Summary
At synapses, sodium-coupled transporters remove released neurotransmitters, thereby recycling them and maintaining a low extracellular concentration of the neurotransmitter. The molecular mechanism underlying sodium-coupled neurotransmitter uptake is not completely understood. Several structures of homologues of human neurotransmitter transporters have been solved with X-ray crystallography. These crystal structures have spurred a plethora of computational and experimental work to elucidate the molecular mechanism underlying sodium-coupled transport. Here, we compare the structures of GltPh, a glutamate transporter homologue, and LeuT, a homologue of neurotransmitter transporters for the biogenic amines and inhibitory molecules GABA and glycine. We relate these structures to data obtained from experiments and computational simulations, to draw conclusions about the mechanism of uptake by sodium-coupled neurotransmitter transporters. We here propose how sodium and substrate binding is coupled and how binding of sodium and substrate opens and closes the gates in these transporters, thereby leading to an efficient coupled transport.
Neurotransmitter transporters
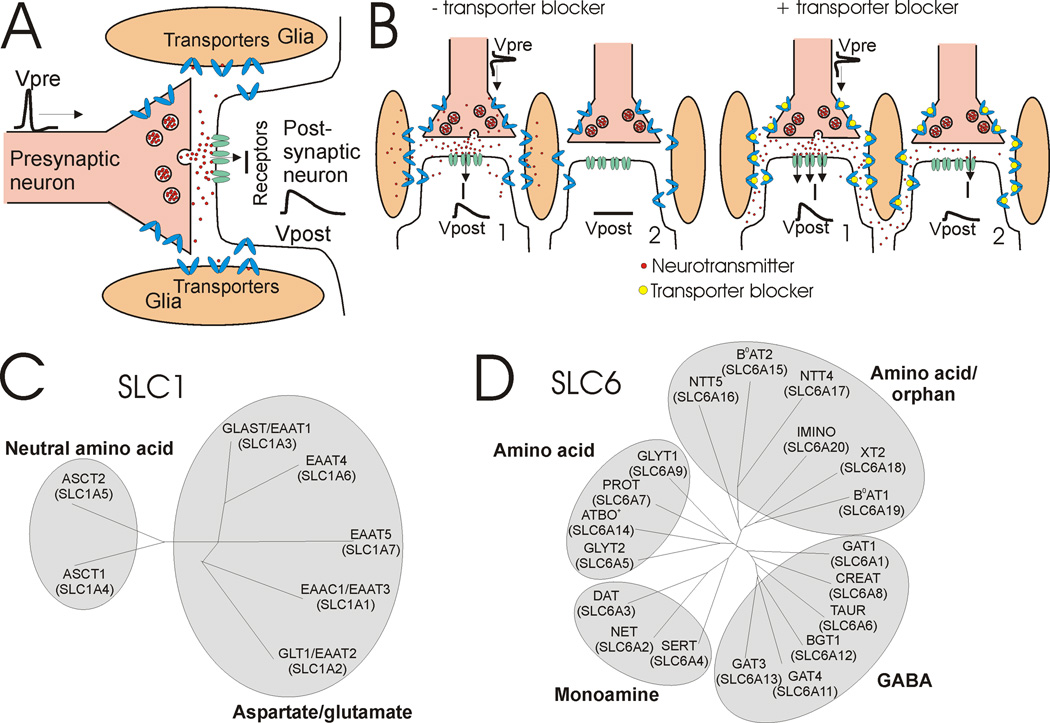
Communication between cells in the nervous system is mainly chemical, through pre-synaptic release of neurotransmitters, diffusion across the synapse, and activation of receptors in the post-synaptic cell (Fig. 1A). The released molecules, for example glutamate, GABA, serotonin, or dopamine, are subsequently removed from the extracellular space and transported back into the neuron or surrounding glial cells by neurotransmitter transporters. Their removal allows for subsequent release to exert full effect, as well as to localize signaling action to a synapse (Fig. 1B). Removal also prevents the prolonged presence of high concentrations of neurotransmitter, which can be detrimental in other ways. For example, high concentrations of extracellular glutamate are neurotoxic - basal extracellular glutamate concentration must be kept low (Danbolt, 2001; Grewer and Rauen, 2005).
Fig. 1. Transporter function and family trees.
A) A presynaptic action potential (Vpre) causes synaptic release of neutrotransmitter that diffuses across the synapse and activates postsynaptic receptors to cause an excitatory postsynaptic potential (Vpost). Subsequently, neurotransmitters diffuse out of the synapse and are taken up by neurotransmitter transporters. B) (left) Neurotransmitter transporters decrease the chance of neurotransmitter spillover by removing neurotransmitters released at one synapse before it has reached a nearby synapse. (right) If neurotransmitter transporters are blocked pharmacologically, then neurotransmitters will have a great chance of reaching nearby synapses and cause a postsynaptic response in nearby synapses (spillover). C) SLC1 family tree containing both aspartate/glutamate and neutral amino acid transporters. D) SLC6 family tree containing amino acid, orphan, monamine, and GABA transporters. For comprehensive descriptions of SLC1 and SLC6 family members, we refer readers to the following excellent reviews (Broer and Gether, 2012; Kanai and Hediger, 2003; Kristensen et al., 2011).
Extracellular glutamate is removed from the synapse by transporters called Excitatory Amino Acid Transporters (EAATs), which are expressed in neurons and glia. EAATs belong to solute carrier family 1 (SLC1; Fig 1C). Serotonin, noradrenaline, dopamine, GABA, and glycine are removed by neurotransmitter sodium symporters (NSS), belonging to solute carrier family 6 (SLC6; Fig. 1D). Due to their crucial role of keeping basal concentrations of neurotransmitters low, malfunction or improper regulation of these transporters contributes to neurological and neuropsychiatric disorders (Gether et al., 2006). For example, during ischemia in the brain caused by stroke, EAATs can malfunction and release glutamate, thereby elevating glutamate levels in the extracellular space to neurotoxic levels and causing massive neuronal death (Grewer and Rauen, 2005; Rossi et al., 2000). In addition, many drugs target the transporters, including drugs of abuse, such as cocaine and amphetamine, as well as drugs to treat depression, anxiety, obesity, and epilepsy (Kristensen et al., 2011).
A number of structures of the transporters GltPh and LeuT, homologous to the mammalian EAATs and NSSs, respectively, have been elucidated in various states by X-ray crystallography (Boudker et al., 2007; Krishnamurthy and Gouaux, 2012; Piscitelli and Gouaux, 2012; Quick et al., 2009; Reyes et al., 2009; Singh et al., 2008; Singh et al., 2007; Verdon and Boudker, 2012; Wang et al., 2012; Yamashita et al., 2005; Yernool et al., 2004; Zhou et al., 2007; Zhou et al., 2009). In-depth analysis of these structures and other proteins with similar structures have been conducted in several excellent reviews (Abramson and Wright, 2009; Boudker and Verdon, 2010; Forrest et al., 2011; Krishnamurthy et al., 2009). Here, we compare side-by-side the structures of GltPh and LeuT and relate these structures to data obtained from experiments and computational simulations, to draw conclusions about the mechanism of uptake by neurotransmitter transporters. We focus on how sodium and substrate binding is coupled and how binding of sodium and substrate affects the gates in these transporters, thereby leading to an efficient coupled transport.
Basic mechanism of secondary active transporters: alternating access
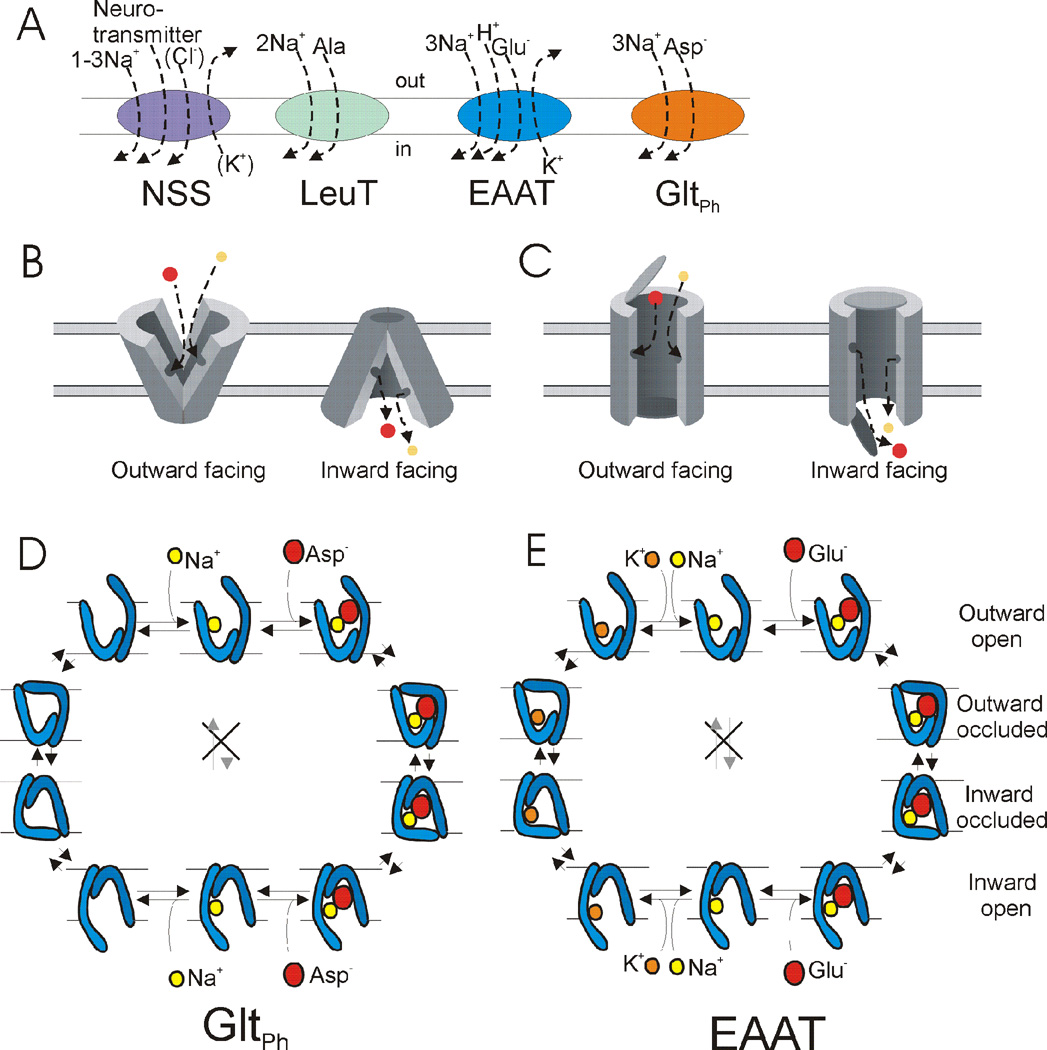
Neurotransmitter transporters are mainly powered by the Na+ gradient across the plasma membrane. The NSS-type transporters co-transport one to three Na+ (depending on the specific transporter), most co-transport one Cl−, and some counter-transport one K+ or H+ per substrate molecule transported; reviewed in (Kristensen et al., 2011) (Fig. 2a). The EAAT-type transporters co-transport three Na+ and one H+, and counter-transport one K+, for each glutamate molecule (Fig. 2a) (Billups et al., 1998; Zerangue and Kavanaugh, 1996). The coupling stoichiometries for the bacterial/archaeal homologues of NSS (LeuT) and EAATs (GltPh) are as follows; LeuT co-transports two Na+ (Yamashita et al., 2005) and GltPh co-transports three Na+ (Groeneveld and Slotboom, 2010) per transported substrate molecule (Fig. 2a).
