Abstract
The HIV gp41 fusion domain plays a critical role in membrane fusion during viral entry. A thorough understanding of the relationship between the structure and activity of the fusion domain in different lipid environments helps to formulate mechanistic models on how it might function in mediating membrane fusion. The secondary structure of the fusion domain in small liposomes composed of different lipid mixtures was investigated by circular dichroism spectroscopy. In membranes containing less than 30 mol% cholesterol the fusion domain formed an α-helix and in membranes containing equal to or more than 30 mol% cholesterol the fusion domain formed β-sheet secondary structure. EPR spectra of spin-labeled fusion domains also indicated different conformations in membranes with and without cholesterol. Power saturation EPR data were further used to determine the orientation and depth of α-helical fusion domains in lipid bilayers. Fusion and membrane perturbation activities of the gp41 fusion domain were measured by lipid mixing and contents leakage. The fusion domain fused membranes in both its helical and β-sheet forms. High cholesterol, which induced β-sheet, promoted fusion, but acidic lipids, which promoted relatively deep membrane insertion as an α-helix, also induced fusion. The results indicate that the structure of the HIV gp41 fusion domain is plastic and depends critically on the lipid environment. Provided their membrane insertion is deep, α-helical and β-sheet conformations contribute to membrane fusion.
Keywords: HIV, fusion domain, structure, lipid bilayers, cholesterol
Introduction
Human immunodeficiency virus (HIV) enters and infects susceptible cells by membrane fusion. The viral particle is enveloped by a lipid bilayer membrane, which harbors the homotrimeric integral membrane glycoprotein gp120/gp41. The gp120 subunit of each monomer recognizes the CD4 receptor and the CCR5 or CXCR4 chemokine co-receptors. The gp41 subunit spans the membrane with a single transmembrane domain near its C-terminus. Binding of gp120 to its receptor and co-receptor alters its interaction with gp41 such that gp41 extends into an elongated pre-hairpin structure. This extended structure delivers a hydrophobic and conserved sequence at the extreme N-terminus of gp41 towards the surface of the target cell, where it inserts into the lipid bilayer of the target cell membrane. Because this reaction is crucial for bridging and eventually fusing the viral and cell membranes, the N-terminal peptide has been called the fusion peptide or fusion domain of gp41. Fusion domain insertion is followed by a refolding of the extended structure between the two membranes into a helical hairpin and the formation of this low energy structure is believed to generate enough force to pull the two membranes together and eventually fuse them, at which point the gp41 fusion and transmembrane domains end up in close proximity in the same membrane. Since there are three copies in a trimer that each fold into a hairpin, the final postfusion structure of gp41 is a six-helix bundle. Interference of six-helix bundle formation has been a successful therapeutic strategy for HIV cell entry inhibition with several peptide products including Fuzeon® on the market or in clinical trials.
How the two membranes deform during fusion and how the fusion and transmembrane domains adapt to and drive this deformation is not well understood. Just as correct six-helix bundle formation is essential for productive fusion of the viral and cellular membranes, a precise insertion of the viral fusion domains into the target membrane is very critical for fusion to proceed in a controlled manner. The HIV-1 gp41 fusion domain comprises approximately 15 apolar residues followed by another 8 moderately polar residues. The overall secondary structure as well as the fine structure of this domain in lipid bilayers has been debated frequently in the literature with many often substantially diverging opinions. Early CD and FTIR experiments favored a model of an obliquely inserted α-helix in the membrane. However, other studies performed with longer peptides or at higher concentrations indicated that membrane association occurred in the form of a β-sheet. Structures of the HIV fusion domain solved by solution NMR in detergent micelles revealed a helical conformation. However, when the same sequences were studied in lipid bilayers by solid-state NMR methods, β-sheet predominated. The β-sheet structure was later refined to being mostly antiparallel with the first 17 residues running as straight strands with a zero- or one-residue off-set against each other 14. Less than 15% of the population formed parallel β-sheets in these experiments, which were performed at −50 °C in bilayers containing 53% PC, 13% PG and 33% cholesterol.
It is clear that peptide concentration, lipid composition, peptide sequence, and measuring temperature can all affect the outcome of these experiments. It was shown early on that increasing peptide concentration and peptide/lipid ratios induced the fusion domains to switch from α-helical to β-sheet structures. Synthetic trimers of the gp41 fusion domain also always favored the β-sheet structures 17. The presence of cholesterol increased the propensity of β-sheet and even without cholesterol the α-helical form was only partially populated under the solid-state NMR conditions 17. An important question then becomes whether the α-helical or β-sheet forms are active in membrane fusion. It appears that both forms can be fusion-active depending on conditions and it has been argued that membrane penetration depth may be a more important determinant of fusion activity than secondary structure18. Since much of the evidence for β-sheet comes from solid-state NMR experiments conducted at very low temperatures, i.e. conditions that potentially could squeeze polypeptides out of fluid membranes, it is important to know whether the fusion domain conformations are still predominantly β-sheet when studied by techniques that can be performed at room or physiological temperatures. To gain more clarity on possible correlations between secondary structure, depth of membrane penetration, and membrane fusion, we studied lipid mixing, pore formation, secondary structure, and membrane insertion of a 23-residue fusion domain construct by relief of lipid FRET, release of a fluorescent content dye, CD spectroscopy, and spin label EPR spectroscopy, respectively, under the same set of lipid and ambient temperature conditions with variable amounts of cholesterol. Cholesterol profoundly affects all parameters in a correlated manner indicating that with up to 20 mol% cholesterol the helical form predominates in promoting membrane fusion whereas at 30 mol% cholesterol the β-sheet form is responsible lipid mixing and extensive membrane perturbation.
