Abstract
Recent studies suggest that plant pectin methylesterases (PMEs) are directly involved in plant defence besides their roles in plant development. However, the molecular mechanisms of PME action on pectins are not well understood. In order to understand how PMEs modify pectins during banana (Musa spp.)–Fusarium interaction, the expression and enzyme activities of PMEs in two banana cultivars, highly resistant or susceptible to Fusarium, were compared with each other. Furthermore, the spatial distribution of PMEs and their effect on pectin methylesterification of 10 individual homogalacturonan (HG) epitopes with different degrees of methylesterification (DMs) were also examined. The results showed that, before pathogen treatment, the resistant cultivar displayed higher PME activity than the susceptible cultivar, corresponding well to the lower level of pectin DM. A significant increase in PME expression and activity and a decrease in pectin DM were observed in the susceptible cultivar but not in the resistant cultivar when plants were wounded, which was necessary for successful infection. With the increase of PME in the wounded susceptible cultivar, the JIM5 antigen (low methyestrified HGs) increased. Forty-eight hours after pathogen infection, the PME activity and expression in the susceptible cultivar were higher than those in the resistant cultivar, while the DM was lower. In conclusion, the resistant and the susceptible cultivars differ significantly in their response to wounding. Increased PMEs and thereafter decreased DMs acompanied by increased low methylesterified HGs in the root vascular cylinder appear to play a key role in determination of banana susceptibility to Fusarium.
Key words: Banana (Musa spp.), degree of pectin methylesterification, Fusarium wilt, host–, pathogen interaction, pectin methylesterases, wound induced.
Introduction
Banana Fusarium wilt caused by Fusarium oxysporum f. sp. cubense (Foc) is one of the major global constraints limiting banana (Musa spp.) production. Cavendish banana varieties (Musa spp. AAA) are highly susceptible to Foc race 4 (van den Berg et al., 2007). The vascular pathogen penetrates banana roots through the wound of the vascular tissues (Sequeira et al., 1958), and colonizes and occludes the xylem vessels. Fungal proliferation in the xylem causes a reddish-brown discoloration of the rhizome and pseudo-stem, leaf wilting, and ultimately death of the whole plant (Saravanan et al., 2003).
The cell wall forms the first barrier for pathogen penetration into the intracellular space in plants. Homogalacturonan (HG) is the major group of cell wall pectins, playing a key role in providing a barrier against environmental stresses including pathogen attacks (Vorwerk et al., 2004). As a strategy to overcome the cell wall barrier, Fusarium oxysporum, a hemibiotrophic and/or necrotrophic pathogen, secretes a substantial array of cell wall-degrading enzymes (CWDEs) such as pectin methylesterases (PMEs), polygalacturonases (PGs), and polymethylgalacturonases (PMGs) to digest the host plant cell wall (Gothoskar et al., 1953; Vorwerk et al., 2004; Cantu et al., 2008).
On the other hand, in response to cell wall damage by pathogens, plants have evolved a battery of defence responses including hydroxylation of plant cell wall components and the CWDEs (Lionetti et al., 2007; Cantu et al., 2008). Plant PMEs have major roles in pectin remodelling and they control the methylesterification status of most abundant pectin polysaccharide HG (Pelloux et al., 2007; Wolf et al., 2009). In plant–pathogen interactions, some cell wall HG-derived compounds act as elicitors whereas others act as defence suppressors (Vorwerk et al., 2004; Osorio et al., 2008). In addition, PME activities (An et al., 2008; Raiola et al., 2011; Volpi et al., 2011), the degree of HG methylesterification (DM) (Wiethölter et al., 2003; Boudjeko et al., 2006; Lionetti et al., 2007, 2012; Pelloux et al., 2007; Curvers et al., 2010; Raiola et al., 2011), and the molecular properties of HG (Wiethölter et al., 2003; Cantu et al., 2008) have been correlated with changes in the susceptibility of plants to pathogens. More recently, some reports have shown that overexpression of PME inhibitor (PMEI) protein reduced the activity or expression level of PME, resulting in higher resistance of plants to pathogens (Lionetti et al., 2007; An et al., 2008; Volpi et al., 2011). On the other hand, silencing of the PMEI gene enhanced susceptibility of plants to pathogens (An et al., 2008). It was reported that PMEs were involved in plant defence by influencing the pectin structure and DM (Lionetti et al., 2007; Osorio et al., 2008; Raiola et al., 2011; Volpi et al., 2011). Molecular mechanisms of PME action on pectins are not well understood, and only very few studies addressed the role of PMEs in plant–pathogen interactions and in the response to wounding. In addition, contradictory data were published on the PME expression level (Raiola et al., 2011; Wojtasik et al., 2011) and cell wall composition changes in response to pathogens in diverse plant species (Simon et al., 2005; Boudjeko et al., 2006).