Fig. 2. Stoichiometry and alternating access of transporters.
A) Stoichiometry of uptake of substrate and ions in NSS, LeuT, EAAT, and GltPh transporters in one uptake cycle. LeuT transports small hydrophobic amino acids and GltPh transporters aspartate. B–C) Alternating access mechanism in B) rocker-switch model and C) two-gated pore model. D) Model of co-transport of sodium (Na+) and aspartate (asp) in GltPh. E) Model of cotransport of sodium (Na+) and glutamate (glu) and counter-transport of potassium (K+) in one uptake cycle of EAATs. Only one Na+ is shown for simplicity in D and E.
How is coupled transport thought to be accomplished by neurotransmitter transporters? The models made for most secondary active transporters involve ‘alternating access’: a binding site for both substrate and transported ions is alternately accessible either to the external or the internal solution, but never to both solutions at the same time (Mitchell, 1957). Two versions have been proposed for alternating access: the rocker-switch (Fig. 2b) (Jardetzky, 1966; Vidaver, 1966) and the two-gated pore (Fig. 2c) (Patlak, 1957). In the rocker-switch, the transporter is composed of two domains able to undergo a rigid-body rocking motion relative to one another so that external access to the binding site is closed and the internal access to the binding site is simultaneously opened, or vice versa (Fig. 2b). In the two-gated pore (Fig. 2c), a pore across the membrane is terminated by a gate at each end. Only one gate is open at any time - both can be closed, but both gates cannot be open simultaneously. Binding of substrate and the ions from the exposed side of the membrane closes the gate on that side. The state with both gates closed around the trapped substrate is referred to as the occluded state. From the occluded state, the gate on the opposite side of the membrane can open and allow the substrate and the ions to diffuse out of the pore, thereby completing the coupled transport (Fig. 2c). Recent models based on crystal structures of GltPh and LeuT combine aspects of both the rocker-switch and the two-gate pore models.
Alternating access can produce either co-transport of ions and substrate (both transported in same direction across membrane, as described above) or counter-transport of ions and substrate (ions and substrate transported in opposite directions across membrane), depending on the postulated rules of switching between the outward-facing to inward-facing conformations. A co-transporter switches from one conformation to the other only when both substrate and coupled ions are bound to the transporter or when neither is bound (Fig. 2d). In co-transport, the transporter with either only substrate bound or only the coupled ions bound does not switch between outward and inward conformations, lest it generates a leak flow of substrate or the coupled ions. This leak flow would alter the measured stoichiometry of substrate and coupled ions and degrade the energy coupling for coupled uptake. EAATs have been shown to have an uncoupled Cl− leak current (Wadiche et al., 1995), but because this current is not coupled to glutamate uptake it does not affect the stoichiometry of coupled uptake. However, some NSSs display Na+ leak currents (Lester et al., 1996) and these would be predicted to degrade the energy for coupled uptake in NSS uptake. A transporter (such as in Figure 2C) functions as a counter-transporter if it can switch between the outward- and inward-facing conformations only when either substrate or ions are bound to the transporter, but not when both substrate and ions are bound or when neither is bound to the transporter (Fig. 2e). More complicated models for co- and counter-transport are possible, but, for example, the EAATs seem to use these simple rules to co-transport Na+/H+/glutamate and counter-transport K+ using two gates that control access to a binding pocket (Fig. 2e).
The transport models can accomplish substrate uptake into the cell by clockwise stepping through the states of the cycle, or substrate release by counter-clockwise stepping through the states (Fig. 2d, 2e). In which direction does the transport normally occur? The requirement for net secondary active transport is that the free energy drop in transporting the coupled ions down their electro-chemical gradient exceeds the free energy required for transporting the substrate against its electro-chemical gradient. An EAAT transporter operating under physiological ionic conditions and voltage is thought to be able to establish a transmembrane glutamate concentration ratio of 106 (10 mM inside/10 nM outside the cell) (Zerangue and Kavanaugh, 1996). The direction of transport or maximal gradient achieved by the transporter is only determined by the stoichiometry of the substrate and transported ions and the thermodynamic gradient for transport, and not the molecular details of transport or the affinity for the different molecules in the different states (but these factors could determine the kinetics of transport).
So what is the structural basis for how neurotransmitter transporters implement alternating access and accomplish a coupled co- or counter-transport necessary for an efficient uptake of neurotransmitters against steep concentration gradients of neurotransmitters? In the next section, we review which states in the transport cycle have been identified for GltPh and LeuT.
Crystallographic structures of archaeal/bacterial homologues
Atomic resolution 3D structural information on sodium-coupled neurotransmitter transporters started to arrive in 2004 and 2005 with reports of the structures of GltPh, an archaeal EAAT homologue from Pyrococcus horikoshii (Yernool et al., 2004), and LeuT, a bacterial NSS homologue from Aquifex aeolicus (Yamashita et al., 2005). Structures of these transporters have now been determined in various conformations, beginning to reveal the structural basis of substrate and ion binding, mechanisms of inhibition, and mechanisms of transport.
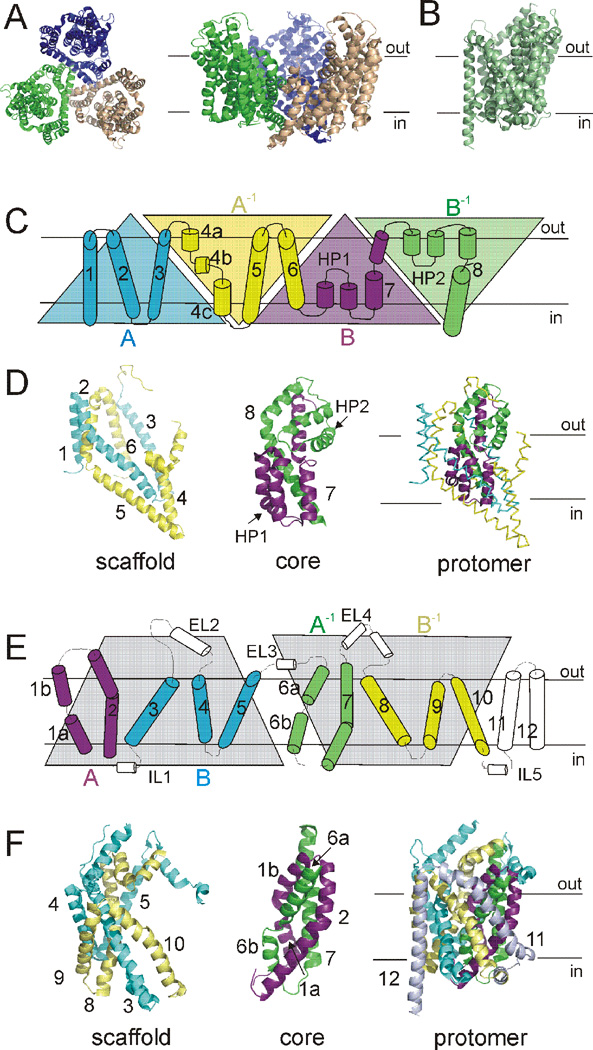
The first published structures revealed substantial differences in the three-dimensional fold of GltPh (Fig. 3A) and LeuT (Fig. 3B), yet presented a common theme of two-fold internal structural symmetry and discontinuous membrane helices (Yamashita et al., 2005; Yernool et al., 2004). GltPh assembles as a bowl-shaped trimer with a large solvent-filled basin open to the extracellular solution (Fig. 3A) (Yernool et al., 2004). Each protomer in GltPh is made up of two sets of inverted repeats (AA−1BB−1: Fig. 3C). The first inverted repeat forms a scaffold domain consisting of six transmembrane segments (TM1-6) folded into a cylinder that houses the second repeat; a core domain (Fig. 3D) consisting of two reentrant helical hairpin loops (HP1 and HP2) and two transmembrane helices (TM7 and TM8) (Fig. 3C).
Fig. 3. Structural makeup of GltPh and LeuT.
A) GltPh assembles as a bowl-shaped trimer. Left, extracellular view. Right, view parallel to the membrane. Individual monomers are colored wheat, blue, green. B) LeuT monomer, viewed parallel to the membrane. C) Primary structure of a GltPh monomer. First inverted repeat (AA−1: blue, yellow) and second inverted repeat (BB−1: magenta, green) displayed as triangles. D) Structural relationship of internal repeat structures in GltPh. Scaffold domain, left. Core domain, middle. Protomer fold, right. TMs colored as in C. E) Primary structure of LeuT. Inverted repeat defined by gray shaded area. TMs 1–2 (A: magenta) and 6–7 (A−1: green), as well as TMs 3–5 (B: blue) and 8–10 (B−1: yellow), are symmetrically related. F) Structural relationship of internal repeat structures in LeuT. Scaffold domain, left. Core domain, middle. Protomer fold, right. TMs colored as in E. TMs 11 and 12 shown only in the protomer fold for clarity.
