Abstract
One of the most potent insecticidal venom peptides described to date is Aps III from the venom of the trapdoor spider Apomastus schlingeri. Aps III is highly neurotoxic to lepidopteran crop pests, making it a promising candidate for bioinsecticide development. However, its disulfide-connectivity, three-dimensional structure, and mode of action have not been determined. Here we show that recombinant Aps III (rAps III) is an atypical knottin peptide; three of the disulfide bridges form a classical inhibitor cystine knot motif while the fourth disulfide acts as a molecular staple that restricts the flexibility of an unusually large β hairpin loop that often houses the pharmacophore in this class of toxins. We demonstrate that the irreversible paralysis induced in insects by rAps III results from a potent block of insect voltage-gated sodium channels. Channel block by rAps III is voltage-independent insofar as it occurs without significant alteration in the voltage-dependence of channel activation or steady-state inactivation. Thus, rAps III appears to be a pore blocker that plugs the outer vestibule of insect voltage-gated sodium channels. This mechanism of action contrasts strikingly with virtually all other sodium channel modulators isolated from spider venoms that act as gating modifiers by interacting with one or more of the four voltage-sensing domains of the channel.
Keywords: voltage-gated sodium channel, neurotoxin, spider-venom peptide, pore blocker, gating modifier, inhibitor cystine knot
1. Introduction
Insects serve as vectors for a wide range of debilitating and potentially lethal human diseases such as malaria, dengue, Chagas disease, and yellow fever [1]. About 3.3 billion people, almost half of the world’s population, are at risk of contracting vector-borne disease [2]. Moreover, despite intensive control measures, insect pests reduce world crop yields by 10–14% annually [3, 4].
Despite the widespread introduction of insect-resistant transgenic crops, chemical insecticides remain the dominant method for controlling insect pests in both the agricultural and public health arenas. These chemicals target a very small number of molecular targets in the insect nervous system [5]. As a result, their widespread use over several decades has promoted the evolution of resistant insect populations, with >600 insects and mites now resistant to one or more classes of chemical insecticides [6]. In addition, key classes of insecticides have been withdrawn from sale or their use has been restricted by regulatory authorities due to growing environmental and human health concerns [7]. Thus, there is an urgent need to develop novel classes of insecticides or alternative methods of insect pest control.
A promising approach in the agricultural sector is to engineer crops to produce insecticidal toxins. By 2010, 148 million hectares of genetically modified (GM) crops had been planted in 29 countries, representing 10% of all cropland [8]. While the introduction of GM crops that express Bacillus thuringiensis (Bt) toxins has provided an alternative and potentially safer method of insect control than chemical insecticides, alternative insect-toxin transgenes are urgently needed as constitutive expression of Bt toxin in transgenic plants is likely to expedite resistance development [9].
There are very few well characterised toxins that could be considered as alternatives or adjuncts to Bt. However, some of the most promising candidates are novel insecticidal peptides that have been isolated from the venom of spiders [7, 10–12], the most successful insect predators on the planet. Most of these peptides are highly stable because they contain an inhibitor cystine knot (ICK) motif [13, 14] that provides them with resistance to extremes of pH, high temperatures, and proteolytic enzymes [7, 15]. One of the first insecticidal spider-venom peptides to be reported was Aps III from the venom of the trapdoor spider Apomastus schlingeri [16]. With a reported LD50 of 133 pmol/g against the tobacco hornworm Manduca sexta, this peptide is one of the most potent insect toxins described to date according to ArachnoServer [17, 18]. Aps III comprises 37-residues with four disulfide-bonds, but its three-dimensional structure and mode of action are unknown.
Here we describe the development of an efficient E. coli expression system that was used to produce recombinant Aps III (rAps III) for functional and structural studies. The 3D solution structure of rAps III determined using NMR spectroscopy revealed an inhibitor cystine knot motif that is commonly found in spider-venom peptides. However, rAps III contains an additional disulfide bridge that is employed as a molecular staple to tie together the ends of a very large β-hairpin loop. We demonstrate that the insecticidal activity of rAps III results from a potent block of insect voltage-gated sodium (Nav) channels in combination with a weaker block of insect voltage-gated calcium (Cav) channels. However, in striking contrast to previously characterised Nav channel blockers from spiders, all of which are gating modifiers [19], rAps III appears to be a pore blocker that plugs the outer vestibule of insect Nav channels.
2. Material and Methods
2.1 Chemicals
All chemicals were purchased from Sigma-Aldrich Australia (Castle Hill, NSW, Australia), Sigma-Aldrich USA (St Louis, MO, USA), or Merck Chemicals (Kilsyth, Victoria, Australia) with the exception of isopropyl-β-d-thiogalactopyranoside (IPTG) and streptomycin (Life Technologies, Victoria, Australia), tetrodotoxin (Alomone Labs, Israel), and HPLC-grade acetonitrile (RCI Labscan, Bangkok, Thailand). 13C6-glucose and 15NH4Cl were from Sigma-Aldrich Australia. Recombinant His6-TEV protease (EC 3.4.22.44) was produced in-house used a published protocol [20].
2.2 Production of recombinant Aps III
A synthetic gene encoding Aps III, with codons optimised for expression in Escherichia coli, was produced and cloned into a variant of the pLIC-MBP expression vector [21] by GeneArt (Invitrogen, Regensburg, Germany). This vector (pLIC-NSB1) encodes a MalE signal sequence for periplasmic export [22], a His6 tag for affinity purification, a maltose binding protein (MBP) fusion tag to aid solubility [23], and a tobacco etch virus (TEV) protease recognition site directly preceding the codon-optimised Aps III gene (Fig. 1A).
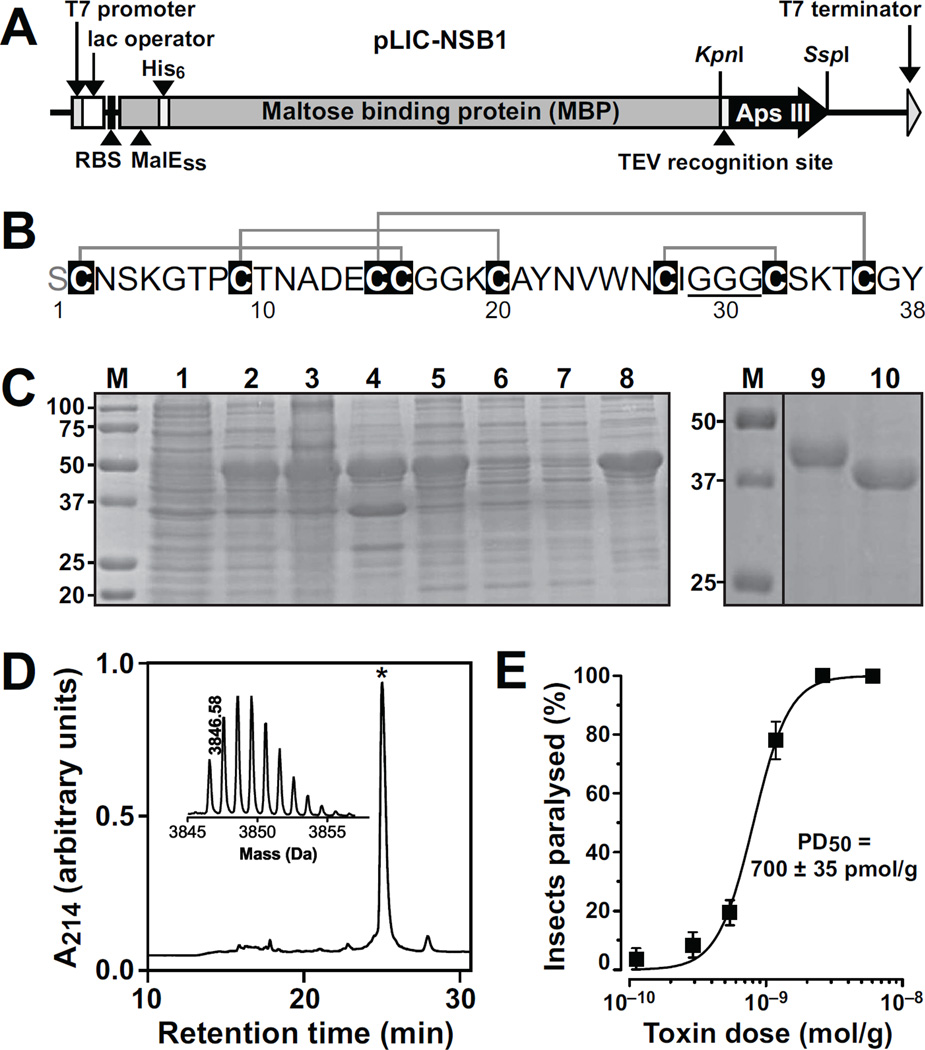
Fig. 1. Production and functional analysis of recombinant Aps III.
(A) Schematic representation of the pLicC-NSB1 vector used for periplasmic expression of Aps III. The coding region includes a MalE signal sequence (MalESS) for periplasmic export, a His6 affinity tag, an MBP fusion tag, and a codon-optimised gene encoding Aps III, with a TEV protease recognition site inserted between the MBP and toxin coding regions. The locations of key elements of the vector are shown, including the ribosome binding site (RBS). (B) Primary structure of rAps III. The non-native N-terminal Ser residue is highlighted in grey and the triglycine sequence is underlined. The disulfide framework of rAps III as determined in the current study is shown above the amino acid sequence. (C) SDS-PAGE gels illustrating different steps in the purification of rAps III. Lanes are as follows: M, molecular weight markers; lane 1, E. coli cell extract prior to IPTG induction; lane 2, E. coli cell extract after IPTG induction; lane 3, lysate resulting from cell disruption; lane 4, soluble periplasmic extract; lane 5, Ni-NTA beads after loading the cell lysate (the His6-MBP-Aps III fusion protein is evident at ~49 kDa); lane 6, eluate from washing Ni-NTA resin with loading buffer; lane 7, eluate from washing Ni-NTA resin with 10 mM imidazole; lane 8, eluate from washing Ni-NTA resin with 500 mM imidazole; lane 9, purified fusion protein before TEV cleavage; lane 10, fusion protein sample after TEV protease cleavage, showing complete cleavage of fusion protein to His6-MBP. (D) RP-HPLC chromatogram showing the final step in the purification of rAps III. The asterisk denotes the peak corresponding to correctly folded rAps III. Inset is a MALDI-TOF MS spectrum showing the [M+H]+ ion for the purified recombinant toxin (obs. = 3846.58 Da; calc. = 3846.49Da). (E) Dose-response curve for the paralytic effects of rAps III determined 24 h after injection into sheep blowflies (L. cuprina).
The plasmid encoding Aps III was transformed into E. coli strain BL21(λDE3) for recombinant toxin production. Protein expression and purification were performed as described previously [24] with minor modifications. Briefly, cultures were grown in Terrific Broth at 37°C with shaking at 120 rpm. Toxin gene expression was induced with 1 mM IPTG at an OD600 of 1.1–1.2, then cells were grown at 18°C for a further 12 h before harvesting by centrifugation for 15 min at 8000 rpm. For production of uniformly 13C/15N-labelled rAps III, cultures were grown in minimal medium supplemented with 13C6-glucose and 15NH4Cl as the sole carbon and nitrogen sources, respectively.
The His6-MBP-toxin fusion protein was extracted from the bacterial periplasm by cell disruption at 26 kPa (TS Series Cell Disrupter, Constant Systems Ltd, Northants, UK), then captured by passing the extract (buffered in 40 mM Tris, 500 mM NaCl, pH 8.0) over Ni-NTA Superflow resin (Qiagen). Proteins bound non-specifically were removed by washing with 10 mM imidazole then the fusion protein was eluted with 500 mM imidazole. The eluted fusion protein was concentrated to 10 ml and the buffer was exchanged to remove imidazole. Reduced and oxidised glutathione were then added to 0.6 mM and 0.4 mM, respectively, to maintain TEV protease activity and promote folding of the protein. Approximately 100 µg of His6-tagged TEV protease was added per mg of rAps III, then the cleavage reaction was allowed to proceed at room temperature for 12 h. The cleaved His6-MBP and His6-TEV were removed by passing the solution over Ni-NTA Superflow resin, while the eluate containing rAps III was collected for further purification using reverse-phase HPLC (RP-HPLC). RP-HPLC was performed on a Vydac C18 column (250 × 4.6 mm, particle size 5 µm) using a flow rate of 1 ml/min and a gradient of 20–40% Solvent B (0.043% trifluoroacetic acid (TFA) in 90% acetonitrile) in Solvent A (0.05% TFA in water) over 20 min. rAps III contains a non-native N-terminal serine residue (a vestige of the TEV protease cleavage site), making it one-residue longer than native Aps III (Fig. 1B).
