Abstract
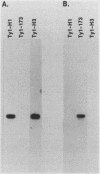
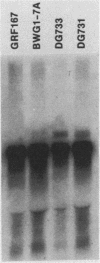
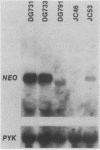
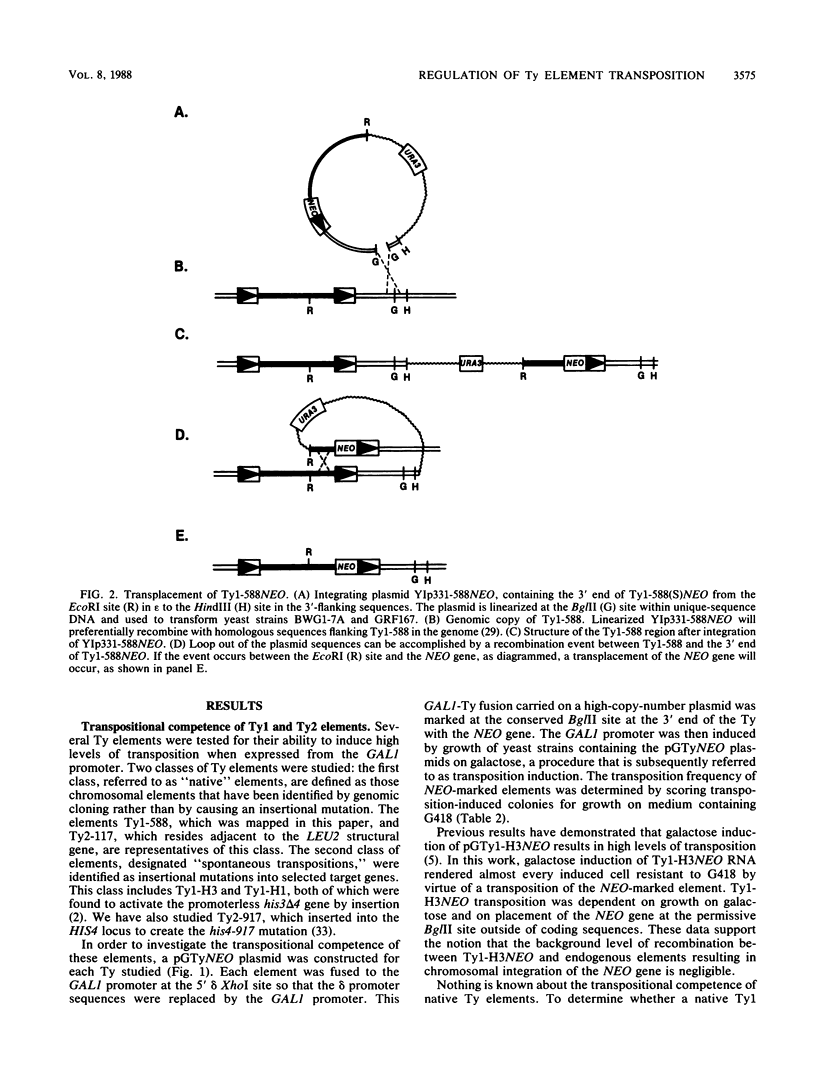
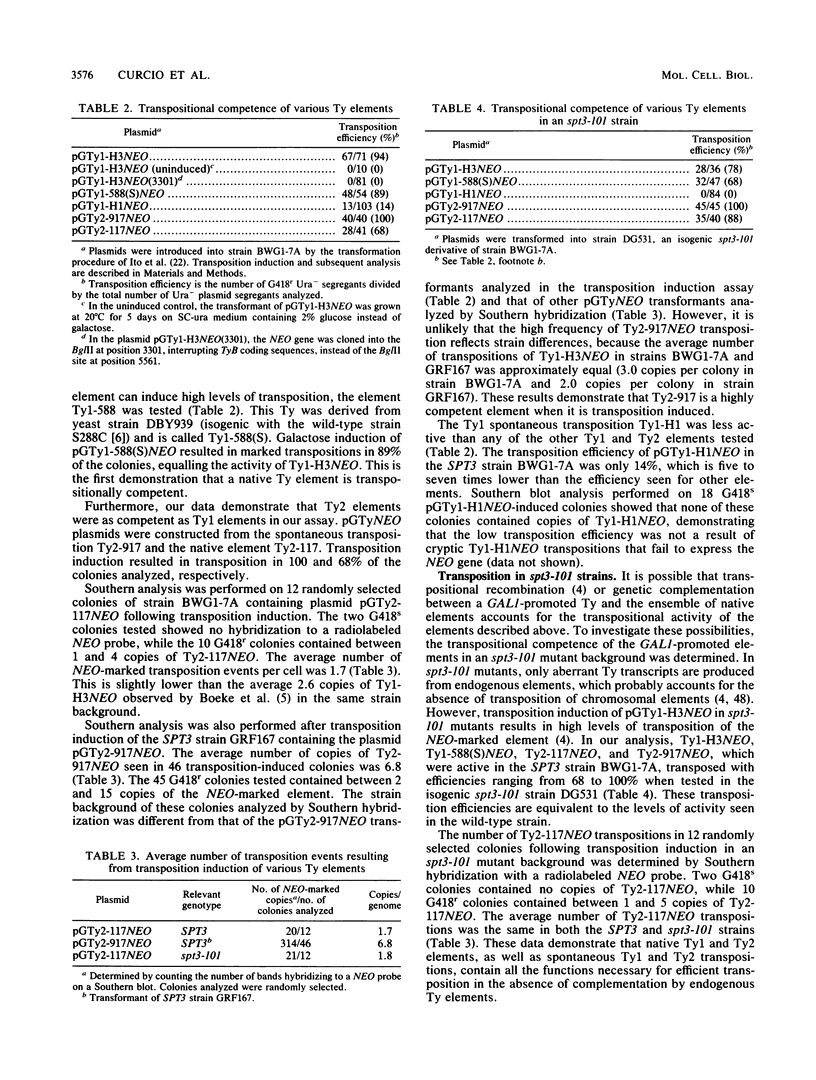
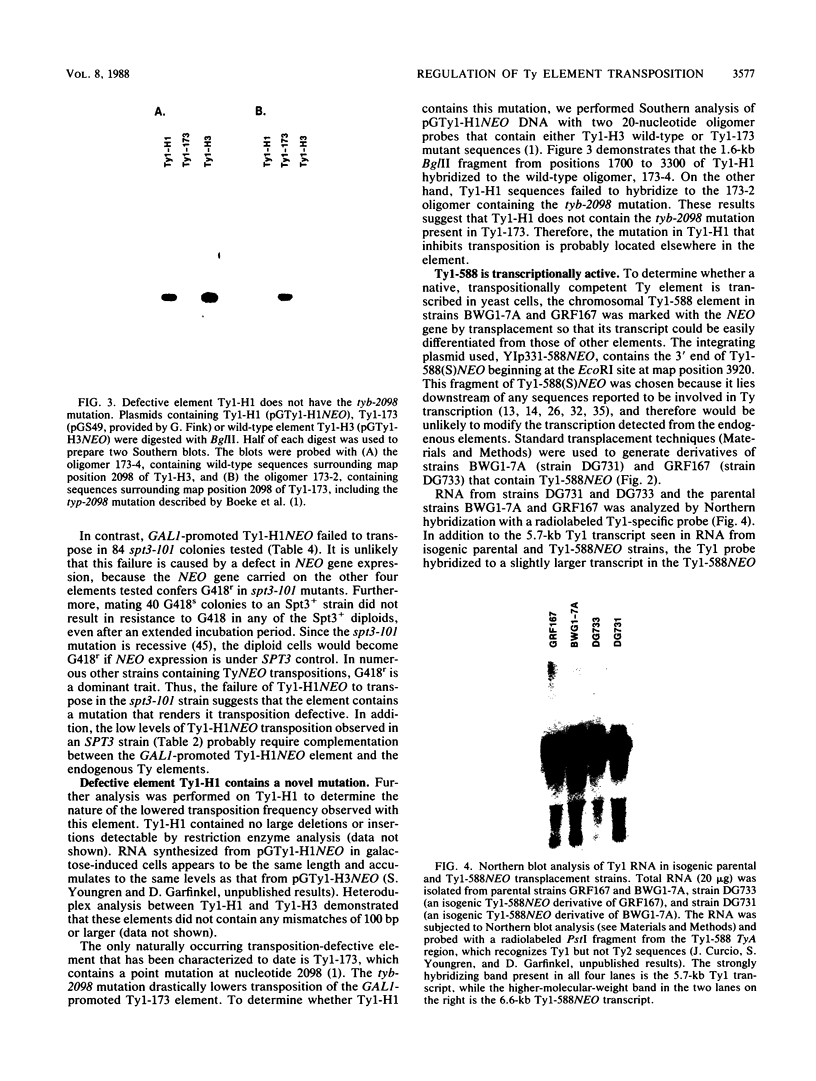
Transposition of Ty elements in the yeast Saccharomyces cerevisiae occurs through an RNA intermediate. Although Ty RNA accounts for 5 to 10% of the total polyadenylated RNA in a haploid cell, the transposition frequency is only 10(-7) to 10(-8) per gene. To determine whether Ty elements native to the yeast genome are transpositionally competent, two elements were fused to the GAL1 promoter and tested for their ability to transpose. These native elements, Ty1-588 and Ty2-117, transposed at high levels when the GAL1 promoter was induced. Three Ty's identified as spontaneous transpositions in specific target genes were also tested. Of these three, Ty2-917 and the previously characterized element Ty1-H3 were shown to be transpositionally competent. The third element, Ty1-H1, was transposition defective. In addition, we marked the chromosomal copy of Ty1-588 with the NEO gene and demonstrated that Ty1-588NEO was actively transcribed in yeast cells. Ty1-588NEO transcription was regulated by the SPT3 and MAT loci in the same manner as that observed for Ty's collectively. These results indicate that the yeast genome contains functional Ty elements. The presence of a transpositionally competent, actively transcribed element suggests that regulation of Ty transposition occurs at a posttranscriptional level.