Results
Fusion and Perturbation of Membranes of Different Lipid Composition by HIV gp41 Fusion Domain
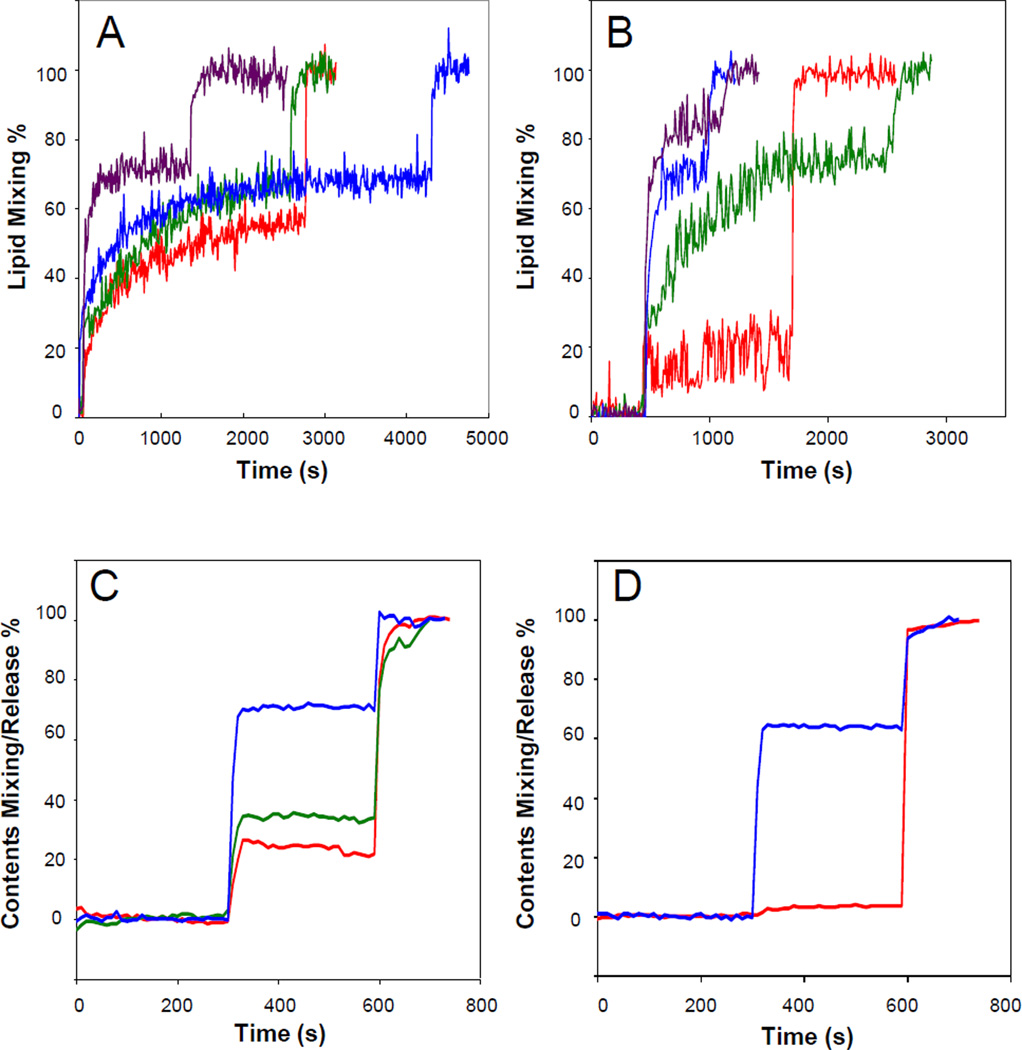
We previously reported that the HIV gp41 fusion domain induces the mixing of lipids between liposomes composed of 80% POPC and 20% POPG 11. This activity that is often interpreted as fusion of the participating liposomes is promoted by the fusion domain up to a concentration of 5 µM at a concentration of 200 µM total lipid. Above 5 µM protein the lipid mixing traces became very noisy and sometimes erratic. FTIR experiments on fully hydrated supported bilayers showed that these higher protein concentrations also formed increased proportions of β-sheet on membranes at the expense of α-helix which predominated at or below 5 µM protein 11. Based on these results, we chose to investigate in more detail how 5 µM HIV gp41 fusion domain would fuse and perturb lipid bilayers of different composition with increasing amounts of cholesterol. This is important because both viral and cellular target membranes contain on the order of 30 mol% cholesterol 19. While the fusion domain induced rapid lipid mixing of POPC:POPG bilayers without cholesterol, increasing the cholesterol content gradually increased the extent of lipid mixing from about 50% to about 70% maximal FRET relief (Figure 1A). Whenever cholesterol was increased, phosphatidylcholine was decreased by an equivalent percentage of total lipid throughout this work. The effect of cholesterol on fusion of bilayers composed of 80% POPC and 20% POPS was more dramatic. Very little fusion was observed in the absence of cholesterol, but the liposomes became 70 to 90% mixed when cholesterol was increased from 10 to 30 mol% in these bilayers (Figure 1B). PS is the predominant acidic lipid species in eukaryotic cell membranes and its headgroup is bulkier compared to POPG and capable of forming strong in-plane ionic headgroup interactions and stronger inter-headgroup hydrogen bonds than POPG. Spacing the phospholipids and disrupting headgroup interactions in the membrane surface by intercalation of cholesterol may well be the reason for the much higher sensitivity of POPS to cholesterol compared to the cholesterol sensitivity of POPG-containing membranes.
Fig. 1.
Effect of cholesterol on HIV gp41 fusion domain-induced lipid mixing (A,B) and release of contents (C,D) between and from liposomes composed of POPC:POPG (4:1) (A,C) or POPC: POPS (4:1) (B,D) plus 0% (red), 10% (green), 20% (blue), and 30% (purple) cholesterol. 10% of the large unilamellar liposomes were labeled with 1% each of NBD-PE and Rhodamine-PE (A,B) or 16.7% were labeled with 15 mM ANTS and 45 mM DPX (C,D) and the rest were unlabeled. Fusion was induced by the addition of 5 µM HIV gp41 fusion domain at the first sharp rise of each curve. Triton X-100 to give a final concentration of 1% disrupting the liposomes was added at the second sharp rise of each curve.
These effects were mirrored when the release of the fluorescent content markers ANTS/DPX from liposomes was measured. Release of contents did occur, albeit with low efficiency from pure POPC:POPG liposomes, but increased when cholesterol was increased up to 20 mol% (Figure 1C). No ANTS/DPX release was measured from POPC:POPS only vesicles, but more than 60% of the fluorescent dye was released from POPC:POPS liposomes containing 20 mol% cholesterol (Figure 1D). In these experiments we cannot distinguish between dye release into unloaded vesicles that fuse with loaded vesicles (contents mixing) or dye release into the surrounding buffer (pore formation). We therefore classify these experiments as measuring lipid perturbation, which could be interpreted as contents mixing or pore formation or a combination of both activities.
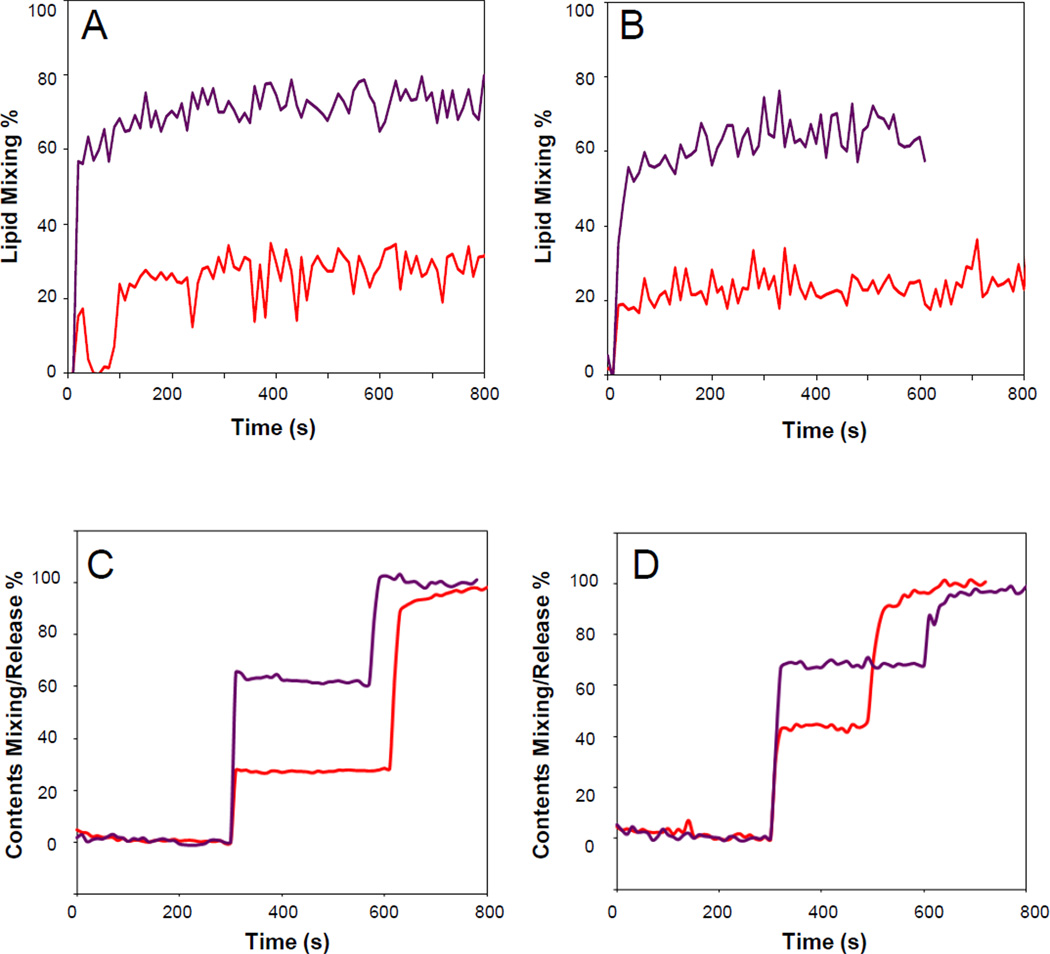
The membranes of infected cells as well as those of the HIV envelope are more complex and contain additional lipid components 19. Besides PS, the most abundant acidic lipid is PI. Therefore, we measured lipid mixing and contents release with vesicles composed of POPC:POPS:PI (12:2:1) with and without 33 mol% cholesterol (Figure 2A and C). Just as with the vesicles containing only PC and PS, cholesterol dramatically increased lipid mixing and contents release. However, basal contents release in the absence of cholesterol was higher than in the vesicles lacking PI. The most complex lipid mixture that we examined in this work was composed of POPC:POPE:SM:POPS:PI (20:5:2:2:1) with and without 33 mol% cholesterol. This mixture with cholesterol should represent the viral and cell membrane compositions quite closely and has been termed “LM3” in several papers from the Weliky laboratory. In addition to the previously discussed lipids, the mixture contains the raft-forming lipid sphingomyelin and the fusion-promoting lipid POPE. Again, basal lipid mixing in the absence of cholesterol was quite low, but increased significantly in the presence of 33 mol% cholesterol (Figure 2B). Contents release from vesicles with this complex lipid mixture was quite significant even in the absence of cholesterol (about 45%), but further increased to about 70% when 33 mol% cholesterol was present in the mixture (Figure 2D). The fusion and contents release results are summarized in Table 1.