2.3 Mass spectrometry
Toxin masses were confirmed by matrix assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) using a Model 4700 Proteomics Bioanalyser (Applied Biosystems, CA, USA). RP-HPLC fractions were mixed (1:1 v:v) with α-cyano-4 hydroxy-cinnamic acid matrix (5 mg/ml in 50/50 acetonitrile/H2O) and MALDI-TOF spectra were acquired in positive reflector mode. All reported masses are for monoisotopic [M+H]+ ions.
2.4 Insecticidal assays
rAps III dissolved in insect-saline [25] was injected into the ventro-lateral thoracic region of sheep blowflies (Lucilia cuprina; mass 19.7–23.9 mg) using a 1.0 ml Terumo Insulin syringe (B-D Ultra-Fine, Terumo Medical Corporation, MD, USA) with a fixed 29 G needle fitted to an Arnold hand micro-applicator (Burkard Manufacturing Co. Ltd., England). A maximum volume of 2 µl was injected per fly. Thereafter, flies were individually housed in 2 ml tubes and the paralytic activity was determined after 24 h. A total of three tests were carried out and for each test seven doses of rAps III (n = 10 flies per dose) and the appropriate control (insect saline; n = 30 flies each) were used. PD50 values were calculated as described previously [26].
2.5 Electrophysiological measurements
2.5.1 Primary cell culture
Dorsal unpaired median (DUM) neurons were isolated from unsexed adult American cockroaches (Periplaneta americana) as described previously [27, 28]. Briefly, terminal abdominal ganglia were removed and placed in normal insect saline (NIS) containing (in mM): NaCl 180, KCl 3.1, N-hydroxyethylpiperazine-N-ethanesulfonic acid (HEPES) 10 and D-glucose 20. Ganglia were then incubated in 1 mg/ml collagenase (type IA) (EC 3.4.24.3) for 40 min at 29°C. Following enzymatic treatment, ganglia were washed three times in NIS and triturated through a fire-polished Pasteur pipette. The resultant cell suspension was then distributed onto 12-mm diameter glass coverslips pre-coated with 2 mg/ml concanavalin A (type IV). DUM neurons were maintained in NIS supplemented with 5 mM CaCl2, 4 mM MgCl2, 5% foetal bovine serum and 1% penicillin and streptomycin, and maintained at 29°C, 100% humidity.
2.5.2 Patch-clamp electrophysiology
Ionic currents were recorded in voltage-clamp mode using the whole-cell patch-clamp technique employing version 10.2 of the pCLAMP data acquisition system (Molecular Devices, Sunnyvale, CA). Data were filtered at 5–10 kHz with a low-pass Bessel filter with leakage and capacitative currents subtracted using P-P/4 procedures. Digital sampling rates were set between 15 and 25 kHz depending of the length of the protocol. Single-use 0.8–2.5 MΩ electrodes were pulled from borosilicate glass and fire-polished prior to current recordings. Liquid junction potentials were calculated using JPCALC [29], and all data were compensated for these values. Cells were bathed in external solution through a continuous pressurised perfusion system at 1 ml/min, while toxin solutions were introduced via direct pressurised application via a perfusion needle at ~50 µl/min (Automate Scientific, San Francisco, CA) to a bath volume of 300 µl. To avoid issues of desensitization or rundown of currents, particularly with CaV channel currents, recording periods were kept as short as possible with the effect of toxin recorded within 5 min of control recordings. Control data was not acquired until at least 20 min after whole-cell configuration was achieved. This was to eliminate the influence of fast time-dependent shifts in steady-state inactivation resulting in current rundown, particularly when recording NaV channel currents (INa). Time-dependent shifts in steady-state NaV channel inactivation are typically of the order of 2–3 mV beyond this period and do not significantly influence current amplitude. Experiments were performed with a single concentration of toxin tested on one cell, which was subsequently repeated in separate experiments using unexposed cells. The number of independent recordings for each type of experiment is provided in the relevant sections of the results. All experiments were performed at ambient room temperature (20–23°C).
To record INa, the external bath solution contained (in mM): NaCl 80, CsCl 5, CaCl2 1.8, tetraethylammonium chloride (TEA-Cl) 50, 4-aminopyridine (4-AP) 5, HEPES 10, NiCl2 0.1, and CdCl2 1, adjusted to pH 7.4 with 1 M NaOH. The pipette solution contained (in mM): NaCl 34, CsF 135, MgCl2 1, HEPES 10, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA) 5, and ATP-Na2 3, adjusted to pH 7.4 with 1 M CsOH. Due to the reported current rundown with calcium as a charge carrier [30], BaCl2 replaced CaCl2 in all experiments on voltage-activated calcium (CaV) channels.
The external bath solution for barium current (IBa) recordings contained (in mM): Na acetate 140, TEA-Br 30, BaCl2 3 and HEPES 10, adjusted to pH 7.4 with 1 M TEA-OH. The external solution also contained 300 nM tetrodotoxin (TTX) to block NaV channels. Pipette solutions contained (in mM): Na acetate 10, CsCl 110, TEA-Br 50, ATP-Na2 2, CaCl2 0.5, EGTA 10 and HEPES 10, adjusted to pH 7.4 with 1 M CsOH. The external bath solution for recording global voltage-activated potassium (KV) channel currents (IK) contained (in mM): NaCl 200, K gluconate 50, CaCl2 5, MgCl2 4, TTX 0.3, HEPES 10 and D-glucose 10, adjusted to pH 7.4 with 1 M NaOH. The pipette solution consisted of (in mM): K gluconate 135, KF 25, NaCl 9, CaCl2 0.1, MgCl2 1, EGTA 1, HEPES 10 and ATP-Na2 3, adjusted to pH 7.4 with 1 M KOH.
To eliminate any influence of differences in osmotic pressure, all internal and external solutions were adjusted to 400 ± 5 mOsmol/l with sucrose. Experiments were rejected if there were large leak currents or currents showed signs of poor space clamping.
2.5.3 Curve-fitting and statistical analyses
Data were analysed using AXOGRAPH X version 1.3 (Molecular Devices). Curve-fitting of I-V data was performed using GraphPad Prism version 5.00d for Macintosh (GraphPad Software, San Diego). Comparisons of two sample means were made using a paired Student's t-test and differences were considered to be significant if p < 0.05. All data are presented as mean ± standard error of the mean (SEM) of n independent experiments.
Concentration-response curves were fitted using the following Logistic equation:
| Equation 1 |
where x is the toxin dose, nH is the Hill coefficient (slope parameter), and IC50 is the median inhibitory concentration to block channel currents.
The following equation was employed to fit current-voltage (I-V) curves:
| Equation 2 |
where I is the amplitude of the current at a given test potential V, gmax is the maximal conductance, V1/2 is the voltage at half-maximal activation, s is the slope factor, and Vrev is the reversal potential.
The voltage dependence of steady-state NaV channel inactivation (h∞/V) data were normalised to the maximum peak current in the control or maximum peak current and fitted using the following Boltzmann equation:
| Equation 3 |
where A is the fraction of control maximal peak INa (value of 1.0 under control conditions), V1/2 is the midpoint of inactivation, k is the slope factor, and V is the prepulse voltage.
2.5.4 Two-electrode voltage-clamp recordings from Xenopus oocytes
To prepare cRNA for oocyte injection, separate plasmids encoding the α-subunit of the NaV1 channel from the German cockroach Blatella germanica (BgNaV1; [31]) and the Drosophila NaV1 auxiliary subunit TipE [32] were linearised with NotI, followed by in vitro transcription using T7 polymerase (mMESSAGE mMACHINE kit, Life Technologies, CA, USA). After Xenopus oocytes were co-injected at 1:5 molar ratio with cRNA encoding BgNaV1 and TipE, they were incubated for 2–3 days at 17°C (in 96 mM NaCl, 2 mM KCl, 5 mM HEPES, 1 mM MgCl2, 1.8 mM CaCl2, 50 µg/ml gentamycin, pH 7.6) prior to recording BgNaV1-mediated currents via two-electrode voltage-clamp recording techniques using an OC-725C Oocyte Clamp Amplifier (Warner Instruments, CT, USA) with a 150-µl recording chamber. Data were filtered at 4 kHz and digitised at 50 kHz using pCLAMP 10. Microelectrode resistances were 0.1–1 MΩ when filled with 3 M KCl. The external recording solution contained (in mM): 96 NaCl, 2 KCl, 5 HEPES, 1 MgCl2 and 1.8 CaCl2, pH 7.6. Experiments were performed at ambient temperature (~22 °C) and leak and background conductance was subtracted by blocking the residual sodium current with TTX.
Voltage–activation relationships were obtained by measuring steady-state currents elicited by stepwise depolarisations of 5 mV from a holding potential of −90 mV and calculating conductance (G) using G = I/(Vm–Erev) in which G is conductance, I is peak inward current, Vm is the test potential, and Erev is the reversal potential. Reversal potentials were individually estimated for each data set [33]. After addition of the toxin to the recording chamber (150 µl), the equilibration between the toxin and the channel was monitored using weak depolarizations (50-ms test pulse to a voltage near the foot of the G-V curve, ~ −30 mV) elicited at intervals of 5 s. We recorded voltage–activation relationships in the absence and presence of toxin. Off-line data analysis was performed using Clampfit 10 (Molecular Devices, USA) and Origin 8 (OriginLab, MA, USA).
2.6 Structure determination
Recombinant 15N/13C-labelled Aps III was dissolved in 20 mM sodium phosphate, pH 6.0 to a final concentration of 450 µM 52H2O was added, then the sample was filtered using a low-protein-binding Ultrafree-MC centrifugal filter (0.22 µm pore size; Millipore, MA, USA) and 300 µL was added to a susceptibility matched 5 mm outer-diameter microtube (Shigemi Inc., Japan). NMR data were acquired at 25°C using a 900 MHz NMR spectrometer (Bruker BioSpin, Germany) equipped with a cryogenically cooled probe. 3D and 4D data used for resonance assignments were acquired using non-uniform sampling (NUS). Sampling schedules that approximated the signal decay in each indirect dimension were generated using sched3D [34]. NUS data were processed using the Rowland NMR toolkit (www.rowland.org/rnmrtk/toolkit.html) and maximum entropy parameters were automatically selected as previously described [35]. 13C- and 15N-edited HSQC-NOESY (mixing time of 200 ms) experiments were acquired using uniform sampling. All experiments were acquired in H2O except for the 13C-edited HSQC-NOESY, which was acquired in D2O.
Dihedral angles (29 Ф, 30 ψ) were derived from TALOS+ chemical shift analysis [36] and the restraint range for structure calculations was set to twice the estimated standard deviation. The Thr6–Pro7 peptide bond was determined to be in the trans conformation on the basis of characteristic NOEs and the Cα and Cβ chemical shifts of the Pro residue.
Six backbone amide protons were identified as being involved in hydrogen-bonds by comparison of 2D 1H-15N HSQC spectra acquired either in H2O or 60 min after reconstitution of lyophilised protein in D2O. The presence of intense NOESY crosspeaks for the hydroxyl proton of Thr 4 indicated that it is also engaged in a hydrogen bond. Hydrogen-bond acceptors and disulfide-bond partners were identified from preliminary structure calculations, and hydrogen-bond and disulfide-bond restraints were applied in subsequent structure calculations as described previously [37]. NOESY spectra were manually peak picked and integrated, then peaklists were automatically assigned, distance restraints extracted, and an ensemble of structures calculated using the torsion angle dynamics package CYANA 3.0 [38]. The tolerances used for CYANA 3.0 were 0.025 ppm in the direct 1H dimension, 0.03 ppm in the indirect 1H dimension, and 0.3 ppm for the heteronucleus (13C/15N). During the automated NOESY assignment/structure calculation process, CYANA assigned ~86% of all NOESY crosspeaks (1127 out of 1313).
