Abstract
Given the rapid rate of population aging and the increased incidence of cognitive decline and neurodegenerative diseases with advanced age, it is important to ascertain the determinants that result in cognitive impairment. It is also important to note that some many of the aged population exhibit ‘successful’ cognitive aging, in which cognitive impairment is minimal. One main goal of normal aging studies is to distinguish the neural changes that occur in unsuccessful (functionally impaired) subjects from those of successful (functionally unimpaired) subjects. In this review, we present some of the structural adaptations that neurons and spines undergo throughout normal aging and discuss their likely contributions to electrophysiological properties and cognition. Structural changes of neurons and dendritic spines during aging, and the functional consequences of such changes, remain poorly understood. Elucidating the structural and functional synaptic age-related changes that lead to cognitive impairment may lead to the development of drug treatments that can restore or protect neural circuits and mediate cognition and successful aging.
Introduction
Aging is a normal physiological process causing changes in neuronal circuitry and, in some individuals, resulting in impaired cognition and behavior. A common misconception about brain aging is that the functional decline seen in some individuals is simply a reflection of significant neuronal death. Rigorous quantitative stereologic studies in rat, rhesus monkey, and human have demonstrated minimal, if any, loss of either excitatory or inhibitory neurons in some neocortical regions and in the hippocampus during normal aging (Hof and Morrison, 2004, Morrison and Baxter, 2012). However, other studies have demonstrated that some neuronal loss occurs with aging in brain regions such as the cerebellum and substantia nigra (Cabello et al., 2002, Andersen et al., 2003, Woodruff-Pak et al., 2010). Thus, the mechanisms that distinguish age-related cognitive and behavioral deficits arising from multiple brain regions from functionally unimpaired aging remain to be elucidated. Research has focused on the many factors that may contribute to the breakdown of neuronal circuits. Various subtle structural changes in neurons and spines have been found to occur in the brain during normal aging, as well as alterations in neurotransmitter receptors and changes in electrophysiological properties (Nakamura et al., 1985, Barnes, 1994, Jacobs et al., 2001, Hof et al., 2002, Duan et al., 2003, Chang et al., 2005). Since spines are the principal sites of glutamatergic synapses and of forms of synaptic plasticity such as long-term potentiation and long-term depression, they likely are important to learning and memory. Hence, loss of spines or changes in the proportion of spine types and distributions along the dendritic shafts may affect synaptic events critical to cognition. This review will discuss the various morphological and functional changes that neurons and spines undergo in the context of aging as a whole, and the association of these changes to neural circuitry, connectivity, and cognition.
Age-related deficits in cognitive function mediated by the prefrontal cortex
The prefrontal cortex (PFC) is responsible for mediation of complex executive functions such as working memory, planning, and goal-directed behavior (for review see (Funahashi and Takeda, 2002, Watanabe and Sakagami, 2007, Chudasama, 2011)), and is most developed in non-human primates and in humans. Because individuals that exhibit age-related cognitive decline tend to show impairments of these executive functions first, it has been postulated that neurons and circuits of the PFC may be particularly vulnerable during normal aging (humans: (Albert, 1993, Salthouse et al., 2003, Fisk and Sharp, 2004, Rhodes, 2004, Rodriguez-Aranda and Sundet, 2006, Sorel and Pennequin, 2008); non-human primates: (Bartus et al., 1979, Rapp, 1990, Lai et al., 1995, Herndon et al., 1997, Steere and Arnsten, 1997, Voytko, 1999, Moore et al., 2003, Moore et al., 2005, Moore et al., 2006). In the rhesus monkey, spatial and object visual reversal tasks have been used predominately to assess executive function. Performance on these tasks provides a metric for cognitive flexibility by testing the ability of the monkey to set-shift from an original stimulus-reinforcement pair to a novel stimulus-reinforcement pair. Several impairments on reversal learning have been observed in aged rhesus monkeys over the years, first as an inability to set-shift and by increased perseveration (Bartus et al., 1979). Increased training time during the initial task acquisition phase was observed in aged macaque monkeys in one study (Rapp et al., 1990). Impairment performance on spatial reversals, but neither object reversals nor task acquisition, was reported in a later study (Lai et al., 1995). However, in agreement with previous work, an increased tendency for perseveration on both spatial and object reversals was observed (Lai et al., 1995). Overall, these findings are consistent with the view that attention deficits likely contribute significantly to cognitive impairment in aged monkeys.
In a comprehensive study of cognitive changes during aging, Herndon et al. (1997) employed a battery of behavioral tests to assess cognitive and mnemonic function in rhesus monkeys at early (19–23 years old), advanced (24–28 years old) and oldest (≥29 years old) stages of aging versus that of young monkeys. With the exception of the delayed recognition span test (DRST)-color, aged groups as a whole demonstrated a significant decline in cognitive performance on all tests. Of these tests, the highest degree of impairment was observed in the DRST in both the early-aged and the oldest- aged monkeys. Thus, deficits in spatial memory were pronounced in aged cohorts. Moreover, impairment rate was shown to increase progressively with age in a number of tests, including the Cognitive Impairment Index (CII); DRST-color condition task; spatial reversal learning tasks; and delayed non-matching-to-sample (DNMS) acquisition task (Herndon et al., 1997). In the DNMS, an animal is first presented with a sample stimulus. After a delay, the sample is shown again along with a novel stimulus; the animal is rewarded for recognizing the new stimulus. The Conceptual Set Shifting Task, a task analogous to the Wisconsin Card Sorting Test employed in human studies, was designed to examine set shifting, in addition to abstraction and concept formation in non-human primates. Performance in both the acquisition and test phases of this task have been shown to be significantly impaired in aged monkeys, and as in visual reversal tasks, aged monkeys as a group displayed increased perseverative tendencies compared to young monkeys (Moore et al., 2005). Performance in the DNMS task, which tests for explicit learning and memory, has also been correlated to changes in spines and synapse densities as well as in synaptic receptor expression and localization (discussed in detail below).
Behavioral data consistently reveal that while considerable deficits in executive functioning can occur in a proportion of monkeys early during the aging process (and become progressively more pronounced with age), many aged monkeys do not show deficits in cognitive performance relative to young monkeys (Rapp, 1990, Rapp and Amaral, 1991, Baxter and Voytko, 1996, Moore et al., 2006). Aged monkeys that show no cognitive impairment can then be assumed to undergo ‘successful’ aging, as opposed to ‘unsuccessful’ aging. Therefore, ‘unsuccessful’ and ‘successful’ aging occurs in non-human primates just as it does in humans. The key question in studies of normal aging, then, is ‘What are the neural changes that occur in subjects that are unsuccessful (functionally impaired) but not in successful (functionally unimpaired) subjects?’ Given that neurons are not substantially lost in many brain regions, it is certainly plausible that detrimental changes to the structural properties of neurons and synapses could be, at least in part, responsible for cognitive decline.