In contrast, LeuT is a monomer made up of twelve transmembrane helices and with a fold resembling a shallow ‘shot glass’ (Fig. 3E–F) (Yamashita et al., 2005). In LeuT, the first ten transmembrane helices constitute an internal structural repeat relating the first five helices to the second five by a pseudo-two-fold axis parallel to the membrane plane (ABA−1B−1: Fig. 3E). TMs 1, 2, 6, and 7 form a centrally located core domain, while TMs 3–5 and 8–10 form a surrounding scaffold domain (Fig. 3F). For both GltPh and LeuT, the inverted repeat structural elements were not a priori predicted from sequence analysis, but only apparent in the crystal structures. The inverted repeats have proven integral to understanding the basic transport mechanisms of GltPh and LeuT and are a common theme for many transporters with different architectures (Abramson and Wright, 2009; Boudker and Verdon, 2010; Forrest et al., 2011; Forrest and Rudnick, 2009; Krishnamurthy et al., 2009). Furthermore, the LeuT fold itself has been found in a number of seemingly unrelated transporters, establishing the two-fold-related “5+5” transmembrane repeat as an integral aspect of numerous transporters (Abramson and Wright, 2009; Boudker and Verdon, 2010; Forrest et al., 2011; Forrest and Rudnick, 2009; Krishnamurthy et al., 2009), while the GltPh-like folds are quite rare by comparison (Johnson et al., 2012).
Substrate and ion-bound “outward-occluded” states
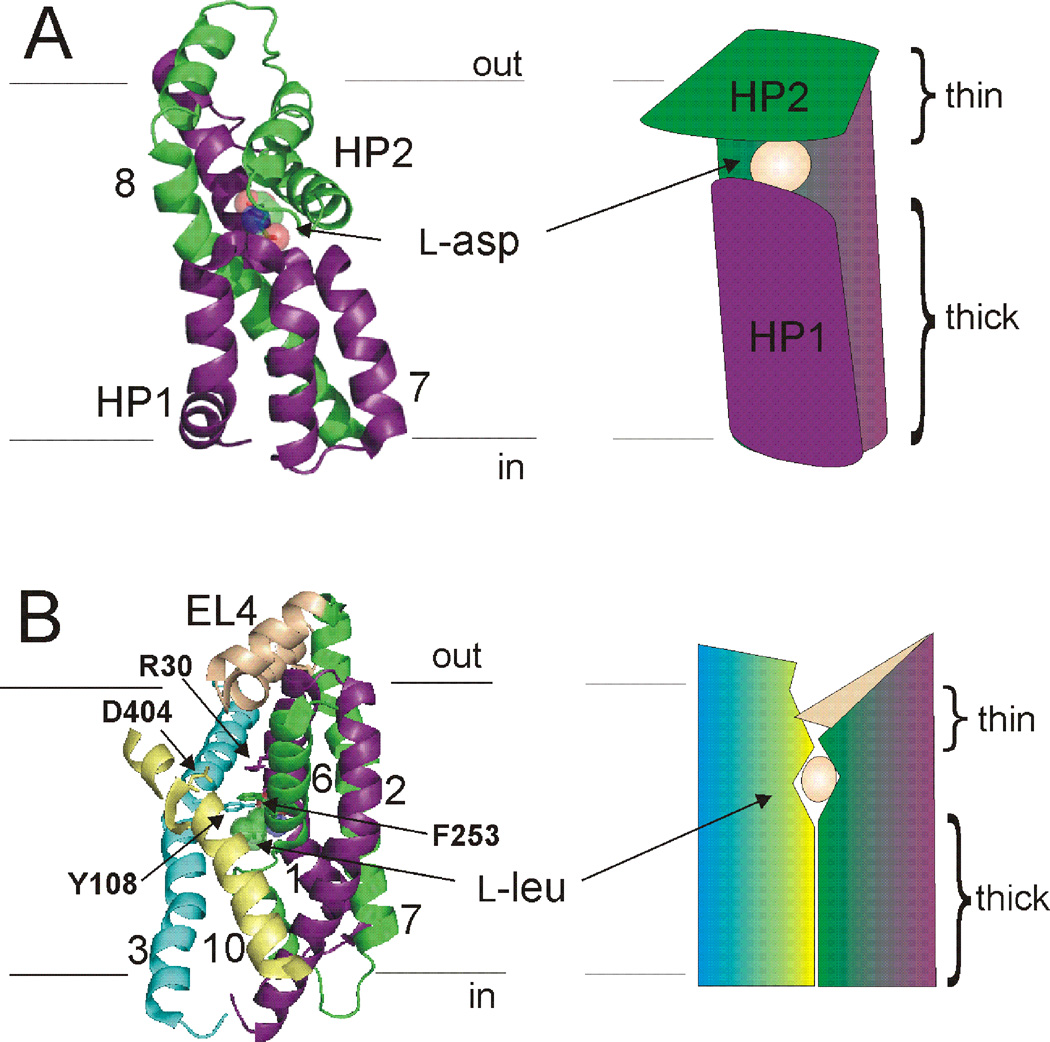
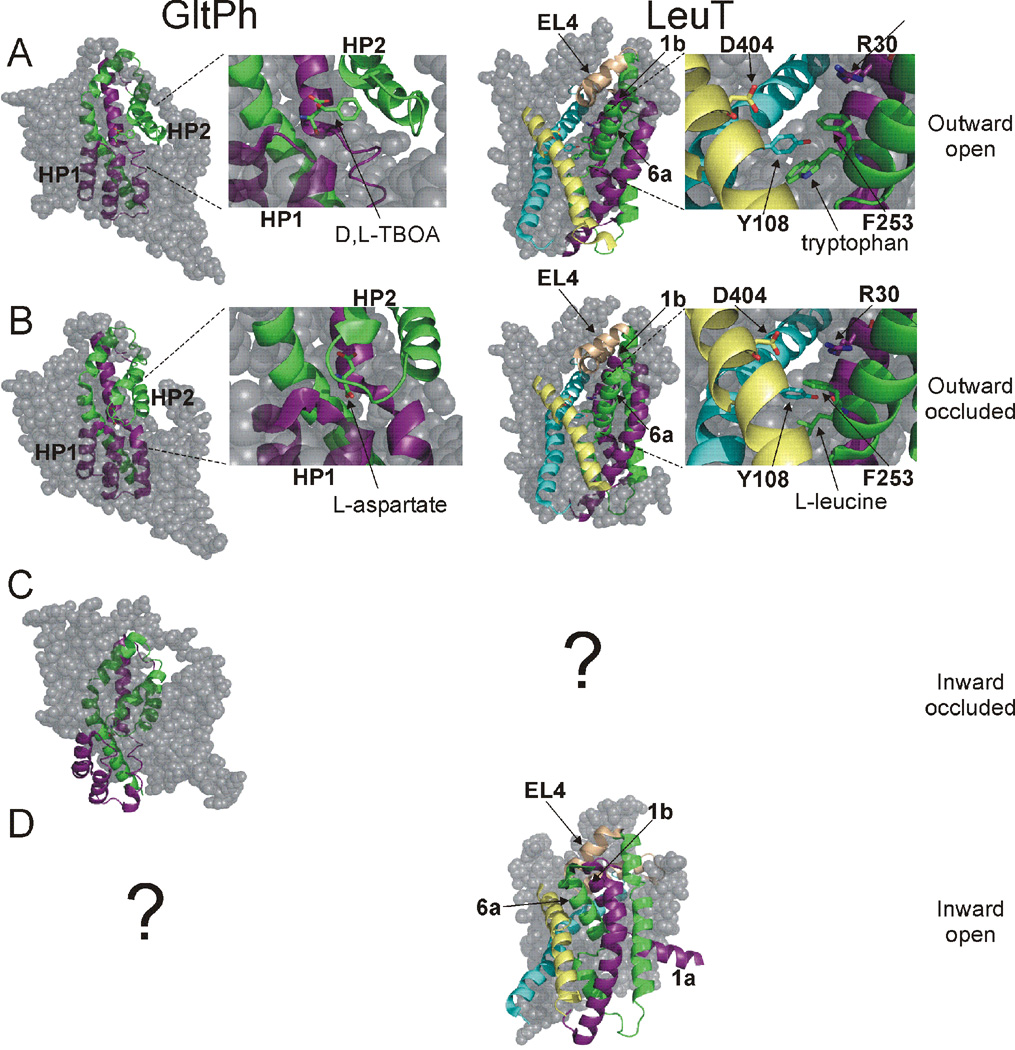
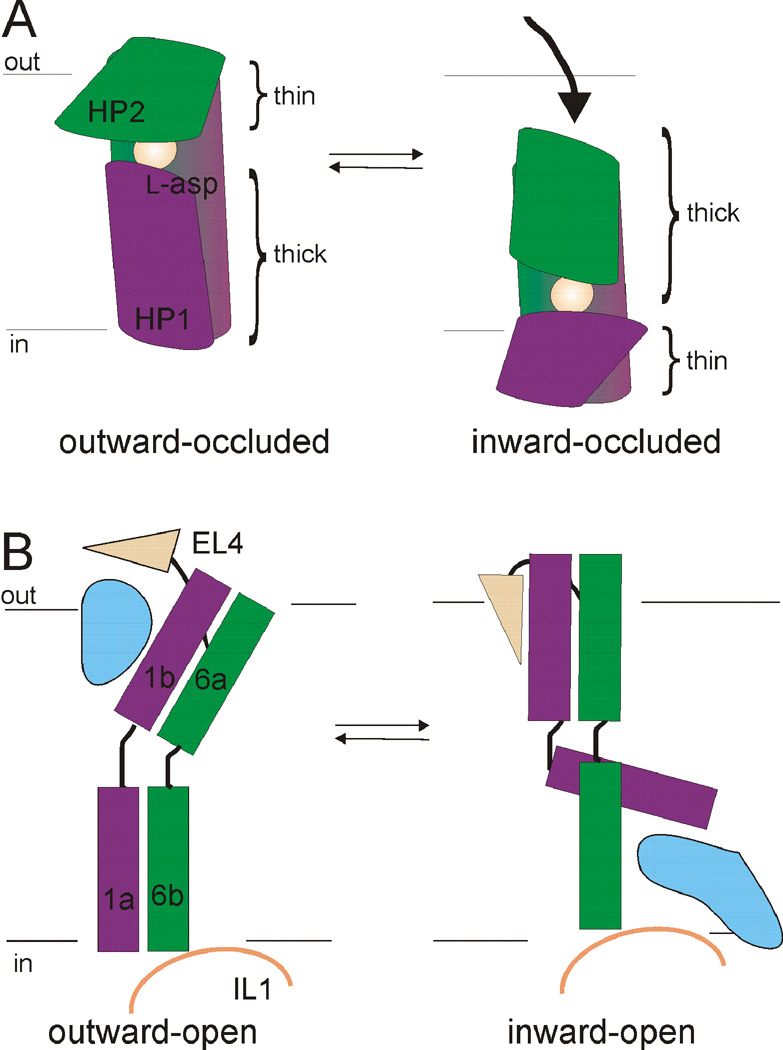
In the first published structures of GltPh and LeuT, the substrates were occluded from solution on both sides of the membrane (Fig. 4) (Yamashita et al., 2005; Yernool et al., 2004). In GltPh, the substrate was occluded by HP2 on the extracellular side (Fig. 4A & 5A), suggesting HP2 forms the extracellular gate (Yernool et al., 2004). In LeuT, Tyr 108 and Phe 253 sequestered the substrate and ion binding sites from the extracellular solution. These residues, together with residues Arg 30 and Asp 404 and the extracellular loop 4 (EL4), were proposed to form the extracellular gate in LeuT (Fig. 4B & 5B) (Yamashita et al., 2005). Additionally, in both structures the proposed extracellular gates were observed to be “thin” sections of protein, while access to the substrate and ion-binding sites from the intracellular side of the membrane was obstructed by 15–20 Å of “thick” sections of protein (Fig. 4) (Krishnamurthy et al., 2009; Yamashita et al., 2005; Yernool et al., 2004). These crystal structures were thus proposed to represent outward-facing occluded states of the transporters with both gates closed.