3. RESULTS
3.1 Production of recombinant Aps III
Recombinant production of venom toxins is often challenging due to the presence of multiple disulfide bonds, which cannot be formed in the cytoplasm of most prokaryotic and eukaryotic cells because of the reducing intracellular environment. An alternative approach that has proved successful for expression of disulfide-rich spider toxins [24, 39, 40] is production in the periplasm of E. coli, where the enzymes involved in disulfide-bond formation are located [41]. Thus, we attempted to produce rAps III using an IPTG-inducible construct (Fig. 1A) that allowed export of a His6-MBP-toxin fusion protein to the E. coli periplasm.
Using this expression system, a significant amount of His6-MBP-toxin fusion protein was recovered in the soluble cell fraction following IPTG induction (Fig. 1C, lanes 1–4). The fusion protein was subsequently purified using nickel affinity chromatography (Fig. 1C, lanes 5–8) then eluted from the column and cleaved with His6-tagged TEV protease (Fig. 1C, lanes 9–10). The His6-tagged MBP and TEV protease were removed by passage over a nickel column, then the eluted toxin was further purified using RP-HPLC (Fig. 1D). rAps III eluted as a single major disulfide-bond isomer with a retention time of ~25 min under the chosen experimental conditions. The purity of recombinant rAps III following RP-HPLC was >98% as assessed by SDS-PAGE and MALDI-TOF mass spectrometry (Fig. 1D, inset), and the final yield was ~1.5 mg of toxin per litre of culture.
3.2 rAps III induces irreversible paralysis in insects
The insecticidal activity of rAps III was tested using the blowfly Lucilia cuprina. This dipteran pest is the causative agent of flystrike, which results in annual economic losses in Australia of ~$280 million [42]. rAps III induced flaccid paralysis in adult L. cuprina, and the PD50 measured 24 h after injection was 700 ± 35 pmol/g (Fig. 1E). The toxicity assay used does not allow measurement of toxic effects for periods extending beyond 24 h as the survival rate in control cohorts begins to decrease, possibly due to confinement of the flies in small tubes. However, flies paralyzed by a high dose of rAps III did not recover but died two days post-injection. We conclude that rAps III produces an irreversible paralysis in blowflies and would most likely produce similar effects in related dipterans such as mosquitoes and tsetse flies that vector human diseases.
3.3 rAps III is a potent blocker of insect NaV channels
The majority of insecticidal spider toxins that have been isolated to date modulate the activity of voltage-gated ion channels [7, 11, 28, 43, 44]. We therefore used patch-clamp electrophysiology to examine the ability of rAps III to modulate the activity of a variety of ion channels in cockroach DUM neurons.
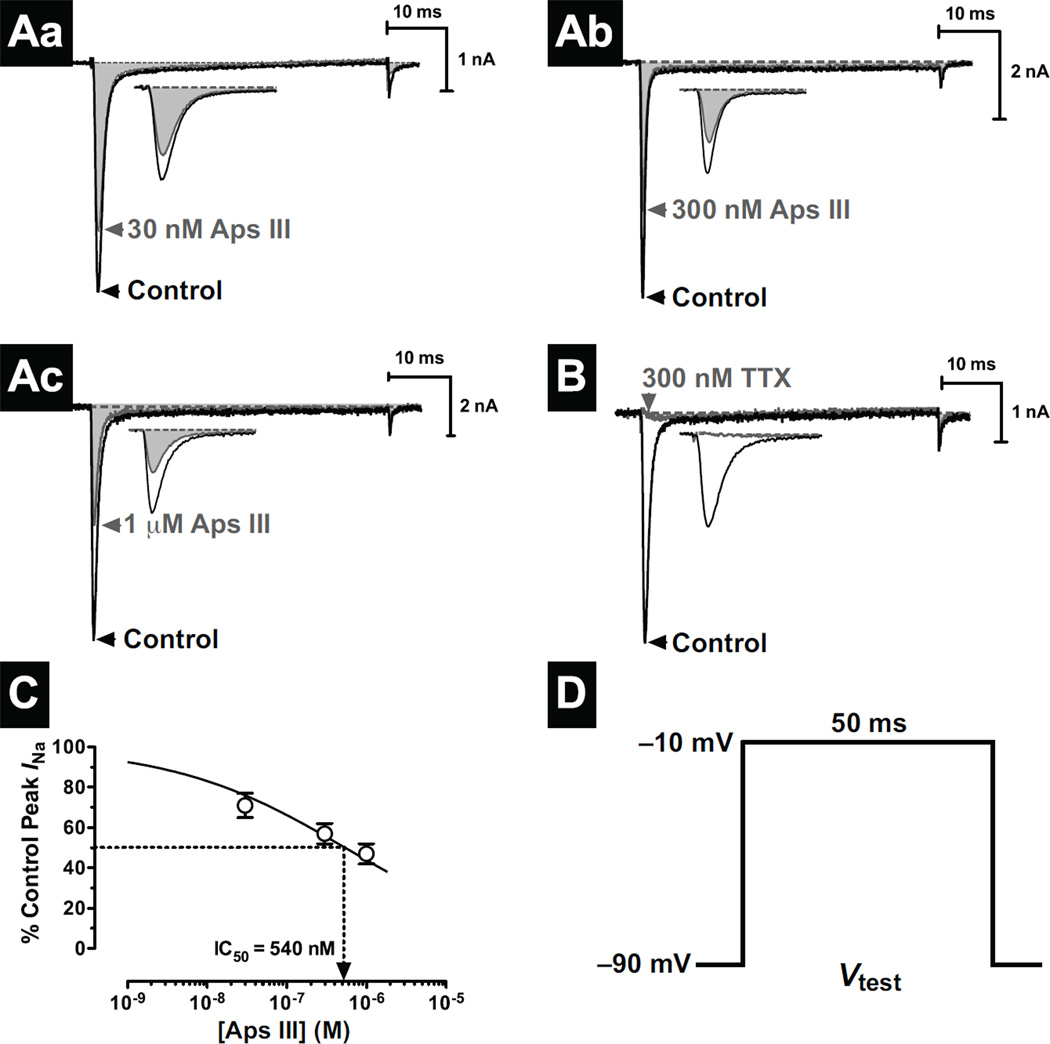
NaV channel currents (INa) in DUM neurons were elicited using 50-ms depolarising test pulses from a holding potential (Vh) of −90 mV to −10 mV every 10 s (0.1 Hz) (Fig. 2D). This elicited a rapidly activating and inactivating ionic current characteristic of classical INa observed previously in DUM neurons (Fig. 2A,B) [28, 45, 46]. This current was confirmed to be mediated by NaV channels following complete INa inhibition with 300 nM TTX (Fig. 2B). In separate experiments, perfusion with rAps III produced a concentration-dependent inhibition of peak INa. This occurred in the absence of any significant changes in the time to peak, time course of inactivation (decay) kinetics, or time course of tail currents at the end of the depolarising test pulse, as shown in the representative current traces in Fig. 2A. At a concentration of 30 nM, rAps III reduced peak INa by 29 ± 6% (n = 5 cells, p < 0.05). In separate experiments, higher concentrations of 300 nM and 1 µM rAps III produced a concentration-dependent reduction in peak INa by 43 ± 5% (n = 9 cells, p < 0.001) and 53 ± 5% (n = 5 cells, p < 0.01), respectively.
Fig. 2. Effects of rAps III on NaV channels in cockroach DUM neurons.
(Aa–c) Typical effects of increasing concentrations of rAps III on INa. Traces show superimposed control (black) and toxin (grey and shaded) current traces elicited by a 50-ms depolarising test pulse (Vtest) shown in panel D. The reduction in INa is highlighted in the inset of panels Aa–c. (B) Complete block of INa by 300 nM TTX, confirming that ionic currents are solely mediated through NaV channels. Dotted lines represent zero current. (C) Concentration-response relationship of rAps III to inhibit peak INa. The percentage block at increasing concentrations of rAps III were fitted with a logistic function (Eq. 1; see Materials and Methods). The median inhibitory concentration (IC50) was determined to be 540 nM. Data points are the mean ± SEM of 5–9 cells.
The half-maximal inhibitory concentration (IC50) for rAps III on DUM neuron INa was estimated to be ~540 nM (Fig. 2C). However, the Hill coefficient was significantly less than unity (shallower slope), which may reflect incomplete block at higher concentrations, as has been observed with certain µ-conotoxin derivatives [47].
3.4 rAps III is a classical pore blocker
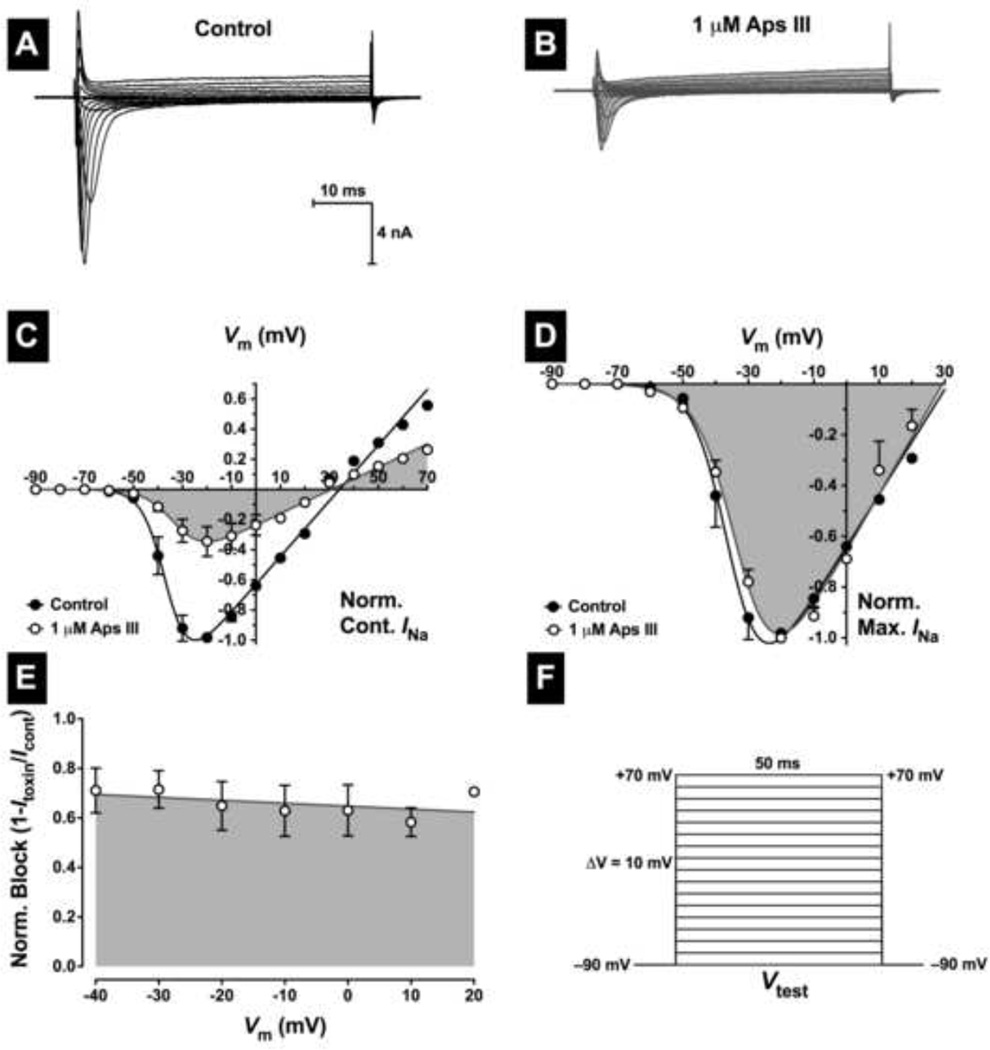
To determine whether toxin inhibition of peak INa was due to a depolarising shift in the voltage dependence of activation, families of INa (Fig. 3A,B) were elicited using a test pulse that depolarised the cell from Vh of −90 mV to +70 mV for 50 ms in 10-mV increments (Fig. 3F). Peak INa were then normalised against the maximum peak INa in the control and plotted against membrane potential (V) to establish an INa-V curve. Peak INa was then fitted to Equation 2 (Materials and Methods) using non-linear regression analysis. In the absence of toxin, INa activated around −60 mV. This threshold did not shift in the presence of any concentration of rAps III tested as shown by the superimposed control and toxin curves around −60 mV (Fig. 3C,D). The voltage at half maximum NaV channel activation (V1/2) in control cells was only marginally shifted (4 mV) in the hyperpolarising direction in the presence of 1 µM rAps III (control V1/2 = −35 ± 1 mV versus toxin V1/2 = −31 ± 3 mV; n = 4 cells, p < 0.005). This is more clearly observed as a lack of any significant shift in the voltage dependence of activation when currents recorded in the presence of toxin were normalised to the peak inward control current (Fig. 3D). No significant shifts in V1/2 were observed with either 30 nM or 300 nM rAps III. Considering the time-dependent hyperpolarising shifts in V1/2 of around 5 mV over a 10−15 min period that occur in whole-cell patch clamp configurations, this would indicate that the toxin does not alter the voltage-dependence of activation. Importantly, only a depolarising shift in the voltage-dependence of NaV channel activation would reduce INa.
