Abstract
Studies of immune system metabolism (“immunometabolism”) segregate along two paths. The first investigates the effects of immune cells on organs that regulate whole body metabolism, such as adipose tissue and liver. The second explores the role of metabolic pathways within immune cells and how this regulates immune response outcome. Distinct metabolic pathways diverge and converge at many levels and cells therefore face choices in how to achieve their metabolic goals. There is interest in fully understanding how and why immune cells commit to particular metabolic fates, and in elucidating the immunologic consequences of reaching a metabolic endpoint by one pathway versus another. This is particularly intriguing since metabolic commitment is influenced not only by substrate availability, but also by signaling pathways elicited by metabolites. Thus metabolic choices in cells enforce fate and function and this area will be the subject of this review.
INTRODUCTION
The immune system encompasses a heterogeneous population of cells that for the most part are relatively quiescent in the steady state, but share the ability to rapidly respond to infection, inflammation, and other perturbations. Responses are regulated by a broad range of cell type specific, and/or shared, activating and inhibitory receptors that are responsive to pathogen-derived or immune system intrinsic signals. The response that is mounted by immune cells typically involves changes in the expression of large numbers of genes and results in the acquisition of new functions, such as the high output production of cytokines, lipid mediators, tissue remodeling enzymes, toxic gases, and the ability to migrate through tissues and/or undergo cellular division. There is a growing appreciation of the fact that transitions between quiescent and activated states require the apportioning of nutrients into different pathways and therefore there is a strong interest in how metabolic pathways are regulated to support or direct functional changes.
The need to produce ATP to provide energy for cellular function is of course essential in both quiescent and activated cells. Glucose can be used to fuel this process through two integrated pathways. The first of these, glycolysis, involves the conversion of glucose to pyruvate in the cytoplasm. In this pathway, phosphates are transferred from glycolytic intermediates to ADP to generate ATP. The second pathway, the tricarboxylic acid (TCA) cycle, generates the reducing equivalents nicotinamide adenine dinucleotide (NADH) and flavin adenine dinucleotide (FADH2), which donate electrons to the electron transport chain to fuel oxidative phosphorylation (OXPHOS), the process by which ATP is generated in the mitochondria. Glycolysis and the TCA cycle can be integrated when pyruvate is converted into acetyl-CoA, which enters the TCA cycle. To differing degrees, cells also have the flexibility to metabolize other substrates, such as glutamine, via glutaminolysis, or fatty acids, via β-oxidation, to replenish the TCA cycle and fuel OXPHOS. Under hypoxic conditions cells can produce ATP solely by the breakdown of glucose via glycolysis, with pyruvate being diverted primarily towards lactate rather than acetyl-CoA. In some cases cells preferentially use glycolysis for ATP generation even when oxygen isn’t limiting, a process known as aerobic glycolysis or Warburg metabolism. Thus, as might be expected, cells have several options for producing ATP, and activity between different metabolic pathways will be influenced to a great extent by the relative availability of glucose, glutamine, and fatty acids, and whether there is sufficient oxygen to utilize OXPHOS. As has been pointed out before, in sufficiently fed metazoan organisms in the steady state nutrient availability is regulated centrally, and the ability of any given cell type to access nutrients will depend on their ability to express appropriate transporters and enzymes within the metabolic pathways that permit utilization of that nutrient (Thompson, 2011). The regulated expression and posttranscriptional control of pathway specific genes and/or proteins therefore assume key roles in dictating the metabolic profile of a cell under specific circumstances. In the immune system, this level of regulation is imposed by growth factor cytokines and by key activating receptors such as toll-like receptors (TLRs) on myeloid cells, and co-stimulatory receptors on T cells.
Interconnections between metabolic pathways are notoriously complex and it is therefore the case that superficially simple choices between aerobic glycolysis, or the oxidation of various substrates in the mitochondria for ATP production will have enormous ramifications on the outcome of key ancillary metabolic processes such as the pentose phosphate pathway (PPP, an offshoot of glycolysis that generates reducing equivalents in the form of nicotinamide adenine dinucleotide phosphate, NADPH, and ultimately is important for the synthesis of pentose sugars), the synthesis of fatty acids, and the production of lipid ligands for nuclear hormone receptors. Thus regulated changes in metabolism are key to function. The focus of this article is not on areas that have been thoroughly addressed in recently published reviews, but rather is centered around the growing interest in determining the extent to which, during changes in immune cell function, metabolic changes are responsive versus instructive. Further, the emphasis on T cells, macrophages and dendritic cells not only reflects our research interests, but also the fact that there is relatively little known, or nothing published, on other immune cell types.
GRANULOCYTES
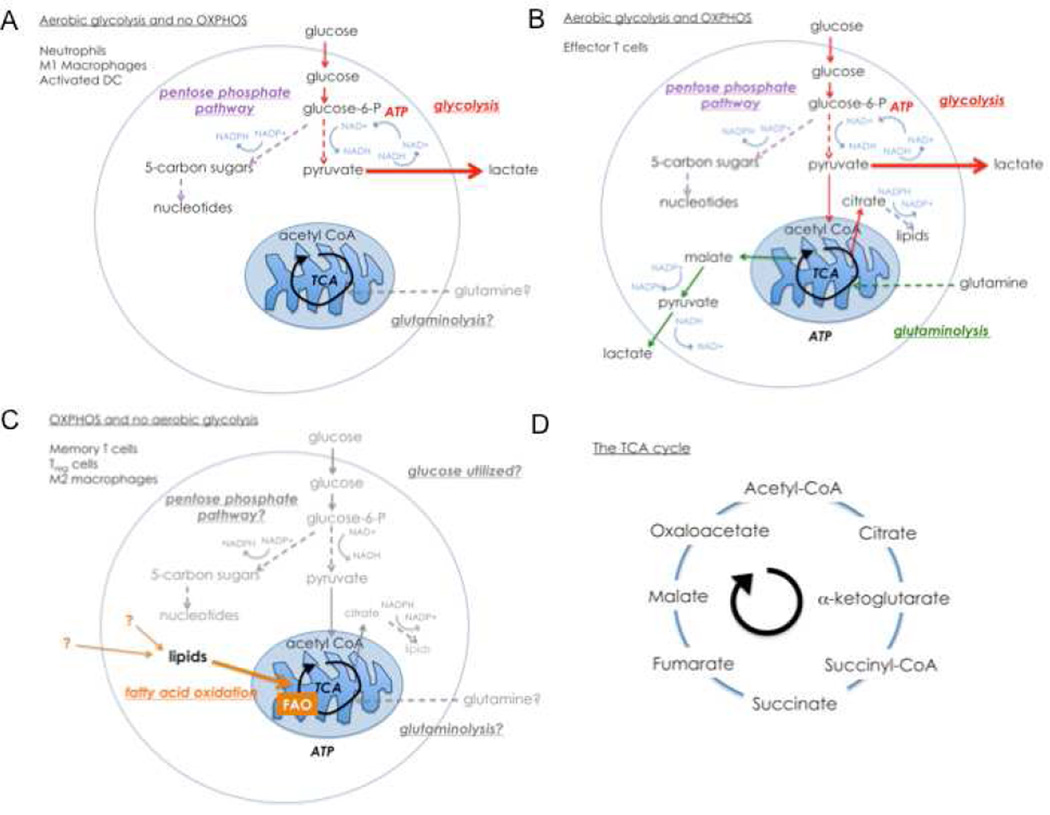
There is a general view that myeloid cells primarily use glycolysis as a source of ATP (Kominsky et al., 2010). A fine example of this axiom is provided by neutrophils, short-lived granulocytes whose primary function is to rapidly enter sites of infection and initiate microbial killing. Studies dating back to 1950s have shown that neutrophils are highly dependent on glucose for ATP production via aerobic glycolysis (Fig. 1A) (Sbarra and Karnovsky, 1959; Valentine and Beck, 1951). Consistent with this, neutrophils have few mitochondria and consume little oxygen (van Raam et al., 2006). Upon activation by TLR agonists, or phagocytosis of antibody-coated particles, neutrophils increase their consumption of glucose and oxygen (Borregaard and Herlin, 1982). However, this is not a reflection of increased oxidation of glucose in the mitochondria, but rather it is a reflection of the Warburg effect and increased activity through the PPP (Fig 1A), which generates NADPH, an essential cofactor for the NADPH oxidase, which consumes oxygen to produce the important neutrophil microbicidal product H2O2 (Dale et al., 2008).