Fig. 2.
Effect of cholesterol on HIV gp41 fusion domain-induced lipid mixing (A,B) and release of contents (C,D) between and from liposomes composed POPC:POPS:PI (12:2:1) (A,C) or POPC:POPE:SM:POPS:PI (20:5:2:2:1) (B,D) without (red) and with 33% (purple) cholesterol. 10% of the large unilamellar liposomes were labeled with 1% each of NBD-PE and Rhodamine-PE (A,B) or 16.7% were labeled with 15 mM ANTS and 45 mM DPX (C,D) and the rest were unlabeled. Fusion was induced by the addition of 5 µM HIV gp41 fusion domain at the first sharp rise of each curve. Triton X-100 to give a final concentration of 1% disrupting the liposomes was added at the second sharp rise of each curve.
Table 1.
Summary of extents of fusion and contents release of vesicles composed of various phospholipids and cholesterol by the HIV gp41 fusion domain and its secondary structure in bilayers of corresponding lipid compositions.
| Lipid Composition |
% Negative Lipids |
% Cholesterol | % Lipid Mixing |
% Contents Mixing/Release |
Predominant Secondary Structure |
|---|---|---|---|---|---|
| POPC:POPG (4:1) | 20 | 0 | 55 | 22 | α-helix |
| 10 | 60 | 33 | α-helix | ||
| 20 | 60 | 70 | α-helix | ||
| 30 | 70 | β-structure | |||
| POPC:POPS (4:1) | 20 | 0 | 15 | 4 | α-helix |
| 10 | 60 | α-helix | |||
| 20 | 65 | 63 | α-helix | ||
| 30 | 80 | β-structure | |||
| POPC:POPS: PI (12:2:1) | 20 | 0 | 30 | 27 | α-helix |
| 33 | 70 | 62 | β-structure | ||
| POPC:POPS: SM:POPS:PI (20:5:2:2:1) | 10 | 0 | 23 | 45 | α-helix |
| 33 | 55 | 68 | β-structure |
Secondary Structure of the Fusion Domain in Bilayers of Different Lipid Composition
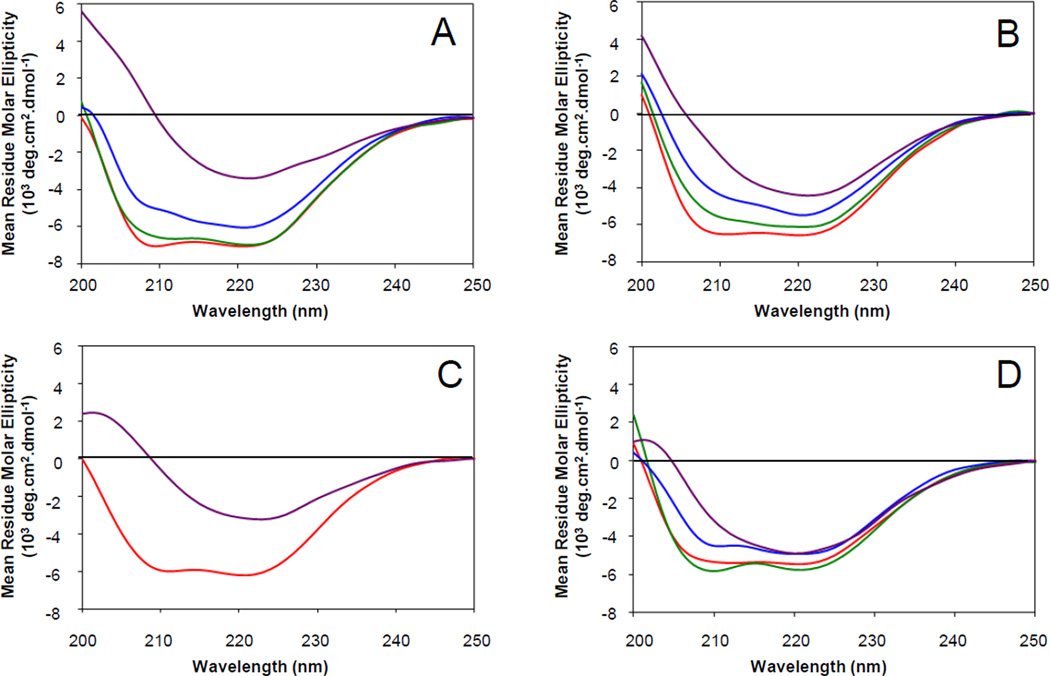
Since the activity of the HIV gp41 fusion domain depends so critically on the lipid composition of the liposomes they interact with, we wanted to know what secondary structures the fusion domains adopt in bilayers of the same composition and whether these structures would correlate with their activities. To this end, we recorded CD spectra of the fusion domains bound to small unilamellar vesicles at a lipid:protein ratio of 100. This ratio is somewhat higher than the nominal ratio of 40 used in the fusion experiments. However, the absolute protein concentration is 20 fold lower in the fusion (5 µM) than in the CD experiments (100 µM), which likely decreases the amount of protein that is actually bound to the membranes in the fusion experiments. Therefore, the actual lipid:protein ratio in the fusion experiments may also be on the order of 100 or even higher. Figure 3A shows CD spectra of the fusion domain bound to bilayers composed of 80% POPC and 20% POPG with increasing mol% cholesterol. The spectrum taken in the absence of cholesterol shows two minima at 208 nm and 222 nm that are typical for α-helical secondary structure. The α-helical character decreases with increasing cholesterol and the spectrum at 30 mol% cholesterol has the characteristics of a mixture of α-helix and β-sheet that is dominated by β-sheet. CD spectra of pure β-sheets typically have a single minimum at 216 nm. It is likely that the 30 mol% cholesterol spectrum also represents some aggregated or fused larger lipid/protein complexes because increased light scattering of such structures can distort and artificially elevate the signal in the very far UV region (200 to 215 nm) of the spectrum. Very similar responses to adding cholesterol were found with bilayers containing 20% POPS instead of 20% POPG (Figure 3B). Adding PI to the POPC:POPS mixture did not change the predominantly α-helical CD signal, which was again changed to a predominantly β-sheet spectrum upon the addition of 33 mol% cholesterol (Figure 3C). The complex biological lipid mixture composed of POPC:POPE:SM:POPS:PI (20:5:2:2:1) still showed a predominantly α-helical CD spectrum in the absence of cholesterol. When cholesterol was added to this mixture, the helical CD spectra changed less dramatically than in the other less complex lipid mixtures, but eventually also converted to a mixture with a significant amount of β-sheet secondary structure (Figure 3D). In cases where we observed mixed α-helical and β-sheet signals, we think that two populations of fusion domains more likely swap entirely between helical and sheet conformations because the sequences are probably too short to harbor both secondary structures at the same time. Qualitative assignments of secondary structures of the HIV gp41 fusion domain bound to membranes of different lipid compositions are listed in Table 1 along with the fusion and contents release results.