Fig. 3. Effects of rAps III on the voltage-dependence of NaV channel activation in cockroach DUM neurons.
Typical families of INa recorded prior to (A), and following (B), application of 1 µM rAps III. Nav channel currents were elicited by the test pulse protocol shown in panel F. (C–D) Normalised peak INa-V relationships. Currents recorded in the presence of 1 µM rAps III were normalised to the maximum inward INa in controls (C), or maximum inward INa (D). Data shows INa before (closed symbols), and after (open symbols and shaded), application of 1 µM rAps III. Data were fitted with Eq. 2 (see Materials and Methods). As can be seen in panel (D), no significant shifts in the voltage dependence of NaV channel activation were observed. (E) Fractional block of INa by 1 µM rAps III showing lack of voltage dependence. Normalised peak INa in the presence of toxin were calculated as a fraction of peak control INa and plotted against the test potential. Data were taken from panel C and fitted using linear regression. All data are expressed as the mean ± SEM of 4 cells.
To determine whether toxin-induced block of INa was voltage-dependent, peak INa in the presence of toxin was calculated as a fraction of the corresponding control INa from the INa-V relationships. Data were then fitted by linear regression and the slope coefficient determined. This revealed that the inhibition of NaV channels by 1 µM rAps III was voltage-independent and the binding of the toxin to the channel was not relieved at increasing membrane potentials (Fig. 3E). The slope of the line did not significantly deviate from zero (p > 0.05, n = 4 cells).
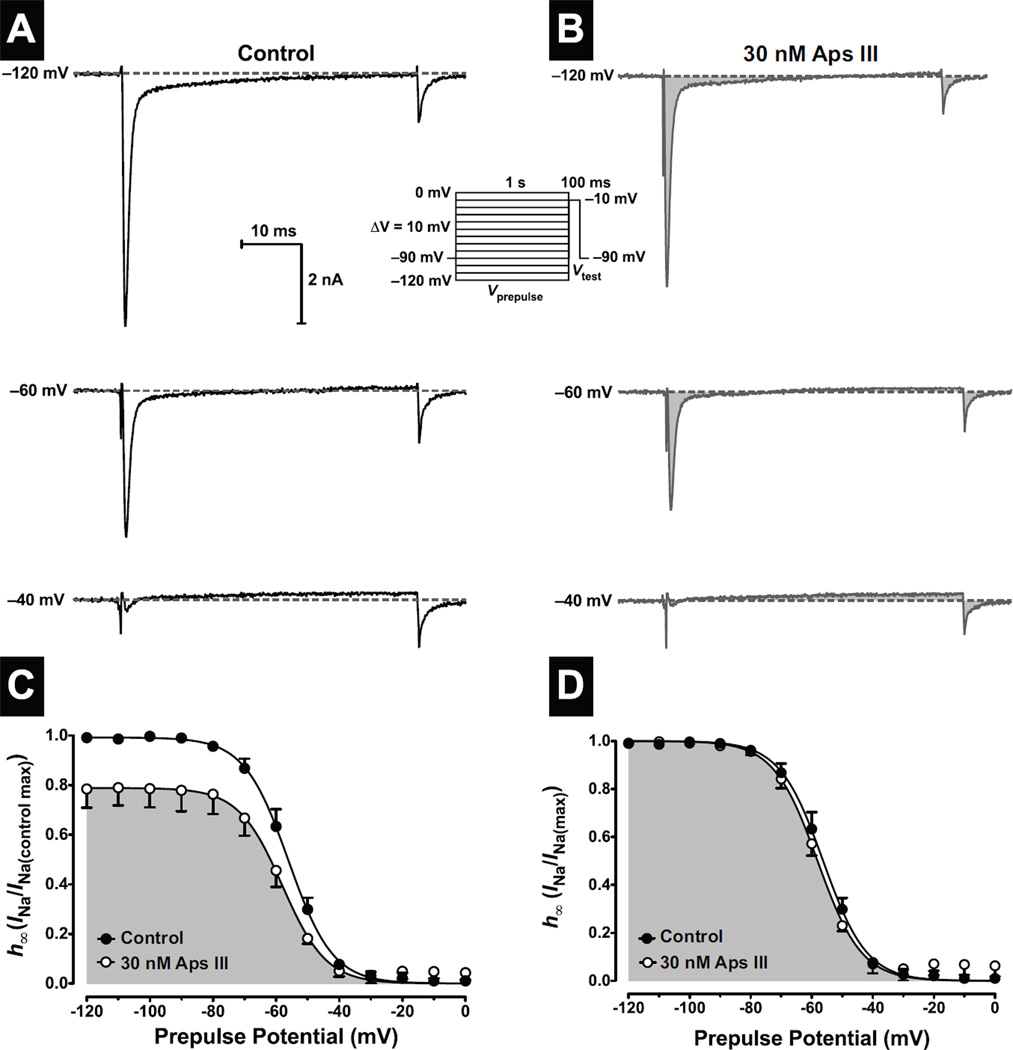
In separate experiments, the effects of rAps III on the voltage-dependence of steady-state inactivation (h∞/V) were examined to determine whether the reduction of peak INa was due to stabilisation of the inactivated (closed) state of the channel, as opposed to a pore blocking mechanism. Accordingly, experiments were conducted using a two-pulse protocol consisting of a 1 s conditioning pre-pulse (Vprepulse) followed by a 50 ms test pulse (Vtest) to −10 mV. The conditioning prepulse clamped the membrane potential from −120 mV to 0 mV in 10-mV increments (see inset to Fig. 4A,B). Due to increasing levels of depolarisation during the conditioning prepulse, channels eventually accrue in the inactivated state and have insufficient time to recover from inactivation before the test pulse. Thus, INa amplitude decreases with increasing prepulse potential (Fig. 4A,C). In the presence of 30 nM rAps III, INa was inhibited to 79 ± 3% (n = 3 cells) of control amplitude (parameter ‘A’ in Equation 3). Normalisation of the toxin data to the maximum peak INa during the test pulse revealed that the curves almost completely overlap (Fig. 4D) with an insignificant 2-mV hyperpolarising shift in h∞/V from −56 ± mV in controls to −58 ± mV in the presence of rAps III (p > 0.05, n = 3 cells; Fig. 3D). Thus the 21% reduction in NaV channel current does not appear to be the result of a reduction in channel availability due to stabilisation of the channels in the inactivated state. Therefore rAps III appears to be a classical pore blocker.
Fig. 4. Effects of rAps III on steady-state NaV channel inactivation (h∞).
Steady-state inactivation was determined using a two-pulse protocol (see inset). (A–B) Typical peak INa recorded during the test pulse (Vtest) are shown following 1-s prepulse potentials (Vprepulse) to −120 mV, −60 mV and −40 mV recorded before (left-hand traces), and following (right-hand shaded traces), perfusion with 30 nM rAps III. Dotted lines represent zero current. (C–D) Peak INa, recorded during Vtest were expressed as a fraction of maximum control INa (C), or normalised to peak INa amplitude (D), and plotted against prepulse potential. Panels show the proportion of INa that is available for activation under control conditions (closed circles), and during perfusion with 30 nM rAps III (open circles and shaded). The h∞/V curves were fitted with Eq. 3 (see Materials and Methods). All data are expressed as the mean ± SEM of 3 cells.
3.5 rAps III is a weak blocker of insect CaV channels
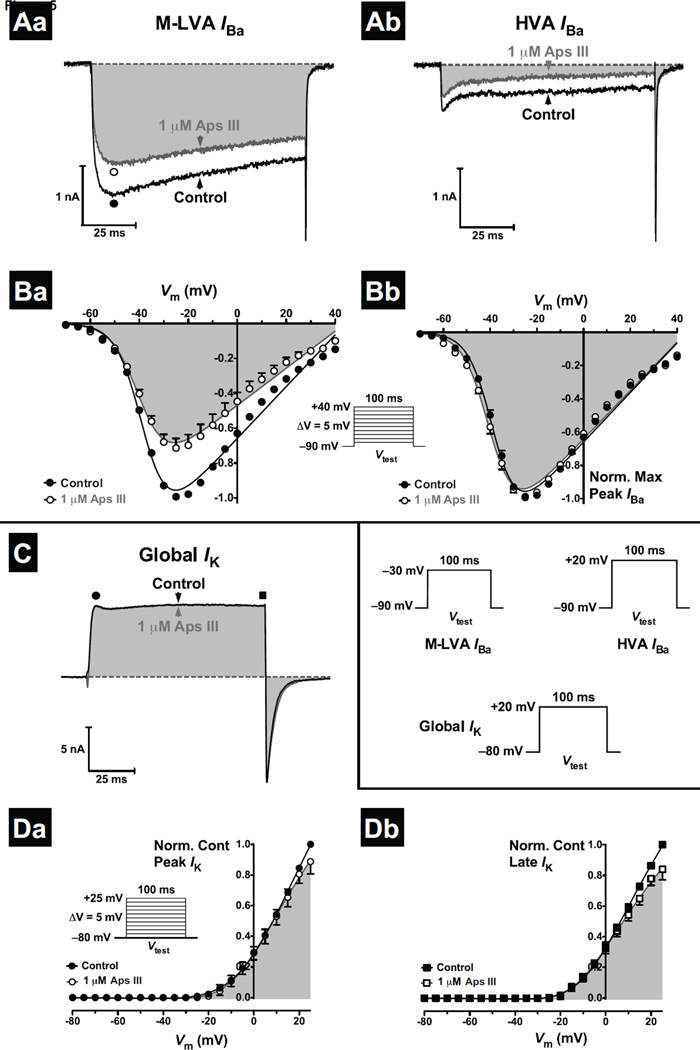
We next tested the ability of rAps III to modulate the activity of insect CaV channels as numerous spider-venom peptides have been demonstrated to inhibit this channel [48, 49]. Two distinct CaV channel subtypes have been previously observed in DUM neurons: mid to low-voltage-activated (M-LVA) and high-voltage activated (HVA) CaV channels [46]. M-LVA and HVA CaV channel barium currents (IBa) were elicited using alternating 100-ms depolarising test pulses to −30 mV (M-LVA IBa) and +20 mV (HVA IBa) from a Vh of −90 mV every 7 s. These elicited inward currents characteristic of classical IBa observed previously in DUM neurons [46]. Perfusion with 1 µM rAps III caused weak inhibition of both M-LVA and HVA CaV channel currents. This occurred in the absence of changes in M-LVA and HVA IBa activation and inactivation kinetics, with no alteration in the time to reach peak or timecourse of current decay (Fig. 5A). The M-LVA CaV channel currents were reduced by 28 ± 5% (p < 0.01, n = 5 cells) and HVA CaV channel currents by 31 ± 7% (p < 0.01, n = 6 cells). The small difference in the block between inhibition of M-LVA and HVA current was statistically insignificant (unpaired Student’s t-test, p > 0.05). Given the weak effects on CaV channel currents at 1 µM rAps III, experiments were not conducted at 30 nM or 300 nM concentrations. In an additional smaller number of cells there was a partial recovery from CaV channel current inhibition. In these cells initial rapid inhibition was followed by partial recovery to a steady-state level. This resulted in a statistically insignificant block of both M-LVA CaV channel currents (18 ± 6% block, p > 0.05, n = 4 cells) and HVA CaV channel currents (19 ± 12%, p > 0.05, n = 3 cells).