Figure 1. Cell fate and function in the immune system is supported by engagement of metabolic pathways.
In this diagram colored arrows represent pathways that have been shown to be used in the cell types indicated, grey arrows indicate pathways that might be used, but have yet to be clearly defined, and dashed arrows indicate multiple steps shown in a single arrow. A. In activated neutrophils, M1 macrophages and iNOS expressing DCs stimulated with TLR agonists, Warburg metabolism dominates. ATP production and cellular survival are dependent on glycolysis, with the majority of pyruvate being converted to lactate. In this pro-glycolytic state, the PPP is active and provides NADPH for key microbicidal pathways regulated by NADPH oxidase. Under these conditions, there is little evidence for OXPHOS, but maintenance of mitochondrial potential and integrity are needed to maintain cell survival. B. Activated T cells engage OXPHOS and glycolysis. Most pyruvate is excreted as lactate, but some also enters the TCA cycle. Glutaminolysis is an important pathway in these cells as glutamine replenishes TCA cycle intermediates as they are withdrawn for biosynthesis. Metabolizing glucose in the PPP can yield both nucleotides and NADPH for lipid synthesis. C. Memory T cells, Treg cells, and alternatively activated macrophages use FAO for survival and to support function. Other pathways are depicted in grey as the extent to which they are used in these cells, if at all, has not been established. D. Overview of the TCA cycle.
The presence of non-functional mitochondria can be dangerous because loss of mitochondrial membrane potential can lead to the release of cytochrome c into the cytoplasm, and the initiation of apoptosis (Galluzzi et al., 2012; van Raam et al., 2006). However, despite the fact that they are not functioning to produce ATP, neutrophil mitochondria maintain their membrane potential through the glycerol-3-phosphate shuttle, a pathway that allows the receipt of electrons from glycolysis by Complex III of the electron transport chain (van Raam et al., 2008). In this way the mitochondria simultaneously contribute to redox balance and promote flux through the glycolytic pathway while avoiding commitment to the apoptotic process. This extreme commitment to Warburg metabolism may underlie the ability of neutrophils to temporarily remain viable during netosis, the process of releasing extracellular traps of mitochondrial DNA and embedded microbicidal products (Remijsen et al., 2011). The importance of glycolysis in the microbicidal functions of neutrophils is emphasized by the fact that netosis is NADPH oxidase-dependent (Kirchner et al., 2012).
Available evidence, albeit scant, indicates that eosinophils and basophils are metabolically similar to neutrophils (Sher et al., 1983; Sumbayev et al., 2009; Venge et al., 2003). A unifying feature of granulocytes is that they are terminally differentiated, and largely incapable of proliferating in the periphery. It is interesting to speculate that this aspect of their biology is directly connected to their relative lack of mitochondrial function.
DENDRITIC CELLS
Dendritic cells (DCs) are heterogeneous and can be categorized into a large and growing number of subsets (Satpathy et al., 2012). These cells are functionally united in standing at the checkpoint between innate and adaptive immunity since activated DCs participate in the initiation of inflammation and play an essential role in priming of T cell responses (Banchereau et al., 2000). Activation can be driven via an array of receptors for pathogen associated molecular patterns (PAMPS) and alarmins that allow DCs to respond to infection or other changes in the environment (Bianchi, 2007). As is the case for macrophages (Geissmann et al., 2010), during inflammation DCs can arise from monocytes, and consistent with this, there are metabolic similarities in the way that monocyte-derived dendritic cells and macrophages respond to certain stimuli. Aspects of this area have been reviewed recently elsewhere (O'Neill and Hardie, 2013).
Many studies on DC biology have utilized cells derived from bone marrow by culture in granulocyte macrophage-colony stimulating factor (GM-CSF) as a model for monocyte-derived and tumor necrosis factor-α (TNF-α) and inducible nitric oxide synthase (iNOS) producing (TiP) DCs. At rest, these cells oxidize glucose in the mitochondria, engage OXPHOS, and consequently produce relatively little lactate. However, after stimulation with TLR agonists they undergo a remarkable metabolic transformation in which they become dependent on Warburg metabolism for survival (Krawczyk et al., 2010). Signaling through phosphatidyl inositol 3-OH kinase (PI3K) and Akt is central to the regulation of glycolytic metabolism (Locasale and Cantley, 2011; Shaw and Cantley, 2006) and consistent with this, PI3K and Akt play an essential role in sustained commitment to glycolysis in activated DCs (Krawczyk et al., 2010). In cells that have been activated for >12 h, despite the fact the glucose consumption increases, glucose carbons no longer enter the TCA cycle and mitochondrial oxygen consumption ceases (Everts et al., 2012; Krawczyk et al., 2010). Rather, lactate production increases substantially and cells survive by aerobic glycolysis alone (Fig. 1A). The reason for the collapse of mitochondrial respiration in these cells is that activation leads to the expression of iNOS, which produces the toxic gas NO from arginine at a high rate (Everts et al., 2012). NO inhibits mitochondrial electron transport by nitrosylation of iron-sulfur containing proteins, including complex I (NADH-ubiquinone oxidoreductase), complex II (succinate-ubiquinone oxidoreductase), and complex IV (cytochrome c oxidase) and therefore blocks oxygen consumption and coupled ATP production (Beltran et al., 2000; Cleeter et al., 1994; Clementi et al., 1998). The general connection between reduced respiratory rate and inflammation has been recognized for some time and high output NO production by cells such as monocyte derived DCs and macrophages (described below) is the underlying cause since NO, which diffuses rapidly, is able to affect bystander cells within inflamed tissue (Rees et al., 1998). At low doses, in brain, liver, kidney and muscle cells, NO stimulates mitochondrial biogenesis, which ultimately results in an overall increase in mitochondrial ATP output (Nisoli et al., 2004). Whether dendritic cells are capable of a similar response to low NO concentrations is unknown. However, it is clear that the commitment to glycolytic metabolism in activated DCs occurs only in DC subsets that express iNOS, and that it is a direct consequence of the inhibition of OXPHOS by NO, and serves a vital survival function to provide ATP in the absence of mitochondrial ATP generation (Everts et al., 2012).
While aerobic glycolysis is clearly important for prolonged survival of activated DCs, it seems additionally likely that a rapid, early increase in flux through this pathway following stimulation with TLR agonists is important for initiating activation. We base this on the fact that 2-Deoxy-D-glucose, which inhibits glycolysis, is able to profoundly prevent early (<6 h, which is prior to the initiation of NO production) manifestations of activation when present at the time of DC stimulation (Krawczyk et al., 2010). It will be important to determine whether rapid changes in metabolism are important in other DC subsets in addition to those derived from cultured bone marrow cells.
DCs also play an important role in maintaining tolerance. This is exemplified by the situation in the gut, where regulation of potential responsiveness to the microbial flora is controlled to a considerable degree by the production of retinoic acid (RA), a small lipid metabolite of vitamin A, by gut associated lymphoid tissue (GALT) CD103+ DCs (Coombes et al., 2007). RA is produced from vitamin A by a process that is depended dependent on retinal dehydrogenases (RALDH), and is a ligand for the nuclear receptors RA receptor (RAR) and retinoid X receptor (RXR) (Nagy et al., 2012). In the steady state RA strongly promotes both transforming growth factor-β (TGF-β) dependent inducible T regulatory (Treg) cell development (Coombes et al., 2007; Mucida et al., 2007; Sun et al., 2007), and the production of IgA by B cells (Mora et al., 2006), which helps maintain intestinal barrier integrity, and therefore plays an important role in immune homeostasis in this organ. These processes are consolidated by the fact that RA induces expression of the gut homing molecules α4β7 and CCR9 in a variety of immune cells, and thereby plays a role in their retention within the intestine (Iwata et al., 2004). Paradoxically, in inflammatory environments RA also plays an important role in promoting the development of effector T cells (Hall et al., 2011). This may reflect the superimposition of inflammation-induced signals onto RAR and RXR signaling, or possibly functionally important differences in the composition of RAR and RXR heterodimers in T cells in steady state vs. inflammatory conditions.