Changes in neuronal morphology with age
As a context for changes in spines with aging, first we briefly review quantitative studies of age-related changes in the morphology of neurons that possess dendritic spines. The dynamics of dendritic trees are of high importance, as they integrate information from thousands of excitatory and hundreds of inhibitory synaptic inputs from within the same region of the brain and between regions. Pyramidal neurons of the neocortex and the hippocampus possess extensive apical and basilar dendritic trees, which undergo significant changes across the lifespan. Many studies have demonstrated that with increasing age, and under non-pathological conditions, dendritic trees undergo progressive regression in dendritic arbors of pyramidal neurons located in the prefrontal, superior temporal, and precentral cortices in humans (Scheibel et al., 1975, Nakamura et al., 1985, de Brabander et al., 1998), nonhuman primates (Peters et al., 1998, Page et al., 2002, Duan et al., 2003, Kabaso et al., 2009), aged dogs (Mervis, 1978), and mice (Shimada et al., 2006), as well as in Purkinje cells of the cerebellar cortex (Zhang et al., 2006).
Neocortex
Non-human primates represent an ideal animal model for studying normal aging (reviewed in (Luebke et al., 2010), and a number of investigators have examined the morphological, physiological and biochemical changes that occur with age in the neocortex of rhesus monkeys. Cupp and Uemura (1980) found that in Golgi-stained sections of area 46 entire segments and branches from apical dendrites were lost with age (Cupp and Uemura, 1980). This aging effect is not limited to the prefrontal cortex (PFC), considering that similar declines in dendritic neuropil are also observed in the motor (Nakamura et al., 1985), frontopolar (area 10), and occipital (area 18) cortices (Jacobs et al., 1997). These observations were confirmed by ultrastructural electron microscopic analysis of areas 46 and 17 that also showed a reduction in number of apical dendritic tuft branches from pyramidal neurons (Peters et al., 1998, Peters et al., 2001). More recent investigations of corticocortically projecting neurons in Patas monkeys and several species of macaque monkeys, revealed reduced complexity of the apical dendrites in aged compared to young monkeys (Page et al., 2002, Duan et al., 2003). While a minor decrease in the length of dendritic segments in aged animals was observed, the outcome was not statistically significant (Page et al., 2002). Duan et al. (2003) extended these findings and did find a significant regression of apical dendrites of neocortical pyramidal neurons in aged macaque monkeys compared to young animals. Sholl analysis of these neurons revealed a reduction in the number of dendrites extending from 140 μm to 180 μm from the soma with significant decreases in dendritic length and segment numbers at second branch order apical dendrites. Overall, these results showed a 25% reduction in both apical and basal dendrites in aged animals compared to controls (Duan et al., 2003). Recently, Kabaso et al. (2009) examined the structural changes in two populations of layer 3 pyramidal neurons of the neocortex of rhesus monkey: ‘long-projecting’ neurons from the superior temporal cortex that project to the PFC, and ‘local-projecting’ neurons within the PFC. Interestingly, this study found that apical dendrites of long-projecting neurons were shorter and less complex with age, while dendrites of local-projecting neurons remained unaltered (summarized in Figure 1A).
Figure 1.
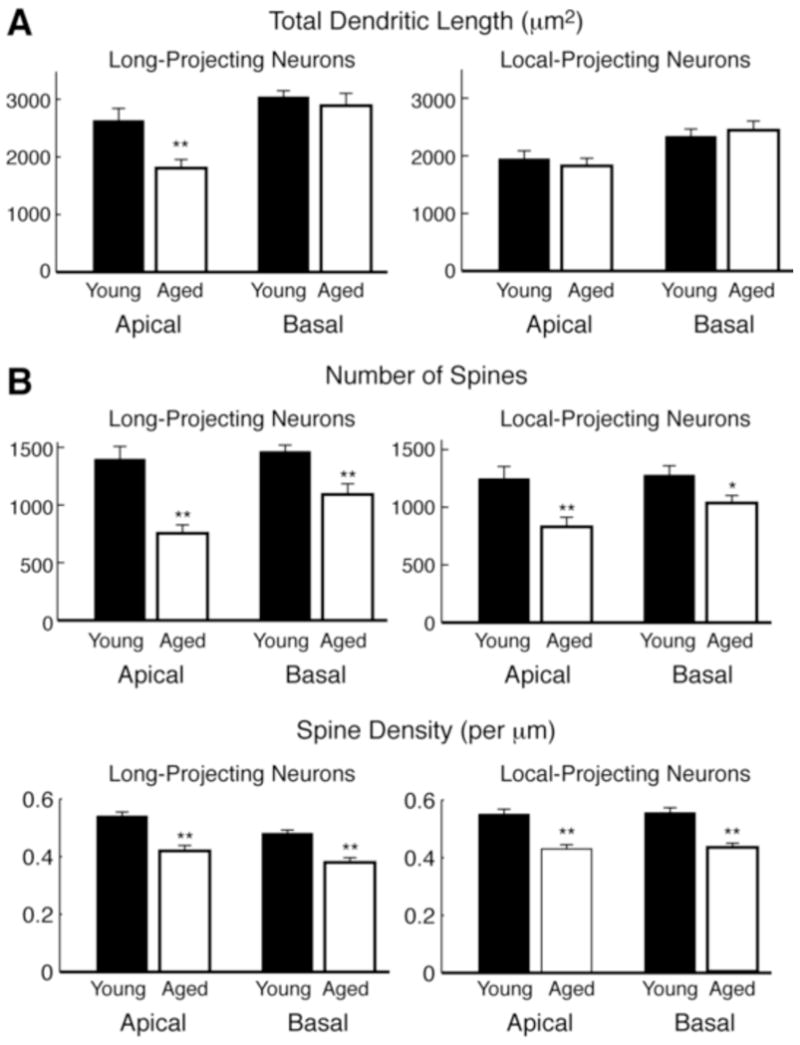
Age-related morphologic changes of layer 3 neocortical pyramidal neurons forming long and local projections in rhesus monkeys. (A) Total length of dendrites from the apical and basal arbors of long projection (from superior temporal cortex to PFC) and locally projecting neurons (within PFC). (B) The total number of spines per neuron (top) and average spine density (bottom) on apical and basal dendrites of long projection and locally projecting neurons. Data from young and aged monkeys are shown in black and white respectively. (*p < 0.05; **p < 0.01). Data summarized from Kabaso et al. (2009).
Hippocampus
A number of key insights into the effects of aging on neuronal morphology in the hippocampus have been gained from rodent studies; however, results have been inconsistent. Early studies looking at hippocampal CA1 neurons in aged rats revealed changes in neuronal morphology. Pyapali and Turner (1996) found that, compared to young rats, aged rats exhibited a variable but overall increase in total neuronal dendritic length, in particular basal length differed compared to apical length. These changes were thought to be attributed to compensatory synaptic reorganization that occurs in successful aging since only 39% of the aged rats exhibited impairment in spatial memory (Pyapali and Turner, 1996). This lack of dendritic regression has also been observed in humans during normal aging where there was a net stability of dendritic extent in CA2/3, CA1, and subiculum (Flood and Coleman, 1990). In contrast, Markham et al. (2005) found age-related regression of apical dendrites with age in CA1 neurons with significant decrease in dendritic complexity in aged (20–24 months) male rats compared to young (3–5 months) male rats. Interestingly, there were no evident changes neuronal in complexity with age in aged female rats (Markham et al., 2005). Senescence-accelerated mice (SAM), an established murine model of aging (Hanada et al., 1991), have also demonstrated results similar to those seen in non-human primates. Senescence-prone strains (SAMP), in particular SAMP10 mice, display a gradual retraction of apical dendrites with relative preservation of overall complexity in the PFC with no alterations occurring in basal dendrites (Shimada et al., 2006). At 12 months of age the total length of apical dendrites decreases by 45% and had a decline in stem thickness compared to 3 months old mice.
