Abstract
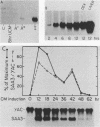
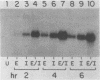
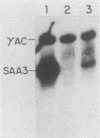
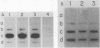
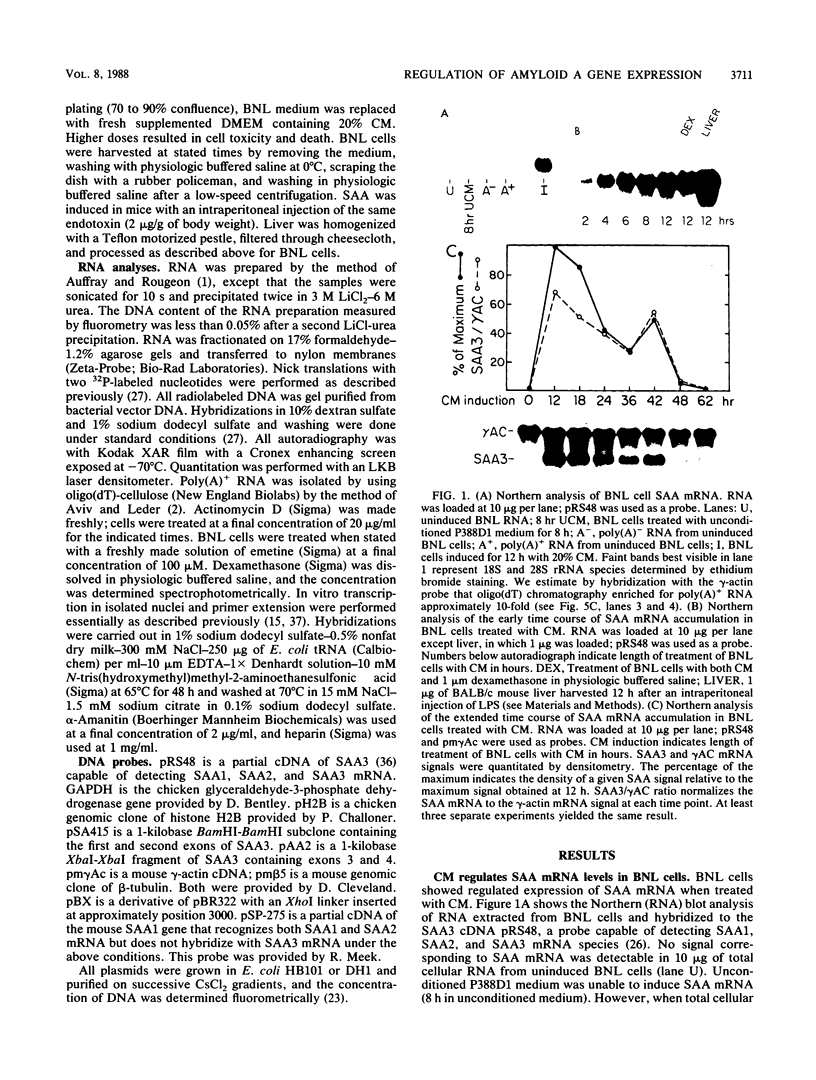
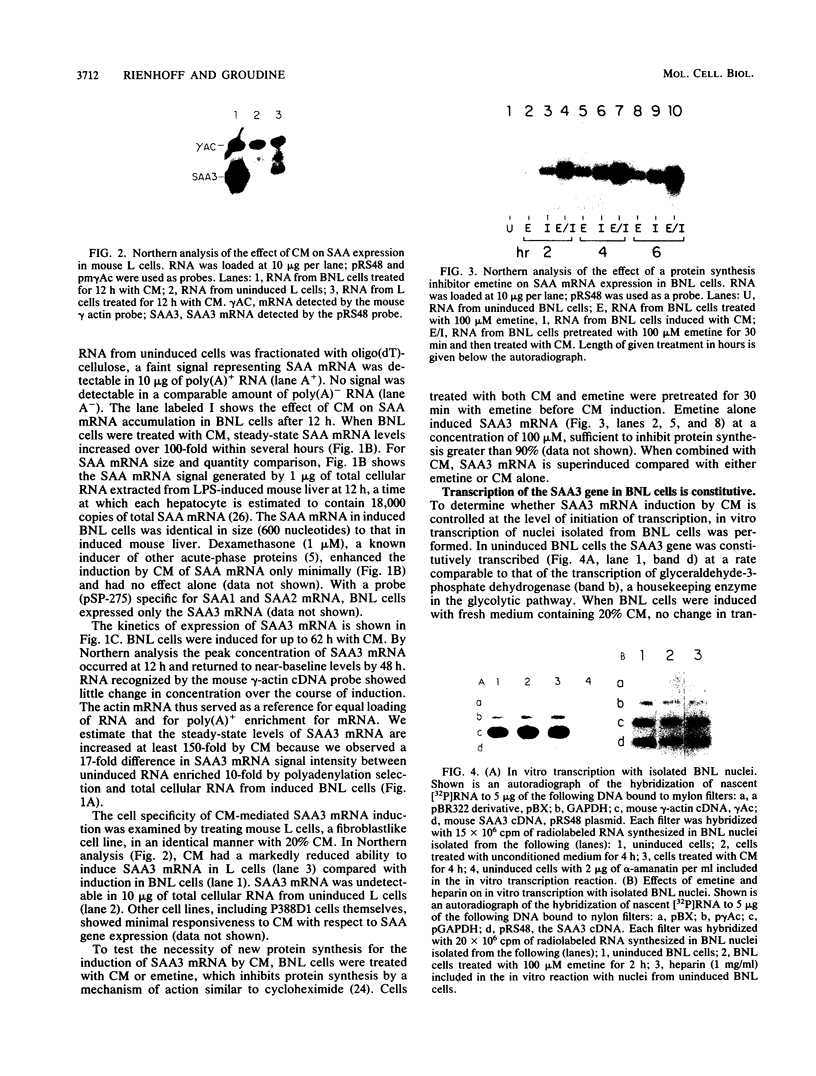
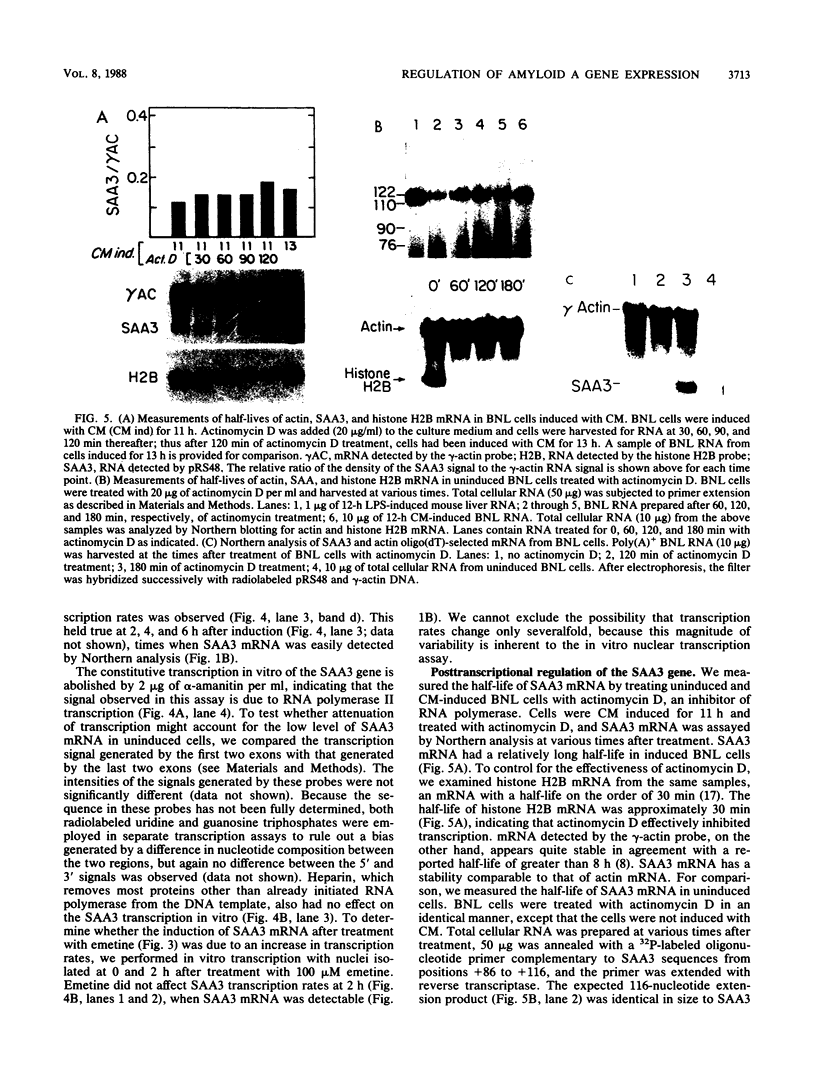
Serum amyloid A (SAA) proteins are secreted by mammalian liver in response to inflammatory stimuli. Both transcriptional and posttranscriptional mechanisms have been shown to regulate the 2,000-fold increase in SAA mRNA after injection of endotoxin into mice. We report here the characterization of a cell line derived from mouse liver (BNL) in which the expression of SAA3 mRNA is regulated. In this model, SAA3 mRNA accumulated in response to conditioned medium from the mouse macrophage P388D1 cell line with kinetics similar to that seen in mouse liver (C. A. Lowell, R. S. Stearman, and J. F. Morrow, J. Biol. Chem. 261:8453-8461, 1986). In in vitro nuclear transcription assays, the SAA3 gene was transcribed equally in induced and uninduced cells. In addition, in steady-state RNA studies treatment with conditioned medium allowed the cells to rapidly accumulate SAA3 mRNA without an apparent change in half-life. These observations suggest that conditioned medium contains a factor(s) that acts directly on hepatocytes to regulate SAA3 mRNA processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Held W. A., Berger F. G. The acute phase response of mouse liver. Genetic analysis of the major acute phase reactants. J Biol Chem. 1984 Jan 10;259(1):566–573. [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Gaines K. C. Synthesis and regulation of acute phase plasma proteins in primary cultures of mouse hepatocytes. J Cell Biol. 1983 Sep;97(3):866–876. doi: 10.1083/jcb.97.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M. D., Scheinberg M. A., Shirahama T., Cathcart E. S., Skinner M. Kinetics of serum amyloid protein A in casein-induced murine amyloidosis. J Clin Invest. 