Fig. 3.
Effect of cholesterol on secondary structure of HIV gp41 fusion domain in lipid bilayers of different lipid compositions. Far-UV circular dichroism spectra of HIV gp41 fusion domain bound to small unilamellar vesicles at a peptide:lipid ratio of 1:100 were recorded at room temperature. The lipid bilayers were composed of (A) POPC:POPG (4:1), (B) POPC:POPS (4:1), (C) POPC:POPS:PI (12:2:1), or (D) POPC:POPE:SM:POPS:PI (20:5:2:2:1) with 0% (red), 10% (green), 20% (blue), 30% (A and B, purple), or 33% (C and D, purple) cholesterol.
Fusion Domain Dynamics and Accessibility by EPR Spectroscopy
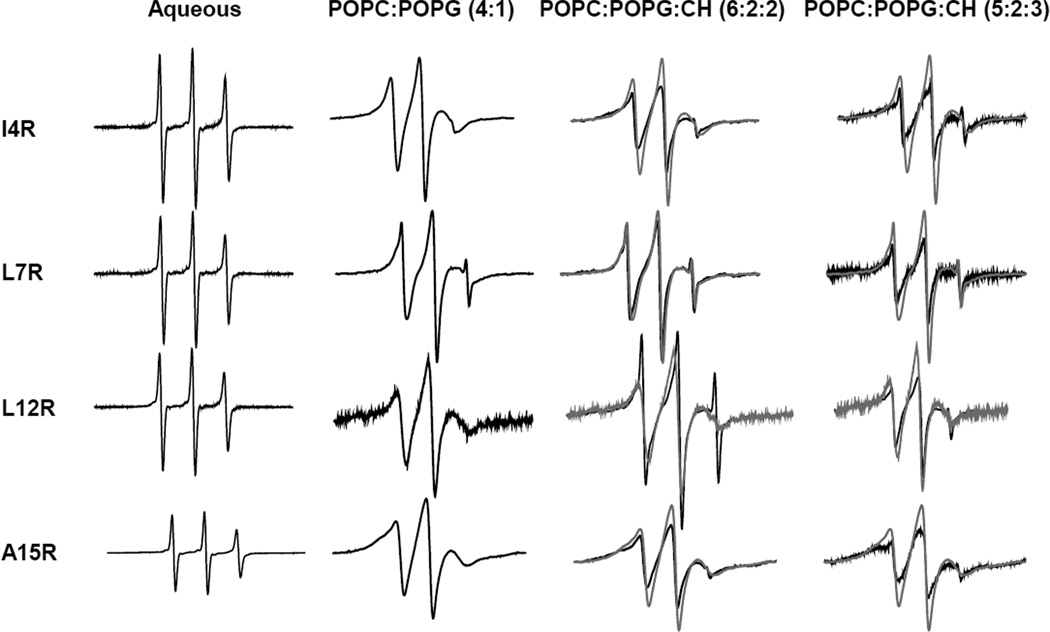
EPR signals of nitroxide spin labels attached to cysteines of membrane-bound proteins are sensitive reporters of the backbone and side-chain dynamics of the labeled location in the polypeptide chain. In order to probe these properties of the HIV gp41 fusion domain in different lipid environments, we individually labeled the fusion domain with the spin label MTSL in four different positions corresponding to residues numbers 4, 7, 12, and 15. All labeled positions are natively hydrophobic residues on the most hydrophobic side of the helical structure of the fusion domain in DPC 11. When EPR spectra of the fusion domain were recorded in aqueous solution, all four sites exhibited sharp lines indicative of fast local and overall tumbling of the nitroxides (Figure 4). When these proteins were bound to bilayers composed of 80% POPC and 20% POPG, all positions exhibited similarly broadened spectra without significant component features in the three resonance lines. This is typical for proteins that bind in a unique conformation to lipid bilayers. For example, we would expect this result if the fusion domains inserted as α-helices as expected from our CD spectra recorded at the same temperature and in the same lipid system. When the fusion domains were bound to bilayers that additionally contained 20 or 30 mol% cholesterol, additional sub-features appeared in the spectra that were furthermore not uniform over all four sites (Figure 4). The least changed spectra are perhaps those of the fusion domain labeled in position 7. The spectra of the proteins that were labeled at the other sites indicated the presence of two components – a sharper and a broader component – that are clearly seen in the high and low field lines of the spectra. Consistent with the CD results in these lipid mixtures, the fusion domains adopted mixed conformations presumably representing co-existing α-helical and β-sheet components.
Fig. 4.
Effect of lipids and cholesterol on EPR spectra of spin-labeled HIV gp41 fusion domain with the MTSL spin label attached at four positions that were mutated to cysteines as indicated. Fusion domains were solubilized in buffer or bound at a peptide:lipid ratio of (at most) 1:900 to 100 mM large unilamellar vesicles composed of POPC:POPG (4:1), POPC:POPG:cholesterol (6:2:2), or POPC:POPG:cholesterol (5:2:3). The gray lines in the spectra taken in the presence of cholesterol are spectra taken in POPC:POPG (4:1) for comparison.
Samples of the same lipid compositions with and without cholesterol were treated with molecular nitrogen or NiEDDA or left untreated, which means that they contained molecular oxygen that is naturally present in untreated solutions. Saturation of the EPR signals was then measured in each of these samples by gradually increasing the microwave power. It is well established that due to Heisenberg spin exchange between nitroxide spin labels and paramagnetic oxygen or nickel, the EPR signals saturate at different microwave power depending on whether the nitroxides are surrounded by these agents or not 22. Since O2 preferentially partitions into the hydrophobic core of lipid bilayers and since NiEDDA is highly water-soluble and excluded from lipid bilayers, the susceptibility of each site to power saturation by Ni2+ or O2 provides a good measure of the insertion depth of that site into the membrane. The insertion depths can be determined quantitatively by measuring the Φ values at each site, which are the ratios of the power saturation change with O2 over the power saturation change with Ni2+. Experimental Φ values can then be converted to actual distances by calibration with lipid spin labels that are introduced in control experiments in the same lipid systems. Experimentally determined Φ values for all four sites of the HIV gp41 fusion domain in lipid bilayers of the three lipid compositions are shown in Table 2 along with calibration values of spin-labeled fatty acids obtained in corresponding lipid systems. It is evident from these data that all four spin-labeled sites penetrate into lipid bilayers that lack cholesterol to a similar depth that approximately corresponds to the level of carbon-5 of nitroxide-labeled control fatty acids. This depth is about 8 Å below the level of the phospholipid phosphates at the bilayer surface. In the presence of 20 mol% cholesterol the penetration depths of all four labeled sites are much shallower and all four sites are found to reside in the headgroup region of the bilayer. Interestingly, the spin probes insert more deeply at 30 mol% than at 20 mol% cholesterol, but not as deep as in the absence of cholesterol when the fusion domain is inserted in an α-helical conformation. In summary, cholesterol appears to squeeze the fusion domains out of the membrane and thereby convert some of its α-helical to a β-sheet conformation. When larger fractions of fusion domains are converted to non-helical structures at higher cholesterol, the larger aggregates reverse their course and again penetrate more deeply into the lipid bilayer.