Subcortical structures
Several subcortical regions also undergo dendritic regression with aging. In cerebellum, studies in aged cats and rats have revealed a lower density of neurons in the cerebellar cortex and granular layer (Zhang et al., 2006). Retraction of Purkinje cell dendritic arbors accompanied by atrophy of their somas have also been reported (Graham and van Ooyen, 2006). Purkinje cell dendrites also exhibit many vacuolar profiles, tortuous dendrites, and membrane swirls (Chen and Hillman, 1999), and substantial loss of distal branches (Quackenbush et al., 1990). Similarly, a study in human substantia nigra showed distorted somas and pronounced dendritic varicosities in aged subjects, in addition to significant dendrite loss (Cruz-Sanchez et al., 1995). One study in the cat striatum found that total and mean dendrite length both decreased by about 40% in aged animals (Levine et al., 1986), although the number of dendrites was unchanged. An exception to the pattern of age-related dendritic regression occurs in amygdala (Rubinow et al., 2009), in which dendrite length increased by 14% in aged rats.
Altogether these studies point to notable morphological alterations and loss of dendritic length that occur in neurons in several brain areas (summarized in Table 1). However, it is highly unlikely that these changes alone can completely account for the impairment of memory function observed in some individuals. For example, data suggest that extensive myelin alterations alter the timing of synaptic transmission (reviewed in (Peters, 2002, Luebke et al., 2010). Rather, cognitive impairment most likely arises from an interaction between sub-lethal changes at the circuit, cellular, and subcellular levels.
Table 1.
Summary of aging studies
| Brain Region | Neuron Type | Species | Gender | Dendritic Regression | Spine Density | Cognitive Decline | Reference |
|---|---|---|---|---|---|---|---|
| PFC | pyramidal | Mouse | male | ~45% decrease in apical length; preservation of complexity | (Shimada et al., 2006) | ||
| PFC | pyramidal | Macaque monkey | 25% reduction of apical dendrites; decrease in complexity | 43% decrease on proximal apical dendrites; 27% loss in basal dendrites | (Duan et al., 2003) | ||
| PFC | Rhesus monkey | 25% loss of spines | (Cupp and Uemura 1980) | ||||
| PFC | pyramidal | Rhesus monkey | 33% loss of spines, with a 50% loss of thin spines; 32% decrease in axospinous synapses | impaired performance on the DNMS task | (Dumitriu et al., 2010) | ||
| Hippocampus (DG) | Rat | reduction in axospinous and perforated synapses | impaired in hippocampus-dependent spatial memory tasks | (Geinisman et al. 1986, 1992) | |||
| Hippocampus (OML of DG) | Rhesus monkey | reduction in perforated synapses | impaired performance on the DNMS task | (Hara et al., 2011) | |||
| Hippocampus (MML of DG; stratum radiatum of CA1) | Rat | male | no change in synapse densities | (Shi et al., 2007; Newton et al., 2008) | |||
| Hippocampus (stratum radiatum in CA1) | pyramidal | Rat | male | regression of apical dendrites; decrease in complexity | no change in spine density, in either sex | (Markham et al., 2005) | |
| female | No change in neuronal complexity | ||||||
| Hippocampus (CA3) | Rat | 24% decrease in synapses | (De Groot and Bierman, 1987) | ||||
| Hippocampus (CA3, stratum lacunosum-moleculare) | Rat | male | decrease in macular axospinous synapses; no change in size | (Adams et al., 2010) | |||
| Cerebellum | Purkinje | Rat | retraction of dendritic arbors; atrophy of soma | (Chen and Hillman, 1999) (Graham and van Ooyen, 2006) | |||
| Cerebellum | Purkinje cells | Rat | male | 33% loss in axospinous synapses | (Glick et al., 1979) | ||
| Cerebellum | Purkinje cells | Rat | male | dendritic regression, especially distal | 17% lower spine density | (Rogers et al., 1984) | |
| Striatum (caudate) | medium-sized spiny | Cat | ~40% decrease in total dendrite length and mean branch length | 40% loss of distal spines; higher proportion of ‘enlarged-head’ spines | (Levine et al., 1986) | ||
| Striatum; Hypothalamus | Rat | male | axospinous synapses fewer in number, but with greater mean area | (Itzev et al., 2001, 2003) | |||
| Substantia Nigra | Types I, II, III | Human | Distorted cell body; varicosities in and loss of dendrites | Types I-II: severe spine loss; remaining spines thinner and longer | (Cruz-Sanchez et al., 1995) | ||
| Amygdala | bitufted and multipolar | Rat | male | spine density unchanged with age | (Marcuzzo et al., 2007) | ||
| Amygdala | principal neurons | Rat | increased dendritic arborization | spine density unchanged with age | (Rubinow et al., 2009) |
Changes in spines with aging
In neuronal circuits, the vast majority of excitatory synaptic transmission occurs at the postsynaptic dendritic spines. Changes in spine size, shape, and density are thought to reflect changes in normative glutamatergic synaptic transmission and in synaptic plasticity that occur during development and aging. It is plausible that such structural changes may lead to perturbations in function and impairments in memory and learning (Dumitriu et al., 2010, Hara et al., 2011, Morrison and Baxter, 2012), but a causal link has not yet been shown. While significant attrition of neuronal dendrites has been observed throughout the aging brain, spine pathologies with aging are less uniform. The degree of spine loss varies by brain region, as do the types of spines and synapses most vulnerable to aging. These changes are reviewed below, and summarized in Table 1.
Neocortex
In addition to subtle changes in neuron morphology in the non-human primate studies of the PFC described above, significant changes in spine density and morphology with aging were also reported. Using Golgi-stained sections, Cupp and Uemura (1980) found that in pyramidal neurons from aged rhesus monkeys that exhibited a loss of dendritic branches, there was also an approximate 25% loss of spines. These findings were supported by electron microscopic examination of areas 46 (Peters et al., 1998) and 17 (Peters et al., 2001) which demonstrated a loss of spines and a 30% reduction in the number of excitatory (asymmetric) synapses. As a different approach, we have used retrograde tract tracing of neocortical pyramidal neurons filled with the dye Lucifer Yellow followed by high-resolution 3-dimensional reconstruction to investigate spine changes in aging (Figure 2). Changes in dendritic spines were found along all levels of dendrites analyzed, with a decreased number of total spines between 28–37% in the basal and apical dendrites of aged animals compared to young animals, and a 23% decrease in spine densities (Page et al., 2002). Duan et al. (2003) confirmed these results and found an approximately 43% decrease in the number of spines on proximal apical dendrites, whereas basal dendritic spine loss was estimated at 27% and occurred primarily on distal branches. Kabaso et al. (2009) also found a significant decrease in spine densities of apical and basal dendrites, in neurons forming both long and local projections (Figure 1B).
Figure 2.

Representative dendritic segments and spines from layer 3 pyramidal neurons from PFC of young (left) and aged (right) rhesus monkeys. Top row shows segments from the apical arbor; bottom row from the basal arbor. These are xy-projections of confocal laser scanning microscopy images (40X, 1.5 digital zoom), after deconvolution.