Fig. 4. Outward occluded states of GltPh and LeuT.
A) Core domain of GltPh (left). Substrate is occluded from extracellular and intracellular solutions by a proposed external gate (HP2) and internal gate (HP1). Right, model of the core domain gates indicating the “thin” and “thick” nature of gates. B) Core domain, TMs 3,10, and EL4 in LeuT (left). Residues proposed to make up the external gate, along with EL4, are indicated. L-leucine is occluded from both sides of the membrane. Right, model depicting the “thin” and “thick” nature of gates in LeuT. In (A) and (B) coloring scheme as in Fig. 3.
Fig. 5. Crystal structures of multiple states in GltPh and LeuT.
A) D,L-TBOA locks GltPh in an outward-facing state by preventing closure of HP2. Tryptophan locks LeuT in an outward-facing state by increasing the distance between aromatic and charged extracellular gating residues. TMs 1a, 6b, and EL4 are outwardly rotated in the presence of tryptophan, widening the extracellular cavity. B) In the outward occluded state of GltPh, substrate is trapped between HP1 and HP2. In the outward occluded state of LeuT, substrate is blocked from the extracellular solution by the extracellular gate comprised of aromatic and charged amino acids, and EL4. C) In the inward-facing occluded state of GltPh, the core domain is moved towards the cytosol, with substrate remaining trapped between HP1 and HP2. D) The inward-open state of LeuT is the result of an inward tilt of TMs 1b and 6a, inwardly directed movement of EL4, and outward movement of TM1a. No crystal structures have been solved for the inward-occluded state of LeuT or the inward-open state of GltPh (indicated by ?).
Mechanisms of inhibition and gating
Direct evidence supporting the proposed nature of the extracellular gates in GltPh and LeuT has been provided by crystal structures solved in complex with various inhibitors. The crystal structure of GltPh in complex with the non-transportable, competitive inhibitor D,L-threo-β-benzyloxyaspartate (TBOA) revealed a structure in which the tip of HP2 was displaced by about 10 Å (Fig. 5A) from its position in an aspartate-bound structure (Fig. 5B) (Boudker et al., 2007). Displacement of HP2 was found to be due to a steric hindrance of HP2 induced by the bulky benzyl group of TBOA (Fig. 5A), with the aspartate moiety of TBOA residing in the substrate-binding pocket (Boudker et al., 2007). The structural basis of TBOA-inhibition therefore was revealed to be a result of competitive inhibition, locking GltPh in an outward-facing state with the extracellular gate (HP2) propped open.
Like TBOA inhibition of GltPh, the competitive inhibitor tryptophan inhibits LeuT by displacing the substrate and trapping the transporter in an outward-facing conformation with the extracellular gate locked open (Fig. 5A) (Singh et al., 2008). This conformation is largely the result of outward rotation of TMs 1b, 6a, and EL4, and an increase in the distance between the extracellular gate residues Y108 and F253 (cf. Fig. 5A and 5B) (Singh et al., 2008). Together these movements result in a widening of the extracellular vestibule and increased solvent accessibility to the substrate-binding site.
In contrast, LeuT crystal structures in complex with tricyclic antidepressants (TCAs) (Singh et al., 2007; Zhou et al., 2007), selective serotonin re-uptake inhibitors (SSRIs) (Zhou et al., 2009), and the detergent octylglucoside (Quick et al., 2009), have all revealed a non-competitive mechanism of transport inhibition. This non-competitive mechanism has been shown to be the result of the bound inhibitor stabilizing the substrate-bound transporter in an outward-facing occluded conformation with the extracellular gate closed (Singh et al., 2007) (Zhou et al., 2007). Taken together, the mechanism for competitive inhibition in GltPh and LeuT appears to be the result of preventing closure of the extracellular gate, while non-competitive inhibitors (evidenced for LeuT alone) function by trapping substrate-bound transporters in an outward-facing occluded state with the extracellular gate closed.
Transport principles revealed by inward-occluded GltPh and inward-open LeuT states
Insight into the conformational changes from the outward- to inward-facing states came from crystal structures of a crosslinked double cysteine GltPh mutant in the inward-facing occluded state (Reyes et al., 2009) and a mutated LeuT in the inward-facing open state (Krishnamurthy and Gouaux, 2012).
The strategy for GltPh was based on previous experimental evidence for the formation of a spontaneous intramolecular disulphide bond between two residues in EAAT1 (Ryan et al., 2004). The homologous positions in GltPh (K55 and A364) were observed to be greater than 25 Å apart in both the outward-open and outward-occluded (Boudker et al., 2007; Yernool et al., 2004) GltPh structures, suggesting that a large movement was required for these residues to come into close proximity. Crystals obtained following crosslinking K55C and A364C with Hg2+ yielded a structure in which the substrate binding site was observed to move approximately 20 Å from its position in the outward-facing structures to a position near the cytoplasm (Fig. 5C & 6A) (Reyes et al., 2009). In the crosslinked structure, bound L-asp and Na+ were observed to be close to the intracellular solution, occluded from the intracellular solution by a “thin” section of protein while occluded from the extracellular solution by a “thick” section of protein (Fig. 6A) (Reyes et al., 2009). This structure was thus interpreted as representing an inward-facing occluded state, with both the extracellular and intracellular gates closed. Superimposition of the outward-open, outward-occluded, and inward-occluded states revealed that TM1, TM2, TM4, and TM5 are invariant in position (Reyes et al., 2009). In contrast, the other parts of the protein, housing the substrate and ion binding sites (TM3, TM6, HP1, TM7, HP2, and TM8), undergo substantial conformational change. GltPh was thus proposed to comprise two structural domains, the ‘trimerization’ domain (TM1, TM2, TM4, TM5) and the ‘transport’ domain (TM3, TM6, HP1, TM7, HP2, and TM8) (Reyes et al., 2009). The trimerization domain consists mainly of the first internal repeat, whereas the transport domain comprises mainly the second internal repeat. This structure suggests that the transition between the outward facing state to the inward facing state involves movement of the transport domain within the frame of a rigid trimerization domain (Fig. 6A) (Reyes et al., 2009). No crystal structure of the inward-facing open state of GltPh has been published, leaving it unclear how ions and substrate obtain access to the cytoplasm (but see below for modeling studies).
Fig. 6. Models of the outward-inward transition in GltPh and LeuT.
A) The transition from the outward-occluded to inward-occluded state in GltPh involves coordinated movement of the transport domain, which leads to a swap of the “thin” and “thick” gates. Shown for clarity is only the core of GltPh as depicted in Fig. 3. B) The transition from the outward-open to inward-open state of LeuT involves a coordinated tilt of TMs 1b and 6a, inwardly directed movement of EL4, and uncoupled outward movement of TM1a. Color scheme is as in Fig. 3. Blue areas in (B) indicate water pathways.
At present, no crystal structure defines a LeuT inward-facing occluded state. However, recently a LeuT structure in the inward-facing open state was published (Fig. 5D) (Krishnamurthy and Gouaux, 2012). Comparison of LeuT structures in the outward-open and outward-occluded states with the inward-open state indicates that the conformational changes are not strictly those of a “rocking bundle” (Fig. 6B) (Krishnamurthy and Gouaux, 2012). The core domain does not move as a rigid body, as in a rocking bundle mechanism. Instead, only a portion of the core moves as a unit, so that symmetry of the inward- and outward-facing conformations is not strictly preserved (Fig. 6B). The transport mechanism in LeuT thus appears to exploit local hinge-like movements that lead to the coordinated opening and closing of both “thin” and “thick” extracellular and intracellular gates (Fig. 6B). In contrast, crystal structures of other transporters with the LeuT fold in outward- and inward-facing states are suggestive of a more strict “rocking bundle” mechanism. The adherence to a strict “rocking-bundle” mechanism or a more local hinge-like mechanism likely depends on the individual transporter in question (Abramson and Wright, 2009; Boudker and Verdon, 2010; Forrest et al., 2011; Forrest and Rudnick, 2009; Jeschke, 2012; Krishnamurthy et al., 2009).
Remarkably, models similar to the crystals structures for the inward-facing states of GltPh and LeuT were generated independently of the crystal structures by utilizing the inverted-topology repeats in GltPh and LeuT and assuming that the inward-facing states could be modeled by threading the sequence of the first part of each repeat on the structure of second part of each repeat and vice versa (Crisman et al., 2009; Forrest et al., 2008).