MACROPHAGES
Macrophages are a key cell type in the immune system. They exist throughout the body as resident components of most tissues. These cells are embryonically derived, seeded into tissues in utero, and maintained by in situ proliferation (Geissmann et al., 2010; Schulz et al., 2012). During inflammation additional macrophages of hematopoietic origin develop from monocytes recruited from the bone marrow (Geissmann et al., 2010). Macrophages play crucial roles in innate immunity and can respond to local immune- and/or pathogen-derived signals to adopt different activation states. Interferon-γ (IFN-γ) in combination with TLR agonists promotes M1 (or classical) activation, whereas the cytokines IL-4 and IL-13 promote M2 (or alternative) activation (Benoit et al., 2008; Gordon, 2003; Murray and Wynn, 2011). From the host defense standpoint, M1 macrophages are inflammatory, secreting mediators such as IL-12, which promotes IFN-γ production by NK calls and T cells, TNF-α, which activates other immune cells, and NO, which is directly cytotoxic; they are implicated in the clearance of many types of microbial infections. In contrast, M2 macrophages make a range of molecules that serve to modulate inflammation, promote tissue repair, and regulate adaptive immunity. M2 activation dominates the response to helminth parasites, and is linked to resistance to these metazoan pathogens (Anthony et al., 2007). Intriguingly, M2 macrophages play another crucial role, linked to metabolic homeostasis, particularly within adipose and liver tissues (Biswas and Mantovani, 2012; Odegaard and Chawla, 2011). In lean animals, resident macrophages in these tissues express genes associated with M2 activation. Functionally, M2 macrophages serve to promote insulin sensitivity by inhibiting M1 activation and associated inflammation. In obese mice with insulin resistance, liver and adipose macrophages are M1-like and M1 products such as TNF-α are instrumental in causing insulin resistance. This area is discussed in detail in the accompanying review.
M1 and M2 activation are characterized by distinct metabolic states, which differ from those of resting macrophages (Rodriguez-Prados et al., 2010). This metabolic difference is most evident in the differential and defining difference in arginine use by these two types of cell, with M1 cells using this amino acid as a substrate for iNOS (expressed in M1, but not M2 cells), while M2 macrophages use it as a substrate for Arginase1 (which is expressed in M2, but not M1 cells) (Murray and Wynn, 2011). Metabolically, M1 macrophages have a very glycolytic metabolism and therefore are similar to activated bone marrow derived dendritic cells (Fig. 1A). Remarkably, reduced mitochondrial respiration serves an important role in the production of reactive oxygen species (ROS) for host defense. Normally we would expect NADPH oxidase to play the dominant role in this process, but it has been shown recently that in macrophages activated by TLRs expressed at the surface membrane, mitochondria are recruited to phagolysosomes in a TNF receptor associated factor 6 (TRAF6) dependent fashion, and produce ROS that are important for killing phagocytosed bacteria (West et al., 2011). The link between inhibition of OXPHOS and this process is emphasized by the fact that treatment of macrophages with the electron transport chain inhibitors rotenone and antimycin-A, mimics the effects of TLR agonists in promoting mitochondrial ROS production (West et al., 2011). Mitochondrial stress that leads to increased ROS production also plays a key role in the activation of the nucleotide-binding domain and leucine rich repeat pyrin-3 (NLRP3) containing inflammasome. In macrophages infected with certain bacteria or viruses, or exposed to danger signals such as monosodium urate or extracellular ATP, or adjuvants such as alum, there are coordinated increases in mitochondrial ROS, IL-1β, and caspase-1 production (Sorbara and Girardin, 2011; Tschopp and Schroder, 2010). The key role of mitochondria in this process has been demonstrated by the inhibition of inflammasome activation, and IL-1β and caspase-1 production, by inhibitors of mitochondrial ROS, but not by inhibitors of NADPH oxidase-dependent ROS (Bulua et al., 2011; van Bruggen et al., 2010; Zhou et al., 2011). Taken together, these findings point to an important role for mitochondria in inflammation and host-defense, a concept further supported by the (metabolically enigmatic) findings that mitochondria are a platform for the retinoic acid induced gene-1 (RIG-I) and mitochondrial anti-viral signaling (MAVS) initiated induction of type 1 interferon production in response to viral infection (Koshiba, 2013; Sun et al., 2006).
The importance of the commitment to a glucose based metabolism in activated M1 macrophages is emphasized by the fact that expression of carbohydrate kinase-like protein (CARKL), which inhibits flux through the PPP, is repressed following stimulation with TLR agonists (Haschemi et al., 2012). The PPP is essential for M1 macrophages not only because it generates NADPH for NADPH oxidase, which is important for ROS production but also because NADPH is required for NO synthesis (Aktan, 2004), and moreover reduces glutathione, which then plays a crucial role minimizing auto-oxidative damage by ROS.
Hypoxia inducible factor-1 (HIF-1) plays an important role in the commitment to glycolysis by orchestrating the decision to commit pyruvate to acetyl-CoA or to lactate (Kim et al., 2006; Papandreou et al., 2006). In order to maintain flux through the glycolysis pathway it is essential that NAD+ be regenerated from NADH. This can occur in one of two ways. In normoxic conditions when OXPHOS is active, the malate aspartate shuttle provides a means for regenerating NAD+ in the cytoplasm while at the same time producing NADH in the mitochondrial matrix to help fuel OXPHOS. However in conditions where OXPHOS is inhibited, the conversion of pyruvate into lactate becomes an essential means of regenerating NAD+ (Locasale and Cantley, 2011) (Fig. 1). HIF-1α, which is induced by hypoxia, but also by TLR agonists and proinflammatory cytokines such as TNF-α and IL-1β, supports this process by promoting the expression of lactate dehydrogenase, which is responsible for the production of lactate from pyruvate, and of pyruvate dehydrogenase kinase, which phosphorylates and thereby inhibits pyruvate dehydrogenase, an enzyme complex that converts pyruvate into acetyl CoA (Imtiyaz and Simon, 2010). Thus under conditions where OXPHOS is inhibited, HIF-1α, promotes aerobic glycolysis. As discussed above, increased glycolytic flux is essential for macrophage and DC activation and consistent with this, HIF isoforms have been shown to be crucial for activation in both cell types (Imtiyaz et al., 2010; Jantsch et al., 2008).