Table 2.
Φ value of spin-labeled HIV gp41 fusion domains and spin-labeled lipids in bilayers with different concentrations of cholesterol
| Spin-labeled protein or lipid |
Cholesterol content in bilayer | ||
|---|---|---|---|
| 0 mol% | 20 mol% | 30 mol% | |
| I4R | 2.31 | 0.098 | 1.76 |
| L7R | 2.13 | −0.53 | 1.14 |
| L12R | 2.11 | −0.073 | 1.19 |
| A15R | 2.2 | 0.15 | 2.34 |
| 5‘-doxyl | 2.23 | 1.88 | 1.73 |
| 7‘-doxyl | 2.51 | 3.8 | 3.69 |
| 10‘-doxyl | 2.78 | 4.37 | 4.42 |
| 12‘-doxyl | 3.29 | 5.56 | 5.64 |
Docking of α-Helical HIV gp41 Fusion Domain to Lipid Bilayers
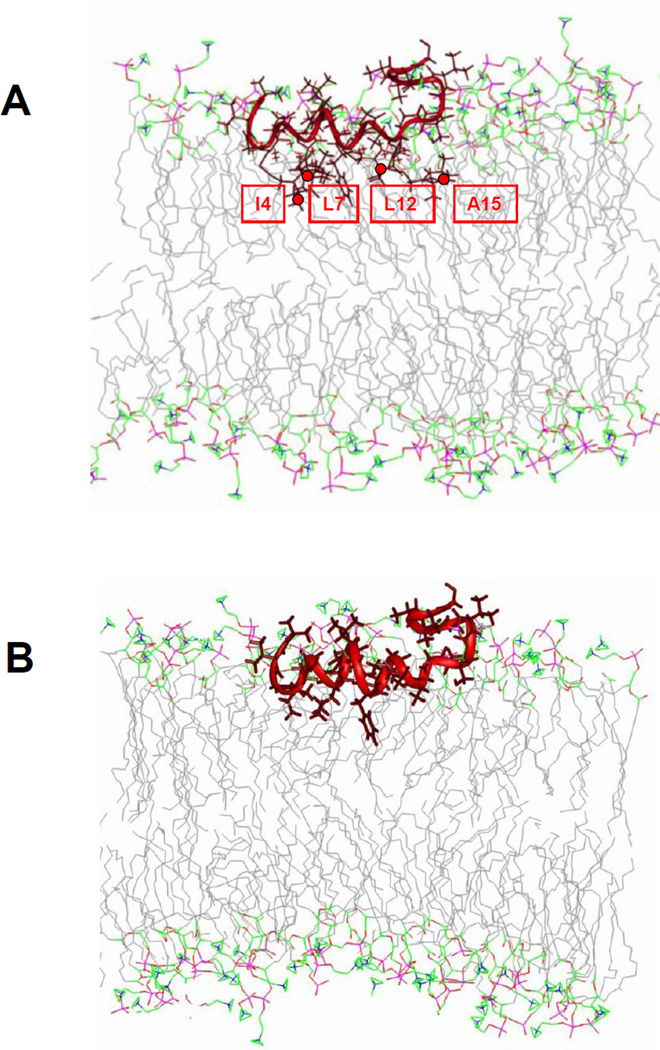
The power saturation data can be used to dock the structure of the HIV gp41 fusion domain to lipid bilayers. Since the fusion domain adopts heterogeneous conformations in the presence of cholesterol, we restrict our docking attempts to the situation in POPC/POPG bilayers without cholesterol where the fusion domain appears to adopt a homogeneously α-helical conformation that is likely very similar to the helical structure observed by NMR in DPC micelles 11. We used the same procedures that previously have proven successful for docking the NMR structures of the influenza hemagglutinin fusion domain and several of its mutants to lipid bilayers. Briefly, the four nitroxides were computationally incorporated into the lowest energy HIV gp41 fusion domain conformer (PDB accession code 2PJV). This structural model was then translated and rotated in a model lipid bilayer until the best fit between the model and the experimentally determined Φ values was found by least squares analysis. The outcome of this docking procedure and the resulting structural model in a lipid model membrane are shown in Figure 5. Not surprisingly, the helix was inserted approximately horizontally into the lipid bilayer and its backbone remained largely submerged below the level of the headgroup phosphates at the bilayer surface with the more hydrophobic side chains reaching into the upper hydrocarbon region of the bilayer.
Fig. 5.
Model of the α-helical HIV gp41 fusion domain docked to a lipid bilayer based on power saturation experiments performed in POPC:POPG (4:1). (A) Docked fusion domain (PDB accession code 2PJV 11) with four cysteines substituted in positions 4, 7, 12 and 15 and with nitroxide spin labels attached in all four positions. (B). Same as in (A), but with restored native HIV gp41 fusion domain sequence. The green lines represent the average positions of lipid phosphate groups relative to the fusion domains. The positions of spin label nitroxides are marked with red circles.
Discussion
Understanding how the fusion domain of HIV gp41 inserts into and perturbs lipid bilayers is important for understanding its function in promoting membrane fusion. A critical prerequisite for formulating gp41-mediated fusion mechanisms is to obtain a clear picture of its structure in lipid bilayers. Unfortunately, this structure has been controversial for many years. The two high-resolution structures of the gp41 fusion domain show the domain in α-helical conformations. However, these structures have been obtained by solution NMR in detergent micelles and there are justified concerns that the micelle environment may not be representative of a bilayer environment and that the structures in bilayers may be different. Most structural work on this domain in lipid bilayers has been conducted by solid-state NMR, which provided strong evidence for predominantly β-sheet secondary structure. However, it is important to note that the solid-state NMR methods required relatively high sample concentrations and low temperatures to obtain interpretable signals. Therefore, it was still not clear which secondary structure is physiologically conducive to membrane fusion. The current work was designed to bring some clarity to this issue by correlating fusion, secondary structure, and membrane penetration depths measurements under very similar conditions.
