439. doi: 10.1016/j.conb.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PRL, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neruosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J. Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Hetzel A, Häckel O, Jones I, Liss B, Roeper J. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008;57:760–773. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Lamont EW, Kokkinidis L. Infusion of the dopamine D1 receptor antagonist SCH 23390 into the amygdala blocks fear expression in a potentiated startle paradigm. Brain Res. 1998;795:128–136. doi: 10.1016/s0006-8993(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Lerner TN, Kreitzer AC. Neuromodulatory control of striatal plasticity and behavior. Curr Opinion Neurobiol. 2011;21:322–327. doi: 10.1016/j.conb.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima SQ, Hromádka T, Znamenskiy P, Zador AM. PINP: A new method of tagging neuronal populations for identification during in vivo electrophysiological recording. PLoS ONE. 2009;4:e6099. doi: 10.1371/journal.pone.0006099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeberg J, Usoskin D, Bengtsson H, Gustafsson A, Kylberg A, Sderstrm S, Ebendal T. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40:67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- Ljungberg T, Apicella P, Schultz W. Responses of monkey dopamine neurons during learning of behavioral reactions. J Neurophysiol. 1992;67:145–163. doi: 10.1152/jn.1992.67.1.145. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell Type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The laterodorsal tegmentum is essential for burst firing of ventral tegmental area dopamine neurons. Proc. Natl. Acad. Sci. U.S.a. 2006;103:5167–5172. doi: 10.1073/pnas.0510715103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–357. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Gallagher K, McGehee DS. Nicotine potentiation of excitatory inputs to ventral tegmental area dopamine neurons. J Neurosci. 2011;31:6710–6720. doi: 10.1523/JNEUROSCI.5671-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Chefer VI, Shippenberg TS, Hjelmstad GO, Fields HL. Kappa opioids selectively control dopaminergic neurons projecting to the prefrontal cortex. Proc. Natl. Acad. Sci. U.S.A. 2006a;103:2938–2942. doi: 10.1073/pnas.0511159103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006b;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Mitchell JM, Ishikawa J, Hjelmstad GO, Fields HL. Midbrain dopamine neurons: projection target determines action potential duration and dopamine D(2) receptor inhibition. J Neurosci. 2008;28:8908–8913. doi: 10.1523/JNEUROSCI.1526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area gabaergic neurons. PLoS ONE. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y. The presence of background dopamine signal converts long-term synaptic depression to potentiation in rat prefrontal cortex. J Neurosci. 2006;26:4803–4810. doi: 10.1523/JNEUROSCI.5312-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui A, Williams JT. Opioid-sensitive GABA inputs from rostromedial tegmental nucleus synapse onto midbrain dopamine neurons. J Neurosci. 2011;31:17729–17735. doi: 10.1523/JNEUROSCI.4570-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–841. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Beeler JA, Roitman MF. Sucrose-predictive cues evoke greater phasic dopamine release than saccharin-predictive cues. Synapse. 2011;66:346–351. doi: 10.1002/syn.21519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirenowicz J, Schultz W. Importance of unpredictability for reward responses in primate dopamine neurons. J Neurophysiol. 1994;72:1024–1027. doi: 10.1152/jn.1994.72.2.1024. [DOI] [PubMed] [Google Scholar]
- Montague PR. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. U.S.A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neurosci. 2008;152:1024–1031. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology. 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- Nicola SM. The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J Neurosci. 2010;30:16585–16600. doi: 10.1523/JNEUROSCI.3958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenberg MJ, Vaughan RA, Uhl GR, Kuhar MJ, Pickel VM. The dopamine transporter is localized to dendritic and axonal plasma membranes of nigrostriatal dopaminergic neurons. J. Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Stricker EM, Zigmond MJ, Berger TW. Long-term effects of dopamine-depleting brain lesions on spontaneous activity of type II striatal neurons: relation to behavioral recovery. Brain Res. 1986;398:221–230. doi: 10.1016/0006-8993(86)91481-2. [DOI] [PubMed] [Google Scholar]