The specific aim of the present study was to understand how PMEs modify specific groups of HGs and to monitor the changes of individual groups of HGs during banana–Fusarium interaction. Expression of the MaPME1 gene (PME1 of Musa accuminata) as well as enzyme activities and immunolocalization of PMEs were compared in two banana cultivars, one highly resistant and one susceptible to Fusarium. Furthermore, the spatial distributions of PMEs and their correlations with pectin methylesterification of 10 individual HGs with different DMs were also examined.
Materials and methods
Plant material
Musa spp. AAA cv. ‘Yueyoukang 1’ and ‘Brazil’ were selected as plant material. ‘Brazil’ is susceptible (S) to Foc race 4 whereas ‘Yueyoukang 1’, a somaclonal variant, is resistant (R) to this pathogen (Chen et al., 2006).
Inoculation of two banana cultivars with pathogen
Tissue cultured banana plants were cultured in liquid rooting medium, incubated at 28±2 °C under light on a reciprocal shaker. Two weeks after induction of roots, one root of each plant was cut off to facilitate the penetration of the pathogen. Afterwards, such treated plants were transferred to medium containing Foc race 4 at a final concentration of 5×102 ml–1. Plants transferred to a medium without fungus served as the cut controls, The samples were collected 6h and 48h after the transfer, respectively. Intact plants collected 6h after transfer to new liquid rooting medium without fungus served as the non-cut control (no differences were observed between 6h and 48h after transfer).
Tissue-specific pathogen diffusion
The protocols for fixation and embedding of samples were carried out as described by Xu et al. (2011a). To reveal the level of the tissue-specific Fusarium diffusion in the R and S banana cultivars, dewaxed sections were stained with Calcofluor White Stain (Sigma 18909) for 10min. Fluorescence was examined with an Olympus BH-2-FRCA microscope.
Enzyme assay
Banana root samples (0.2g) were washed in distilled water and homogenized on ice with 1.5ml of extraction buffer (0.25M NaCl, 0.1M acetic acid-sodium acetate buffer, pH 5.0). The tissue extracts were centrifuged at 10 000rpm for 20min at 4 °C. PME activity of the supernatant was determined using a modification of the method developed by Marcus and Schejter (1983). Aliquots of the supernatant (0.3ml) were added to a 5ml tube containing 3ml of substrate (1% citrus pectin). PME activity was measured at pH 6.5 by continuous automatic titration with 0.01M NaOH of the carboxyl groups released during the enzyme reaction. The activity unit of PME was defined as the number of microequivalents of carboxyl groups cleaved by 1mg of enzyme min–1, at 30 °C and pH 6.5. The protein content was determined by the method of Bradford (1976).
qPCR analysis of MaPME1 expression
Recently, several loci of the banana genome were identified to have putative pectinesterase activity (D’Hont et al., 2012). Of them, the full length of MaPME1 was successfully cloned in our lab (accession no. KC492743) corresponding to the partial cDNA sequence (FJ264505) reported by Mbéguié-A-Mbéguié et al. (2009). Therefore, the relative expression levels of this isoform in the banana–Foc interaction were investigated. Total RNA was extracted from the samples using the RNAOUT kit (Tiandz, Beijing). RNase-free DNase I (TaKaRa, Japan) was used for removing genomic DNA residues, and RNase-free columns (Tiandz, Beijing) were used for purifying total RNA. The quality and concentration of the total RNA were checked by RNAse-free agarose gel electrophoresis and measured by a spectrophotometer at 260nm and 280nm (Eppendorf, Germany). Only those RNA samples whose 260nm/280nm ratio was between 1.8 and 2.0 were used for subsequent analyses. Subsequently, first-strand cDNA was synthesized from total RNA (2 µg) following the manufacturer’s instructions of the ReverTra Ace qPCR RT Kit (TOYOBO, Japan). PCR analysis was performed with the cDNA extracted from different samples as a template. The transcript levels of MaPME1 were analysed using quantitative real-time PCR with THUNDERBIRD qPCR mix (TOYOBO, Japan) and the iCycler iQ™ Real-time Detection system (Bio-Rad Laboratories, USA), according to the manufacturers’ instructions. Samples were subjected to thermal cycling conditions of DNA polymerase activation at 95 °C for 1min, 40 cycles of 10 s at 95 °C, 30 s at 60 °C. The melting curve was designed to increase 0.3 °C every 10 s from 62 °C. All quantitative PCRs (qPCRs) were normalized using the Ct value corresponding to the ubiquitin gene. The relative expression levels of target genes were calculated with the formula 2–ΔΔCT (Livak and Schmittgen, 2001). The primers for qPCR are listed in Supplementary Table S1 available at JXB online.