Perhaps surprisingly we found that both, the α-helical and β-sheet forms of the gp41 fusion domain can promote membrane fusion. The key factor that fine-tunes the conformation in lipid bilayers appears to be cholesterol. At physiological concentrations of cholesterol, the β-sheet form dominates although a fraction is still present in α-helical form. At lower cholesterol concentrations the fusion domain inserts as an approximately horizontal α-helix into lipid bilayers (Figure 5). Since the cholesterol distribution in cellular membranes is not uniform and since cholesterol is often concentrated in lipid rafts in cell membranes, the question arises as to whether fusion is initiated by the helical form in cholesterol-poor non-raft regions or by the sheet form in the more ordered cholesterol-rich raft regions of the host cell membrane. Although the experiments presented here clearly show that both forms can promote fusion, they do not provide a final answer to which form is the physiologically more relevant form. An intriguing possibility is that both forms may physiologically contribute to fusion if fusion was initiated at phase boundaries between raft and non-raft lipid domains in the cell membrane. For example, a possible scenario might be that fusion domains assemble on raft regions in sheet form, but then penetrate and traverse the bilayer in adjacent more fluid regions in their helical form. Such a scenario seems plausible because it is relatively straight-forward and energetically not too costly to traverse a lipid bilayer as an α-helix. However, it would be energetically very difficult to traverse a lipid bilayer as an open β-sheet because bare polypeptide backbones and open hydrogen bonds at the edges are energetically very costly to transfer into the hydrophobic core of a lipid bilayer 27. For example, stable integral β-sheet membrane proteins and pore-forming β-sheet toxins always form closed β-barrels when fully inserted into lipid bilayers. However, there is so far no evidence for a β-barrel structure of the HIV or any other viral fusion domains in lipid bilayers.
The plasticity and potential switching of the HIV fusion domain from a β-sheet to an α-helical structure in the course of membrane fusion would also make sense in the framework of fusion models that have implicated the involvement of the transmembrane domain at a later stage in fusion, such as, for example, for the better studied mechanism of influenza virus membrane fusion 28. In this model it has been hypothesized that the transmembrane domain interacts with the fusion domain in the merged membrane when the six helix-bundle forms at a late stage in fusion. Although there is so far no direct proof for fusion domain-transmembrane domain interaction in HIV gp41 or any other viral fusion protein, it would be difficult to envisage this interaction to take place if the fusion domain were still in a β-sheet conformation at this stage of fusion. Therefore, a conformational switch to an α-helical deeply inserted and eventually transmembrane-oriented conformation of the HIV gp41 fusion domain could facilitate a plausible fusion mechanism even if the fusion domain first assembles and inserts into membranes in a β-sheet and only later transforms to an α-helical conformation.
Conclusion
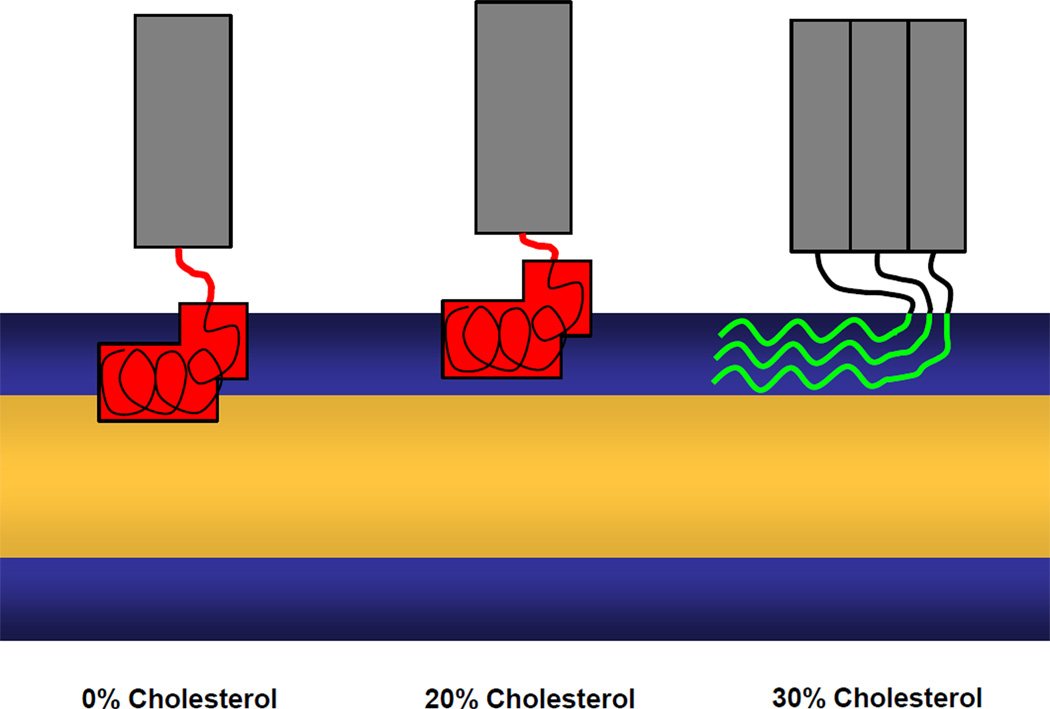
Cholesterol plays an important role in determining the secondary structure, membrane insertion and fusogenicity of HIV gp41 fusion domains. While α-helical and β-sheet promote membrane fusion, the α-helical form clearly predominates in the absence of cholesterol and the β-sheet form predominates at high cholesterol concentrations although fractions of α-helix are still found under these conditions. Intermediate levels of cholesterol produce mixtures of the two secondary structures and squeeze aggregates out of the membrane. In agreement with an earlier study, which however focused on the β-sheet form 18, depth of membrane penetration is an important factor determining the fusogenicity and membrane perturbation activity of the HIV gp41 fusion domain. An intriguing possibility is that fusion domains first assemble as β-sheets on membrane surfaces, but later convert to α-helices to complete fusion.
Materials and Methods
Materials
HIV-1 gp41 fusion domains with the sequence AVGIGALFLGFLGAAGSTMGAAS-GGGKKKKK and the four single Cys replacement mutants I4C, L7C, L12C, A15C were synthesized by solid phase synthesis by the Biomolecular Research Facility at the University of Virginia or by AnaSpec (San Jose, CA). The first 23 residues correspond to the N-terminal sequence of LAVmal strain HIV-1 gp41. The last 9 residues are a solubilization tag that has proved very useful in numerous hydrophobic fusion domain studies. Cholesterol was purchased from Sigma (St. Louis, MO). All other lipids and spin-labeled fatty acids were purchased form Avanti Polar Lipids (Alabaster, AL). ANTS and DPX were from Invitrogen (Grand Island, NY) and MTSL was purchased from Toronto Research Chemicals (Toronto, ON).
Liposomes
For liposomes containing no PI, desired amounts of lipids from chloroform stock solutions were mixed and the solvent was evaporated under a stream of nitrogen. The dispersions were dried at the bottom of glass test tubes overnight under vacuum. For liposomes containing PI, desired amount of lipids from chloroform stock solutions were mixed and first dried for 1 hour in a Rotavapor RE111 (Büchi, Switzerland), then dried overnight under vacuum. HEPES/MES buffer (5 mM HEPES, 10 mM MES, pH 7) was added to resuspend the dispersion. To prepare SUVs, the suspensions were sonicated in an ice-water bath using a titanium tip ultrasonicator (Branson) for 30 minutes at 50% duty cycle until the solution was transparent. To prepare LUVs, the suspensions were freeze-thawed 5 times before passed through two polycarbonate membranes (100 nm pore size) 19 times using a Liposofast extruder (Avestin, Ottawa, ON).
Circular dichroism spectroscopy
0.1 mg/ml fusion domains were added to SUVs in HEPES/MES buffer at a ratio of 1:100 protein:lipid and degassed for 5 min at room temperature before measurement. CD spectra were collected at 25°C on an AVIV model 215 spectropolarimeter. Two or three scans at 0.5 nm resolution were averaged for each spectrum. The signals from pure SUVs were subtracted from the sample spectra as blanks. Relative contents of secondary structures were estimated from the relative shapes and intensities at 208 and 222 nm for minima of α-helices and 216 nm for the minimum of β-sheets. Since samples may not be completely monodisperse and since it is difficult to measure ellipticities below 200 nm, we avoided using spectral deconvolution to attempt to extract precise amounts of secondary structure because solutions using these procedures are not unique.