- OLDS J, MILNER P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J Comp Physiol Psychol. 1954;47:419–427. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Bell R, Sesack SR. Lateral habenula projections to dopamine and GABA neurons in the rat ventral tegmental area. Eur J Neurosci. 2009;30:1239–1250. doi: 10.1111/j.1460-9568.2009.06924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owesson-White CA, Roitman MF, Sombers LA, Belle AM, Keithley RB, Peele JL, Carelli RM, Wightman RM. Sources contributing to the average extracellular concentration of dopamine in the nucleus accumbens. J Neurochem. 2012;121:252–262. doi: 10.1111/j.1471-4159.2012.07677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling as a neural correlate of consciousness. Neurosci. 2011;198:213–220. doi: 10.1016/j.neuroscience.2011.06.089. [DOI] [PubMed] [Google Scholar]
- Parkinson JA, Dalley JW, Cardinal RN, Bamford A, Fehnert B, Lachenal G, Rudarakanchana N, Halkerston KM, Robbins TW, Everitt BJ. Nucleus accumbens dopamine depletion impairs both acquisition and performance of appetitive Pavlovian approach behaviour: implications for mesoaccumbens dopamine function. Behav Brain Res. 2002;137:149–163. doi: 10.1016/s0166-4328(02)00291-7. [DOI] [PubMed] [Google Scholar]
- Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2011;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- Pawlak V, Kerr JND. Dopamine receptor activation is required for corticostriatal spike-timing-dependent plasticity. J Neurosci. 2008;28:2435–2446. doi: 10.1523/JNEUROSCI.4402-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault ML, Hasbi A, O’Dowd BF, George SR. The dopamine D1–D2 receptor heteromer in striatal medium spiny neurons: evidence for a third distinct neuronal pathway in basal ganglia. Front. Neuroanat. 2011;5:31. doi: 10.3389/fnana.2011.00031. doi:10.3389/fnana.2011.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- Phillips GD, Salussolia E, Hitchcott PK. Role of the mesoamygdaloid dopamine projection in emotional learning. Psychopharmacology. 2010;210:303–316. doi: 10.1007/s00213-010-1813-z. [DOI] [PubMed] [Google Scholar]
- Phillips PEM, Stuber GD, Heien MLAV, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J. Comp. Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Prensa L, Parent A. The nigrostriatal pathway in the rat: A single-axon study of the relationship between dorsal and ventral tier nigral neurons and the striosome/matrix striatal compartments. J Neurosci. 2001;21:7247–7260. doi: 10.1523/JNEUROSCI.21-18-07247.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimondo JV, Kay L, Ellender TJ, Akerman CJ. Optogenetic silencing strategies differ in their effects on inhibitory synaptic transmission. Nat Neruosci. 2012;15(8):1102–4. doi: 10.1038/nn.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Vautrelle N, Reynolds JNJ. Functional properties of the basal ganglia’s re-entrant loop architecture: selection and reinforcement. Neurosci. 2011;198:138–151. doi: 10.1016/j.neuroscience.2011.07.060. [DOI] [PubMed] [Google Scholar]
- Reynolds JNJ, Wickens JR. Dopamine-dependent plasticity of corticostriatal synapses. Neural Netw. 2002;15:507–521. doi: 10.1016/s0893-6080(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Richfield EK, Penney JB, Young AB. Anatomical and affinity state comparisons between dopamine D1 and D2 receptors in the rat central nervous system. Neurosci. 1989;30:767–777. doi: 10.1016/0306-4522(89)90168-1. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Functions of dopamine in the dorsal and ventral striatum. Sem Neurosci. 1992;4:119–127. [Google Scholar]
- Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: commentary on Berridge (2006) Psychopharmacology. 2007;191:433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- Roesch MR, Calu DJ, Schoenbaum G. Dopamine neurons encode the better option in rats deciding between differently delayed or sized rewards. Nat Neurosci. 2007;10:1615–1624. doi: 10.1038/nn2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF. Dopamine operates as a subsecond modulator of food seeking. J Neurosci. 2004;24:1265–1271. doi: 10.1523/JNEUROSCI.3823-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Wheeler RA, Wightman RM, Carelli RM. Real-time chemical responses in the nucleus accumbens differentiate rewarding and aversive stimuli. Nat Neurosci. 2008;11:1376–1377. doi: 10.1038/nn.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine-mediated modulation of odour-evoked amygdala potentials during pavlovian conditioning. Nature. 