Measurement of pectin DM using a colorimetric method
The alcohol-insoluble residue (AIR) was prepared as described by Louvet et al. (2011). In brief, samples were washed three times with 70% ethanol (v/v) at 70 °C for 30min after homogenization. The supernatant was removed after centrifugation. The pellet was crushed in liquid nitrogen and freeze-dried. The DM was calculated as the ratio of methanol content to 1mol of galacturonic acid (GalA). The methanol was determined colorimetrically as described in Xu et al. (2011b). In detail, 5mg of AIR was saponified in 2ml of 0.25M KOH at room temperature for 1h and neutralized with phosphoric acid (to pH 7.5). After centrifugation at 10 000 g for 10min, 1ml of supernatant was loaded into a 15ml tube. Alcohol oxidase (1ml, 1U ml–1, diluted in distilled water, Sigma) was added to each tube. After gently mixing, the tube was incubated at room temperature for 20min. Thereafter, 2ml of a mixture containing 0.1% 2, 4-pentanedione in 1M ammonium acetate and 0.14% acetic acid was added. Following incubation for 15min at 60 °C, samples were cooled on ice and the absorbance was measured at 420nm (Hitachi, U-2900). Methanol in potassium phosphate buffer (pH 7.5) was used as a standard. The GalA content was also determined as described previously (Xu et al., 2011b ). In detail, 5mg of AIR was hydrolysed in 125 µl of 13M sulphuric acid at room temperature for 30min. A second hydrolysis was performed. The sample was diluted five times with distilled water and saponified at a final concentration of 1M NaOH at 100 °C for 2h. The supernatant was adjusted to pH 8 with NaOH and neutralized with HCl. After centrifugation at 10 000 g for 10min, 0.5ml of supernatant was loaded into a 15ml tube. Pre-cooled samples (in an ice–water bath) were supplemented with 1.5ml of pre-cooled 0.025M sodium tetraborate buffer in concentrated sulphuric acid and incubated at 100 °C for 5min. After cooling in an ice–water bath, 25 µl of 0.15% meta-hydroxydiphenyl in 0.5% NaOH was added to the samples. Following 10min incubation at room temperature, absorbance was recorded at 520nm. GalA (0–100mg l–1 range) was used as a standard. Three replicates were made for each treatment and each experiment was repeated twice. GalA was calculated as micrograms of GalA per gram of AIR.
Immunofluorescence labelling of HGs at different DMs and of PMEs in the root cell walls
The protocol for fixation, embedding of samples, and immunolabelling was carried out as described by Xu et al. (2011a). The antibodies used were the same as those described for immunodot assay (Supplementary Table S2 at JXB online). In addition, PME monoclonal antibody binding to PMEs of banana was also used. The anti-mouse IgG (whole molecule)–FITC (fluorescein isothiocyanate; Sigma F9006) was used as secondary antibody for 2F4, PME, and CCRM antibodies, while the anti-rat IgG (whole molecule)–FITC (Sigma F6258) was used for the remaining antibodies.
Quantification of immunofluorescence signal and dot blots
For quantification of immunofluorescence signal, the mean fluorescence intensity (n=3 sections of roots, in three biological replicates) and dot blot signal were measured with Image J 1.44 software.
Statistical analysis
Statistical analyses were performed using analysis of variance (ANOVA) by the statistical program SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA). At least three replicates were used in the experiments. Data are expressed as the mean ±SD. Multiple differences among means were evaluated using Duncan’s multiple range tests at a 5% probability level.