Hippocampus
Initial studies by Geinisman and colleagues (1986) using behaviorally characterized aged rats and electron microscopy showed that animals impaired in hippocampus-dependent spatial memory tasks had a reduced density of perforated synapses in the dentate gyrus (DG), while rats with intact spatial memory had no changes in these types of synapses. A follow-up stereologic study from the same group reported a 20% decline in the number of axospinous synapses in the middle and inner molecular layer of the DG from (young) 5- to (aged) 28-month old rats (Geinisman et al., 1992). More recent studies in aged rhesus monkeys corroborate these findings, and found a reduction in the number of perforated synapses in the outer molecular layer of the DG (Hara et al., 2012a). These changes also correlated with impaired performance on the DNMS task. However, studies evaluating caloric restriction on the aging process found no changes in synapse densities across lifespan in the middle molecular layer of the DG (Newton et al., 2008) or in the striatum radiatum of the CA1 region (Shi et al., 2007), in either the control or caloric restriction groups. Studies in the CA3 region of the hippocampus, a region thought to be uniquely sensitive and vulnerable to aging, also show changes in spines and synapses. An early stereologic study reported a 24% overall decrease in CA3 synapses in rats between 3 and 12 months of age (De Groot and Bierman, 1987). More recently, Adams et al. (2010) found age-related synapse loss in the stratum lacunosum-moleculare of the CA3 region (Adams et al., 2010). This observation can be corroborated to previous studies which have shown significantly lower levels of synaptophysin in aged, learning-impaired rats compared to either young or aged unimpaired rats (Smith et al., 2000).
Subcortical structures
While studied most often in the neocortex and hippocampus, the effects of aging on dendritic spines have also been examined in the cerebellum and other subcortical structures. In cerebellar Purkinje cells of the rat, spine density decreases with aging. Glick and Bondareff (1979) reported a 33% loss of axospinous synapses (Glick and Bondareff, 1979) of granule cells onto Purkinje cells in 25-month versus 12-month old rats. Similarly, Rogers et al. (1984) found that Purkinje cell spine density decreased 17% in 26-month versus 6-month old rats.
In the caudate region of the striatum of cat, spine density of distal dendrites decreased with aging, to a total loss of about 50% by 15–18 years of age (Levine et al., 1986). Changes in spine density began to occur before any changes in dendritic morphology (described above). The same study also reported that the relative proportion of spine shapes was changed with aging: 20–30% of dendritic spines in aged cats had enlarged heads (and therefore were presumably mushroom), higher than the 5% proportion observed in 1–3 year old cats. Consistent with these findings, Itzev et al. (2001) published an electron microscopy study in the rat neostriatum in which aged animals had fewer spines, but that the mean area of spines increased. The same research group also studied aging synapses in rat hypothalamus (Itzev et al., 2003), with similar results: reduced spine density in aged animals, with increased mean area of individual spines. In the substantia nigra of human, severe spine loss was observed in aged (70–91 years) compared to middle-aged subjects (Cruz-Sanchez et al., 1995). Yet in contrast to striatum, remaining spines of the aged subjects were thinner and more elongated than those in the younger groups.
Interestingly, in principal neurons of the rat amygdala, two different studies report no change in spine density (Marcuzzo et al., 2007, Rubinow et al., 2009). Thus, the age-related increase of dendrite length and unchanged spine densities reported in amygdala oppose results from the brain regions studied most commonly in aging. Evidence that amygdalar function is unimpaired with aging (reviewed in (Leigland et al., 2004)) is consistent with these morphometric studies.
To date, many studies have revealed spine changes in normal aging, and many have demonstrated cognitive changes, but direct correlations between spine loss and cognitive decline are lacking. Moreover, it still remains to be determined if this spine loss is global or if it is limited to specific spine types. This issue was recently addressed by assessing spine changes in layer 3 PFC pyramidal neurons from behaviorally characterized rhesus monkeys (Dumitriu et al., 2010). These investigators found a 33% age-related loss of spines on pyramidal cells and a 32% decrease in the density of axospinous synapses. In addition, morphometric analysis of spines showed a specific loss of almost 50% of thin spines on these neurons during normal aging, accounting for almost all of the total spine loss. By contrast, other morphological spine subtypes (mushroom and stubby) were reported to be relatively spared. These results are consistent with those of aging striatum and hypothalamus described above. Given that thin spines contain synapses that are highly plastic, it is possible that loss of this spine type in particular could negatively affect synaptic plasticity events essential to normal cognition (Morrison and Baxter, 2012). Indeed, a strong correlation between thin spine density and the ability of aged monkeys to learn a DNMS task was found (Dumitriu et al., 2010). Further studies which quantify spine density, spine subtype densities, and spatial distributions in cognitively-tested animals are clearly needed to determine whether this finding is ubiquitous in different neuron classes, areas of the aging brain, and different types of executive function.
Age-related alterations in glutamatergic synaptic events associated with dendritic spines
Empirical and computational modeling studies have revealed that the morphological properties of neurons play an important role in determining their electrotonic properties, which are in turn fundamental determinants of synaptic integration and neural firing patterns (Mainen and Sejnowski, 1996, Euler and Denk, 2001, Vetter et al., 2001, Krichmar et al., 2002, Weaver and Wearne, 2008, Amatrudo et al., 2012). Dendritic spines are the locus of the vast majority of excitatory glutamatergic synapses onto neocortical pyramidal cells. Thus, the significant age-related dendritic regression and loss of dendritic spines would be expected to have a major impact on the functional properties of individual neurons. It has often been postulated that age-related changes in dendritic spines likely lead to changes in excitatory synaptic signaling in aged neurons. To date however, while many studies have shown that dendritic spines are lost in the neocortex, none have correlated this loss of spines directly with altered electrophysiological properties of the same neurons; thus the impact of spine changes on synaptic function is currently entirely speculative. For example, it is reasonable to propose that spine loss likely leads to decreased excitatory synaptic responses or that selective loss of thin spines leads to reduced synaptic plasticity (e.g., (Morrison and Baxter, 2012), but empirical evidence for these hypotheses is not yet available. Thus, further empirical and computational modeling studies are urgently needed to determine the functional consequences of spine loss to basic synaptic signaling as well as to synaptic plasticity changes in the neocortex during normal aging.
There is electrophysiological evidence for altered synaptic transmission during normal aging, and that such alterations plausibly could be due to changes in the density of dendritic spines. In the only study to directly assess glutamatergic synaptic signaling in neocortical pyramidal cells during normal aging, Luebke et al. (2004) reported a significant age-related decrease in the frequency of non-N-methyl-D-aspartate (NMDA)-receptor mediated synaptic currents in layer 3 pyramidal neurons (in which other studies showed have a reduced spine density, see above) in the monkey prefrontal cortex. These spontaneous excitatory postsynaptic currents (PSCs) represent the postsynaptic cells’ response to both action potential-dependent and action potential-independent release of glutamate from presynaptic nerve terminals. This decrease could be due to reduced glutamate release from presynaptic nerve terminals or to a reduction of postsynaptic structures, the majority of which are dendritic spines. Given that there is a reported increase in excitability (action potential firing rates) of layer 3 neurons of the aged monkey PFC (Chang et al., 2005), the latter possibility seems more likely. That said, a significant decrease in excitability and release of glutamate from neurons located in other layers or brain areas and forming synapses on layer 3 pyramidal cells cannot be ruled out. While the frequency of non-NMDA synaptic currents in pyramidal cells in the primate PFC is reduced with age, available evidence suggests that the postsynaptic non-NMDA glutamate receptors, although reported to be present in lower numbers (Hof et al., 2002), may be functionally intact. For example, neither the amplitude distribution of miniature excitatory PSCs nor their kinetics (which reflect the distribution or composition of receptor subunits) are changed with age (Luebke et al., 2004). As with the relationship between spine loss and cognition, further studies showing the relationship of spine loss to synaptic physiology are also needed to determine, empirically, the functional consequences of the significant spine loss and change seen during normal aging.