In addition to changes in core ATP generating pathways, TLR-initiated signaling induces alterations in macrophage lipid metabolism that are of interest because they are both extensive and integral to the induction and resolution of inflammation (Andreyev et al., 2010a; Andreyev et al., 2010b; Anthony et al., 2007). For example, M1 activation leads to increased metabolism of arachidonic acid, with the production of eicosanoids including the generally inflammatory leukotrienes and prostanoids (prostaglandins, prostacyclins and thromboxanes) (Greene et al., 2011). TLR agonists also lead to increased metabolism of eicosapentaenoic and docosahexaenoic acids with the synthesis of nonclassic eicosanoids like resolvins and lipoxins, which tend to have anti-inflammatory properties (Serhan et al., 2011). The eicosanoids exert their diverse effects on other cells via G-protein coupled receptors (GPCRs) that are differentially expressed (Hirata and Narumiya, 2012; Serhan et al., 2011). IL-4 can counter some of these effects by inhibiting, for example, the TLR agonist induced expression of cyclooxygenase-2 (COX2), which along with constitutively expressed COX1 catalyzes one of the first steps in prostanoid synthesis (Endo et al., 1998; Raetz et al., 2006). Thus a balance of external signals, coupled with temporal changes in the pattern of expression of different mediators from lipid substrates, will have profound effects on immune cell functions and the successful initiation and conclusion of inflammation. The magnitude of the effects mediated by these pathways is attested to by the clinical importance of drugs that target them for the treatment of, for example, pain and asthma (reviewed in (Greene et al., 2011)). Recent insights have revealed additional effects of lipid metabolism on inflammation induced by TLR agonists. In M1 macrophages there are increases in the concentration of the cholesterol precursor desmosterol. Suppression of expression of DHCR24, which catalyzes the synthesis of desmosterol, is found to result in increased expression of inflammatory genes associated with M1 activation in mice fed a high fat diet (Spann et al., 2012). Desmosterol is a ligand for the nuclear receptor LXR, and the intrinsic anti-inflammatory effects of desmosterol has been found to be at least in part LXR-dependent. This is consistent with earlier reports that LXR can inhibit TLR4-initated macrophage activation by preventing epigenetic remodeling of TLR4-responsive regulatory elements (Ghisletti et al., 2007; Kidani and Bensinger, 2012).
M2 macrophages are the ying to the M1 yang. They have higher basal mitochondrial oxygen consumption rates than either resting macrophages or, of course, M1 macrophages. This reflects IL-4 and signal transducer and activator of transcription-6 (STAT6) driven expression of PGC-1β, an important transcriptional regulator of oxidative metabolism genes (Vats et al., 2006). Consistent with the induction of OXPHOS by IL-4, M2 macrophage development is profoundly prevented by inhibition of mitochondrial OXPHOS, and specifically by inhibition of fatty acid oxidation (FAO). Thus M2 metabolism is skewed heavily towards the use of FAO and mitochondrial respiration to meet functional needs (Vats et al., 2006) (Fig. 1C). A fundamental difference between M1 and M2 macrophages is that, under certain circumstances, M2 macrophages are able to proliferate (Jenkins et al., 2011), and although the issue has not been addressed experimentally, it makes sense to consider the metabolic differences between the two activation states as being instrumental in this divergence of function. As discussed in more depth below, mitochondrial ROS are important for antigen-driven T cell proliferation, and a similar relationship between proliferative capacity and mitochondrial activity in M2 macrophage proliferation would be consistent with the differences in OXPHOS between these and M1 cells. Additionally however proliferating macrophages must face metabolic demands specific to the requirements of daughter cell production, which clearly would require anabolic pathways. It is currently unclear how this fits with the facts that: 1) M2 macrophages primarily use catabolic pathways such as FAO, and 2) IL-4 induces the expression of CARKL, which inhibits flux through the PPP (Haschemi et al., 2012). This is an interesting area for future study.
There has been considerable interest in the role of peroxisome proliferator-activated receptors (PPARs) in M2 macrophages. PPARs are a family of nuclear receptors for fatty acid ligands. They heterodimerize with RXR and interact with transcriptional coactivators to control the expression of genes involved in fatty acid metabolism (Nagy et al., 2012). Consistent with the noted importance of FAO in M2 macrophages, PPARδ, which is responsible for promoting the expression of genes involved in substrate oxidation and oxidative phosphorylation, has been found to be essential for M2 differentiation (Kang et al., 2008; Odegaard et al., 2008). Expression of this PPAR is increased in M2 macrophages from mice exposed to parasitic helminths, and these cells additionally contain high amounts of PGI2, an eicosanoid ligand for PPARδ (Thomas et al., 2012). It seems therefore that M2 activation can be consolidated metabolically through the production of endogenous ligands for PPARδ. Moreover, PPAR response elements are abundant and over-represented in genes induced in M2 macrophages, supporting the view that PPARs are important transcription factors that drive this activation state (Thomas et al., 2012).
T CELLS
Given the critical nature of T cells in clearing or controlling infections and cancer, as well as mediating protective immunity over the long-term, it is logical that a considerable effort is made to target these cells for therapeutic purposes. However, while metabolism underlies the fate and function of T cells, or of any immune cell for that matter, metabolic interventions for manipulating immunity are rare and can be considered to represent a largely untapped opportunity. T cells differ from innate cells in many aspects, but perhaps their ability to extensively and rapidly proliferate upon activation is what sets them apart from other immune cells, at least for the purposes of this discussion. These attributes of activated T cells has led to their ‘metabolic’ comparison with tumor cells, as both cells types have been shown to engage Warburg metabolism when proliferating (Fox et al., 2005; Jones and Thompson, 2007; van Bruggen et al., 2010; Vander Heiden et al., 2009) (in contrast to most innate cells which engage Warburg metabolism upon activation, but do not proliferate). Another characteristic specific to cells of the adaptive immune system is their ability to generate long-lived antigen-specific memory cells that mediate protection against re-infection or tumor re-emergence. Memory T cells have a very different metabolism from their activated effector T cell counterparts in that they do not use aerobic glycolysis, but rely on mitochondrial FAO for development and persistence (Figure 1) (van der Windt et al., 2012).
When a naïve T cell recognizes antigen in the context of proper co-stimulation it undergoes a developmental program characterized by rapid growth, proliferation, and acquisition of specialized effector functions. This is an energetically demanding process that requires metabolic reprogramming. For cells to grow and proliferate they must change from a catabolic metabolism to an anabolic metabolism because unlike in a resting cell, nutrients will no longer be utilized for maintenance and homeostasis, but rather will be incorporated into biomass for new daughter cells. Similarly, T cells destined to become memory cells must maintain, or adopt, a catabolic metabolism, a feature that underlies their quiescence and longevity, and may even serve to impede their terminal differentiation. There is a growing appreciation for the importance of metabolic reprogramming in immune cells. How molecules like mammalian target of rapamycin (mTOR), P13K, Akt, Myc, and HIF link immune signals and metabolic cues for the activation, development, function, and maintenance of T cells, has been reviewed in-depth elsewhere (Hirata and Narumiya, 2012; Seth et al., 2006; Waickman and Powell, 2012; Wang and Green, 2012a, b). More specifically, metabolic pathways can influence the development of various T helper subsets and this has also been the subject of several reviews (Chi, 2012; Gerriets and Rathmell, 2012; Zemirli and Arnoult, 2012). For example, Treg cells predominantly use OXPHOS and mitochondrial FAO for development and survival (Michalek et al., 2011), while the generation of T helper-17 (Th17) cells requires glycolysis (Shi et al., 2011). While these findings are well documented, it remains unclear precisely why these cells must adopt these very different metabolic phenotypes for their differentiation. In this discussion we hope to provide insight into why changes in metabolism occur in T cells, the challenges to consider, and what remains unanswered in this developing field.
While the use of heavy-labeled substrates has allowed a first look into how T cells utilize nutrients, much work still needs to be done to understand 1) what specific nutrients are actually providing to the cells, and 2) how substrate utilization occurs in vivo. For example, while it known that both glucose and glutamine are critical nutrients for T cells, precisely what they provide to the T cell, especially in vivo during an immune response, is not completely understood. Glucose can be used as an example. As introduced above, the breakdown of glucose in the glycolysis pathway generates ATP in the cytoplasm, but its oxidation in the mitochondria can generate even more ATP through the production of reducing equivalents (NADH and FADH2) to power OXPHOS. Once glucose enters the TCA cycle (as pyruvate-derived acetyl CoA), it can also act as a precursor for biosynthesis. For example, glucose-derived citrate can be exported from the mitochondria to the cytosol for the synthesis of fatty acids. In addition, glucose can also enter the PPP at a branch point early in the glycolysis pathway. The PPP is important for the production of 5-carbon sugars for nucleotide biosynthesis, as well as for the production of NADPH, which is a critical coenzyme for fatty acid synthesis, and for the regeneration of reduced glutathione. For the continued breakdown of glucose in the glycolysis pathway NAD+ is needed. Pyruvate can be excreted as lactate from the cell as way to regenerate cytosolic NAD+, which can then be utilized to support the glycolysis pathway. However, NAD+ can also be exported from the mitochondria via specialized shuttles, and in theory, the continued breakdown of glucose could also be maintained without the excretion of lactate. Therefore, by considering the fate of glucose in the cell, even in this most basic way, it becomes readily apparent that precisely how this substrate is utilized is exceedingly complex. Now if we consider not only how, but also why, a specific substrate is metabolized into a particular pathway the picture becomes even more complicated. For example, glucose may be directed to the PPP for the purpose of NAPDH production or for nucleotide synthesis. If nucleotides are not needed, then is NADPH produced because the cell needs to elongate fatty acids, make cholesterol, or to reduce glutathione? We can further layer these types of questions with the complexities that are inherent in vivo during an immune response, such as differences in substrate availability in various tissues, inflammatory signals, competition for substrates from other immune and non-immune cells, and how each of these alters the local balance of nutrients and growth factor signals. Thus, even though the basic principles have been elucidated, the question of how metabolic processes influence functional and developmental outcomes remains far from clear. Given the fact that metabolism controls the function and fate of T cells, continued research in this area is warranted.