1977 Mar;59(3):412–417. doi: 10.1172/JCI108654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D., Kirschner M. Autoregulatory control of the expression of alpha- and beta-tubulins: implications for microtubule assembly. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):171–183. doi: 10.1101/sqb.1982.046.01.020. [DOI] [PubMed] [Google Scholar]
- Cox R. F. Quantitation of elongating form A and B RNA polymerases in chick oviduct nuclei and effects of estradiol. Cell. 1976 Mar;7(3):455–465. doi: 10.1016/0092-8674(76)90176-8. [DOI] [PubMed] [Google Scholar]
- Dente L., Ciliberto G., Cortese R. Structure of the human alpha 1-acid glycoprotein gene: sequence homology with other human acute phase protein genes. Nucleic Acids Res. 1985 Jun 11;13(11):3941–3952. doi: 10.1093/nar/13.11.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale B. E., Zacharchuk C. M., Okajima M., Shin H. S. Assay of a cytocidal protein excreted by activated macrophages. Methods Enzymol. 1986;132:549–555. doi: 10.1016/s0076-6879(86)32040-8. [DOI] [PubMed] [Google Scholar]
- Georgiev O., Birnstiel M. L. The conserved CAAGAAAGA spacer sequence is an essential element for the formation of 3' termini of the sea urchin H3 histone mRNA by RNA processing. EMBO J. 1985 Feb;4(2):481–489. doi: 10.1002/j.1460-2075.1985.tb03654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner G. G. Amyloid deposits and amyloidosis. The beta-fibrilloses (first of two parts). N Engl J Med. 1980 Jun 5;302(23):1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- Graves R. A., Pandey N. B., Chodchoy N., Marzluff W. F. Translation is required for regulation of histone mRNA degradation. Cell. 1987 Feb 27;48(4):615–626. doi: 10.1016/0092-8674(87)90240-6. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. L., Munford R. S. Enzymatic deacylation of the lipid A moiety of Salmonella typhimurium lipopolysaccharides by human neutrophils. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6671–6675. doi: 10.1073/pnas.80.21.