2002;417:282–287. doi: 10.1038/417282a. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Saunders BT, Robinson TE. The role of dopamine in the accumbens core in the expression of Pavlovian-conditioned responses. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu. Rev. Neurosci. 2007;30:259–288. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Funct. 2010;6:24. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annu. Rev. Neurosci. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, Ljungberg T. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J. Neurosci. 1993;13:900–913. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Ceccatelli S, Schalling M, Hökfelt T, Frey P, Walsh J, Dockray G, Brown J, Buchan A, Goldstein M. A subpopulation of dopaminergic neurons in rat ventral mesencephalon contains both neurotensin and cholecystokinin. Brain Res. 1988;455:88–98. doi: 10.1016/0006-8993(88)90117-5. [DOI] [PubMed] [Google Scholar]
- Seroogy K, Schalling M, Brené S, Dagerlind A, Chai SY, Hökfelt T, Persson H, Brownstein M, Huan R, Dixon J. Cholecystokinin and tyrosine hydroxylase messenger RNAs in neurons of rat mesencephalon: peptide/monoamine coexistence studies using in situ hybridization combined with immunocytochemistry. Exp Brain Res. 1989;74:149–162. doi: 10.1007/BF00248288. [DOI] [PubMed] [Google Scholar]
- Seroogy KB, Gall CM. Expression of neurotrophins by midbrain dopaminergic neurons. Exp. Neurol. 1993;124:119–128. doi: 10.1006/exnr.1993.1182. [DOI] [PubMed] [Google Scholar]
- Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Sombers LA, Beyene M, Carelli RM, Mark Wightman R. Synaptic overflow of dopamine in the nucleus accumbens arises from neuronal activity in the ventral tegmental area. J Neurosci. 2009;29:1735–1742. doi: 10.1523/JNEUROSCI.5562-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparta DR, Stamatakis AM, Phillips JL, oslash NH, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protocols. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat Neurosci. 2012;15(8):1105–7. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker RE, Jacobs BL. Substantia nigra dopaminergic unit activity in behaving cats: effect of arousal on spontaneous discharge and sensory evoked activity. Brain Res. 1985;361:339–350. doi: 10.1016/0006-8993(85)91304-6. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. doi: 10.1523/JNEUROSCI.1754-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Song WJ, Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16:6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res. Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Tai L-H, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in the value of competing actions. Nat Neurosci. 2012;15(9):1281–9. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouèbe G, Deisseroth K, Tye KM, Lüscher C. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73:1173–1183. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecuapetla F, Patel JC, Xenias H, English D, Tadros I, Shah F, Berlin J, Deisseroth K, Rice ME, Tepper JM, Koos T. Glutamatergic signaling by mesolimbic dopamine neurons in the nucleus accumbens. J Neurosci. 2010;30:7105–7110. doi: 10.1523/JNEUROSCI.0265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307:1642–1645. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Tong ZY, Overton PG, Clark D. Stimulation of the prefrontal cortex in the rat induces patterns of activity in midbrain dopaminergic neurons which resemble natural burst events. Synapse. 1996;22:195–208. doi: 10.1002/(SICI)1098-2396(199603)22:3<195::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Ding JB, Sabatini BL. Dopaminergic neurons inhibit striatal output via non-canonical release of GABA. Society for Neuroscience. 2012 doi: 10.1038/nature11466. abstract 87.07/OO17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, Deisseroth K. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012;13(4):251–66. doi: 10.1038/nrn3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim S-Y, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Tye LD, Cone JJ, Hekkelman EF, Janak PH, Bonci A. Methylphenidate facilitates learning-induced amygdala plasticity. Nat Neurosci. 2010;13:475–481. doi: 10.1038/nn.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Grace AA. Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 2012;35(7):422–30. doi: 10.1016/j.tins.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP. Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science. 2004;303:2040–2042. doi: 10.1126/science.1093360. [DOI] [PubMed] [Google Scholar]