Neurochemical changes in the aging brain
Given the structural changes occurring to spines with age and the impairment in neuronal transmission it is not surprising that there are significant alterations in the expression and distribution of various neurotransmitters. These changes in turn, may impact the functions of the cholinergic, serotonergic, dopaminergic, and glutamatergic systems; however, there is still a lack of consistency in the data on the changes that occur in these systems with aging (Morrison and Hof, 1997). Early studies by Wenk et al. (1989) found age-associated changes in the levels of choline acetyltransferase (ChAT) activity, as well as endogenous levels of serotonin, norepinephrine, and homovanillic acid in the motor, parietal and occipital cortices of aged rhesus monkeys compared to young (age range 2–37 years) in the prefrontal cortex and the precentral gyrus (Wenk et al., 1989). This was later confirmed by another study in aged monkeys, which also found marked decreases ChAT in the occipital cortex but not in the caudate cingulate cortex. Similar regional reductions in ChAT activity have also been observed in humans (Perry et al., 1987). Additional neurotransmitters including acetylcholine, somatostatin, and neuropeptide Y have also been reported to be decreased in the occipital poles (Beal et al., 1991). Levels of acetylcholine are also decreased in the neocortex, hippocampus, and striatum of aged rats (Wu et al., 1988). Subsequent studies also found a significant decrease in NMDA in cortical and subcortical regions in aged rats and aged rhesus monkeys compared to younger controls (Wenk et al., 1991). It has also been noted that levels of glutamate synthesis are also decreased with age (Tyce and Wong, 1980, Dawson et al., 1989). Dopamine, which affects working memory functions, is also altered with age. Biochemical measurements of dopamine and its metabolite HVA in area 46 of aged monkeys show a 50% decrease between the ages of 10 to 18 years (Goldman-Rakic and Brown, 1981, Wenk et al., 1989).
More recent quantitative immunohistological studies in post-mortem tissue have demnstrated that the number of neurons expressing certain synaptic proteins, such as glutamate receptors (GluR) and NMDA receptor (NMDAR) subunits, are significantly reduced during aging in non-human primates (Gazzaley et al., 1996, Hof et al., 2002). In particular, quantitative analysis of the distributions of GluR2 and NMDAR1 in long and short corticocortical connections in young and old macaque and Patas monkeys revealed a downregulation of the expression of both receptors with aging. GluR2 expression was decreased to a greater extent in the prefrontal cortex compared to other areas, such as the temporal cortex, whereas significant reductions in NMDAR1 occurred primarily in the long corticocortical projections from the superior temporal cortex (Hof et al., 2002). These have demonstrated region specific alterations in the many neurotransmitters involved in cognition and memory and suggest that the changes observed in the corticocortical association pathways can account for the cognitive decline that occurs with age.
It is important to note that in all the studies above, while demonstrating significant changes in neurotransmitter levels with age, there is no association to the distribution of neurotransmitters in spines and synapses and how this distribution may affect cognition. Studies have shown that the protein kinase C (PKC) family of kinases is implicated in structural plasticity, and that overactivation of PKC results in spine loss and altered spine morphology (Calabrese and Halpain, 2005). Moreover, it has been shown that PKC is crucial for hippocampal memory formation (Mathis et al., 1992, Yang and Lee, 1993, Serrano et al., 1994, Zhao et al., 1994), and that dysregulation of PKC contributed to deficits in hippocampal mediated memory in aged animals (Battaini et al., 1990, Battaini et al., 1995, Colombo et al., 1997, Colombo and Gallagher, 2002). In the aging PFC, working memory performance and dendritic integrity are inversely related to the activity of PKC: PKC activation within the medial PFC of aged rats predicted poorer working memory performance, and decreased basal dendritic length (Brennan et al., 2009). Cyclic-AMP (cAMP) signaling is also important in network connectivity, and variations in cAMP signaling can strengthen or weaken neuronal firing and working memory by altering the state of hyperpolarization-activated cyclic nucleotide-gated (HCN) channels located on spines (Arnsten et al., 2010). These cAMP-HCN channels are located in high concentrations on thin spines, and as such, the disinhibition of cAMP signaling that occurs with age may render thin spines more vulnerable and may account for the preferential loss of thin spines with aging (Wang et al., 2011).
Recently, Hara et al. (2012) examined the synaptic distribution of GluA2 and the atypical PKC ζ isoform (PKCζ) in the DG of aged monkeys that were previously tested on the DNMS recognition memory test. They found that aged monkeys had a lower density of GluA2 labeled synapses compared to young and correlated with poor performance on the DNMS task (Hara et al., 2012b). Moreover, spines that express high levels of both GluA2 and PKCζ are important in retaining memory, and these populations of spines decline with age.
Experience-dependent plasticity of spines in the aging brain
So far we have described aging-associated alterations in neuronal and dendritic spine morphology. While such information in important in understanding potential changes in neuronal communication during the normal aging process, it is known that spines are highly plastic structures that undergo rapid changes in a large variety of experiential and synaptic circumstances. Whether the aging process can directly affect the capacity for experience-dependent spine plasticity is still unresolved. An example of an experience-dependent condition is stress. Many studies in rats have shown that exposure to acute or chronic-restrained stress results in the inability to perform set-shifting tasks, retraction of apical dendrites, and changes in synaptic plasticity in medial PFC (Radley et al., 2004, Radley and Morrison, 2005, Liston et al., 2006, Radley et al., 2008, Goldwater et al., 2009). Such set-shifting PFC-dependent tasks have also been shown to be impaired in aged rats (Barense et al., 2002). Given that both stress and aging result in a decline in behavioral performance, it is possible that exposure to stress may accelerate cognitive aging and decline.
Recently, Bloss et al. (2010) examined the effect of stress on neuronal resilience on layer 3 medial PFC neurons in young (3 months), middle-aged (12 months), and aged (20 months) rats and found that both middle-aged and aged rats exhibited stress-induced apical dendritic atrophy (~20%) that was not reversible after a recovery period compared to young rats (Bloss et al., 2010). In agreement with other aging studies, control young and aged rats exhibited a progressive age-related decrease in spine density (~30%) with spine loss occurring along the entire apical and basal dendritic trees. While there was a decrease in spine density and a shift in spine morphology with age, assessment of spines from stressed rats revealed that dendritic spines become less plastic with age, since in response to repeated exposure to stress there was no significant change in spine density or morphology in middle aged and aged animals (Bloss et al., 2011). Taken together, it appears that aging selectively impairs the neuronal resilience and synaptic plasticity in response to experience-dependent stimuli such as stress and that co-existence of both conditions may be additive and exacerbate the aging process.