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz N., Sive H. L., Roeder R. G. Regulation of human histone gene expression: kinetics of accumulation and changes in the rate of synthesis and in the half-lives of individual histone mRNAs during the HeLa cell cycle. Mol Cell Biol. 1983 Apr;3(4):539–550. doi: 10.1128/mcb.3.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman J. S., Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Murine tissue amyloid protein AA. NH2-terminal sequence identity with only one of two serum amyloid protein (ApoSAA) gene products. J Exp Med. 1984 Feb 1;159(2):641–646. doi: 10.1084/jem.159.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Kushner I. The phenomenon of the acute phase response. Ann N Y Acad Sci. 1982;389:39–48. doi: 10.1111/j.1749-6632.1982.tb22124.x. [DOI] [PubMed] [Google Scholar]
- Kyle R. A., Bayrd E. D. Amyloidosis: review of 236 cases. Medicine (Baltimore) 1975 Jul;54(4):271–299. doi: 10.1097/00005792-197507000-00001. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Potter D. A., Stearman R. S., Morrow J. F. Structure of the murine serum amyloid A gene family. Gene conversion. J Biol Chem. 1986 Jun 25;261(18):8442–8452. [PubMed] [Google Scholar]
- Lowell C. A., Stearman R. S., Morrow J. F. Transcriptional regulation of serum amyloid A gene expression. J Biol Chem. 1986 Jun 25;261(18):8453–8461. [PubMed] [Google Scholar]
- McAdam K. P., Sipe J. D. Murine model for human secondary amyloidosis: genetic variability of the acute-phase serum protein SAA response to endotoxins and casein. J Exp Med. 1976 Oct 1;144(4):1121–1127. doi: 10.1084/jem.144.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek R. L., Benditt E. P. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986 Dec 1;164(6):2006–2017. doi: 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. F., Stearman R. S., Peltzman C. G., Potter D. A. Induction of hepatic synthesis of serum amyloid A protein and actin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4718–4722. doi: 10.1073/pnas.78.8.