Fig. 5. Effect of rAps III on KV and CaV channel currents in cockroach DUM neurons.
(A) Whole-cell M-LVA and HVA IBa in the absence (black traces), and presence (dark grey and shaded traces), of 1 µM rAps III. M-LVA and HVA IBa were activated by a 100 ms Vtest to −30 mV and +20 mV, respectively, as shown in the inset in panel C. Perfusion with 1 µM rAps III partially blocked M-LVA (Aa) and HVA (Ab) CaV channel currents. Dotted lines represent zero current. (B) Effects of 1 µM rAps III on the voltage-dependence of CaV channel activation. Families of CaV channel currents were generated by the Vtest protocol shown in the inset of panel C. Currents recorded in the presence of 1 µM rAps III were normalised to the maximum inward IBa in control (Ba) or maximum inward IBa in toxin (Bb). IBa-V relationships show current recorded before (closed circles), and after (open circles and shaded), perfusion with 1 µM rAps III. Normalised I–V relationships were fitted using Eq. 2. (C) Typical superimposed global IK recorded prior to (black traces), and following (dark grey and shaded traces), application of 1 µM rAps III. Currents were generated by 100-ms depolarising test pulses (Vtest) as shown in the as shown in the inset in panel C. (D) Effects of rAps III on the voltage-dependence of global KV channel activation. Families of outward IK were recorded before (closed circles), and after (open circles and shaded), application of 1 µM rAps III. Families of IK were generated by the Vtest protocol shown in the inset of panel C. Global IK-V relationships show effects of the toxin on peak (Da) and late (Db) global IK. Late currents were measured at 100 ms. All data are expressed as the mean ± SEM of 4 cells.
To investigate whether the weak block of CaV channels by rAps III was due to a shift in the threshold of CaV channel activation, the IBa-V relationship was examined. The IBa-V relationships were established from families of IBa generated by 100-ms depolarising test potentials from Vh of −90 mV to +40 mV, at 5-mV increments every 7 s. Families of peak inward IBa were normalised against the maximum control inward peak IBa and plotted against the membrane potential (Fig. 5Ba). CaV channels activated around −60 mV, and this threshold was not altered in the presence of 1 µM rAps III. Additionally, there were no significant shifts in the V1/2 of channel activation (p > 0.05, n = 4 cells) and currents were essentially superimposable when normalised to the peak IBa (Fig. 5Bb). The partial block of CaV channels by rAps III was also voltage-independent (p > 0.05, n = 4 cells, data not shown).
3.6 rAps III does not modulate the activity of insect KV channels
The major outward KV channel current subtypes present in cockroach DUM neurons include a slowly activating, non-inactivating delayed-rectifier [IK(DR)], transient “A-type” [IK(A)], and large-conductance Ca2+-activated [IBK(Ca)] KV channel currents [28, 50]. To determine the effects of 1 µM rAps III on KV channels, global KV channel currents (IK) were generated by 100-ms depolarising test pulses to +25 mV from a Vh of −80 mV, every 5 s (0.2 Hz). This generated a large outward IK that displayed fast activation and partial inactivation, consistent with global IK previously observed in cockroach DUM neurons [27, 28]. Global IK were measured at the peak and at the end of the test pulse (100 ms). The early peak global IK results mainly from the contribution of rapidly activating IK(A) and IK(Ca), while the late global IK results from the slowly activating IK(DR) and slow inactivating component of the IK(Ca).
Application of 1 µM rAps III caused minimal inhibition of global IK. rAps III reduced the peak global IK by only 2 ± 2% (p > 0.05, n = 4 cells; Fig. 5C) while late global IK were inhibited only slightly by 5 ± 2% (p < 0.05, n = 4 cells; Fig. 5C). This lack of overt activity was mirrored by the lack of effect on the voltage-dependence of global IK activation. Global IK-V relationships for early and late currents were not significantly altered with no marked differences in V1/2 values between the IK-V for controls (V1/2 = 3 mV for early and −4 mV for late IK) versus toxin (V1/2 = −4 mV for early and −9 mV for late IK) (Fig. 5D). Due to the lack of any overt activity of 1 µM rAps III on global KV channel currents, the effect of rAps III on individual KV channel sub-types was not pursued. We conclude that rAps III does not modulate the activity of delayed-rectifier, ‘A-type’ or BKCa potassium channels that are the major contributors to the global outward Kv channel current in DUM neurons [51].
3.7 rAps III is a potent blocker of cloned cockroach NaV channels
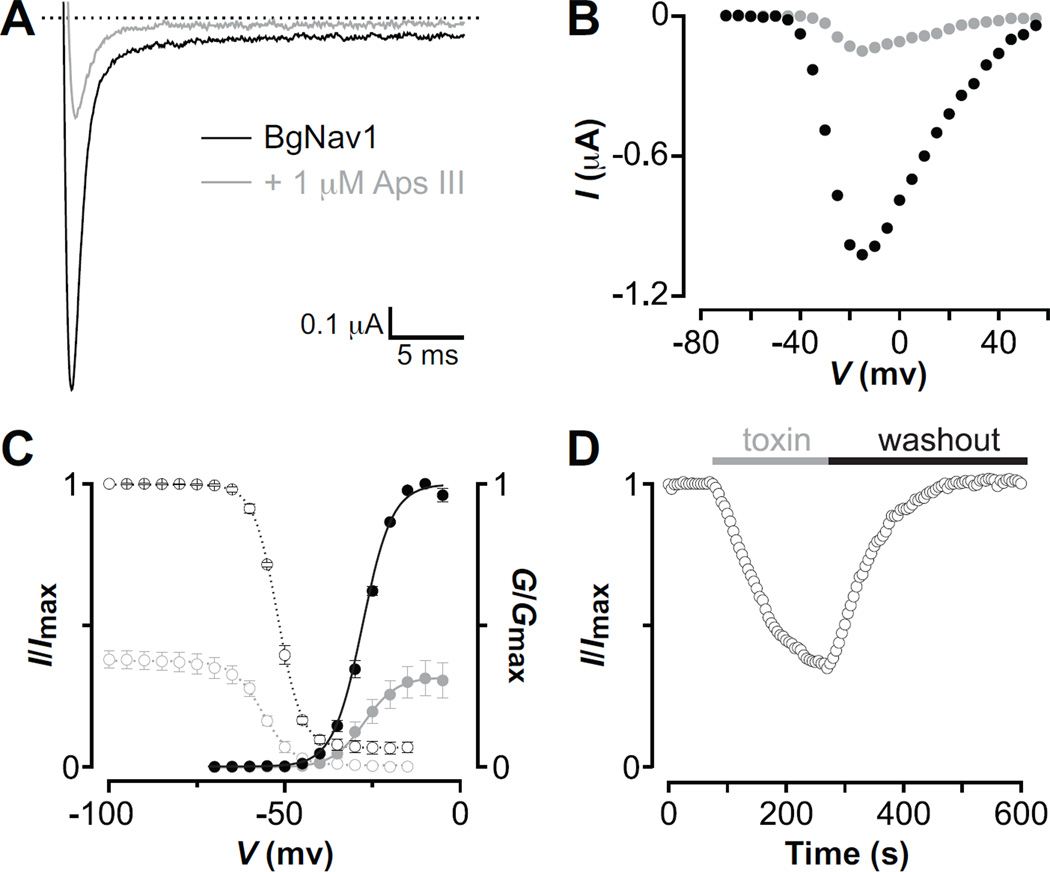
To further investigate the ability of rAps III to inhibit insect NaV channels, we applied the toxin to Xenopus oocytes expressing the cloned BgNaV1 channel [31]. At 1 µM concentration, rAps III strongly inhibited BgNaV1-mediated sodium currents over a wide voltage range (Fig. 6A,B). Boltzmann fits of conductance-voltage relationships before (V1/2 = −27 ± 1 mV; slope factor = 4.1 ± 0.2) and after (V1/2 = −27 ± 1 mV; slope factor = 4.6 ± 0.3; n = 3 cells) toxin addition as well as the steady-state inactivation relationships (control: V1/2 = −53 ± 1 mV; slope factor = 4.3 ± 0.1 and toxin: V1/2 = −55 ± 1mV; slope factor = 4.3 ± 0.2; n = 3 cells) revealed no significant changes in the midpoints or slope factors (Fig. 6C). The onset of rAps III action is rapid, and there is a fast and complete recovery of channel current upon toxin washout (Fig. 6D). In contrast to gating-modifier toxins that inhibit channel opening by interacting with one or more of the four NaV channel voltage sensors [52], rAps III decreased INa without shifting the midpoint of activation to more depolarised voltages (Fig. 6B). Together with the experiments reported above on DUM neurons (Fig. 3), this is consistent with a pore-blocking activity.
Fig. 6. rAps III inhibits BgNaV1-mediated sodium currents.
(A) Inhibition of BgNaV1-mediated sodium currents by 1 µM rAps III at a depolarization to −20 mV from a holding voltage of −90 mV. (B) Representative INa-V relationship for BgNaV1 before (black) and after (grey) addition of 1 µM rAps III. Currents were elicited by 5-mV step depolarizations from a holding voltage of −90 mV. (C) Comparison of the gating properties of BgNaV1 before (black) and after (grey) addition of 1 µM rAps III. Shown are the deduced conductance (G)–voltage (filled circles) and steady-state inactivation (open circles) relationships. Error bars denote SEM, with n = 3 cells. (D) Onset of BgNaV1-mediated INa inhibition by 1 µM rAps III at depolarizations to −20 mV (holding voltage was −90 mV) followed by a complete recovery after toxin washout.
3.8 High-resolution solution structure of rAps III
The development of an efficient bacterial expression system allowed us to produce uniformly 13C/15N-labelled rAps III for structure determination using heteronuclear NMR. 1HN, 15N, 13Cα, 13Cβ, and 13C′ resonance assignments for the toxin were obtained from analysis of amideproton strips in 3D HNCACB, CBCA(CO)NH, and HNCO spectra. Sidechain 1H and 13C chemical shifts were obtained using a 4D HCC(CO)NH-TOCSY experiment, which has the advantage of providing sidechain 1H-13C connectivities [34]. Complete chemical shift assignments have been deposited in BioMagResBank (Accession Number 18946).
CYANA was used for automated NOESY assignment and structure calculation [38]. The disulfide-bond pattern (1–4, 2–5, 3–8, 6–7; see Fig. 1B) was unambiguously determined from preliminary structures calculated without disulfide-bond restraints [53]; this disulfide framework is notably different from the 1–4, 2–5, 3–6, 7–8 framework predicted in the UniProt entry for rAps III (P49268). Disulfide-bond and hydrogen-bond restraints were used in the final round of structure calculations. 200 structures were calculated from random starting conformations, then the 20 conformers with highest stereochemical quality as judged by MolProbity [54] were selected to represent the solution structure of rAps III. Coordinates for the final ensemble of structures are available from the Protein Data Bank (Accession Number 2M36).
Statistics highlighting the high precision and stereochemical quality of the ensemble of rAps III structures are shown in Table 1. The average MolProbity score of 1.67 places the ensemble in the 90th percentile relative to all other structures ranked by MolProbity. The high stereochemical quality of the ensemble stems from a complete absence of bad close contacts, very few unfavourable sidechain rotamers (5%), and reasonably high Ramachandran plot quality (85% of residues in the most favoured region). The structural ensemble is also highly precise with backbone and heavy-atom RMSD values over all residues of 0.32 ± 0.09 Å and 0.54 ± 0.07 Å, respectively. The ensemble of rAps III structures ranks as “high resolution” based on these measures of precision and stereochemical quality [55].