The crystal structures of transporters in different states indicate what type of conformational changes the transporters undergo. However, one also needs to understand the principles by which the binding of ions and substrate are coupled to these conformational changes. In this review, we focus on how binding of substrate and Na+ are coupled to the conformational changes in the transport cycle. On the role of other ions in neurotransmitter transporters, see a number of excellent reviews (Danbolt, 2001; Grewer and Rauen, 2005; Kristensen et al., 2011).
Substrate and ion binding
Substrate
In a high-resolution structure of GltPh crystallized in the presence of L-aspartate (Boudker et al., 2007), aspartate is located deep in the transport domain, between the tips of HP1 and HP2, the non α-helix region of TM7, and polar residues of TM8 (Fig 7A). Several residues that contribute to the substrate binding site in this GltPh structure have also been implicated in substrate binding by mammalian EAATs. For example, R397 interacts with the β carboxylate of aspartate in the crystal structures of GltPh (Fig. 7A) (Boudker et al., 2007). The homologous R447 in EAATs has been suggested to interact with the γ carboxylate of the acidic substrate (Bendahan et al., 2000). Interestingly, the residue homologous to R447 is a neutral residue in the related ASCT neutral amino acid transporters (Bendahan et al., 2000; Boudker et al., 2007).
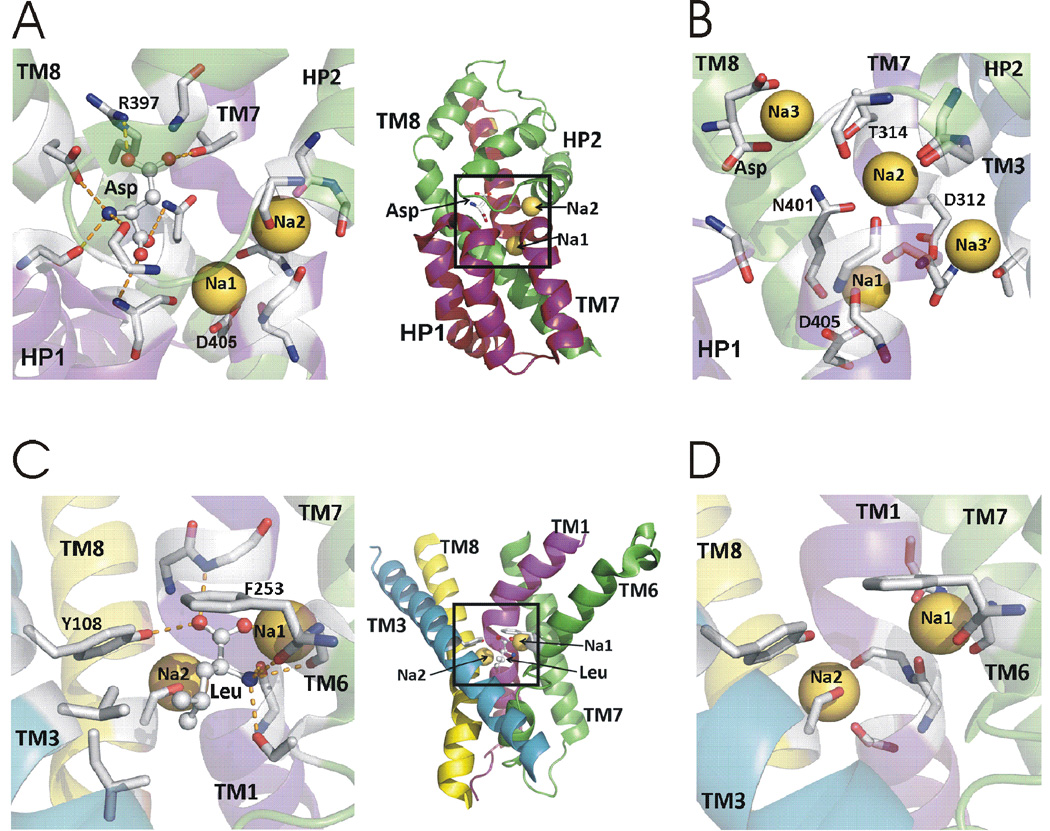
Fig. 7. Substrate and sodium binding sites in GltPh and LeuT.
A) Aspartate and two thallium ions in the crystal structure of GltPh. B) Potential Na+ sites in simulations of GltPh. C) Leucine and two Na+ in the crystal structure of LeuT. D) Two Na+ in LeuT. Leucine removed for clarity. Side chains and backbones interacting with substrate and cations are shown in A-D as stick.
All LeuT structures with bound substrate show a substrate molecule accommodated in a common site (termed S1) together with two Na+ ions (Fig. 7C) (Yamashita et al., 2005). The substrate and the two Na+ are located in a central cavity formed by a four-helix bundle comprised of TM1, TM3, TM6, and TM8 (Yamashita et al., 2005). In the substrate binding site the polar α-amino and α-carboxylate groups of the amino acid substrate interacts with a phenolic hydroxyl moiety from Tyr108 in TM3, one Na+, and the unwound regions of TM1 and TM6. The substrate is stabilized by hydrogen bonds with the backbone of TM1 and TM6 and by interacting with the ends of these α-helices (Fig. 7C). The hydrophobic side chain of the substrate is accommodated by hydrophobic side chains from TM3, TM6, and TM8. The residues forming the S1 substrate binding site are conserved between LeuT and the NSS family of transporters and several S1 residues are important for substrate selectivity and affinity in NSS transporters (Kristensen et al., 2011), suggesting that the S1 site is conserved in NSS transporters.
Comparison of the substrate binding sites in GltPh and LeuT shows that the substrate binding site in GltPh is entirely within the core of the transport domain, whereas in LeuT the substrate binding site is on the interface between the transport and scaffold domains. But, GltPh and LeuT share commonalities in the way the substrates are bound, including the heavy involvement of the non-helical regions of the trans-membrane segments and involvement of backbone polar groups in substrate coordination.
Na+
Na+ was replaced with Tl+ in a GltPh crystal structure for its higher anomalous scattering signal (Boudker et al., 2007). Two Tl+ ions observed bound to GltPh were labeled Na1 and Na2 (Boudker et al., 2007). Na1 is buried deeply in the transporter structure and is mainly coordinated by residue D405 in TM8 (Fig. 7A). Na2 is coordinated by backbone oxygen atoms of the tip of HP2 and the unwound section in the center of TM7, close to extracellular solution (Fig. 7A). Experimental studies lend support that the two Tl+ in the crystal structure represent Na+ binding sites (Boudker et al., 2007; Tao et al., 2008; Teichman et al., 2009).
However, three Na+ are cotransported with one substrate in each GltPh transport cycle (Groeneveld and Slotboom, 2010), similar to EAATs (Zerangue and Kavanaugh, 1996). This raises the question of where the third Na+ ion binds and what role this Na+ plays in the transport mechanism. Computations and experiments have not yet produced a consensus regarding the location of the third Na+ site (Teichman et al., 2012) (Bastug et al., 2012; Bendahan et al., 2000; Holley and Kavanaugh, 2009; Huang et al., 2009; Larsson et al., 2010; Rosental et al., 2006; Ryan et al., 2009; Shrivastava et al., 2008; Tao et al., 2010). For example D312 in TM7 and T92 in TM3 have been suggested to form a Na3 site that binds Na+ prior to substrate binding (Fig. 7B) (Bastug et al., 2012; Huang and Tajkhorshid, 2010; Tao et al., 2010). Another study proposed this site to be a transiently occupied site (called Na3’), because a Na+ bound at this location was electrostatically destabilized by a Na+ bound at Na1 site (Larsson et al., 2010). This study proposed another Na3 site, involving the side chains of T314 and N401 and, interestingly, the charged β–carboxylate group of the bound substrate (Fig. 7B) (Larsson et al., 2010). Direct substrate-ion contact had been previously suggested by electrostatic mapping (Holley and Kavanaugh, 2009) and is similar to what has been found in LeuT (Yamashita et al., 2005). Direct contact of Na3 with the substrate could explain the observation that the apparent affinity for different acidic amino acids depends on the nature of the cotransported cation (Menaker et al., 2006) and the findings that mutants of residues homologous to N401 and T314 both alter cation and substrate selectivity (Larsson et al., 2010; Teichman et al., 2012).
With evidence for more Na+ sites than the number of transported Na+, we propose that the three transported Na+ go through one or more intermediate binding sites before finalizing their binding positions. Consistent with this idea, another site, formed by a conserved aspartate residue together with a tyrosine and located at the external end of the binding pocket, is proposed to be a transient Na+ site, through which one or more of the Na+ have to pass on their way to other binding sites (Rosental et al., 2011). To further determine the location and function of the different proposed Na+ binding sites additional functional and/or computational approaches are required, including techniques allowing for manipulation of the backbone carbonyls which contribute extensively to Na+ binding in GltPh and likely in EAATs.
Structures of LeuT obtained in the presence of substrate and Na+ reveal two distinct Na+ sites, termed Na1 and Na2, in the S1 binding pocket (Yamashita et al., 2005). The Na1 and Na2 sites coordinate Na+ by six and five oxygen ligands, respectively. (Fig. 7D). The Na1 site is highly conserved among the NSS family. In the Na2 site, residues G20, V23, A351 and S355 in TM1 and TM8 are also well conserved in eukaryotic SLC6 transporters. The Na+ at Na1 interacts directly with the substrate α-carboxyl group and, according to computer simulations (Noskov et al., 2008), the strong electrostatic field generated by the carboxylate of leucine appears to control the selectivity for Na+ at the Na1 site. Na1 is suggested to bind Na+ before substrate binding (Yamashita et al., 2005). Consistent with this binding order, in a recent Na+-bound, substrate-free structure of LeuT in which the “thin” extracellular gate is open, Na+-binding at Na1 is observed to impart a stabilizing effect on TMs 1b and 6a, proposed to be an important aspect for subsequent substrate-binding (Krishnamurthy and Gouaux, 2012). Selectivity for Na2 seems to be driven by the local structure constraints of the cavity created by the five neutral coordinating oxygen ligands (Noskov et al., 2008). Although Na+ binding sites similar to Na1 and Na2 exist in the mammalian NSS transporters, the precise role of Na+ occupation of these sites for substrate binding and translocation remains largely unknown in mammalian NSSs. More functional data addressing the role of each site in the individual NSS members is needed. Interestingly, in other sodium-coupled transporters that belong to different families (other than NSS) and have low overall sequence similarity, but share the LeuT-type fold, biochemical and structural studies have identified Na2 as being highly conserved, whereas Na1 is less conserved (Khafizov et al., 2012). This suggests that sodium binding to Na2 is integral to the sodium-coupling mechanism in transporters with the LeuT-type fold (see below for more details on possible role of Na2).