In addition to generating energy and reductive power, and supporting biosynthesis, metabolic pathways also control other key cellular processes. In this context, recent studies have shed light on the requirements for mitochondrial processes versus cytosolic metabolic pathways in fueling T cell activation, survival, proliferation, and effector functions. An example illustrating this idea is found in a recent study that shows a critical role for mitochondrial ROS in T cell activation (Sena et al., 2013). T cells from mice with a T-cell specific reduction in a subunit of the mitochondrial electron transport chain complex III, which leads to a reduction in ROS from this complex, is required for the activation of NFAT and subsequent IL-2 production. Interestingly, T cells in these mice were not able to undergo antigen-specific expansion in response to infection, even though they were able to proliferate in a lymphopenic environment. These observations allowed the authors conclude that cells with reduced mitochondrial ROS are not lacking bioenergetically, as they were able to undergo homeostatic proliferation, but rather lack ROS-dependent signaling mechanisms needed for antigen-specific T cell activation and subsequent clonal expansion. These results highlight how metabolic pathways can act as signaling mechanisms to control cellular processes other than energy production or biosynthesis directly. Emerging work from our groups also suggests that a switch to, or induction of, aerobic glycolysis is not a requirement for T cell activation, and that this process actually requires mitochondrial OXPHOS. The observation that proliferating cells, such as tumor cells and activated T cells (and bacteria and yeast), use aerobic glycolysis has contributed to the idea that engagement of this pathway is a prerequisite for, or occurs hand in hand with, cellular proliferation. However, an overlooked fact is that most cells in the innate immune system adopt Warburg metabolism upon activation, but do not proliferate (as discussed above).
While there has been considerable interest in the metabolic demands of T cell activation, less time has been spent addressing how changes in metabolism contribute to the contraction of antigen-specific cell populations after the peak of the effector response and the generation of stable memory T cells. Our published work has shown that memory T cells rely on mitochondrial FAO for their development and long-term survival (Pearce, 2010; Pearce et al., 2009; van der Windt et al., 2012; van der Windt and Pearce, 2012). In contrast to effector T cells, which use glycolysis and have reduced mitochondrial mass, memory T cells maintain greater mitochondrial mass, which supports their oxidative metabolism (van der Windt et al., 2012). These findings led us to propose a model in which most effector T cells die during contraction due to their bioenergetic instability, i.e. their lack of mitochondrial maintenance renders them unable to support OXPHOS when infection associated signals that maintain glycolysis decline. One picture that seems to emerge from these findings is that substantial mitochondrial respiratory capacity (due to increased mitochondrial mass, maintenance, or simply the reliance on mitochondrial pathways?) is linked to cellular longevity. Preliminary studies from our laboratory indicate that memory T cells do have more mitochondrial mass than their naïve counterparts and that this enhanced mitochondrial mass imparts memory T cells with a bioenergetic advantage that rapidly fuels subsequent re-activation. These findings would be consistent with the idea that T cell activation requires OXPHOS. Given that memory T cells maintain more mitochondria than naïve T cells it will be interesting to determine where stem cell memory T cells fall in this range (Gattinoni et al., 2011; Zhang et al., 2005). Stem cell memory T cells are more differentiated than naïve T cells, but less than central or effector memory T cells (Gattinoni et al., 2012), therefore we might speculate that they have substantial mitochondrial spare respiratory capacity that supports their persistence and ability for self-renewal. If this were the case then increased mitochondrial biogenesis, which is known to occur immediately after T cell activation (D'Souza et al., 2007) might represent one of the first developmental steps in the generation of stem cell memory T cells, whereas those cells that continue to rapidly differentiate toward the effector state would not maintain mitochondrial numbers or capacity to the same extent or in the same way. In this context, future investigation of mitochondrial dynamics will be needed to determine whether terminal differentiation controls, or is simply marked by bioenergetic imbalance.
When considering these issues it is also worth drawing attention to the fact that effector T cells do also maintain OXPHOS in conjunction with glycolysis, even after full activation is attained (Sena et al., 2013; van der Windt et al., 2012; Wang et al., 2011). However, whether this is a requirement for continued proliferation or function is not clear. In addition, it is not known whether OXPHOS is engaged in these cells is only for the ROS signaling that is needed for proliferation (Sena et al., 2013; Weinberg et al., 2010), or also for other purposes such as powering ATP production directly, the regeneration of NADH to NAD+ to support TCA cycling and production of biosynthetic precursors, or to maintain mitochondrial integrity. Establishing the all of the reasons why particular metabolic pathways are used will be key to developing techniques that target metabolism for immunotherapy.
B CELLS
Our discussion of metabolism in the adaptive immune system has focused on T cells. While B cells do share certain fundamental metabolic characteristics with T cells, such as their increased glucose uptake and induction of glycolysis after activation (Doughty et al., 2006; Dufort et al., 2007), much less is known about how metabolism directs the fate of normal B cells (a considerable amount of work has been done to investigate metabolic pathways in cancerous B cells). Where gaps do exist in the knowledge about B cell metabolism, one might be able to fill in some blanks by considering parallel events in T cells. For example, can we speculate that short-lived plasma cells and memory B cells have similar metabolic profiles as effector T cells and memory T cells, respectively? Given the similarity in the functional capacity and life span of these cell types this would seem a reasonable assumption. However, during an immune response, B cells can also differentiate into long-lived plasma cells, which survive within the bone marrow for years, continuously synthesizing large amounts of antibody to maintain physiologically relevant concentrations in plasma. Our models of metabolism in immune cells do not easily explain how plasma cells are able to combine longevity and high output biosynthesis in this way, and these cells clearly represent an important, although challenging subject for further work.
TO WHAT EXTENT DO MICROENVIRONMENTS RESTRICT CELLULAR METABOLIC CHOICES?