Table 1.
Structural statistics for the ensemble of rAps III structures.
| Experimental restraints2 | ||
| Interproton distance restraints | ||
| Intraresidue | 111 | |
| Sequential | 159 | |
| Medium range (i−j < 5) | 93 | |
| Long range (i−j ≥5) | 165 | |
| Hydrogen-bond restraints3 | 14 | |
| Disulfide-bond restraints | 12 | |
| Dihedral-angle restraints (φ,ψ) | 59 | |
| Total number of restraints per residue | 16.1 | |
| R.m.s. deviation from mean coordinate structure (Å) | ||
| All backbone atoms (residues 1–38) | 0.33 ± 0.08 | |
| All heavy atoms (residues 1–38) | 0.54 ± 0.07 | |
| Backbone atoms (residues 2–27, 32–38) | 0.13 ± 0.03 | |
| Heavy atoms (residues 2–27, 32–38) | 0.43 ± 0.06 | |
| Stereochemical quality4 | ||
| Residues in most favored Ramachandran region (%) | 84.5 ± 2.8 | |
| Ramachandran outliers (%) | 2.4 ± 1.4 | |
| Unfavorable sidechain rotamers (%) | 5.0 ± 2.1 | |
| Clashscore, all atoms5 | 0.0 ± 0.0 | |
| Overall MolProbity score | 1.67 ± 0.15 |
All statistics are given as mean ± S.D.
Only structurally relevant restraints, as defined by CYANA, are included.
Two restraints were used per hydrogen bond.
According to MolProbity (http://molprobity.biochem.duke.edu)
Defined as the number of steric overlaps >0.4 Å per thousand atoms
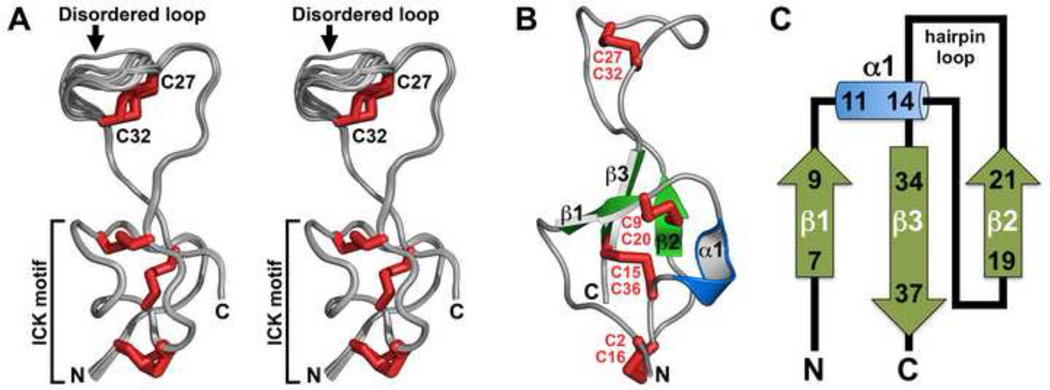
Fig. 7A shows a backbone overlay of the ensemble of 20 rAps III structures, while a schematic of the top-ranked structure highlighting key secondary structure elements is shown in Fig. 7B. Three of the four disulfide bonds in rAps III form a classical ICK motif in which the Cys2–16 and Cys9–20 disulfide bonds and the intervening sections of polypeptide backbone form a 13-residue ring that is pierced by the Cys15–Cys36 disulfide bond (Fig. 7A). This region forms a highly structured, disulfide-rich core from which emerges an unusual β-hairpin with a very large hairpin loop (Fig. 7B). The two β-strands of the hairpin are formed by residues 19–21 (β2) and 34–37 (β3), while the loop comprises residues 22–33. Residues 7–9 form a third β-strand (β1) and residues 11–14 form a single turn of 310-helix (α1) (Fig. 7B). A summary of the secondary structure of rAps III as judged by PDBsum [56] is shown in Fig. 7C.
Fig. 7. Three-dimensional structure of rAps III.
(A) Stereoview of an overlay of the ensemble of 20 rAps III structures. Disulfide bonds are highlighted in red and the N- and C-termini are labelled. The structures are overlaid over the backbone atoms of residues 2–27 and 32–38 in order to highlight the disordered nature of the triglycine loop (residues 27–32) relative to the well-structured ICK region of the toxin. (B) Ribbon representation of the rAps III structure highlighting the various secondary structure elements and disulfide bonds. The views in panels (B) and (C) are related by a ~180° rotation around the long axis of the molecule. (C) Topology map of the secondary structure of rAps III. The N- and C-termini are labelled.
While the compact ICK region of the rAps III structure is similar to previously determined structures of knottin peptides [57], the protruding β-hairpin loop, which often houses the pharmacophore in this class of spider toxins [15], is highly unusual. First, this hairpin is unusually large, comprising 13 residues (Fig. 7A,B). In comparison, the average size of the β-hairpin loop in the 35 spider-venom ICK toxins listed in ArachnoServer [17, 18] is 4.8 ± 1.8 residues. Second, the hairpin loop contains a glycine triplet (Gly29–Gly30–Gly31; underlined in Fig. 1B) that is rare in this class of toxins. Third, the glycine-rich portion of the loop is poorly defined in the ensemble of rAps II structures (Fig. 7A). Gly30 and to a lesser extent Gly32 consistently fall into unfavorable regions of the Ramachandran plot, suggesting that this region is highly dynamic in solution. Nevertheless, the dynamics of the triglycine loop is likely to be limited by the Cys27–Cys32 disulfide bond (Fig. 7A,B) which forms a molecular staple that serves to isolate this loop from the remainder of the β-hairpin, which in contrast is well structured.
It should be noted that the disulfide framework has not been experimentally established for native Aps III. However, the fact that rAps III is insecticidal and that it contains an ICK motif that is the defining structural characteristic of this class of toxins suggests that the recombinant peptide has the same disulfide framework and 3D fold as the native peptide.
4. DISCUSSION
4.1 rAps III potently inhibits insect NaV channels
In the present study, the effect of recombinantly produced Aps III was examined on three families of insect voltage-activated ion channels: NaV, CaV and KV channels. The main effect of rAps III was to produce a concentration-dependent inhibition of insect NaV channels with an estimated IC50 of 540 nM. The pharmacological properties of rAps III resemble those of ‘pore blocking’ toxins that target NaV channels such as the guanidinium compounds TTX and saxitoxin as well as µ-conotoxins from marine cone snail venoms that reduce peak INa [58].
rAps III displays similar activity to µ-TRTX-Hhn2b (hainantoxin-I) and µ-TRTX-Hh1a (huwentoxin-III), depressant neurotoxins from the venom of the Chinese tarantulas Haplopelma hainanum and H. huwenum, respectively. These toxins have been postulated to block insect NaV channels via binding to neurotoxin receptor site-1 near the mouth of the channel [59, 60]. Both toxins block insect NaV channels more potently than vertebrate NaV channels. For example, µ-TRTX-Hhn2b blocks Drosophila melanogaster NaV channels expressed in Xenopus oocytes with an IC50 of 4.5 µM compared with a 15-fold higher IC50 of 68 ± 6 µM for block of rat NaV1.2 channels [59]. µ-TRTX-Hh1a did not block any of the vertebrate NaV channel subtypes expressed in rat dorsal root ganglion neurons but it inhibited insect NaV channel currents in cockroach DUM neurons with an IC50 of 1.1 µM [60]. Thus, although both µ-TRTX-Hhn2b and µ-TRTX-Hh1a selectively block insect NaV channels, rAps III targets the insect channel with higher affinity, consistent with its high level of lethality (LD50 = 133 pmol/g) in larvae of the tobacco hornworm Manduca sexta [16].
One mechanism that can lead to inhibition of INa is an increase in the number of NaV channels stabilised in the inactivated state. µ-TMTX-Hme1a (Hm-1) from the venom of the spider Heriaeus melloteei inhibits mammalian NaV channels expressed in Xenopus oocytes without an alteration in the activation or inactivation kinetics of the channel [61]. The reduction in peak INa produced by µ-TMTX-Hme1a results from a shift in steady-state NaV channel inactivation in the hyperpolarising direction [61]. Thus, the number of closed channels available for opening is reduced, resulting in flaccid paralysis. Unlike µ-TMTX-Hme1a, neither rAps III nor µ-TRTX-Hh1a produce a significant shift in the voltage-dependence of steady-state NaV channel inactivation. Thus rAps III does not share a common mode of action with µ-TMTX-Hme1a, despite its ability to inhibit INa.
Interestingly, rAps III displays weak sequence homology with a range of spider δ-toxins (41% homology). All δ-toxins are gating modifiers that delay NaV channel inactivation via an interaction with the voltage sensor of channel domain IV [62–67]. As a result, cells generate spontaneous and repetitive action potentials at, or near, the resting membrane potential to induce contractile paralysis [65]. A similar effect has been noted with spider β-toxins that shift the voltage-dependence of activation in the hyperpolarising direction primarily via an interaction with the voltage sensor of channel domain II [52, 68, 69]. However, the lack of any significant shifts in the voltage-dependence of NaV channel activation or slowing of NaV channel inactivation kinetics indicates that the effect of rAps III on NaV channels is not due a modification of channel gating. Other toxins, such as the depressant toxin β-TRTX-Cm1a (ceratotoxin-1) from the straight-horned tarantula Ceratogyrus marshalli, can inhibit INa by shifting the voltage dependence of activation in the depolarising direction to produce a depressant phenotype [70]. In contrast, rAps III inhibits insect NaV channels conductance in the absence of any depolarising shift in the voltage-dependence of activation. In summary, rAps III does not cause a hyperpolarising shift (as seen for excitatory spider β-toxins) or a depolarising shift (as observed for depressant spider β-toxins) in the voltage-dependence of NaV channel activation, nor does it slow the kinetics of NaV channel inactivation as observed for spider δ-toxins.
Based on the pharmacology described here, Aps III should be renamed µ-cyrtautoxin-As1a (µ-CUTX-As1a) based on the rational nomenclature recently proposed for spider-venom peptides [71]. This is consistent with its action to induce flaccid paralysis in M. sexta by inhibiting neuronal excitability [16], in contrast with the spastic paralysis observed with δ-toxins and excitatory β-toxins.
4.2 Promiscuous activity of rAps III on insect NaV and CaV channels
While rAps III inhibits insect NaV channels with higher potency than other insect-selective spider neurotoxins such as µ-TRTX-Hhn2b and µ-TRTX-Hh1a, the IC50 value (540 nM) is still relatively high compared to spider toxins that inhibit vertebrate NaV channels [45]. This suggests that rAps III may also target other voltage-activated channels. Spider toxins can interact with more than one target often across voltage-activated ion channel families and/or across channel subtypes to elicit multiple functions. This non-selective activity is due to the common structural elements shared between voltage-activated ion channels that are recognised by these toxins [72]. NaV and CaV channels, in particular, are closely related and contain shared structural motifs and functional domains. Thus it is not surprising to find toxins with promiscuous activity across these channel families [49, 67]. Promiscuous activity of spider toxins with high affinity for both NaV and KV, or NaV and CaV, channels is not without precedence and has been previously demonstrated by vertebrate active spider toxins including the NaV/KV channel toxins µ/ω-TRTX-Hh1a [73] and β/κ-TRTX-Cj1a [74] and the NaV/CaV channel toxin β/ω-TRTX-Tp2a (ProTx-II) [75]. Moreover, spider toxins targeting CaV channels with additional low affinity for NaV channels have also been described including ω-TRTX-Hg1a (SNX482) [76], ω-agatoxin-Aa4a (ω-Aga-IVA)[77] and ω-hexa-toxin-Ar1a[46].