- van Zessen R, Phillips JL, Budygin EA, Stuber GD. Activation of VTA GABA neurons disrupts reward consumption. Neuron. 2012;73:1184–1194. doi: 10.1016/j.neuron.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PEM, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ. NMDA Receptors in Dopaminergic Neurons Are Crucial for Habit Learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassum KM, Ostlund SB, Maidment NT. Phasic mesolimbic dopamine signaling precedes and predicts performance of a self-initiated action sequence task. Biol Psychiatry. 2012;71:846–854. doi: 10.1016/j.biopsych.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu L, Ogawa SK, Vamanrao A, Uchida N. Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron. 2012;74:858–873. doi: 10.1016/j.neuron.2012.03.017. [DOI] [PubMed] [Google Scholar]
- White NM, Milner PM. The psychobiology of reinforcers. Annu. Rev. Psychol. 1992;43:443–471. doi: 10.1146/annurev.ps.43.020192.002303. [DOI] [PubMed] [Google Scholar]
- Wheeler RA, Aragona BJ, Fuhrmann KA, Jones JL, Day JJ, Cacciapaglia F, Wightman RM, Carelli RM. Cocaine cues drive opposing context-dependent shifts in reward processing and emotional state. Biol Psychiatry. 2011;69:1067–1074. doi: 10.1016/j.biopsych.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Horvitz JC, Costa RM, Killcross S. Dopaminergic mechanisms in actions and habits. J Neurosci. 2007;27:8181–8183. doi: 10.1523/JNEUROSCI.1671-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JNJ, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opinion Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Wickersham IR, Lyon DC, Barnard RJO, Mori T, Finke S, Conzelmann K-K, Young JAT, Callaway EM. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron. 2007;53:639–647. doi: 10.1016/j.neuron.2007.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Wise RA. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2006;361:1149–1158. doi: 10.1098/rstb.2006.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten IB, Steinberg EE, Lee SY, Davidson TJ, Zalocusky KA, Brodsky M, Yizhar O, Cho SL, Gong S, Ramakrishnan C, Stuber GD, Tye KM, Janak PH, Deisseroth K. Recombinase-driver rat lines: tools, techniques, and optogenetic application to dopamine-mediated reinforcement. Neuron. 2011;72:721–733. doi: 10.1016/j.neuron.2011.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Driscoll JR, Wilbrecht L, Margolis EB, Fields HL, Hjelmstad GO. Nucleus Accumbens Medium Spiny Neurons Target Non-Dopaminergic Neurons in the Ventral Tegmental Area. J Neurosci. 2011;31:7811–7816. doi: 10.1523/JNEUROSCI.1504-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Sheen W, Morales M. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Wang H-L, Li X, Ng TH, Morales M. Mesocorticolimbic glutamatergic pathway. J Neurosci. 2011;31:8476–8490. doi: 10.1523/JNEUROSCI.1598-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Electrophysiological studies of neurons in the ventral tegmental area of Tsai. Brain Res. 1980;181:301–313. doi: 10.1016/0006-8993(80)90614-9. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011a;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011b;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahm DS, Cheng AY, Lee TJ, Ghobadi CW, Schwartz ZM, Geisler S, Parsely KP, Gruber C, Veh RW. Inputs to the midbrain dopaminergic complex in the rat, with emphasis on extended amygdala-recipient sectors. J. Comp. Neurol. 2011;519:3159–3188. doi: 10.1002/cne.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetterström T, Herrera-Marschitz M, Ungerstedt U. Simultaneous measurement of dopamine release and rotational behaviour in 6-hydroxydopamine denervated rats using intracerebral dialysis. Brain Res. 1986;376:1–7. doi: 10.1016/0006-8993(86)90893-0. [DOI] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat. Rev. Neurosci. 2007a;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia. 2012;2:5–13. doi: 10.1016/j.baga.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang L-P, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007b;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhou F-M, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA Receptors in Dopamine Neurons for Plasticity and Addictive Behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJY, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweifel LS, Fadok JP, Argilli E, Garelick MG, Jones GL, Dickerson TMK, Allen JM, Mizumori SJY, Bonci A, Palmiter RD. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011;14:620–626. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]