Results
Banana roots were selected for the experiment because Foc is a soil-borne disease and wounding was considered to be necessary for the successful penetration of the pathogen (Sequeira et al., 1958). The transversal sections of the differentiation/elongating zone of the root were used. A representative transversal section showing the structure of banana roots is presented in Supplementary Fig. S1 at JXB online, while Fig. S2 clearly documents the distribution and abundance of Fusarium pathogen in relation to the relative resistance or susceptibility of two banana cultivars.
Changes in banana PMEs in response to injury and Fusarium attack
Plant PMEs play major roles in pectin remodelling since they control the methylesterification status of HG, the most abundant pectin polysaccharide (Pelloux et al., 2007; Wolf et al., 2009), and they affect the resistance of plants to diseases. To reveal the possible role of PMEs in banana–Foc interaction, the temporal changes in PME activities, the spatial distribution of PMEs, and pectin DMs were monitored in both S and R cultivars in response to injury and pathogen attack. In addition, the expression levels of one PME isoform, MaPME1, were also investigated.
Changes in the activities of PMEs and pectin DM
The PME activity of the R cultivar was significantly higher compared with that of the S cultivar before root wounding and infection. The infection of R plants caused a continuous decrease in PME activities throughout the whole experiment, while no significant changes were observed in the cut control plants. On the other hand, a significant increase in PME activities was observed in the S cultivar after injury, while the pathogen effect led to minor changes in the S cultivar after 6h. Substantially lower PME activities were observed 48h after infection. Overall, pathogen infection caused a decrease in PME activities in both cultivars (Fig. 1A). Additionally, similar dynamics in the activities of PGs and PMGs were observed in infected banana roots (Supplementary Fig. S3 at JXB online).
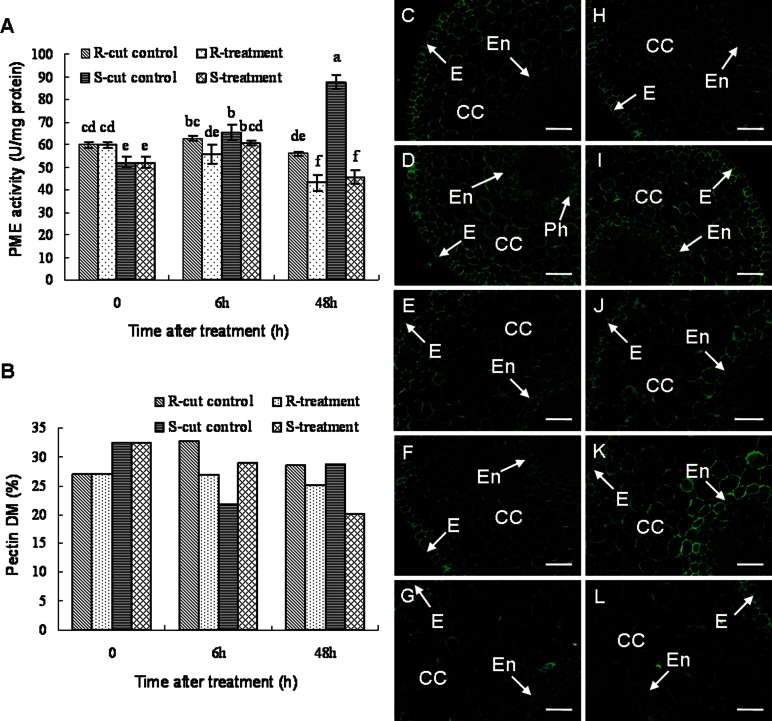
Fig. 1.
The changes in PME activities (A), pectin DM (B), and immunolocalization by a pan-specific PME antibody recognizing more PME isoforms (C–L) in banana (Musa spp. AAA) roots after infection with Fusarium oxysporum f. sp. cubense. For immunolocalization of PME, cross-sections through roots are presented. (C, H) The control of the non-treated resistant (C) and susceptible cultivar (H). (D, I) The resistant (D) and susceptible cultivar (I) 6h after wounding. (E, J) The resistant (E) and susceptible cultivar (J) 6h after infection. (F, K) The resistant (F) and susceptible cultivar (K) 48h after wounding. (G, L) The resistant (G) and susceptible cultivar (L) 48h after infection. Bars represent 50 µm. 0, non-cut control; CC, cortical cells; DM, degree of pectin methylesterification; E, epidermis; En, endodermis; Ph, phloem; PME, pectin methylesterase; R, resistant cultivar ‘Yueyoukang 1’; S, susceptible cultivar ‘Brazil’. Data represent an average of three replicates ±SD. Values followed by the same letter are not significantly different using Duncan’s multiple range test at P < 0.05 after angular transformation of the data. Data of the DM were expressed as the ratio of mean methanol content (in moles; n=3) to 1mol GalA (n=3). (This figure is available in colour at JXB online.)