In addition to its effect on NaV channels, 1 µM rAps III produced a modest 30% block of M-LVA and HVA CaV channel currents without any change in the voltage-dependence of CaV channel activation or inactivation kinetics. Most spider ω-toxins block M-LVA and HVA CaV channels in DUM neurons with IC50 values in the range 270–1000 nM (Table 2). Thus, CaV channels are unlikely to be the primary target of rAps III, but this pharmacology might contribute to the depressant phenotype by blocking Ca2+ entry into nerve terminals and thus inhibiting excitatory neurotransmitter release.
Table 2.
Block of cockroach DUM neuron CaV channels by spider neurotoxins.
| CaV channel subtype |
|||
|---|---|---|---|
| Toxin | M-LVA IC50 (nM) | HVA IC50 (nM) | Reference |
| ω-HXTX-Hv1a | 279 | 1080 | [46] |
| ω-HXTX-Ar1a | 692 | 644 | [46] |
| ω-CNTX-Cs1a* | 467 | 274 | [79] |
Insecticidal toxin also active on mammalian CaV channels
4.3 Advantages of complementary pharmacologies
rAps III produces marked inhibition of NaV channels to inhibit action potential generation and propagation as well as partial block of CaV channels to inhibit neurotransmitter release. Both of these pharmacological sensitivities would cause inhibition of neurotransmission, consistent with the flaccid paralysis induced by rAps III in insect toxicity assays. While the inhibition of CaV channels by rAps III is not as potent as other spider ω-toxins with highly selective actions on a single target, the combined inhibition of both NaV and both CaV channel subtypes by rAps III is likely responsible for its potent insecticidal activity. It should be noted that insects have a much smaller repertoire of CaV channels than vertebrates, with only a single ortholog of the vertebrate CaV1, CaV2 and CaV3 subtypes [48]. Thus, even moderate inhibition of CaV channels can be lethal to insects [48].
4.4 The unusual structure of rAps III might contribute to its novel pharmacology
In addition to its novel mode of action, the 3D structure of rAps III diverges significantly from typical spider-venom ICK toxins in that it contains an unusually large β-hairpin loop. The upper portion of this loop is stapled together by an additional disulfide bridge that serves to limit the solution dynamics of the unusual triglycine region of the loop. Although additional studies will be required to determine the precise mechanism of action and binding site of rAps III on insect NaV channels, the current data suggests that it might bind in the outer vestibule of the channel rather than interact with one of the voltage-sensor domains. The large β-hairpin loop increases the footprint of this region of the toxin compared with other ICK-containing spider-venom toxins, and computer modelling (E. Deplazes and G.F. King, unpublished) indicates that the β-hairpin loop is an ideal size to fit into the turret region of the recently determined crystal structures of bacterial NaV channels [78]. Thus, in addition to being a useful bioinsecticide lead, rAps III might prove to be a valuable pharmacological tool for the study of invertebrate NaV channels.
Acknowledgments
We thank Ke Dong (Michigan State University) for the BgNav1 and TipE clones, Geoff Brown (Department of Agriculture, Fisheries and Forestry, Brisbane) for the supply of blowflies, and the Queensland NMR Network for access to the 900 MHz NMR spectrometer at the University of Queensland. This work was supported by grants from the Australian Research Council (Discovery Grant DP1095728 to G.F.K.) and the National Institute of Neurological Disorders And Stroke of the National Institutes of Health (Award Number R00NS073797 to F.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest.
References
- 1.Tedford HW, Sollod BL, Maggio F, King GF. Australian funnel-web spiders: master insecticide chemists. Toxicon. 2004;43:601–618. doi: 10.1016/j.toxicon.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. World Malaria Report. Geneva, Switzerland: WHO Press; 2009. [Google Scholar]
- 3.Oerke EC. Crop losses to pests. J Agric Sci. 2006;144:31–43. [Google Scholar]
- 4.Pimental D. Pesticides and pest control. In: Peshin R, Dhawan AK, editors. Integrated pest management: innovation-development process. Dordrecht: Springer Verlag; 2009. pp. 83–87. [Google Scholar]
- 5.Tedford HW, Gilles N, Ménez A, Doering CJ, Zamponi GW, King GF. Scanning mutagenesis of ω-atracotoxin-Hv1a reveals a spatially restricted epitope that confers selective activity against invertebrate calcium channels. J Biol Chem. 2004;279:44133–44140. doi: 10.1074/jbc.M404006200. [DOI] [PubMed] [Google Scholar]
- 6.Bass C, Field LM. Gene amplification and insecticide resistance. Pest Manag Sci. 2011;67:886–890. doi: 10.1002/ps.2189. [DOI] [PubMed] [Google Scholar]
- 7.King GF, Hardy MC. Spider-venom peptides: structure, pharmacology, and potential for control of insect pests. Annual review of entomology. 2013;58:475–496. doi: 10.1146/annurev-ento-120811-153650. [DOI] [PubMed] [Google Scholar]
- 8.Gatehouse AM, Ferry N, Edwards MG, Bell HA. Insect-resistant biotech crops and their impacts on beneficial arthropods. Philos Trans R Soc Lond B – Biol Sci. 2011;366:1438–1452. doi: 10.1098/rstb.2010.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates SL, Zhao JZ, Roush RT, Shelton AM. Insect resistance management in GM crops: past, present and future. Nat Biotechnol. 2005;23:57–62. doi: 10.1038/nbt1056. [DOI] [PubMed] [Google Scholar]
- 10.Maggio F, Sollod BL, Tedford HW, Herzig V, King GF. Spider toxins and their potential for insect control. In: LI G, Gill SS, editors. Insect Pharmacology: Channels, Receptors, Toxins and Enzymes. London: Academic Press; 2010. pp. 101–123. [Google Scholar]
- 11.Kuhn-Nentwig L, Stöcklin R, Nentwig W. In: Venom composition and strategies in spiders: is everything possible? Casas J, editor. Elsevier: Spider Physiology and Behaviour—Physiology; 2011. pp. 1–86. [Google Scholar]
- 12.Windley MJ, Herzig V, Dziemborowicz SA, Hardy MC, King GF, Nicholson GM. Spider-venom peptides as bioinsecticides. Toxins. 2012;4:191–227. doi: 10.3390/toxins4030191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallaghy PK, Nielsen KJ, Craik DJ, Norton RS. A common structural motif incorporating a cystine knot and a triple-stranded β-sheet in toxic and inhibitory polypeptides Protein Sci. 1994;3:1833–1839. doi: 10.1002/pro.5560031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King GF, Tedford HW, Maggio F. Structure and function of insecticidal neurotoxins from Australian funnel-web spiders. J Toxicol Toxin Rev. 2002;21:359–389. [Google Scholar]
- 15.Saez NJ, Senff S, Jensen JE, Er SY, Herzig V, Rash LD, et al. Spider-venom peptides as therapeutics. Toxins. 2010;2:2851–2871. doi: 10.3390/toxins2122851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skinner WS, Dennis PA, Li JP, Quistad GB. Identification of insecticidal peptides from venom of the trap-door spider, Aptostichus schlingeri (Ctenizidae) Toxicon. 1992;30:1043–1050. doi: 10.1016/0041-0101(92)90049-b. [DOI] [PubMed] [Google Scholar]
- 17.Wood DL, Miljenovic T, Cai S, Raven RJ, Kaas Q, Escoubas P, et al. ArachnoServer: a database of protein toxins from spiders. BMC Genomics. 2009;10:375. doi: 10.1186/1471-2164-10-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzig V, Wood DLA, Newell F, Chaumeil PA, Kaas Q, Binford GJ, et al. ArachnoServer 2.0, an updated online resource for spider toxin sequences and structures Nucleic Acids Res. 2011;39:D653–D657. doi: 10.1093/nar/gkq1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klint JK, Senff S, Rupasinghe DB, Er SY, Herzig V, Nicholson GM, et al. Spider-venom peptides that target voltage-gated sodium channels: pharmacological tools and potential therapeutic leads. Toxicon. 2012;60:478–491. doi: 10.1016/j.toxicon.2012.04.337. [DOI] [PubMed] [Google Scholar]
- 20.Fang L, Jia KZ, Tang YL, Ma DY, Yu M, Hua ZC. An improved strategy for high-level production of TEV protease in Escherichia coli and its purification and characterization. Protein expression and purification. 2007;51:102–109. doi: 10.1016/j.pep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 21.Cabrita LD, Dai W, Bottomley SP. A family of E. coli expression vectors for laboratory scale and high throughput soluble protein production. BMC Biotechnol. 2006;6:12. doi: 10.1186/1472-6750-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bassford JP. Export of the periplasmic maltose-binding protein of Escherichia coli. J Bioenerg Biomembr. 1990;22:401–439. doi: 10.1007/BF00763175. [DOI] [PubMed] [Google Scholar]
- 23.Kapust RB, Waugh DS. Escherichia coli maltose-binding protein is uncommonly effective at promoting the solubility of polypeptides to which it is fused. Protein Sci. 1999;8:1668–1674. doi: 10.1110/ps.8.8.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saez NJ, Mobli M, Bieri M, Chassagnon IR, Malde AK, Gamsjaeger R, et al. A dynamic pharmacophore drives the interaction between psalmotoxin-1 and the putative drug target acid-sensing ion channel 1a. Mol Pharmacol. 2011;80:796–808. doi: 10.1124/mol.111.072207. [DOI] [PubMed] [Google Scholar]
- 25.Eitan M, Fowler E, Herrmann R, Duval A, Pelhate M, Zlotkin E. A scorpion venom neurotoxin paralytic to insects that affects sodium current inactivation: purification, primary structure, and mode of action. Biochemistry. 1990;29:5941–5947. doi: 10.1021/bi00477a009. [DOI] [PubMed] [Google Scholar]
- 26.Herzig V, Hodgson WC. Neurotoxic and insecticidal properties of venom from the Australian theraphosid spider Selenotholus foelschei. Neurotoxicology. 2008;29:471–475. doi: 10.1016/j.neuro.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Gunning SJ, Maggio F, Windley MJ, Valenzuela SM, King GF, Nicholson GM. The Janus-faced atracotoxins are specific blockers of invertebrate Kca channels. FEBS J. 2008;275:4045–4059. doi: 10.1111/j.1742-4658.2008.06545.x. [DOI] [PubMed] [Google Scholar]
- 28.Windley MJ, Escoubas P, Valenzuela SM, Nicholson GM. A novel family of insect-selective peptide neurotoxins targeting insect large-conductance calcium-activated K+ channels isolated from the venom of the theraphosid spider Eucratoscelus constrictus. Mol Pharmacol. 2011;80:1–13. doi: 10.1124/mol.110.070540. [DOI] [PubMed] [Google Scholar]
- 29.Barry PH. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 30.Wicher D, Penzlin H. Ca2+ currents in central insect neurons: electrophysiological and pharmacological properties. J Neurophysiol. 1997;77:186–199. doi: 10.1152/jn.1997.77.1.186. [DOI] [PubMed] [Google Scholar]
- 31.Tan J, Liu Z, Nomura Y, Goldin AL, Dong K. Alternative splicing of an insect sodium channel gene generates pharmacologically distinct sodium channels. J Neurosci. 2002;22:5300–5309. doi: 10.1523/JNEUROSCI.22-13-05300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng G, Deak P, Chopra M, Hall LM. Cloning and functional analysis of TipE, a novel membrane protein that enhances Drosophila para sodium channel function. Cell. 1995;82:1001–10011. doi: 10.1016/0092-8674(95)90279-1. [DOI] [PubMed] [Google Scholar]