The amino acid sequence and three-dimensional structures of GltPh and LeuT are unrelated, yet they share similarities in the local protein organization and the coordination of the Na+ sites (Boudker et al., 2007). This suggests that Na+-dependent transporters might possess a common Na+-binding motif, for example the unwound transmembrane segments of GltPh (TM7) and LeuT (TM1) (Boudker et al., 2007). In addition, both Na1 and Na2 are formed, in part, by coordinating carbonyl oxygen atoms occupying nearly equivalent positions in GltPh and LeuT.
Alternating access model meets structure
Starting from crystal structures of GltPh and LeuT obtained in various conformations, both computational studies and structure-based experiments are beginning to uncover the dynamics of the transport by EAAT and NSS. We here focus on how Na+ and substrate binding are coupled to the gates of the transporter and the transitions in the transport cycle.
Dynamics of the extracellular gate
If a co-transporter with either only substrate or ions bound is allowed to switch from outward-to-inward facing (Fig. 2D and 2E), it would allow a leakage, with consequences for the stoichiometry and energy efficiency of transport. In both EAATs and NSSs, the binding of at least one Na+ precedes the binding of substrate (Grewer and Rauen, 2005; Krishnamurthy and Gouaux, 2012; Larsson et al., 2004). What are the structural implications of Na+-binding prior to substrate binding? As mentioned above, in both LeuT and GltPh one of the Na+ seems to form part of the substrate binding site. In addition, Na+ binding also seems to have effects on the gates, in ways that prevent leakage.
Molecular dynamics simulations of GltPh (Huang and Tajkhorshid, 2008; Shrivastava et al., 2008) showed fluctuations of the extracellular gate (HP2) between the closed and open conformations in the apo-state and in the presence of Na+ alone without substrate (Huang and Tajkhorshid, 2008). However, electron paramagnetic resonance experiments on spin-labeled GltPh suggested that in the presence of Na+ HP2 is biased toward a more open conformation, whereas in the apo state HP2 occupies a more closed conformation (Focke et al., 2011), consistent with crosslinking studies on GltPh in the apo-state (Reyes et al., 2009). A recent simulation study suggested that the binding of Na+ stabilizes a state of HP2 intermediate between open and closed (Grazioso et al., 2012). A possible interpretation of these experiments and simulations is that HP2 fluctuates between closed and open. In the apo-state, HP2 is biased towards the closed state and, in the Na+-bound state, HP2 is biased towards an open state (Fig. 8A). The stabilization of HP2 in the open state (Fig. 8A) would prevent isomerization to the inward-facing state in the presence of Na+ alone (in the absence of substrate). Interestingly, similar findings have been reported for LeuT. For LeuT in the apo-state, simulations show fluctuations in the extracellular gate (Celik et al., 2008; Claxton et al., 2010), with a bias for the inward-facing state (Shi et al., 2008). The binding of Na+ in the absence of substrate has been shown to promote an outward-open conformation of the extracellular gate of LeuT (Fig. 8B) (Celik et al., 2008; Claxton et al., 2010; Shi et al., 2008). A recent crystal structure of LeuT in the presence of Na+ alone, in which the extracellular gate is open, further supports the idea that Na+ binding stabilizes the open gate (Krishnamurthy and Gouaux, 2012).
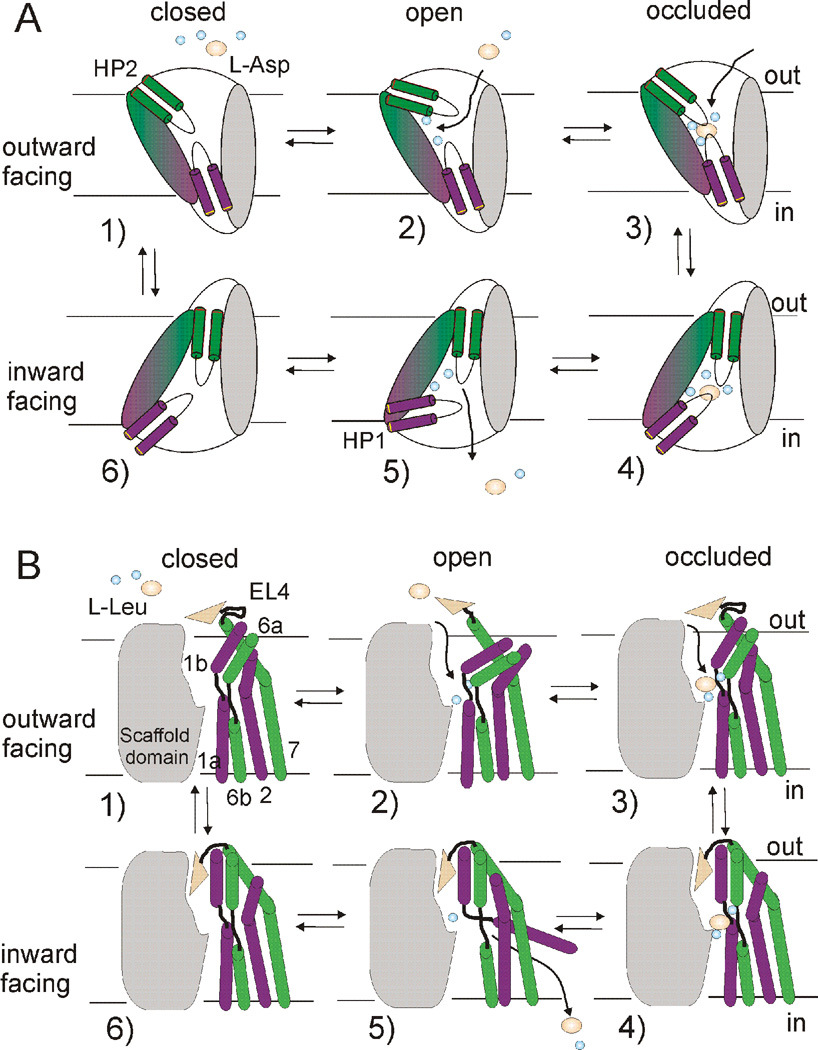
Fig. 8. Models of the transport mechanism in GltPh and LeuT.
In the outward-facing apo state (1) external gate movements (HP2 in GltPh and TMs1b, 6a and EL4 in LeuT) allow for sodium binding, which stabilizes an outward-facing open state (2). This sets up a binding site for substrate and additional ion(s), leading to formation of an outward-occluded state (3). Transition to an inward-facing occluded state (4) involves piston-like motion of the transport domain (magenta and green) relative to the trimerization domain (grey) in GltPh and inwardly-directed movement of EL4 and a coordinated tilt of TMs 1b,6a in LeuT. Release of the first sodium (Na2) leads to opening of the intracellular gate (HP1 in GltPh and TM1a in LeuT) (5) and subsequent release of substrate and additional ion(s). In the inward-facing apo-state (6), the intracellular gate closes to allow for transition to the outward-facing apo-state.
This model is a structural correlate to the model of cotransport in which isomerization of the transporter from an outward- to an inward-facing state is prevented when only Na+ is bound (Fig. 8). The subsequent binding of substrate (and additional ions) is predicted to bias the transporter toward a closed conformation (Fig. 8). Indeed, in GltPh, substrate (aspartate) binding is observed computationally and experimentally to induce a closure of the extracellular gate (Focke et al., 2011; Grazioso et al., 2012; Huang and Tajkhorshid, 2008), with Na+ binding to the last Na2 site in the presence of substrate is predicted to stabilize the closed state (Fig. 8A) (Grazioso et al., 2012; Huang and Tajkhorshid, 2008; Shrivastava et al., 2008). Similarly, in LeuT, substrate binding in the presence of Na+ shifts the conformation from an outward-facing open state to an outward-facing occluded state (Fig. 8B), reducing extracellular gate fluctuations and preventing access to the binding sites (Celik et al., 2008; Claxton et al., 2010; Shi et al., 2008).
Outward-to-inward facing transition
In both GltPh and LeuT, the transition between the outward- and inward-facing conformation is largely due to the movements of the core domain relative to the scaffold domain (Figs. 6 & 8).
Computational studies in GltPh suggest that the transition from the outward facing to the inward facing state involves unequal movements of the trimerization and transport domain in opposite directions, together with a tilt in the transport domain with respect to the membrane (Stolzenberg et al., 2012). These motions result in significant changes in contacts between the trimerization and transport domains and are in line with a recent crystal structure of GltPh captured in an intermediate state (Verdon and Boudker, 2012). This study also observed a conformational change involving the TM3-4 loop during the transport process, consistent with a recent experimental study that suggested that the TM3-4 loop is essential for transport in GltPh (Compton et al., 2010). Another simulation study (Lezon and Bahar, 2012) suggests that the lateral pressure of the membrane on the transporter help guide the protein in the inward-to-outward isomerization in GltPh. Consistent with experimental evidence in EAATs and GltPh crystal structures, both simulation studies observed structural asymmetry of the different subunits, providing further evidence of the independent nature of individual subunits (Grewer et al., 2005; Groeneveld and Slotboom, 2007; Koch et al., 2007; Koch and Larsson, 2005; Leary et al., 2007). In summary, the modeling studies on GltPh are consistent with the large piston-like movement of the transport domain with respect to the trimerization domain in the transition between outward-facing to inward-facing states (Figs. 6A & 8A) (Reyes et al., 2009).