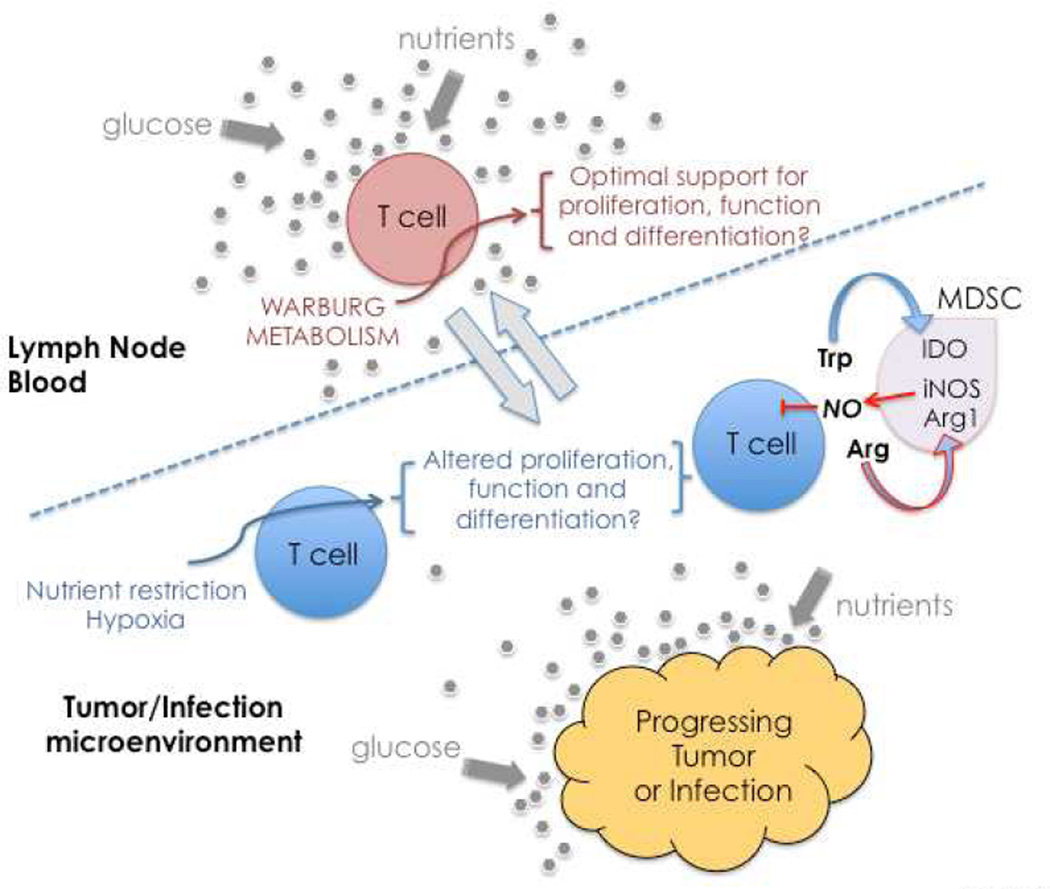
It has long been appreciated that unlike bacteria or yeast, which respond directly to nutrients in their environments, mammalian cells must be instructed by growth factors in order to efficiently utilize nutrients. This represents a fundamental mechanism to regulate cell growth in metazoans. A classic example of this in the immune system is how IL-2 promotes glucose transporter expression and as such effectively directs activated T cells towards a glycolytic metabolism (Fox et al., 2005; Rathmell et al., 2001; Wofford et al., 2008). However, it is equally important to consider that available nutrients, substrates, or other resources may also become limited or imbalanced in immune cell niches, affecting immune cell metabolism and thereby cell function and fate. A well-characterized example of this is tryptophan catabolism by tumors or antigen presenting cells expressing indoleamine-2,3-dioxygenase (IDO). The utilization of this pathway depletes tryptophan in the microenvironment, and together with the production of immunosuppressive catabolites, negatively effects T cells function and survival (Mellor and Munn, 2004; Munn and Mellor, 2013; Platten et al., 2012; Serhan et al., 2011; Zhao et al., 2012). Competition for arginine may also fall into this general category, since myeloid derived suppressor cell populations typically express arginase and iNOS, which together are capable of rapidly depleting this amino acid from the environment (Kidani and Bensinger, 2012; Norris et al., 2013). Given these findings it would seem likely that substrate availability, whether concentrations of the nutrient itself or of the growth factors that allows its acquisition, fluctuates dramatically depending on the location of the immune cell (Figure 2). Of course, oxygen may also be limiting in damaged tissue sites, especially if perfusion is diminished (Lewis et al., 1999).
Figure 2. Model for competition for substrates and production of regulatory metabolites at sites of infection or within tumors.
In the lymph nodes and blood, nutrients and other supportive signals are abundant, and T cells are able to engage Warburg metabolism and attain full effector status. However, conflicting metabolic signals may inhibit T cell function and fate within diseased tissues. T cells may face metabolic restrictions such as reduced local glucose and/or oxygen concentrations due to rapid tumor and/or pathogen growth, and reduced vascular perfusion. Additionally, tumors and sites of infection can be infiltrated by suppressive myeloid cells, which aggressively deplete important substrates such as tryptophan and arginine, and further produce toxic gases (such as NO) that can inhibit OXPHOS.
If lymph nodes and bone marrow are considered to represent nutrient replete environments, would it also be reasonable to assume that nutrient concentrations are precisely the same in all other tissues, especially during infection or inflammation? For example, as a T cell moves from the blood into the airways, as occurs during influenza infection, does this cell experience a nutrient restriction that influences metabolism and hampers its ability to persist or function? Similarly, as immune cells infiltrate a tumor or site of infection, are they effectively in competition with tumor cells or pathogens for all key substrates? Nutrient limitations leading to declines in ATP effectively trigger AMPK activation, which is a major regulator of cellular activation due to its ability to suppress anabolic and promote catabolic pathways; this area has been reviewed recently (O'Neill and Hardie, 2013). Activated AMPK promotes the oxidation of substrates in mitochondria, and coincidentally limits the glycolytic capacity of cells. While this is consistent with the requirements for the development of M2 macrophages, memory T cells, and regulatory T cells, it is not supportive of the types of aggressive effector functions that are usually required to deal with microbial infections or tumors. Moreover, there is evidence that inflamed tissues are deprived of oxygen, creating an environment where aerobic glycolysis is required for survival (Lewis et al., 1999). Thus the combination of the relative availability of nutrients and oxygen within a diseased site and the ability of infiltrating immune cells to utilize these nutrients would be expected to have a profound effect on the outcome of the immune response.
CONCLUSIONS AND FUTURE DIRECTIONS
In this review we discussed how metabolic pathway choices affect immune cell function and fate. Despite considerable progress over the last 10 years, this area remains ripe for increased investigation, especially since virtually nothing is currently known about the metabolism of many of the cell types in the immune system. Research on metabolomics has been difficult for the non-expert to access, in part because many of the experimental approaches require specialized instrumentation. Moreover, metabolomics, the identification of relative amounts of “all metabolites” is technically challenging and therefore not widely available. Thus access to hypothesis generating, unbiased global metabolite datasets analogous to the gene expression profiling that can be generated through microarrays or RNA-seq is limited. Nevertheless, this situation is changing (e.g. (Karnovsky et al., 2012)) and we can expect considerable steps forward in our understanding of metabolism in immunity in the near future.
Other exciting prospects for the future include increased understanding of how metabolites initiate signaling pathways to induce changes in cellular function. For example, it is now clear that there is a large number of GPCRs, previously categorized as orphan receptors, that are, in fact, receptors for metabolic intermediates and energy substrates (Blad et al., 2012). There is growing evidence that these types of receptors play important roles in immune cells. For example, succinate working through the succinate receptor GPR91, is able to directly promote chemotaxis, and potentiate activation initiated by TLR agonists in DCs (Rubic et al., 2008). Moreover, adenosine, through the A2B and A2A adenosine receptors, strongly promotes IL-4 driven M2 activation in macrophages (Csoka et al., 2012). When considering how cells respond to metabolites it seems reasonable to consider whether pathogens or tumor cells produce unusual metabolites that affect immune cell function. In this regard, it is interesting that VγδT cells expressing Vγ9 and Vδ2 receptors are specifically responsive to the microbial metabolite (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP) (Eberl and Moser, 2009). It seems likely that we are aware of just the tip of the iceberg when it comes to these types of interactions, and it will be exciting to see progress in this area in the future.