Full text
PDF

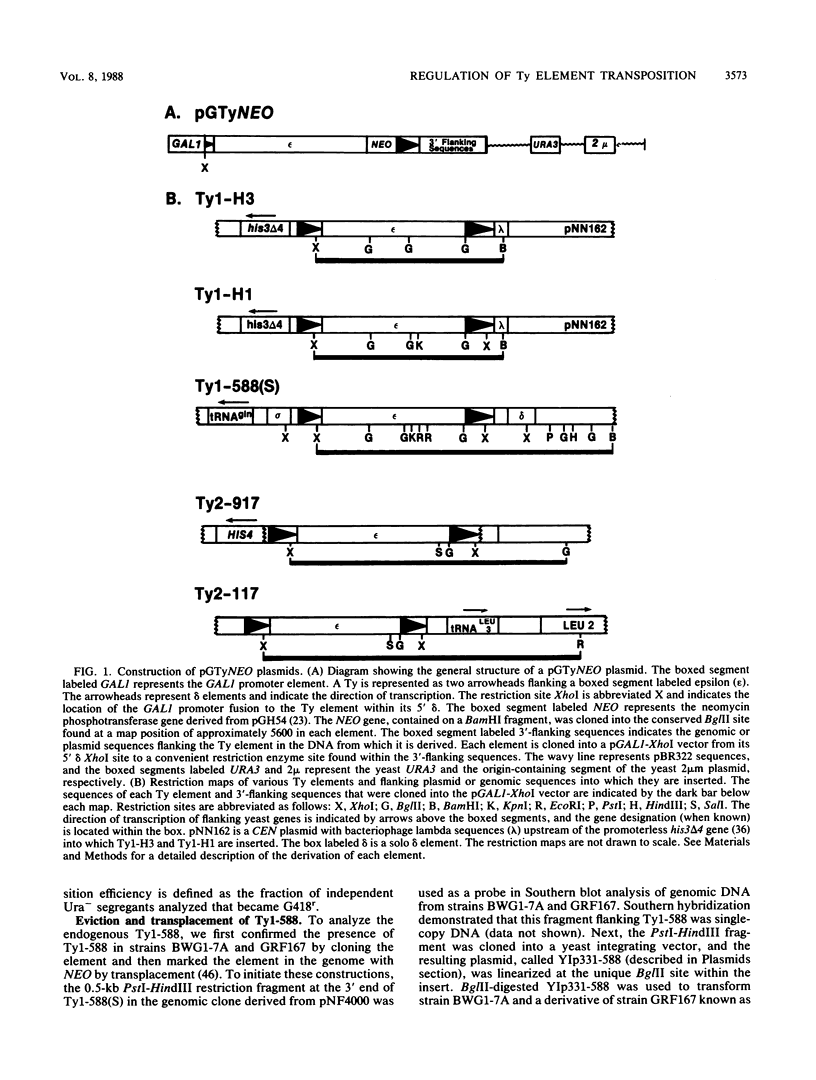





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boeke J. D., Eichinger D., Castrillon D., Fink G. R. The Saccharomyces cerevisiae genome contains functional and nonfunctional copies of transposon Ty1. Mol Cell Biol. 1988 Apr;8(4):1432–1442. doi: 10.1128/mcb.8.4.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., LaCroute F., Fink G. R. A positive selection for mutants lacking orotidine-5'-phosphate decarboxylase activity in yeast: 5-fluoro-orotic acid resistance. Mol Gen Genet. 1984;197(2):345–346. doi: 10.1007/BF00330984. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Styles C. A., Fink G. R. Saccharomyces cerevisiae SPT3 gene is required for transposition and transpositional recombination of chromosomal Ty elements. Mol Cell Biol. 1986 Nov;6(11):3575–3581. doi: 10.1128/mcb.6.11.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeke J. D., Xu H., Fink G. R. A general method for the chromosomal amplification of genes in yeast. Science. 1988 Jan 15;239(4837):280–282. doi: 10.1126/science.2827308. [DOI] [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Ciriacy M., Williamson V. M. Analysis of mutations affecting Ty-mediated gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1981;182(1):159–163. doi: 10.1007/BF00422784. [DOI] [PubMed] [Google Scholar]
- Clare J., Farabaugh P. Nucleotide sequence of a yeast Ty element: evidence for an unusual mechanism of gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2829–2833. doi: 10.1073/pnas.82.9.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. J., Bilanchone V. W., Haywood L. J., Dildine S. L., Sandmeyer S. B. A yeast sigma composite element, TY3, has properties of a retrotransposon. J Biol Chem. 1988 Jan 25;263(3):1413–1423. [PubMed] [Google Scholar]
- Dubois E., Jacobs E., Jauniaux J. C. Expression of the ROAM mutations in Saccharomyces cerevisiae: involvement of trans-acting regulatory elements and relation with the Ty1 transcription. EMBO J. 1982;1(9):1133–1139. doi: 10.1002/j.1460-2075.1982.tb01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder R. T., St John T. P., Stinchcomb D. T., Davis R. W., Scherer S., Davis R. W. Studies on the transposable element Ty1 of yeast. I. RNA homologous to Ty1. II. Recombination and expression of Ty1 and adjacent sequences. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):581–591. doi: 10.1101/sqb.1981.045.01.075. [DOI] [PubMed] [Google Scholar]
- Errede B., Cardillo T. S., Sherman F., Dubois E., Deschamps J., Wiame J. M. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell. 1980 Nov;22(2 Pt 2):427–436. doi: 10.1016/0092-8674(80)90353-0. [DOI] [PubMed] [Google Scholar]