Lipid mixing
Lipid mixing of membrane fusion was measured by FRET using a Fluorolog-3 spectrofluorometer (Jobin-Yvon, Edison, NJ). For each lipid composition, unlabeled and labeled LUVs were prepared. To prepare the labeled LUVs, 1% each of NBD-egg-POPE and Rhodamine-egg-POPE were included in the lipid mixture before drying the lipids in the liposome preparation procedure. Unlabeled and labeled LUVs were mixed at a 9:1 ratio in HEPES/MES buffer. The total lipid concentration was 0.2 mM. After equilibration of the vesicles, an appropriate amount of protein was added from a concentrated stock solution to give a 5 µM final concentration. 10% Triton X-100 was added to achieve a 1% final concentration after fusion was complete. Fluorescence intensities before the addition of fusion domains and after the addition of Triton X-100 were defined as 0 and 100% fusion, respectively. All experiments were performed at least 3 times and representative curves are shown.
Contents mixing/release
Contents mixing and leakage was measured by fluorescence dequenching using a Fluorolog-3 spectrofluorometer. To prepare LUVs with a fluorescent contents dye, lipid dispersions were resuspended with HEPES/MES buffer containing 15 mM ANTS and 45 mM DPX. After extrusion, the vesicles were dialysed in a Slide-A-Lyzer dialysis cassette with a 3500 MW cutoff size (Thermo Scientific, Rockford, IL) against HEPES/MES buffer to remove ANTS and DPX from the solution surrounding the vesicles. Lipid concentrations after dialysis were determined using an organic phosphate assay 30. Unlabeled and labeled LUVs were mixed at a 5:1 ratio in HEPES/MES buffer. The total lipid concentration was 0.5 mM. Appropriate amounts of HIV fusion domains were added to give 5 µM final concentrations. 10% Triton X-100 was added to achieve a 1% final concentration after fusion was complete. Fluorescence intensities before the addition of fusion domains and after the addition of Triton X-100 were defined as 0 and 100 % fusion, respectively. All experiments were performed at least 3 times and representative curves are shown.
Spin labeling and EPR spectroscopy
Single-cysteine fusion domain mutants were labeled with MTSL and purified as previously described 26. Briefly, 1 mg protein dissolved in 350 µl labeling buffer (HEPS/MES buffer, pH 8) and a 10 fold molar excess of MTSL in acetonitrile were mixed at a 1:1 v:v ratio and incubated at room temperature overnight in the dark. The spin-labeled proteins were purified by reversephase HPLC on a Vydac C18 column. Fractions containing the spin-labeled proteins were lyophilized. The labeling was confirmed by MALDI-mass spectrometry and was 25 to 70% efficient. Desired amounts of spin-labeled fusion domains were weighed and dissolved in HEPES/MES buffer. Final concentrations were determined by quantitative amino acid analysis. 100 µM fusion domains were mixed with LUVs at a 1:1000 ratio in HEPES/MES buffer and incubated for 15 min before collecting spectra in a Varian E-line Centuries Series spectrometer at room temperature. Power saturation EPR experiments were performed in the presence of N2, air and 20 mM NiEDDA respectively. The parameter Φ was calculated from the equation
where P1/2 is the microwave power required to reduce the resonance amplitude to half of its unsaturated value, and dHpp is the peak-to-peak line width for the central resonance of the EPR spectra. P1/2 values were obtained from fits of plots of central peak amplitudes versus applied microwave power. Φ values for 1% spin-labeled fatty acids in bilayers of 79:20 POPC:POPG were obtained in the same way.
Molecular modeling and docking of NMR structure to EPR depth data
Cysteines and MTSL were grafted onto the HIV fusion domain NMR structure (PDB accession code: 2PJV) with the program Insight II (Accelrys Inc, San Diego, CA) as previously described 21. The dihedral angles of the spin label χ1, χ2 and χ3 were set to 300°, 300°, and +90° or −90°, respectively 31. Dihedral angles χ4 and χ5 were determined using the local energy minimization function in Insight II. The membrane insertion depths of each nitroxide on the protein and the overall rotational angles and the z-axis translational distance of the protein from the membrane surface were determined by fitting by least-squares minimization the Φ values of each nitroxide on the spin-labeled fusion domain and the Φ value and membrane insertion depth of the nitoxides on spin-labeled fatty acids to a calibration curve described by equation Φ=A*tanh[B(z-C)]+D, where z is the membrane insertion depth and A, B, C, and D are adjustable fit parameters. The docked structure of the protein with the best-fit rotational angles and distance was visualized using the program Insight II.
Fig. 6.
Schematic representations of the modes of HIV gp41 fusion domain insertion into lipid bilayers containing different amounts of cholesterol based on the current work. Red, fusion domains in α-helical conformation; green, fusion domains in β-sheet conformation; gray, gp41 ectodomain.
Highlights.
HIV gp41 fusion domain is helical in lipid bilayers without cholesterol
HIV gp41 fusion domain helix inserts parallel ~8 Å below membrane surface
HIV gp41 fusion domain forms β-structure in membranes with ≥30 mol% cholesterol
α-helical and β-sheet conformations of HIV gp41 fusion domain can be fusion active
Deep membrane insertion is required for membrane fusion activity
Acknowledgements
Support for this work from grant R37 AI30557 from the National Institutes of Health is gratefully acknowledged. We thank David Cafiso (University of Virginia) for helpful comments and access to the EPR spectrometers. We dedicate this work to Harden McConnell (Stanford University) whose half-century long interest in the role of cholesterol as a physiological modulator of membrane structure and function has stimulated this work.