ACKNOWLEDGEMENTS
We would like to thank Chi-Hao Chang, Bart Everts, Stanley Huang, David O’Sullivan, Rianne van der Windt, Maxim Artyomov and Russell Jones for many stimulating discussions that have contributed to the ideas in this review. The authors’ research is supported by grants from NCI and NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aktan F. iNOS-mediated nitric oxide production and its regulation. Life sciences. 2004;75:639–653. doi: 10.1016/j.lfs.2003.10.042. [DOI] [PubMed] [Google Scholar]
- Andreyev AY, Fahy E, Guan Z, Kelly S, Li X, McDonald JG, Milne S, Myers D, Park H, Ryan A, et al. Subcellular organelle lipidomics in TLR-4-activated macrophages. J Lipid Res. 2010a;51:2785–2797. doi: 10.1194/jlr.M008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreyev AY, Shen Z, Guan Z, Ryan A, Fahy E, Subramaniam S, Raetz CR, Briggs S, Dennis EA. Application of proteomic marker ensembles to subcellular organelle identification. Mol Cell Proteomics. 2010b;9:388–402. doi: 10.1074/mcp.M900432-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nature reviews Immunology. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annual review of immunology. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- Beltran B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. Journal of leukocyte biology. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell metabolism. 2012;15:432–437. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Blad CC, Tang C, Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nature reviews Drug discovery. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- Borregaard N, Herlin T. Energy metabolism of human neutrophils during phagocytosis. The Journal of clinical investigation. 1982;70:550–557. doi: 10.1172/JCI110647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulua AC, Simon A, Maddipati R, Pelletier M, Park H, Kim KY, Sack MN, Kastner DL, Siegel RM. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) The Journal of experimental medicine. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nature reviews Immunology. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS letters. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csoka B, Selmeczy Z, Koscso B, Nemeth ZH, Pacher P, Murray PJ, KepkaLenhart D, Morris SM, Jr, Gause WC, Leibovich SJ, Hasko G. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza AD, Parikh N, Kaech SM, Shadel GS. Convergence of multiple signaling pathways is required to coordinately up-regulate mtDNA and mitochondrial biogenesis during T cell activation. Mitochondrion. 2007;7:374–385. doi: 10.1016/j.mito.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale DC, Boxer L, Liles WC. The phagocytes: neutrophils and monocytes. Blood. 2008;112:935–945. doi: 10.1182/blood-2007-12-077917. [DOI] [PubMed] [Google Scholar]
- Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, Chiles TC. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood. 2006;107:4458–4465. doi: 10.1182/blood-2005-12-4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort FJ, Bleiman BF, Gumina MR, Blair D, Wagner DJ, Roberts MF, AbuAmer Y, Chiles TC. Cutting edge: IL-4-mediated protection of primary B lymphocytes from apoptosis via Stat6-dependent regulation of glycolytic metabolism. J Immunol. 2007;179:4953–4957. doi: 10.4049/jimmunol.179.8.4953. [DOI] [PubMed] [Google Scholar]
- Eberl M, Moser B. Monocytes and gammadelta T cells: close encounters in microbial infection. Trends in immunology. 2009;30:562–568. doi: 10.1016/j.it.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Endo T, Ogushi F, Kawano T, Sone S. Comparison of the regulations by Th2-type cytokines of the arachidonic-acid metabolic pathway in human alveolar macrophages and monocytes. Am J Respir Cell Mol Biol. 1998;19:300–307. doi: 10.1165/ajrcmb.19.2.2915. [DOI] [PubMed] [Google Scholar]
- Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nature reviews Immunology. 2005;5:844–852. doi: 10.1038/nri1710. [DOI] [PubMed] [Google Scholar]
- Galluzzi L, Kepp O, Kroemer G. Mitochondria: master regulators of danger signalling. Nature reviews. Molecular cell biology. 2012;13:780–788. doi: 10.1038/nrm3479. [DOI] [PubMed] [Google Scholar]
- Gattinoni L, Klebanoff CA, Restifo NP. Paths to stemness: building the ultimate antitumour T cell. Nature reviews Cancer. 2012;12:671–684. doi: 10.1038/nrc3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, et al. A human memory T cell subset with stem cell-like properties. Nature medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerriets VA, Rathmell JC. Metabolic pathways in T cell fate and function. Trends in immunology. 2012;33:168–173. doi: 10.1016/j.it.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nature reviews Immunology. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Greene ER, Huang S, Serhan CN, Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins & other lipid mediators. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Cannons JL, Grainger JR, Dos Santos LM, Hand TW, Naik S, Wohlfert EA, Chou DB, Oldenhove G, Robinson M, et al. Essential role for retinoic acid in the promotion of CD4(+) T cell effector responses via retinoic acid receptor alpha. Immunity. 2011;34:435–447. doi: 10.1016/j.immuni.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschemi A, Kosma P, Gille L, Evans CR, Burant CF, Starkl P, Knapp B, Haas R, Schmid JA, Jandl C, et al. The sedoheptulose kinase CARKL directs macrophage polarization through control of glucose metabolism. Cell metabolism. 2012;15:813–826. doi: 10.1016/j.cmet.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Narumiya S. Prostanoids as regulators of innate and adaptive immunity. Adv Immunol. 2012;116:143–174. doi: 10.1016/B978-0-12-394300-2.00005-3. [DOI] [PubMed] [Google Scholar]
- Imtiyaz HZ, Simon MC. Hypoxia-inducible factors as essential regulators of inflammation. Current topics in microbiology and immunology. 2010;345:105–120. doi: 10.1007/82_2010_74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. The Journal of clinical investigation. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, Volke M, Glasner J, Warnecke C, Wiesener MS, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180:4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- Jenkins SJ, Ruckerl D, Cook PC, Jones LH, Finkelman FD, van Rooijen N, MacDonald AS, Allen JE. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Thompson CB. Revving the engine: signal transduction fuels T cell activation. Immunity. 2007;27:173–178. doi: 10.1016/j.immuni.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Kang K, Reilly SM, Karabacak V, Gangl MR, Fitzgerald K, Hatano B, Lee CH. Adipocyte-derived Th2 cytokines and myeloid PPARdelta regulate macrophage polarization and insulin sensitivity. Cell metabolism. 2008;7:485–495. doi: 10.1016/j.cmet.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karnovsky A, Weymouth T, Hull T, Tarcea VG, Scardoni G, Laudanna C, Sartor MA, Stringer KA, Jagadish HV, Burant C, et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics. 2012;28:373–380. doi: 10.1093/bioinformatics/btr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y, Bensinger SJ. Liver X receptor and peroxisome proliferatoractivated receptor as integrators of lipid homeostasis and immunity. Immunological reviews. 2012;249:72–83. doi: 10.1111/j.1600-065X.2012.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Kirchner T, Moller S, Klinger M, Solbach W, Laskay T, Behnen M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediators of inflammation. 2012:849136. doi: 10.1155/2012/849136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiba T. Mitochondrial-mediated antiviral immunity. Biochim Biophys Acta. 2013;1833:225–232. doi: 10.1016/j.bbamcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE. Macrophage responses to hypoxia: relevance to disease mechanisms. Journal of leukocyte biology. 1999;66:889–900. doi: 10.1002/jlb.66.6.889. [DOI] [PubMed] [Google Scholar]