- Errede B., Company M., Ferchak J. D., Hutchison C. A., 3rd, Yarnell W. S. Activation regions in a yeast transposon have homology to mating type control sequences and to mammalian enhancers. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5423–5427. doi: 10.1073/pnas.82.16.5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B., Company M., Hutchison C. A., 3rd Ty1 sequence with enhancer and mating-type-dependent regulatory activities. Mol Cell Biol. 1987 Jan;7(1):258–265. doi: 10.1128/mcb.7.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farabaugh P. J., Fink G. R. Insertion of the eukaryotic transposable element Ty1 creates a 5-base pair duplication. Nature. 1980 Jul 24;286(5771):352–356. doi: 10.1038/286352a0. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Boeke J. D., Fink G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell. 1985 Sep;42(2):507–517. doi: 10.1016/0092-8674(85)90108-4. [DOI] [PubMed] [Google Scholar]
- Geliebter J., Zeff R. A., Schulze D. H., Pease L. R., Weiss E. H., Mellor A. L., Flavell R. A., Nathenson S. G. Interaction between Kb and Q4 gene sequences generates the Kbm6 mutation. Mol Cell Biol. 1986 Feb;6(2):645–652. doi: 10.1128/mcb.6.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux C. N., Mis J. R., Pierce M. K., Kohalmi S. E., Kunz B. A. DNA sequence analysis of spontaneous mutations in the SUP4-o gene of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Feb;8(2):978–981. doi: 10.1128/mcb.8.2.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L., Yocum R. R., Gifford P. A GAL10-CYC1 hybrid yeast promoter identifies the GAL4 regulatory region as an upstream site. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7410–7414. doi: 10.1073/pnas.79.23.7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating hormone. J Cell Biol. 1980 Jun;85(3):811–822. doi: 10.1083/jcb.85.3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauber J., Nelböck-Hochstetter P., Feldmann H. Nucleotide sequence and characteristics of a Ty element from yeast. Nucleic Acids Res. 1985 Apr 25;13(8):2745–2758. doi: 10.1093/nar/13.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman A. J., Gimlich R. L., Clarke L., Chinault A. C., Carbon J. Sequence variation in dispersed repetitive sequences in Saccharomyces cerevisiae. J Mol Biol. 1981 Feb 5;145(4):619–632. doi: 10.1016/0022-2836(81)90306-5. [DOI] [PubMed] [Google Scholar]
- Klein H. L., Petes T. D. Genetic mapping of Ty elements in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Feb;4(2):329–339. doi: 10.1128/mcb.4.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X. B., Clare J. J., Farabaugh P. J. The upstream activation site of a Ty2 element of yeast is necessary but not sufficient to promote maximal transcription of the element. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8520–8524. doi: 10.1073/pnas.84.23.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor J., Fulton S. M., Dobson M. J., Wilson W., Kingsman S. M., Kingsman A. J. A retrovirus-like strategy for expression of a fusion protein encoded by yeast transposon Ty1. Nature. 1985 Jan 17;313(5999):243–246. doi: 10.1038/313243a0. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquin C. E., Williamson V. M. Temperature effects on the rate of ty transposition. Science. 1984 Oct 5;226(4670):53–55. doi: 10.1126/science.226.4670.53. [DOI] [PubMed] [Google Scholar]