Aging-induced hormonal changes and spine plasticity
The contributing role of hormones to cognitive decline with aging is still controversial; however, several studies have shown detrimental effects of decreasing levels of estrogen on memory and learning and the incidence of dementia (for more in depth review see (Bailey et al., 2011, Morrison and Baxter, 2012)). In regards to changes in spines with age, most studies are based on data from surgically menopausal non-human primates. When assessing which spines are lost in the absence of estrogen, findings are similar to those with normal aging where there is a significant decrease in spine density. In both intact and ovariectomized (OVX) monkeys, the density of thin spines is significantly reduced (Hao et al., 2007, Dumitriu et al., 2010). On hormone replacement with 17β-estradiol, aged OVX monkeys exhibit an increase in spine density in the dorsolateral PFC similar to densities in young OVX monkeys receiving the same treatment or vehicle. This effect was not seen in OVX monkeys, which received vehicle (Tang et al., 2004, Hao et al., 2006, Hao et al., 2007). Moreover, this increase in spine density was accompanied by improvements in performance in DNMS tasks (Rapp et al., 2003). Similar results in the preservation of thin spines are observed in the CA1 region. In vitro application of estrogen to rat CA1 hippocampal neurons resulted in the increase in the number of thin spines and filopodia without changing the numbers of mushroom or stubby spines (Mukai et al., 2007). In contrast, other studies in rats have shown that 17β-estradiol treatment induces spines in the CA1 of young OVX female rats but fails to do so in aged OVX females (Adams et al., 2001).
Concluding remarks
Here we have highlighted the morphological changes neurons undergo during normal aging, such as reduced dendritic length and arborization, reduction in spine and synapse density and changes in spine subtype distributions. We have also discussed electrophysiological changes with age, as well as cognitive impairments exhibited by ‘unsuccessful’ aging subjects. The loss of spines and/or changes in the proportion of spine subtypes likely impact excitatory synaptic events in neuronal circuits that play an important role in normal cognitive processing. Much remains to be resolved with regard to the molecular mechanisms that underlie substantial morphological alterations associated with normal aging, and the relationship of these changes to single neuron and network function. Understanding the mechanisms that contribute to age-related structural changes will shed key insight into differences between successful and unsuccessful cognitive aging, and ultimately may lead to approaches designed to preserve communication within neuronal circuits and ameliorate cognitive decline during normal aging.
Highlights.
Effects of normal aging on neuronal loss, dendrites, and spines are region-specific.
In many brain areas, normal aging is accompanied by dendritic regression and spine loss.
Aging likely affects stubby, mushroom, and thin spines differently.
Spine changes may contribute to cognitive decline, but direct evidence is lacking.
Acknowledgments
Acknowledgements, grant support.
We thank all the members of the Dickstein, Luebke, Weaver, and Hof laboratories for their participation in these studies. This work was supported by NIH grants MH071818, AG035071, AG025062, and AG00001.
Abbreviations
- DG
dentate gyrus
- DNMS
delayed non-matching-to-sample
- DRST
delayed recognition span test
- HCN
hyperpolarization-activated cyclic nucleotide-gated
- NMDA
N-methyl-D-aspartate
- PFC
prefrontal cortex
- PKC
protein kinase C
- PSCs
postsynaptic currents
- SAM
Senescence-accelerated mice
- SAMP
Senescence-prone strains
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Dara L. Dickstein, Email: dara.dickstein@mssm.edu.
Christina M. Weaver, Email: christina.weaver@fandm.edu.
Jennifer I. Luebke, Email: jluebke@bu.edu.
Patrick R. Hof, Email: patrick.hof@mssm.edu.
References
- Adams MM, Donohue HS, Linville MC, Iversen EA, Newton IG, Brunso-Bechtold JK. Age-related synapse loss in hippocampal CA3 is not reversed by caloric restriction. Neuroscience. 2010;171:373–382. doi: 10.1016/j.neuroscience.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Shah RA, Janssen WG, Morrison JH. Different modes of hippocampal plasticity in response to estrogen in young and aged female rats. Proc Natl Acad Sci U S A. 2001;98:8071–8076. doi: 10.1073/pnas.141215898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. Neuropsychological and neurophysiological changes in healthy adult humans across the age range. Neurobiol Aging. 1993;14:623–625. doi: 10.1016/0197-4580(93)90049-h. [DOI] [PubMed] [Google Scholar]
- Amatrudo J, Weaver CM, Crimins JL, Hof PR, Rosene DL, Luebke JI. Influence of highly distinctive structural properties on the excitability of pyramidal neurons in monkey visual and prefrontal cortices. J Neurosci. 2012 doi: 10.1523/JNEUROSCI.2581-12.2012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BB, Gundersen HJ, Pakkenberg B. Aging of the human cerebellum: a stereological study. J Comp Neurol. 2003;466:356–365. doi: 10.1002/cne.10884. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey ME, Wang AC, Hao J, Janssen WG, Hara Y, Dumitriu D, Hof PR, Morrison JH. Interactive effects of age and estrogen on cortical neurons: implications for cognitive aging. Neuroscience. 2011;191:148–158. doi: 10.1016/j.neuroscience.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Normal aging: regionally specific changes in hippocampal synaptic transmission. Trends Neurosci. 1994;17:13–18. doi: 10.1016/0166-2236(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, 3rd, Fleming DL. Aging in the rhesus monkey: effects on visual discrimination learning and reversal learning. J Gerontol. 1979;34:209–219. doi: 10.1093/geronj/34.2.209. [DOI] [PubMed] [Google Scholar]
- Battaini F, Del Vesco R, Govoni S, Trabucchi M. Regulation of phorbol ester binding and protein kinase C activity in aged rat brain. Neurobiol Aging. 1990;11:563–566. doi: 10.1016/0197-4580(90)90118-j. [DOI] [PubMed] [Google Scholar]
- Battaini F, Elkabes S, Bergamaschi S, Ladisa V, Lucchi L, De Graan PN, Schuurman T, Wetsel WC, Trabucchi M, Govoni S. Protein kinase C activity, translocation, and conventional isoforms in aging rat brain. Neurobiol Aging. 1995;16:137–148. doi: 10.1016/0197-4580(94)00154-5. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Voytko ML. Spatial orienting of attention in adult and aged rhesus monkeys. Behav Neurosci. 1996;110:898–904. doi: 10.1037//0735-7044.110.5.898. [DOI] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AR, Yuan P, Dickstein DL, Rocher AB, Hof PR, Manji H, Arnsten AF. Protein kinase C activity is associated with prefrontal cortical decline in aging. Neurobiol Aging. 2009;30:782–792. doi: 10.1016/j.neurobiolaging.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello CR, Thune JJ, Pakkenberg H, Pakkenberg B. Ageing of substantia nigra in humans: cell loss may be compensated by hypertrophy. Neuropathol Appl Neurobiol. 2002;28:283–291. doi: 10.1046/j.1365-2990.2002.00393.x. [DOI] [PubMed] [Google Scholar]
- Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Chen S, Hillman DE. Dying-back of Purkinje cell dendrites with synapse loss in aging rats. J Neurocytol. 1999;28:187–196. doi: 10.1023/a:1007015721754. [DOI] [PubMed] [Google Scholar]