Abbreviations Used
- ANTS
8-aminonaphthalene-1,3,6-trisulfonic acid
- CD
circular dichroism spectroscopy
- DPX
p-xylene-bis-pyridiniumbromide
- EPR
electron paramagnetic resonance
- FRET
Förster resonance energy transfer
- FTIR
Fourier transform infrared spectroscopy
- HEPES
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid
- HIV
human immunodeficiency virus
- LUV
large unilamellar vesicle
- MES
2-(N-morpholino)ethanesulfonic acid
- MTSL
(S-(2,2,5,5-tetramethyl-2,5-dihydro-1H-pyrrol-3-yl)methyl methanesulfonothioate)
- NBD
7-nitrobenz-2-oxa-1,3-diazole
- NiEDDA
Ni(II)-ethylenediaminediacetate
- NMR
nuclear magnetic resonance
- PI
porcine brain phosphatidylinositol
- POPC
1-palmitoyl-2-oleoyl-sn-gycero-3-phosphocholine
- POPE
1-palmitoyl-2-oleoyl-sn-gycero-3-phosphoethanolamine
- POPG
1-palmitoyl-2-oleoyl-sn-gycero-3-phosphoglycerol
- POPS
1-palmmitoyl-2-oleoyl-sn-gycero-3-phosphoserine
- SM
porcine brain sphingomyelin
- SUV
small unilamellar vesicle
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai AL, Li Y, Tamm LK. Interplay of protein and lipids in virus entry by membrane fusion. In: Tamm LK, editor. Protein-Lipid Interactions. Wiley-VCH: 2005. pp. 279–303. [Google Scholar]
- 3.Melikyan GB. Membrane fusion mediated by human immunodeficiency virus envelope glycoprotein. Curr Top Membr. 2011;68:81–106. doi: 10.1016/B978-0-12-385891-7.00004-0. [DOI] [PubMed] [Google Scholar]
- 4.Lalezari JP, DeJesus E, Northfelt DW, Richmond G, Wolfe P, Haubrich R, Henry D, Powderly W, Becker S, Thompson M, Valentine F, Wright D, Carlson M, Riddler S, Haas FF, DeMasi R, Sista PR, Salgo M, Delehanty J. A controlled Phase II trial assessing three doses of enfuvirtide (T-20) in combination with abacavir, amprenavir, ritonavir and efavirenz in non-nucleoside reverse transcriptase inhibitor-naive HIV-infected adults. Antivir Ther. 2003;8:279–287. [PubMed] [Google Scholar]
- 5.Ashkenazi A, Wexler-Cohen Y, Shai Y. Multifaceted action of Fuzeon as virus-cell membrane fusion inhibitor. Biochim Biophys Acta. 2011;1808:2352–2358. doi: 10.1016/j.bbamem.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Rafalski M, Lear JD, Degrado WF. Phospholipid interactions of synthetic peptides representing the N-terminus of HIV gp41. Biochemistry. 1990;29:7917–7922. doi: 10.1021/bi00486a020. [DOI] [PubMed] [Google Scholar]
- 7.Martin I, Schaal H, Scheid A, Ruysschaert JM. Lipid membrane fusion induced by the human immunodeficiency virus type 1 gp41 N-terminal extremity is determined by its orientation in the lipid bilayer. J Virol. 1996;70:298–304. doi: 10.1128/jvi.70.1.298-304.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nieva JL, Nir S, Muga A, Goni FM, Wilschut J. Interaction of the HIV-1 fusion peptide with phospholipid-vesicles - different structural requirements for fusion and leakage. Biochemistry. 1994;33:3201–3209. doi: 10.1021/bi00177a009. [DOI] [PubMed] [Google Scholar]
- 9.Peisajovich SG, Epand RF, Pritsker M, Shai Y, Epand RM. The polar region consecutive to the HIV fusion peptide participates in membrane fusion. Biochemistry. 2000;39:1826–1833. doi: 10.1021/bi991887i. [DOI] [PubMed] [Google Scholar]
- 10.Jaroniec CP, Kaufman JD, Stahl SJ, Viard M, Blumenthal R, Wingfield PT, Bax A. Structure and dynamics of micelle-associated human immunodeficiency virus gp41 fusion domain. Biochemistry. 2005;44:16167–16180. doi: 10.1021/bi051672a. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Tamm L. Structure and plasticity of the human immunodeficiency virus gp41 fusion domain in lipid micelles and bilayers. Biophys J. 2007;93:876–885. doi: 10.1529/biophysj.106.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang J, Gabrys CM, Weliky DP. Solid-state nuclear magnetic resonance evidence for an extended beta strand conformation of the membrane-bound HIV-1 fusion peptide. Biochemistry. 2001;40:8126–8137. doi: 10.1021/bi0100283. [DOI] [PubMed] [Google Scholar]
- 13.Qiang W, Bodner ML, Weliky DP. Solid-state NMR spectroscopy of human immunodeficiency virus fusion peptides associated with host-cell-like membranes: 2D correlation spectra and distance measurements support a fully extended conformation and models for specific antiparallel strand registries. J Am Chem Soc. 2008;130:5459–5471. doi: 10.1021/ja077302m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmick SD, Weliky DP. Major antiparallel and minor parallel beta Sheet populations detected in the membrane-associated human immunodeficiency virus fusion peptide. Biochemistry. 2010;49:10623–10635. doi: 10.1021/bi101389r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saez-Cirion A, Nieva JL. Conformational transitions of membrane-bound HIV-1 fusion peptide. Biochim Biophys Acta. 2002;1564:57–65. doi: 10.1016/s0005-2736(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 16.Gordon LM, Mobley PW, Pilpa R, Sherman MA, Waring AJ. Conformational mapping of the N-terminal peptide of HIV-1 gp41 in membrane environments using (13)C-enhanced Fourier transform infrared spectroscopy. Biochim Biophys Acta. 2002;1559:96–120. doi: 10.1016/s0005-2736(01)00443-6. [DOI] [PubMed] [Google Scholar]
- 17.Qiang W, Weliky DP. HIV fusion peptide and its cross-linked oligomers: efficient syntheses, significance of the trimer in fusion activity, correlation of beta strand conformation with membrane cholesterol, and proximity to lipid headgroups. Biochemistry. 2009;48:289–301. doi: 10.1021/bi8015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiang W, Sun Y, Weliky DP. A strong correlation between fusogenicity and membrane insertion depth of the HIV fusion peptide. Proc Natl Acad Sci U S A. 2009;106:15314–15319. doi: 10.1073/pnas.0907360106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci U S A. 2006;103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Columbus L, Hubbell WL. A new spin on protein dynamics. Trends Biochem Sci. 2002;27:288–295. doi: 10.1016/s0968-0004(02)02095-9. [DOI] [PubMed] [Google Scholar]
- 21.Tamm LK, Lai AL, Li Y. Combined NMR and EPR spectroscopy to determine structures of viral fusion domains in membranes. Biochim Biophys Acta. 2007;1768:3052–3060. doi: 10.1016/j.bbamem.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altenbach C, Greenhalgh DA, Khorana HG, Hubbell WL. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: application to spin-labeled mutants of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1994;91:1667–1671. doi: 10.1073/pnas.91.5.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han X, Bushweller JH, Cafiso DS, Tamm LK. Membrane structure and fusion-triggering conformational change of the fusion domain from influenza hemagglutinin. Nat Struct Biol. 2001;8:715–720. doi: 10.1038/90434. [DOI] [PubMed] [Google Scholar]
- 24.Li Y, Han X, Lai AL, Bushweller JH, Cafiso DS, Tamm LK. Membrane structures of the hemifusion-inducing fusion peptide mutant G1S and the fusion-blocking mutant G1V of influenza virus hemagglutinin suggest a mechanism for pore opening in membrane fusion. J Virol. 2005;79:12065–12076. doi: 10.1128/JVI.79.18.12065-12076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai AL, Park H, White JM, Tamm LK. Fusion peptide of influenza hemagglutinin requires a fixed angle boomerang structure for activity. J Biol Chem. 2006;281:5760–5770. doi: 10.1074/jbc.M512280200. [DOI] [PubMed] [Google Scholar]
- 26.Lai AL, Tamm LK. Locking the kink in the influenza hemagglutinin fusion domain structure. J Biol Chem. 2007;282:23946–23956. doi: 10.1074/jbc.M704008200. [DOI] [PubMed] [Google Scholar]
- 27.Jayasinghe S, Hristova K, White SH. Energetics, stability, and prediction of transmembrane helices. J Mol Biol. 2001;312:927–934. doi: 10.1006/jmbi.2001.5008. [DOI] [PubMed] [Google Scholar]
- 28.Tamm LK. Hypothesis: spring-loaded boomerang mechanism of influenza hemagglutinin-mediated membrane fusion. Biochim Biophys Acta. 2003;1614:14–23. doi: 10.1016/s0005-2736(03)00159-7. [DOI] [PubMed] [Google Scholar]
- 29.Han X, Tamm LK. A host-guest system to study structure-function relationships of membrane fusion peptides. Proc Natl Acad Sci U S A. 2000;97:13097–130102. doi: 10.1073/pnas.230212097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ames BN. Assay of inorganic phosphate, total phosphate, and phosphatases. Methods Enzymol. 1966;8:115–118. [Google Scholar]
- 31.Langen R, Oh KJ, Cascio D, Hubbell WL. Crystal structures of spin labeled T4 lysozyme mutants: implications for the interpretation of EPR spectra in terms of structure. Biochemistry. 2000;39:8396–8405. doi: 10.1021/bi000604f. [DOI] [PubMed] [Google Scholar]