- Perkins D. D. Biochemical Mutants in the Smut Fungus Ustilago Maydis. Genetics. 1949 Sep;34(5):607–626. doi: 10.1093/genetics/34.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pure G. A., Robinson G. W., Naumovski L., Friedberg E. C. Partial suppression of an ochre mutation in Saccharomyces cerevisiae by multicopy plasmids containing a normal yeast tRNAGln gene. J Mol Biol. 1985 May 5;183(1):31–42. doi: 10.1016/0022-2836(85)90278-5. [DOI] [PubMed] [Google Scholar]
- Rathjen P. D., Kingsman A. J., Kingsman S. M. The yeast ROAM mutation--identification of the sequences mediating host gene activation and cell-type control in the yeast retrotransposon, Ty. Nucleic Acids Res. 1987 Sep 25;15(18):7309–7324. doi: 10.1093/nar/15.18.7309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Farabaugh P. J., Chaleff D. T., Fink G. R. The origins of gene instability in yeast. Science. 1980 Sep 19;209(4463):1375–1380. doi: 10.1126/science.6251544. [DOI] [PubMed] [Google Scholar]
- Roeder G. S., Rose A. B., Pearlman R. E. Transposable element sequences involved in the enhancement of yeast gene expression. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5428–5432. doi: 10.1073/pnas.82.16.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Mann C., Davis R. W. Reversion of a promoter deletion in yeast. Nature. 1982 Aug 26;298(5877):815–819. doi: 10.1038/298815a0. [DOI] [PubMed] [Google Scholar]
- Simchen G., Winston F., Styles C. A., Fink G. R. Ty-mediated gene expression of the LYS2 and HIS4 genes of Saccharomyces cerevisiae is controlled by the same SPT genes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2431–2434. doi: 10.1073/pnas.81.8.2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Sahashi Y. The PET18 locus of Saccharomyces cerevisiae: a complex locus containing multiple genes. Yeast. 1985 Dec;1(2):159–171. doi: 10.1002/yea.320010204. [DOI] [PubMed] [Google Scholar]
- Warmington J. R., Anwar R., Newlon C. S., Waring R. B., Davies R. W., Indge K. J., Oliver S. G. A 'hot-spot' for Ty transposition on the left arm of yeast chromosome III. Nucleic Acids Res. 1986 Apr 25;14(8):3475–3485. doi: 10.1093/nar/14.8.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmington J. R., Waring R. B., Newlon C. S., Indge K. J., Oliver S. G. Nucleotide sequence characterization of Ty 1-17, a class II transposon from yeast. Nucleic Acids Res. 1985 Sep 25;13(18):6679–6693. doi: 10.1093/nar/13.18.6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson V. M. Transposable elements in yeast. Int Rev Cytol. 1983;83:1–25. doi: 10.1016/s0074-7696(08)61684-8. [DOI] [PubMed] [Google Scholar]
- Winston F., Chaleff D. T., Valent B., Fink G. R. Mutations affecting Ty-mediated expression of the HIS4 gene of Saccharomyces cerevisiae. Genetics. 1984 Jun;107(2):179–197. doi: 10.1093/genetics/107.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- Winston F., Durbin K. J., Fink G. R. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984 Dec;39(3 Pt 2):675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- Youngren S. D., Boeke J. D., Sanders N. J., Garfinkel D. J. Functional organization of the retrotransposon Ty from Saccharomyces cerevisiae: Ty protease is required for transposition. Mol Cell Biol. 1988 Apr;8(4):1421–1431. doi: 10.1128/mcb.8.4.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]