- Chudasama Y. Animal models of prefrontal-executive function. Behav Neurosci. 2011;125:327–343. doi: 10.1037/a0023766. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Gallagher M. Individual differences in spatial memory among aged rats are related to hippocampal PKCgamma immunoreactivity. Hippocampus. 2002;12:285–289. doi: 10.1002/hipo.10016. [DOI] [PubMed] [Google Scholar]
- Colombo PJ, Wetsel WC, Gallagher M. Spatial memory is related to hippocampal subcellular concentrations of calcium-dependent protein kinase C isoforms in young and aged rats. Proc Natl Acad Sci U S A. 1997;94:14195–14199. doi: 10.1073/pnas.94.25.14195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Sanchez FF, Cardozo A, Tolosa E. Neuronal changes in the substantia nigra with aging: a Golgi study. J Neuropathol Exp Neurol. 1995;54:74–81. doi: 10.1097/00005072-199501000-00009. [DOI] [PubMed] [Google Scholar]
- Cupp CJ, Uemura E. Age-related changes in prefrontal cortex of Macaca mulatta: quantitative analysis of dendritic branching patterns. Exp Neurol. 1980;69:143–163. doi: 10.1016/0014-4886(80)90150-8. [DOI] [PubMed] [Google Scholar]
- de Brabander JM, Kramers RJ, Uylings HB. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- De Groot DMG, Bierman EPB. Numerical changes in rat hippocampal synapses: An effect of “aging”? Acta Stereol. 1987;6:53–58. [Google Scholar]
- Duan H, Wearne SL, Rocher AB, Macedo A, Morrison JH, Hof PR. Age-related dendritic and spine changes in corticocortically projecting neurons in macaque monkeys. Cereb Cortex. 2003;13:950–961. doi: 10.1093/cercor/13.9.950. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Denk W. Dendritic processing. Curr Opin Neurobiol. 2001;11:415–422. doi: 10.1016/s0959-4388(00)00228-2. [DOI] [PubMed] [Google Scholar]
- Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26:874–890. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Flood DG, Coleman PD. Hippocampal plasticity in normal aging and decreased plasticity in Alzheimer’s disease. Prog Brain Res. 1990;83:435–443. doi: 10.1016/s0079-6123(08)61267-4. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Takeda K. Information processes in the primate prefrontal cortex in relation to working memory processes. Rev Neurosci. 2002;13:313–345. doi: 10.1515/revneuro.2002.13.4.313. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Glick R, Bondareff W. Loss of synapses in the cerebellar cortex of the senescent rat. J Gerontol. 1979;34:818–822. doi: 10.1093/geronj/34.6.818. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Brown RM. Regional changes of monoamines in cerebral cortex and subcortical structures of aging rhesus monkeys. Neuroscience. 1981;6:177–187. doi: 10.1016/0306-4522(81)90053-1. [DOI] [PubMed] [Google Scholar]
- Graham BP, van Ooyen A. Mathematical modelling and numerical simulation of the morphological development of neurons. BMC Neurosci. 2006;7(Suppl 1):S9. doi: 10.1186/1471-2202-7-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Katoh H, Hosokawa T, Hosono M, Takeda T. Immune responses in newly developed short-lived SAM mice. IV. Chromosomal location of a gene controlling defective helper T-cell activity. Immunology. 1991;74:160–164. [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Park CS, Janssen WG, Roberts MT, Morrison JH, Rapp PR. Synaptic correlates of memory and menopause in the hippocampal dentate gyrus in rhesus monkeys. Neurobiol Aging. 2012a;33:421, e417–428. doi: 10.1016/j.neurobiolaging.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Punsoni M, Yuk F, Park CS, Janssen WG, Rapp PR, Morrison JH. Synaptic distributions of GluA2 and PKMzeta in the monkey dentate gyrus and their relationships with aging and memory. J Neurosci. 2012b;32:7336–7344. doi: 10.1523/JNEUROSCI.0605-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara Y, Rapp PR, Morrison JH. Neuronal and morphological bases of cognitive decline in aged rhesus monkeys. Age (Dordr) 2011 doi: 10.1007/s11357-011-9278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87:25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hof PR, Duan H, Page TL, Einstein M, Wicinski B, He Y, Erwin JM, Morrison JH. Age-related changes in GluR2 and NMDAR1 glutamate receptor subunit protein immunoreactivity in corticocortically projecting neurons in macaque and patas monkeys. Brain Res. 2002;928:175–186. doi: 10.1016/s0006-8993(01)03345-5. [DOI] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Itzev DE, Lolov SR, Usunoff KG. Aging and synaptic changes in the paraventricular hypothalamic nucleus of the rat. Acta Physiol Pharmacol Bulg. 2003;27:75–82. [PubMed] [Google Scholar]
- Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: a quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- Jacobs B, Schall M, Prather M, Kapler E, Driscoll L, Baca S, Jacobs J, Ford K, Wainwright M, Treml M. Regional dendritic and spine variation in human cerebral cortex: a quantitative golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- Kabaso D, Coskren PJ, Henry BI, Hof PR, Wearne SL. The electrotonic structure of pyramidal neurons contributing to prefrontal cortical circuits in macaque monkeys is significantly altered in aging. Cereb Cortex. 2009;19:2248–2268. doi: 10.1093/cercor/bhn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krichmar JL, Nasuto SJ, Scorcioni R, Washington SD, Ascoli GA. Effects of dendritic morphology on CA3 pyramidal cell electrophysiology: a simulation study. Brain Res. 2002;941:11–28. doi: 10.1016/s0006-8993(02)02488-5. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiol Aging. 1995;16:947–954. doi: 10.1016/0197-4580(95)02014-4. [DOI] [PubMed] [Google Scholar]
- Leigland LA, Schulz LE, Janowsky JS. Age related changes in emotional memory. Neurobiol Aging. 2004;25:1117–1124. doi: 10.1016/j.neurobiolaging.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Levine MS, Adinolfi AM, Fisher RS, Hull CD, Buchwald NA, McAllister JP. Quantitative morphology of medium-sized caudate spiny neurons in aged cats. Neurobiol Aging. 1986;7:277–286. doi: 10.1016/0197-4580(86)90008-4. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125:277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Weaver CM, Rocher AB, Rodriguez A, Crimins JL, Dickstein DL, Wearne SL, Hof PR. Dendritic vulnerability in neurodegenerative disease: insights from analyses of cortical pyramidal neurons in transgenic mouse models. Brain Struct Funct. 2010;214:181–199. doi: 10.1007/s00429-010-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowski TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Marcuzzo S, Dall’oglio A, Ribeiro MF, Achaval M, Rasia-Filho AA. Dendritic spines in the posterodorsal medial amygdala after restraint stress and ageing in rats. Neurosci Lett. 2007;424:16–21. doi: 10.1016/j.neulet.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Markham JA, McKian KP, Stroup TS, Juraska JM. Sexually dimorphic aging of dendritic morphology in CA1 of hippocampus. Hippocampus. 2005;15:97–103. doi: 10.1002/hipo.20034. [DOI] [PubMed] [Google Scholar]
- Mathis C, Lehmann J, Ungerer A. The selective protein kinase C inhibitor, NPC 15437, induces specific deficits in memory retention in mice. Eur J Pharmacol. 1992;220:107–110. doi: 10.1016/0014-2999(92)90020-5. [DOI] [PubMed] [Google Scholar]