- Locasale JW, Cantley LC. Metabolic flux and the regulation of mammalian cell growth. Cell metabolism. 2011;14:443–451. doi: 10.1016/j.cmet.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature reviews Immunology. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186:3299–3303. doi: 10.4049/jimmunol.1003613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, et al. Generation of guthoming IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends in immunology. 2013;34:137–143. doi: 10.1016/j.it.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy L, Szanto A, Szatmari I, Szeles L. Nuclear hormone receptors enable macrophages and dendritic cells to sense their lipid environment and shape their immune response. Physiological reviews. 2012;92:739–789. doi: 10.1152/physrev.00004.2011. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Falcone S, Tonello C, Cozzi V, Palomba L, Fiorani M, Pisconti A, Brunelli S, Cardile A, Francolini M, et al. Mitochondrial biogenesis by NO yields functionally active mitochondria in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16507–16512. doi: 10.1073/pnas.0405432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris BA, Uebelhoer LS, Nakaya HI, Price AA, Grakoui A, Pulendran B. Chronic but Not Acute Virus Infection Induces Sustained Expansion of Myeloid Suppressor Cell Numbers that Inhibit Viral-Specific T Cell Immunity. Immunity. 2013;38:309–321. doi: 10.1016/j.immuni.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- Odegaard JI, Chawla A. Alternative macrophage activation and metabolism. Annual review of pathology. 2011;6:275–297. doi: 10.1146/annurev-pathol-011110-130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegaard JI, Ricardo-Gonzalez RR, Red Eagle A, Vats D, Morel CR, Goforth MH, Subramanian V, Mukundan L, Ferrante AW, Chawla A. Alternative M2 activation of Kupffer cells by PPARdelta ameliorates obesity-induced insulin resistance. Cell metabolism. 2008;7:496–507. doi: 10.1016/j.cmet.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell metabolism. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Pearce EL. Metabolism in T cell activation and differentiation. Current opinion in immunology. 2010;22:314–320. doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platten M, Wick W, Van den Eynde BJ. Tryptophan catabolism in cancer: beyond IDO and tryptophan depletion. Cancer research. 2012;72:5435–5440. doi: 10.1158/0008-5472.CAN-12-0569. [DOI] [PubMed] [Google Scholar]
- Raetz CR, Garrett TA, Reynolds CM, Shaw WA, Moore JD, Smith DC, Jr, Ribeiro AA, Murphy RC, Ulevitch RJ, Fearns C, et al. Kdo2-Lipid A of Escherichia coli, a defined endotoxin that activates macrophages via TLR-4. J Lipid Res. 2006;47:1097–1111. doi: 10.1194/jlr.M600027-JLR200. [DOI] [PubMed] [Google Scholar]
- Rathmell JC, Farkash EA, Gao W, Thompson CB. IL-7 enhances the survival and maintains the size of naive T cells. J Immunol. 2001;167:6869–6876. doi: 10.4049/jimmunol.167.12.6869. [DOI] [PubMed] [Google Scholar]
- Rees DD, Monkhouse JE, Cambridge D, Moncada S. Nitric oxide and the haemodynamic profile of endotoxin shock in the conscious mouse. British journal of pharmacology. 1998;124:540–546. doi: 10.1038/sj.bjp.0701815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remijsen Q, Kuijpers TW, Wirawan E, Lippens S, Vandenabeele P, Vanden Berghe T. Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell death and differentiation. 2011;18:581–588. doi: 10.1038/cdd.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- Rubic T, Lametschwandtner G, Jost S, Hinteregger S, Kund J, Carballido-Perrig N, Schwarzler C, Junt T, Voshol H, Meingassner JG, et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature immunology. 2008;9:1261–1269. doi: 10.1038/ni.1657. [DOI] [PubMed] [Google Scholar]
- Satpathy AT, Wu X, Albring JC, Murphy KM. Re(de)fining the dendritic cell lineage. Nature immunology. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbarra AJ, Karnovsky ML. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. The Journal of biological chemistry. 1959;234:1355–1362. [PubMed] [Google Scholar]
- Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SE, Pollard JW, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. doi: 10.1126/science.1219179. [DOI] [PubMed] [Google Scholar]
- Sena LA, Li S, Jairaman A, Prakriya M, Ezponda T, Hildeman DA, Wang CR, Schumacker PT, Licht JD, Perlman H, et al. Mitochondria Are Required for Antigen-Specific T Cell Activation through Reactive Oxygen Species Signaling. Immunity. 2013;38:225–236. doi: 10.1016/j.immuni.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Krishnamoorthy S, Recchiuti A, Chiang N. Novel antiinflammatory--pro-resolving mediators and their receptors. Curr Top Med Chem. 2011;11:629–647. doi: 10.2174/1568026611109060629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth RB, Sun L, Chen ZJ. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Sher R, Wadee A, Joffe M. The enhancement of eosinophil function by lymphocyte supernatants. Clinical and experimental immunology. 1983;51:525–534. [PMC free article] [PubMed] [Google Scholar]
- Shi LZ, Wang R, Huang G, Vogel P, Neale G, Green DR, Chi H. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. The Journal of experimental medicine. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21:558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, Reichart D, Fox JN, Shaked I, Heudobler D, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbayev VV, Nicholas SA, Streatfield CL, Gibbs BF. Involvement of hypoxia-inducible factor-1 HiF(1alpha) in IgE-mediated primary human basophil responses. European journal of immunology. 2009;39:3511–3519. doi: 10.1002/eji.200939370. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Sun L, Liu HH, Chen X, Seth RB, Forman J, Chen ZJ. The specific and essential role of MAVS in antiviral innate immune responses. Immunity. 2006;24:633–642. doi: 10.1016/j.immuni.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Thomas GD, Ruckerl D, Maskrey BH, Whitfield PD, Blaxter ML, Allen JE. The biology of nematode- and IL4Ralpha-dependent murine macrophage polarization in vivo as defined by RNA-Seq and targeted lipidomics. Blood. 2012;120:e93–e104. doi: 10.1182/blood-2012-07-442640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CB. Rethinking the regulation of cellular metabolism. Cold Spring Harbor symposia on quantitative biology. 2011;76:23–29. doi: 10.1101/sqb.2012.76.010496. [DOI] [PubMed] [Google Scholar]
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nature reviews Immunology. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- Valentine WN, Beck WS. Biochemical studies on leucocytes. I. Phosphatase activity in health, leucocytosis, and myelocytic leucemia. The Journal of laboratory and clinical medicine. 1951;38:39–55. [PubMed] [Google Scholar]
- van Bruggen R, Koker MY, Jansen M, van Houdt M, Roos D, Kuijpers TW, van den Berg TK. Human NLRP3 inflammasome activation is Nox1-4 independent. Blood. 2010;115:5398–5400. doi: 10.1182/blood-2009-10-250803. [DOI] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunological reviews. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raam BJ, Sluiter W, de Wit E, Roos D, Verhoeven AJ, Kuijpers TW. Mitochondrial membrane potential in human neutrophils is maintained by complex III activity in the absence of supercomplex organisation. PloS one. 2008;3:e2013. doi: 10.1371/journal.pone.0002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Raam BJ, Verhoeven AJ, Kuijpers TW. Mitochondria in neutrophil apoptosis. International journal of hematology. 2006;84:199–204. doi: 10.1532/IJH97.06131. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1beta attenuate macrophage-mediated inflammation. Cell metabolism. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venge P, Moberg L, Bjornsson E, Bergstrom M, Langstrom B, Hakansson L. Mechanisms of basal and cytokine-induced uptake of glucose in normal human eosinophils: relation to apoptosis. Respiratory medicine. 2003;97:1109–1119. doi: 10.1016/s0954-6111(03)00143-4. [DOI] [PubMed] [Google Scholar]
- Waickman AT, Powell JD. mTOR, metabolism, and the regulation of Tcell differentiation and function. Immunological reviews. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Dillon CP, Shi LZ, Milasta S, Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger J, Green DR. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic checkpoints in activated T cells. Nature immunology. 2012a;13:907–915. doi: 10.1038/ni.2386. [DOI] [PubMed] [Google Scholar]
- Wang R, Green DR. Metabolic reprogramming and metabolic dependency in T cells. Immunological reviews. 2012b;249:14–26. doi: 10.1111/j.1600-065X.2012.01155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg F, Hamanaka R, Wheaton WW, Weinberg S, Joseph J, Lopez M, Kalyanaraman B, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:8788–8793. doi: 10.1073/pnas.1003428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 promotes Glut1 trafficking and glucose uptake via STAT5-mediated activation of Akt to support T-cell survival. Blood. 2008;111:2101–2111. doi: 10.1182/blood-2007-06-096297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemirli N, Arnoult D. Mitochondrial anti-viral immunity. Int J Biochem Cell Biol. 2012;44:1473–1476. doi: 10.1016/j.biocel.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nature medicine. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Kuang DM, Wu Y, Xiao X, Li XF, Li TJ, Zheng L. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumorassociated macrophages. J Immunol. 2012;188:1117–1124. doi: 10.4049/jimmunol.1100164. [DOI] [PubMed] [Google Scholar]
- Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]