- 33.Zhou W, Goldin AL. Use-dependent potentiation of the NaV1.6 sodium channel. Biophys J. 2004;87:3862–3872. doi: 10.1529/biophysj.104.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mobli M, Stern AS, Bermel W, King GF, Hoch JC. A non-uniformly sampled 4D HCC(CO)NH-TOCSY experiment processed using maximum entropy for rapid protein sidechain assignment. J Magn Reson. 2010;204:160–164. doi: 10.1016/j.jmr.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mobli M, Maciejewski MW, Gryk MR, Hoch JC. An automated tool for maximum entropy reconstruction of biomolecular NMR spectra. Nat Meth. 2007;4:467–468. doi: 10.1038/nmeth0607-467. [DOI] [PubMed] [Google Scholar]
- 36.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fletcher JI, Smith R, O'Donoghue SI, Nilges M, Connor M, Howden MEH, et al. The structure of a novel insecticidal neurotoxin, ω-atracotoxin-HV1, from the venom of an Australian funnel web spider. Nat Struct Biol. 1997;4:559–566. doi: 10.1038/nsb0797-559. [DOI] [PubMed] [Google Scholar]
- 38.Güntert P. Automated NMR structure calculation with CYANA. Methods Mol Biol. 2004;278:353–378. doi: 10.1385/1-59259-809-9:353. [DOI] [PubMed] [Google Scholar]
- 39.Vetter I, Davis JL, Rash LD, Anangi R, Mobli M, Alewood PF, et al. Venomics: a new paradigm for natural products-based drug discovery. Amino Acids. 2011;40:15–28. doi: 10.1007/s00726-010-0516-4. [DOI] [PubMed] [Google Scholar]
- 40.Meng E, Cai TF, Li WY, Zhang H, Liu YB, Peng K, et al. Functional expression of spider neurotoxic peptide huwentoxin-I in E. coli. PLoS One. 2011;6:e21608. doi: 10.1371/journal.pone.0021608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heras B, Shouldice SR, Totsika M, Scanlon MJ, Schembri MA, Martin JL. DSB proteins and bacterial pathogenicity. Nat Rev Microbiol. 2009;7:215–225. doi: 10.1038/nrmicro2087. [DOI] [PubMed] [Google Scholar]
- 42.Sackett D, Holmes P, Abbott K, Jephcott S, Barber M. Assessing the economic cost of endemic disease on the profitability of Australian beef cattle and sheep producers. Sydney: Meat & Livestock Australia; 2006. [Google Scholar]
- 43.Vassilevski A, Kozlov S, Grishin E. Molecular diversity of spider venom. Biochemistry (Moscow) 2009;74:1505–1534. doi: 10.1134/s0006297909130069. [DOI] [PubMed] [Google Scholar]
- 44.Bosmans F, Escoubas P, Nicholson GM. Spider venom peptides as leads for drug and insecticide design. In: de Lima ME, Pimenta AMC, Martin-Eauclaire MF, Zingali RB, Rochat H, editors. Animal Toxins: State of the Art Perspectives In Health and Biotechnology. Belo Horizonte: Federal University of Minas Gerais Press; 2009. pp. 269–290. [Google Scholar]
- 45.Yamaji N, Little MJ, Nishio H, Billen B, Villegas E, Nishiuchi Y, et al. Synthesis, solution structure, and phylum selectivity of a spider δ-toxin that slows inactivation of specific voltage-gated sodium channel subtypes. J Biol Chem. 2009;284:24568–24582. doi: 10.1074/jbc.M109.030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chong Y, Hayes JL, Sollod B, Wen S, Wilson DT, Hains PG, et al. The ω-atracotoxins: selective blockers of insect M-LVA and HVA calcium channels. Biochemical pharmacology. 2007;74:623–638. doi: 10.1016/j.bcp.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 47.Zhang MM, Han TS, Olivera BM, Bulaj G, Yoshikami D. µ-conotoxin KIIIA derivatives with divergent affinities versus efficacies in blocking voltage-gated sodium channels. Biochemistry. 2010;49:4804–4812. doi: 10.1021/bi100207k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.King GF. Modulation of insect Cav channels by peptidic spider toxins. Toxicon. 2007;49:513–530. doi: 10.1016/j.toxicon.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 49.King GF, Escoubas P, Nicholson GM. Peptide toxins that selectively target insect Nav and Cav channels. Channels. 2008;2:100–116. doi: 10.4161/chan.2.2.6022. [DOI] [PubMed] [Google Scholar]
- 50.Grolleau F, Lapied B. Separation and identification of multiple potassium currents regulating the pacemaker activity of insect neurosecretory cells (DUM neurons) J Neurophysiol. 1995;73:160–171. doi: 10.1152/jn.1995.73.1.160. [DOI] [PubMed] [Google Scholar]
- 51.Wicher D, Walther C, Wicher C. Non-synaptic ion channels in insects—basic properties of currents and their modulation in neurons and skeletal muscles. Prog Neurobiol. 2001;64:431–525. doi: 10.1016/s0301-0082(00)00066-6. [DOI] [PubMed] [Google Scholar]
- 52.Bosmans F, Swartz KJ. Targeting voltage sensors in sodium channels with spider toxins. Trends Pharmacol Sci. 2010;31:175–182. doi: 10.1016/j.tips.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mobli M, King GF. NMR methods for determining disulfide-bond connectivities. Toxicon. 2010;56:849–854. doi: 10.1016/j.toxicon.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucl Acids Res. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwan AH, Mobli M, Gooley PR, King GF, Mackay JP. Macromolecular NMR spectroscopy for the non-spectroscopist. FEBS J. 2011;278:687–703. doi: 10.1111/j.1742-4658.2011.08004.x. [DOI] [PubMed] [Google Scholar]
- 56.Laskowski RA. PDBsum new things. Nucleic Acids Res. 2009;37:D355–D359. doi: 10.1093/nar/gkn860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gracy J, Le-Nguyen D, Gelly JC, Kaas Q, Heitz A, Chiche L. KNOTTIN: the knottin or inhibitor cystine knot scaffold in 2007. Nucleic Acids Res. 2008;36:D314–D319. doi: 10.1093/nar/gkm939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shon KJ, Olivera BM, Watkins M, Jacobsen R, Gray WR, Floresca CZ, et al. µ-Conotoxin PIIIA, a new peptide for discriminating among tetrodotoxin-sensitive Na channel subtypes. J Neurosci. 1998;18:4473–4481. doi: 10.1523/JNEUROSCI.18-12-04473.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li D, Xiao Y, Hu W, Xie J, Bosmans F, Tytgat J, et al. Function and solution structure of hainantoxin-I, a novel insect sodium channel inhibitor from the Chinese bird spider Selenocosmia hainana. FEBS Lett. 2003;555:616–622. doi: 10.1016/s0014-5793(03)01303-6. [DOI] [PubMed] [Google Scholar]
- 60.Wang RL, Yi S, Liang SP. Mechanism of action of two insect toxins huwentoxin-III and hainantoxin-VI on voltage-gated sodium channels. J Zhejiang Univ Sci B. 2010;11:451–457. doi: 10.1631/jzus.B0900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billen B, Vassilevski A, Nikolsky A, Tytgat J, Grishin E. Two novel sodium channel inhibitors from Heriaeus melloteei spider venom differentially interacting with mammalian channel's isoforms. Toxicon. 2008:309–317. doi: 10.1016/j.toxicon.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 62.Fletcher JI, Chapman BE, Mackay JP, Howden MEH, King GF. The structure of versutoxin (δ-atracotoxin-Hv1) provides insights into the binding of site 3 neurotoxins to the voltage-gated sodium channel. Structure. 1997;5:1525–1535. doi: 10.1016/s0969-2126(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 63.Little MJ, Wilson H, Zappia C, Cestele S, Tyler MI, Martin-Eauclaire MF, et al. δ-atracotoxins from Australian funnel-web spiders compete with scorpion α-toxin binding on both rat brain and insect sodium channels. FEBS Lett. 1998:246–252. doi: 10.1016/s0014-5793(98)01378-7. [DOI] [PubMed] [Google Scholar]
- 64.Little MJ, Zappia C, Gilles N, Connor M, Tyler MI, Martin-Eauclaire MF, et al. δ-Atracotoxins from Australian funnel-web spiders compete with scorpion a-toxin binding but differentially modulate alkaloid toxin activation of voltage-gated sodium channels. J Biol Chem. 1998;273:27076–27083. doi: 10.1074/jbc.273.42.27076. [DOI] [PubMed] [Google Scholar]
- 65.Grolleau F, Stankiewicz M, Birinyi-Strachan L, Wang X, Nicholson GM, Pelhate M, et al. Electrophysiological analysis of the neurotoxic action of a funnel-web spider toxin, δ-atracotoxin-Hv1a, on insect voltage-gated Na+ channels. J Exp Biol. 2001;204:711–721. doi: 10.1242/jeb.204.4.711. [DOI] [PubMed] [Google Scholar]
- 66.Matavel A, Cruz JS, Penaforte CL, Araujo DA, Kalapothakis E, Prado VF, et al. Electrophysiological characterization and molecular identification of the Phoneutria nigriventer peptide toxin PnTx2–6. FEBS Lett. 2002:523. doi: 10.1016/s0014-5793(02)02988-5. [DOI] [PubMed] [Google Scholar]
- 67.Nicholson GM. Insect-selective spider toxins targeting voltage-gated sodium channels. Toxicon. 2007;49:490–512. doi: 10.1016/j.toxicon.2006.11.027. [DOI] [PubMed] [Google Scholar]
- 68.Zeng X, Deng M, Lin Y, Yuan C, Pi J, Liang S. Isolation and characterization of Jingzhaotoxin-V, a novel neurotoxin from the venom of the spider Chilobrachys jingzhao. Toxicon. 2007;49:388–399. doi: 10.1016/j.toxicon.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 69.Bosmans F, Martin-Eauclaire MF, Swartz KJ. Deconstructing voltage sensor function and pharmacology in sodium channels. Nature. 2008;456:202–208. doi: 10.1038/nature07473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosmans F, Rash L, Zhu S, Diochot S, Lazdunski M, Escoubas P, et al. Four novel tarantula toxins as selective modulators of voltage-gated sodium channel subtypes. Mol Pharmacol. 2006;69:419–429. doi: 10.1124/mol.105.015941. [DOI] [PubMed] [Google Scholar]
- 71.King GF, Gentz MC, Escoubas P, Nicholson GM. A rational nomenclature for naming peptide toxins from spiders and other venomous animals. Toxicon. 2008;52:264–276. doi: 10.1016/j.toxicon.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 72.Li-Smerin Y, Swartz KJ. Gating modifier toxins reveal a conserved structural motif in voltage-gated Ca2+ and K+ channels. Proc Natl Acad Sci USA. 1998;95:8585–8589. doi: 10.1073/pnas.95.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang M, Guan X, Liang S. The cross channel activities of spider neurotoxin huwentoxin-I on rat dorsal root ganglion neurons. Biochem Biophys Res Commun. 2007;357:579–583. doi: 10.1016/j.bbrc.2007.02.168. [DOI] [PubMed] [Google Scholar]
- 74.Liao Z, Yuan C, Peng K, Xiao Y, Liang S. Solution structure of Jingzhaotoxin-III, a peptide toxin inhibiting both Nav1.5 and Kv2.1 channels. Toxicon. 2007;50:135–143. doi: 10.1016/j.toxicon.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 75.Edgerton GB, Blumenthal KM, Hanck DA. Inhibition of the activation pathway of the T-type calcium channel CaV3.1 by ProTxII. Toxicon. 2010;56:624–636. doi: 10.1016/j.toxicon.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arroyo G, Aldea M, Fuentealba J, Albillos A, García AG. SNX482 selectively blocks P/Q Ca2+ channels and delays the inactivation of Na+ channels of chromaffin cells. Eur J Pharmacol. 2003;475:11–18. doi: 10.1016/s0014-2999(03)02084-3. [DOI] [PubMed] [Google Scholar]
- 77.Wicher D, Penzlin H. ω-Toxins affect Na+ currents in neurosecretory insect neurons. Receptors & Channels. 1998;5:355–366. [PubMed] [Google Scholar]
- 78.Catterall WA. Voltage-gated sodium channels at 60: structure, function and pathophysiology. J Physiol. 2012;590:2577–2589. doi: 10.1113/jphysiol.2011.224204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kubista H, Mafra RA, Nicholson GM, Beirão PS, Cruz JS, Boehm S, et al. CSTX-1, a toxin from the venom of the hunting spider Cupiennius salei, is a selective blocker of L-type calcium channels in mammalian neurons. Neuropharmacol. 2007;52:1650–1662. doi: 10.1016/j.neuropharm.2007.03.012. [DOI] [PubMed] [Google Scholar]