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paek I., Axel R. Glucocorticoids enhance stability of human growth hormone mRNA. Mol Cell Biol. 1987 Apr;7(4):1496–1507. doi: 10.1128/mcb.7.4.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patek P. Q., Collins J. L., Cohn M. Transformed cell lines susceptible or resistant to in vivo surveillance against tumorigenesis. Nature. 1978 Nov 30;276(5687):510–511. doi: 10.1038/276510a0. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Pearson W. R., Morrow J. F. Regulation of synthesis of amyloid A-related protein. Ann N Y Acad Sci. 1982;389:106–115. doi: 10.1111/j.1749-6632.1982.tb22128.x. [DOI] [PubMed] [Google Scholar]
- Stearman R. S., Lowell C. A., Peltzman C. G., Morrow J. F. The sequence and structure of a new serum amyloid A gene. Nucleic Acids Res. 1986 Jan 24;14(2):797–809. doi: 10.1093/nar/14.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. A., Rowe L. Genes for serum amyloid A proteins map to Chromosome 7 in the mouse. Mol Gen Genet. 1984;195(3):491–499. doi: 10.1007/BF00341452. [DOI] [PubMed] [Google Scholar]
- Vaessen R. T., Houweling A., van der Eb A. J. Post-transcriptional control of class I MHC mRNA expression in adenovirus 12-transformed cells. Science. 1987 Mar 20;235(4795):1486–1488. doi: 10.1126/science.3823900. [DOI] [PubMed] [Google Scholar]
- Windle J. J., Shin H. S., Morrow J. F. Induction of interleukin 1 messenger RNA and translation in oocytes. J Immunol. 1984 Mar;132(3):1317–1322. [PubMed] [Google Scholar]
- Woloski B. M., Fuller G. M. Identification and partial characterization of hepatocyte-stimulating factor from leukemia cell lines: comparison with interleukin 1. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1443–1447. doi: 10.1073/pnas.82.5.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Migita S. Complete primary structures of two major murine serum amyloid A proteins deduced from cDNA sequences. Proc Natl Acad Sci U S A. 1985 May;82(9):2915–2919. doi: 10.1073/pnas.82.9.2915. [DOI] [PMC free article] [PubMed] [Google Scholar]