- Mervis R. Structural alterations in neurons of aged canine neocortex: a Golgi study. Exp Neurol. 1978;62:417–432. doi: 10.1016/0014-4886(78)90065-1. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Impairment in abstraction and set shifting in aged rhesus monkeys. Neurobiol Aging. 2003;24:125–134. doi: 10.1016/s0197-4580(02)00054-4. [DOI] [PubMed] [Google Scholar]
- Moore TL, Killiany RJ, Herndon JG, Rosene DL, Moss MB. Executive system dysfunction occurs as early as middle-age in the rhesus monkey. Neurobiol Aging. 2006;27:1484–1493. doi: 10.1016/j.neurobiolaging.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Moore TL, Schettler SP, Killiany RJ, Herndon JG, Luebke JI, Moss MB, Rosene DL. Cognitive impairment in aged rhesus monkeys associated with monoamine receptors in the prefrontal cortex. Behav Brain Res. 2005;160:208–221. doi: 10.1016/j.bbr.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Mukai H, Tsurugizawa T, Murakami G, Kominami S, Ishii H, Ogiue-Ikeda M, Takata N, Tanabe N, Furukawa A, Hojo Y, Ooishi Y, Morrison JH, Janssen WG, Rose JA, Chambon P, Kato S, Izumi S, Yamazaki T, Kimoto T, Kawato S. Rapid modulation of long-term depression and spinogenesis via synaptic estrogen receptors in hippocampal principal neurons. J Neurochem. 2007;100:950–967. doi: 10.1111/j.1471-4159.2006.04264.x. [DOI] [PubMed] [Google Scholar]
- Nakamura S, Akiguchi I, Kameyama M, Mizuno N. Age-related changes of pyramidal cell basal dendrites in layers III and V of human motor cortex: a quantitative Golgi study. Acta Neuropathol (Berl) 1985;65:281–284. doi: 10.1007/BF00687009. [DOI] [PubMed] [Google Scholar]
- Newton IG, Forbes ME, Linville MC, Pang H, Tucker EW, Riddle DR, Brunso-Bechtold JK. Effects of aging and caloric restriction on dentate gyrus synapses and glutamate receptor subunits. Neurobiol Aging. 2008;29:1308–1318. doi: 10.1016/j.neurobiolaging.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page TL, Einstein M, Duan H, He Y, Flores T, Rolshud D, Erwin JM, Wearne SL, Morrison JH, Hof PR. Morphological alterations in neurons forming corticocortical projections in the neocortex of aged Patas monkeys. Neurosci Lett. 2002;317:37–41. doi: 10.1016/s0304-3940(01)02428-4. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Peters A, Moss MB, Sethares C. The effects of aging on layer 1 of primary visual cortex in the rhesus monkey. Cereb Cortex. 2001;11:93–103. doi: 10.1093/cercor/11.2.93. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C, Moss MB. The effects of aging on layer 1 in area 46 of prefrontal cortex in the rhesus monkey. Cereb Cortex. 1998;8:671–684. doi: 10.1093/cercor/8.8.671. [DOI] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA. Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiol Aging. 1996;17:601–611. doi: 10.1016/0197-4580(96)00034-6. [DOI] [PubMed] [Google Scholar]
- Quackenbush LJ, Ngo H, Pentney RJ. Evidence for nonrandom regression of dendrites of Purkinje neurons during aging. Neurobiol Aging. 1990;11:111–115. doi: 10.1016/0197-4580(90)90043-y. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Recognition memory deficits in a subpopulation of aged monkeys resemble the effects of medial temporal lobe damage. Neurobiol Aging. 1991;12:481–486. doi: 10.1016/0197-4580(91)90077-w. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin card sorting test: a meta-analytic review. Psychol Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Aranda C, Sundet K. The frontal hypothesis of cognitive aging: factor structure and age effects on four frontal tests among healthy individuals. J Genet Psychol. 2006;167:269–287. doi: 10.3200/GNTP.167.3.269-287. [DOI] [PubMed] [Google Scholar]
- Rubinow MJ, Drogos LL, Juraska JM. Age-related dendritic hypertrophy and sexual dimorphism in rat basolateral amygdala. Neurobiol Aging. 2009;30:137–146. doi: 10.1016/j.neurobiolaging.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. J Exp Psychol Gen. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Lindsay RD, Tomiyasu U, Scheibel AB. Progressive dendritic changes in aging human cortex. Exp Neurol. 1975;47:392–403. doi: 10.1016/0014-4886(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Serrano PA, Beniston DS, Oxonian MG, Rodriguez WA, Rosenzweig MR, Bennett EL. Differential effects of protein kinase inhibitors and activators on memory formation in the 2-day-old chick. Behav Neural Biol. 1994;61:60–72. doi: 10.1016/s0163-1047(05)80045-7. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Tsuzuki M, Keino H, Satoh M, Chiba Y, Saitoh Y, Hosokawa M. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: a model of cerebral degeneration. Neuropathol Appl Neurobiol. 2006;32:1–14. doi: 10.1111/j.1365-2990.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorel O, Pennequin V. Aging of the planning process: the role of executive functioning. Brain Cogn. 2008;66:196–201. doi: 10.1016/j.bandc.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Steere JC, Arnsten AF. The alpha-2A noradrenergic receptor agonist guanfacine improves visual object discrimination reversal performance in aged rhesus monkeys. Behav Neurosci. 1997;111:883–891. doi: 10.1037//0735-7044.111.5.883. [DOI] [PubMed] [Google Scholar]
- Tang Y, Janssen WG, Hao J, Roberts JA, McKay H, Lasley B, Allen PB, Greengard P, Rapp PR, Kordower JH, Hof PR, Morrison JH. Estrogen replacement increases spinophilin-immunoreactive spine number in the prefrontal cortex of female rhesus monkeys. Cereb Cortex. 2004;14:215–223. doi: 10.1093/cercor/bhg121. [DOI] [PubMed] [Google Scholar]
- Vetter P, Roth A, Hausser M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–937. doi: 10.1152/jn.2001.85.2.926. [DOI] [PubMed] [Google Scholar]
- Voytko ML. Impairments in acquisition and reversals of two-choice discriminations by aged rhesus monkeys. Neurobiol Aging. 1999;20:617–627. doi: 10.1016/s0197-4580(99)00097-4. [DOI] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Sakagami M. Integration of cognitive and motivational context information in the primate prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i101–109. doi: 10.1093/cercor/bhm067. [DOI] [PubMed] [Google Scholar]
- Weaver CM, Wearne SL. Neuronal firing sensitivity to morphologic and active membrane parameters. PLoS Comput Biol. 2008;4:e11. doi: 10.1371/journal.pcbi.0040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL, Pierce DJ, Struble RG, Price DL, Cork LC. Age-related changes in multiple neurotransmitter systems in the monkey brain. Neurobiol Aging. 1989;10:11–19. doi: 10.1016/s0197-4580(89)80005-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Foy MR, Akopian GG, Lee KH, Zach J, Nguyen KP, Comalli DM, Kennard JA, Agelan A, Thompson RF. Differential effects and rates of normal aging in cerebellum and hippocampus. Proc Natl Acad Sci U S A. 2010;107:1624–1629. doi: 10.1073/pnas.0914207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Lee EH. Protein kinase C activation facilitates memory retention in rats. Chin J Physiol. 1993;36:115–123. [PubMed] [Google Scholar]
- Zhang C, Hua T, Zhu Z, Luo X. Age-related changes of structures in cerebellar cortex of cat. J Biosci. 2006;31:55–60. doi: 10.1007/BF02705235. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Sedman GL, Gibbs ME, Ng KT. Effect of PKC inhibitors and activators on memory. Behav Brain Res. 1994;60:151–160. doi: 10.1016/0166-4328(94)90142-2. [DOI] [PubMed] [Google Scholar]