For LeuT, the transition from outward-to-inward facing is more complicated and controversial. A model for the inward-facing state of LeuT based on structural repeats initially suggested the possibility of a “rocking bundle” mechanism of alternating access (Forrest et al., 2008). However, recent crystal structures suggest that LeuT may not strictly adhere to a rocking bundle mechanism, but that the transition from outward-to-inward involves a combination of rocking bundle and two-gated pore models (Fig. 6B & 8B) (Krishnamurthy and Gouaux, 2012). In addition, computational and experimental studies have proposed that the binding of a second substrate molecule at a secondary site (S2) is required for the outward-to-inward transition and subsequent substrate and ion release (Shan et al., 2011; Shi et al., 2008; Zhao et al., 2010; Zhao et al., 2011). At the present time it is unclear whether binding at the S2 site is necessary or not (Piscitelli and Gouaux, 2012; Quick et al., 2012; Wang et al., 2012). Nonetheless, recent reports point to a complex transport mechanism: a combination of local conformational change and rigid body movements in LeuT (Krishnamurthy and Gouaux, 2012; Shaikh and Tajkhorshid, 2010; Zhao and Noskov, 2011; Zhao et al., 2011) .
Dynamics of the intracellular gate
How are ions and substrate released to the cytoplasm in GltPh and LeuT? The inward facing crystal structure of GltPh is in an occluded state (Reyes et al., 2009), with no access of the substrate to the cytosol. A computational model of the inward-facing open state, in which HP1 is assumed to be the intracellular gate, was generated based on the TBOA-bound structure of GltPh and the assumption that HP1 in the inward-facing open state adopts the same conformation as HP2 in the TBOA-bound outward-facing open state (Crisman et al., 2009). Two recent simulation studies point to the dissociation of Na2 as the trigger for intracellular release (DeChancie et al., 2011; Grazioso et al., 2012). Following dissociation of Na2, HP1 is observed to allow water molecules to enter the region and initiate release of the substrate and ions via an increase in the flexibility of HP1 (Fig. 8A). Following the release of Na1, HP1 undergoes large fluctuations similar to what is observed for HP2 in the outward-facing apo-state. These fluctuations in HP1 are hypothesized to allow HP1 to close and thereby enable the binding pocket to translocate back across the membrane (Fig. 8A), consistent with a proposal for GltPh based on EPR measurements (Focke et al., 2011).
The structure of LeuT in the inward-facing open state suggests a potential mechanism of how ion release is coupled to transporter opening to the cytoplasm: the release of Na2 and a conformational change involving hinge movement in TM1a create conditions favorable for subsequent substrate and ion release (Figs. 6B & 8B). Computational models of the inward-facing state(s) supports that hydration of Na2 play a prominent role in the substrate/ion release in LeuT, similar to the proposed role of Na2 in substrate release in GltPh (Shaikh and Tajkhorshid, 2010; Zhao and Noskov, 2011).
General mechanisms
Even if crystal structures show structural differences between GltPh and LeuT, we think that general mechanistic principles shared by sodium-coupled neurotransmitter transporters can be drawn from GltPh and LeuT (Fig. 8). We propose the following general mechanism for sodium-coupled neurotransmitter uptake. In the outward-facing apo state, the extracellular gate fluctuates, allowing access for Na+ to its binding sites. The binding of extracellular Na+ to the transporter stabilizes the outward-facing open state, preventing Na+ leakage and creating a high-affinity substrate-binding site. At least one of the bound Na+ seems to become part of the substrate binding site in both GltPh and LeuT. Following substrate (and potentially additional ion) binding, the extracellular gate closes and a transition to the inward-facing occluded state occurs. In GltPh this process involves a piston-like movement of the transport domain with respect to the trimerization domain, whereas in LeuT the core domain undergoes a combination of rocking bundle and local hinge movements with respect to the scaffold domain. In the inward-facing state, the initial event triggering substrate release appears to be the release of Na2. Following release of Na2, the closed state of the intracellular gate is destabilized, leading to release of further ions and substrate. In both GltPh and LeuT, we propose that in the inward-facing apo-state the intracellular gate continuously opens and closes, resulting in the ability of the intracellular gate to close so that the transporter can transit from the inward-to-outward facing state and, thereby, complete the transport cycle (Fig. 8).
Conclusions (future considerations)
Recent crystal structures of bacterial transporter homologues and recent functional and computational studies of neurotransmitter transporters and their archaeal/bacterial homologues have generated hypotheses for how the EAATs and the NSS transporters accomplish Na+-coupled neurotransmitter uptake. However, not all states in the transporter cycles have yet been identified in the archaeal/bacterial transporters, and still no crystal structure is available for any eukaryotic neurotransmitter transporter. In addition, many of the states proposed from existing crystal structures still need to be verified by other methods, such as EPR and FRET. Controversies have erupted in both the fields of NSS and EAATs. For example, which site is the real third Na+ site in EAATs? And, are there one or two substrate sites in NSS transporters? More crystal structures might help in solving these controversies. But, also longer molecular dynamic simulations (and other computer simulation techniques) of transporters, as well as structural measurements from for example DEER EPR and new FRET techniques, will help in generating models of the complete transport cycle in these transporters. Finally, knowledge of the different states in the transporter cycles at a molecular level will help in developing new drugs that target neurotransmitter transporters to cure or alleviate disorders and diseases.
Highlights.
Review of sodium coupling and conformational change in neurotransmitter transporter
Side-by-side structural comparison of transporter homologues GltPh and LeuT
Relating crystal structures of GltPh and LeuT to functional data and simulations
Structural and functional implications of sodium coupling in these transporters
Acknowledgements
We thank Drs. W. Nonner and S. Noskov for comments on the manuscript. This work was supported by a predoctoral AHA grant 11PRE7990050 (XW), postdoctoral AHA grant 12POST11910068 (PF), and NHLBI R01-HL095920 (HPL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abramson J, Wright EM. Structure and function of Na(+)-symporters with inverted repeats. Curr Opin Struct Biol. 2009;19:425–432. doi: 10.1016/j.sbi.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastug T, Heinzelmann G, Kuyucak S, Salim M, Vandenberg RJ, Ryan RM. Position of the third Na+ site in the aspartate transporter GltPh and the human glutamate transporter, EAAT1. PloS one. 2012;7:e33058. doi: 10.1371/journal.pone.0033058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahan A, Armon A, Madani N, Kavanaugh MP, Kanner BI. Arginine 447 plays a pivotal role in substrate interactions in a neuronal glutamate transporter. J Biol Chem. 2000;275:37436–37442. doi: 10.1074/jbc.M006536200. [DOI] [PubMed] [Google Scholar]
- Billups B, Rossi D, Oshima T, Warr O, Takahashi M, Sarantis M, Szatkowski M, Attwell D. Physiological and pathological operation of glutamate transporters. Prog Brain Res. 1998;116:45–57. doi: 10.1016/s0079-6123(08)60429-x. [DOI] [PubMed] [Google Scholar]
- Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Coupling substrate and ion binding to extracellular gate of a sodium-dependent aspartate transporter. Nature. 2007;445:387–393. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- Boudker O, Verdon G. Structural perspectives on secondary active transporters. Trends Pharmacol Sci. 2010;31:418–426. doi: 10.1016/j.tips.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer S, Gether U. The solute carrier 6 family of transporters. Br J Pharmacol. 2012;167:256–278. doi: 10.1111/j.1476-5381.2012.01975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celik L, Schiott B, Tajkhorshid E. Substrate binding and formation of an occluded state in the leucine transporter. Biophys J. 2008;94:1600–1612. doi: 10.1529/biophysj.107.117580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claxton DP, Quick M, Shi L, de Carvalho FD, Weinstein H, Javitch JA, McHaourab HS. Ion/substrate-dependent conformational dynamics of a bacterial homolog of neurotransmitter:sodium symporters. Nat Struct Mol Biol. 2010;17:822–829. doi: 10.1038/nsmb.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton EL, Taylor EM, Mindell JA. The 3–4 loop of an archaeal glutamate transporter homolog experiences ligand-induced structural changes and is essential for transport. Proc Natl Acad Sci U S A. 2010;107:12840–12845. doi: 10.1073/pnas.1003046107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisman TJ, Qu S, Kanner BI, Forrest LR. Inward-facing conformation of glutamate transporters as revealed by their inverted-topology structural repeats. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0908570106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- DeChancie J, Shrivastava IH, Bahar I. The mechanism of substrate release by the aspartate transporter GltPh: insights from simulations. Mol Biosyst. 2011;7:832–842. doi: 10.1039/c0mb00175a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focke PJ, Moenne-Loccoz P, Larsson HP. Opposite movement of the external gate of a glutamate transporter homolog upon binding cotransported sodium compared with substrate. J Neurosci. 2011;31:6255–6262. doi: 10.1523/JNEUROSCI.6096-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Kramer R, Ziegler C. The structural basis of secondary active transport mechanisms. Biochim Biophys Acta. 2011;1807:167–188. doi: 10.1016/j.bbabio.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Forrest LR, Rudnick G. The rocking bundle: a mechanism for ion-coupled solute flux by symmetrical transporters. Physiology (Bethesda) 2009;24:377–386. doi: 10.1152/physiol.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest LR, Zhang YW, Jacobs MT, Gesmonde J, Xie L, Honig BH, Rudnick G. Mechanism for alternating access in neurotransmitter transporters. Proc Natl Acad Sci U S A. 2008;105:10338–10343. doi: 10.1073/pnas.0804659105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Grazioso G, Limongelli V, Branduardi D, Novellino E, De Micheli C, Cavalli A, Parrinello M. Investigating the mechanism of substrate uptake and release in the glutamate transporter homologue Glt(Ph) through metadynamics simulations. J Am Chem Soc. 2012;134:453–463. doi: 10.1021/ja208485w. [DOI] [PubMed] [Google Scholar]
- Grewer C, Balani P, Weidenfeller C, Bartusel T, Tao Z, Rauen T. Individual subunits of the glutamate transporter EAAC1 homotrimer function independently of each other. Biochemistry. 2005;44:11913–11923. doi: 10.1021/bi050987n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Rauen T. Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling. J Membr Biol. 2005;203:1–20. doi: 10.1007/s00232-004-0731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneveld M, Slotboom DJ. Rigidity of the subunit interfaces of the trimeric glutamate transporter GltT during translocation. J Mol Biol. 2007;372:565–570. doi: 10.1016/j.jmb.2007.06.067. [DOI] [PubMed] [Google Scholar]
- Groeneveld M, Slotboom DJ. Na(+):aspartate coupling stoichiometry in the glutamate transporter homologue Glt(Ph) Biochemistry. 2010;49:3511–3513. doi: 10.1021/bi100430s. [DOI] [PubMed] [Google Scholar]
- Holley DC, Kavanaugh MP. Interactions of alkali cations with glutamate transporters. Philosophical transactions of the Royal Society of London. 2009;364:155–161. doi: 10.1098/rstb.2008.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Ryan RM, Vandenberg RJ. The role of cation binding in determining substrate selectivity of glutamate transporters. J Biol Chem. 2009;284:4510–4515. doi: 10.1074/jbc.M808495200. [DOI] [PubMed] [Google Scholar]
- Huang Z, Tajkhorshid E. Dynamics of the extracellular gate and ionsubstrate coupling in the glutamate transporter. Biophys J. 2008;95:2292–2300. doi: 10.1529/biophysj.108.133421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Tajkhorshid E. Identification of the third Na+ site and the sequence of extracellular binding events in the glutamate transporter. Biophys J. 2010;99:1416–1425. doi: 10.1016/j.bpj.2010.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966;211:969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Jeschke G. A comparative study of structures and structural transitions of secondary transporters with the LeuT fold. Eur Biophys J. 2012 doi: 10.1007/s00249-012-0802-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZL, Cheong CG, Lee SY. Crystal structure of a concentrative nucleoside transporter from Vibrio cholerae at 2.4 A. Nature. 2012;483:489–493. doi: 10.1038/nature10882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479:237–247. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Khafizov K, Perez C, Koshy C, Quick M, Fendler K, Ziegler C, Forrest LR. Investigation of the sodium-binding sites in the sodium-coupled betaine transporter BetP. Proc Natl Acad Sci U S A. 2012;109:E3035–E3044. doi: 10.1073/pnas.1209039109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HP, Brown RL, Larsson HP. The glutamate-activated anion conductance in excitatory amino acid transporters is gated independently by the individual subunits. J Neurosci. 2007;27:2943–2947. doi: 10.1523/JNEUROSCI.0118-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch HP, Larsson HP. Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters. J Neurosci. 2005;25:1730–1736. doi: 10.1523/JNEUROSCI.4138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Gouaux E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature. 2012;481:469–474. doi: 10.1038/nature10737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy H, Piscitelli CL, Gouaux E. Unlocking the molecular secrets of sodium-coupled transporters. Nature. 2009;459:347–355. doi: 10.1038/nature08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Andersen J, Jorgensen TN, Sorensen L, Eriksen J, Loland CJ, Stromgaard K, Gether U. SLC6 neurotransmitter transporters: structure, function, and regulation. Pharmacol Rev. 2011;63:585–640. doi: 10.1124/pr.108.000869. [DOI] [PubMed] [Google Scholar]
- Larsson HP, Tzingounis AV, Koch HP, Kavanaugh MP. Fluorometric measurements of conformational changes in glutamate transporters. Proc Natl Acad Sci U S A. 2004;101:3951–3956. doi: 10.1073/pnas.0306737101. Epub 2004 Ma 3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson HP, Wang X, Lev B, Baconguis I, Caplan DA, Vyleta NP, Koch HP, Diez-Sampedro A, Noskov SY. Evidence for a third sodium-binding site in glutamate transporters suggests an ion/substrate coupling model. Proc Natl Acad Sci U S A. 2010;107:13912–13917. doi: 10.1073/pnas.1006289107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary GP, Stone EF, Holley DC, Kavanaugh MP. The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J Neurosci. 2007;27:2938–2942. doi: 10.1523/JNEUROSCI.4851-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester HA, Cao Y, Mager S. Listening to neurotransmitter transporters. Neuron. 1996;17:807–810. doi: 10.1016/s0896-6273(00)80213-5. [DOI] [PubMed] [Google Scholar]
- Lezon TR, Bahar I. Constraints imposed by the membrane selectively guide the alternating access dynamics of the glutamate transporter GltPh. Biophys J. 2012;102:1331–1340. doi: 10.1016/j.bpj.2012.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menaker D, Bendahan A, Kanner BI. The substrate specificity of a neuronal glutamate transporter is determined by the nature of the coupling ion. J Neurochem. 2006;99:20–28. doi: 10.1111/j.1471-4159.2006.04003.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. A general theory of membrane transport from studies of bacteria. Nature. 1957;180:134–136. doi: 10.1038/180134a0. [DOI] [PubMed] [Google Scholar]
- Patlak CS. Contributions to the theory of active transport. II. The gate type non-carrier mechanism and generalizations concerning tracer flow, efficiency, and measurement of energy expenditure. Bull. Math. Biophys. 1957;19:209–235. [Google Scholar]
- Piscitelli CL, Gouaux E. Insights into transport mechanism from LeuT engineered to transport tryptophan. EMBO J. 2012;31:228–235. doi: 10.1038/emboj.2011.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Shi L, Zehnpfennig B, Weinstein H, Javitch JA. Experimental conditions can obscure the second high-affinity site in LeuT. Nat Struct Mol Biol. 2012;19:207–211. doi: 10.1038/nsmb.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick M, Winther AM, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proc Natl Acad Sci U S A. 2009;106:5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes N, Ginter C, Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462:880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosental N, Bendahan A, Kanner BI. Multiple consequences of mutating two conserved beta-bridge forming residues in the translocation cycle of a neuronal glutamate transporter. J Biol Chem. 2006;281:27905–27915. doi: 10.1074/jbc.M600331200. [DOI] [PubMed] [Google Scholar]
- Rosental N, Gameiro A, Grewer C, Kanner BI. A conserved aspartate residue located at the extracellular end of the binding pocket controls cation interactions in brain glutamate transporters. J Biol Chem. 2011;286:41381–41390. doi: 10.1074/jbc.M111.291021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Oshima T, Attwell D. Glutamate release in severe brain ischaemia is mainly by reversed uptake. Nature. 2000;403:316–321. doi: 10.1038/35002090. [DOI] [PubMed] [Google Scholar]
- Ryan RM, Compton EL, Mindell JA. Functional characterization of a Na+-dependent aspartate transporter from Pyrococcus horikoshii. J Biol Chem. 2009;284:17540–17548. doi: 10.1074/jbc.M109.005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Mitrovic AD, Vandenberg RJ. The chloride permeation pathway of a glutamate transporter and its proximity to the glutamate translocation pathway. J Biol Chem. 2004;279:20742–20751. doi: 10.1074/jbc.M304433200. Epub 22004 Feb 20724. [DOI] [PubMed] [Google Scholar]
- Shaikh SA, Tajkhorshid E. Modeling and dynamics of the inward-facing state of a Na+/Cl- dependent neurotransmitter transporter homologue. PLoS Comput Biol. 2010;6 doi: 10.1371/journal.pcbi.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PloS one. 2011;6:e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Molecular cell. 2008;30:667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava IH, Jiang J, Amara SG, Bahar I. Time-resolved mechanism of extracellular gate opening and substrate binding in a glutamate transporter. J Biol Chem. 2008;283:28680–28690. doi: 10.1074/jbc.M800889200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Piscitelli CL, Yamashita A, Gouaux E. A competitive inhibitor traps LeuT in an open-to-out conformation. Science. 2008;322:1655–1661. doi: 10.1126/science.1166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448:952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Stolzenberg S, Khelashvili G, Weinstein H. Structural intermediates in a model of the substrate translocation path of the bacterial glutamate transporter homologue GltPh. The journal of physical chemistry. 2012;116:5372–5383. doi: 10.1021/jp301726s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Gameiro A, Grewer C. Thallium ions can replace both sodium and potassium ions in the glutamate transporter excitatory amino acid carrier 1. Biochemistry. 2008;47:12923–12930. doi: 10.1021/bi8017174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Rosental N, Kanner BI, Gameiro A, Mwaura J, Grewer C. Mechanism of cation binding to the glutamate transporter EAAC1 probed with mutation of the conserved amino acid residue T101. J Biol Chem. 2010 doi: 10.1074/jbc.M110.121798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman S, Qu S, Kanner BI. The equivalent of a thallium binding residue from an archeal homolog controls cation interactions in brain glutamate transporters. Proc Natl Acad Sci U S A. 2009;106:14297–14302. doi: 10.1073/pnas.0904625106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichman S, Qu S, Kanner BI. Conserved asparagine residue located in binding pocket controls cation selectivity and substrate interactions in neuronal glutamate transporter. J Biol Chem. 2012;287:17198–17205. doi: 10.1074/jbc.M112.355040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon G, Boudker O. Crystal structure of an asymmetric trimer of a bacterial glutamate transporter homolog. Nat Struct Mol Biol. 2012;19:355–357. doi: 10.1038/nsmb.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver GA. Inhibition of parallel flux and augmentation of counter flux shown by transport models not involving a mobile carrier. J Theor Biol. 1966;10:301–306. doi: 10.1016/0022-5193(66)90128-7. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Wang H, Elferich J, Gouaux E. Structures of LeuT in bicelles define conformation and substrate binding in a membrane-like context. Nat Struct Mol Biol. 2012;19:212–219. doi: 10.1038/nsmb.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437:215–223. doi: 10.1038/nature03978. [DOI] [PubMed] [Google Scholar]
- Yernool D, Boudker O, Jin Y, Gouaux E. Structure of a glutamate transporter homologue from Pyrococcus horikoshii. Nature. 2004;431:811–818. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- Zhao C, Noskov SY. The role of local hydration and hydrogen-bonding dynamics in ion and solute release from ion-coupled secondary transporters. Biochemistry. 2011;50:1848–1856. doi: 10.1021/bi101454f. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Terry D, Shi L, Weinstein H, Blanchard SC, Javitch JA. Single-molecule dynamics of gating in a neurotransmitter transporter homologue. Nature. 2010;465:188–193. doi: 10.1038/nature09057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Terry DS, Shi L, Quick M, Weinstein H, Blanchard SC, Javitch JA. Substrate-modulated gating dynamics in a Na+-coupled neurotransmitter transporter homologue. Nature. 2011;474:109–113. doi: 10.1038/nature09971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Goetz RM, Law CJ, Reith ME, Wang DN. LeuT-desipramine structure reveals how antidepressants block neurotransmitter reuptake. Science. 2007;317:1390–1393. doi: 10.1126/science.1147614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Zhen J, Karpowich NK, Law CJ, Reith ME, Wang DN. Antidepressant specificity of serotonin transporter suggested by three LeuT-SSRI structures. Nat Struct Mol Biol. 2009;16:652–657. doi: 10.1038/nsmb.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]