The constitutive (before the experiment) DM of pectins in the roots of the R cultivar was lower than that of the S cultivar (Fig. 1B). This is consistent with higher demethylesterification in the R cultivar, as found by PME activities. Though pathogen infection resulted in a decreased pectin DM in the R plants, resulting also from the pathogen, the DM remained almost unchanged during the experiment, while a slight increase of pectin DM was observed in the controls. In contrast, wounding-induced injury resulted in a decrease of DM in the S cultivar, while lower pectin DM was observed only 48h after infection in pathogen-treated plants. Furthermore, at 48h, the DM of the infected S plants was lower than that of the R plants (Fig. 1B).
Spatio-temporal distribution of PMEs
Before the treatment, PME immunolabelling was distributed over the whole root section. Stronger labelling was observed in the epidermis while weaker labelling appeared in the cells enclosed by the endodermis, the vascular cylinder. Immunolabelling in the R cultivar was slightly stronger when compared with that of the S cultivar (Fig. 1C, H; Supplementary Fig. S4 at JXB online), being in agreement with the PME activities. At 6h after being cut, stronger PME labelling was observed in the R cultivar, mainly in the cortical cells and cells close to the endodermis, such as phloem (Fig. 1C, D; Supplementary Fig. S4), and it was even stronger in the S cultivar (Fig. 1H, I; Supplementary Fig. S4). Subsequently, control roots of the R cultivar displayed lower PME signal intensity 48h after cutting (Fig. 1C, D, F; Supplementary Fig. S4). In contrast, the PME labelling was stronger in S control plants at this time point (if compared with the non-cut control) but was weaker than that in the roots 6h after cutting, especially in the cells close to the endodermis (Fig. 1H, I, K; Supplementary Fig. S4). At 6h and 48h after infection, the PME labelling in the pathogen-infected plants decreased in both cultivars, especially in the cortical cells and endodermal cells of the susceptible cultivar (Fig. 1D–G, I–L; Supplementary Fig. S4), though 6h is too short for the germination and penetration of the pathogen hyphae.
Changes in relative expression levels of MaPME1
The transcript levels of MaPME1 were investigated during the infection with Foc race 4 in banana roots (Fig. 2). Consistent with the PME activity measurements, the transcript levels of MaPME1 were significantly higher in the R cultivar than in the S cultivar before treatment. The transcript level of MaPME1 in the R control plants remained almost unchanged during the experiment. On the other hand, the levels rapidly decreased in the pathogen-infected R plants throughout the experiment. However, the transcript level of MaPME1 in the S control plants dramatically increased 6h after cutting, although this was not the case in the roots 48h after cutting. Infection caused an increase in MaPME1 transcript levels after 6h in S plants. Later, they dramatically decreased after 48h. In brief, the transcript levels of the S cultivar were normally higher than those of the R cultivar after infection. MaPME1 mRNA showed a tendency similar to those of both PME activities and PME expression levels as revealed by immunolabelling.
Fig. 2.
The changes in transcript levels of MaPME1 in banana (Musa spp. AAA) roots after infection with Fusarium oxysporum f. sp. cubense. 0, non-cut control; R, resistant cultivar ‘Yueyoukang 1’; S, susceptible cultivar ‘Brazil’. Data represent an average of three replicates ±SD. Values followed by the same letter are not significantly different using Duncan’s multiple range test at P < 0.05 after angular transformation of the data.
Changes in subcellular distribution and relative levels of HGs with different DMs during host–pathogen interactions
As mentioned above, the methylesterification status of HGs in non-treated plants was controlled by PMEs (Pelloux et al., 2007; Wolf et al., 2009). To understand how PMEs modify specific groups of HGs, the changes in the levels of HG components with different DMs were monitored together with the dynamic changes in PMEs.
2F4
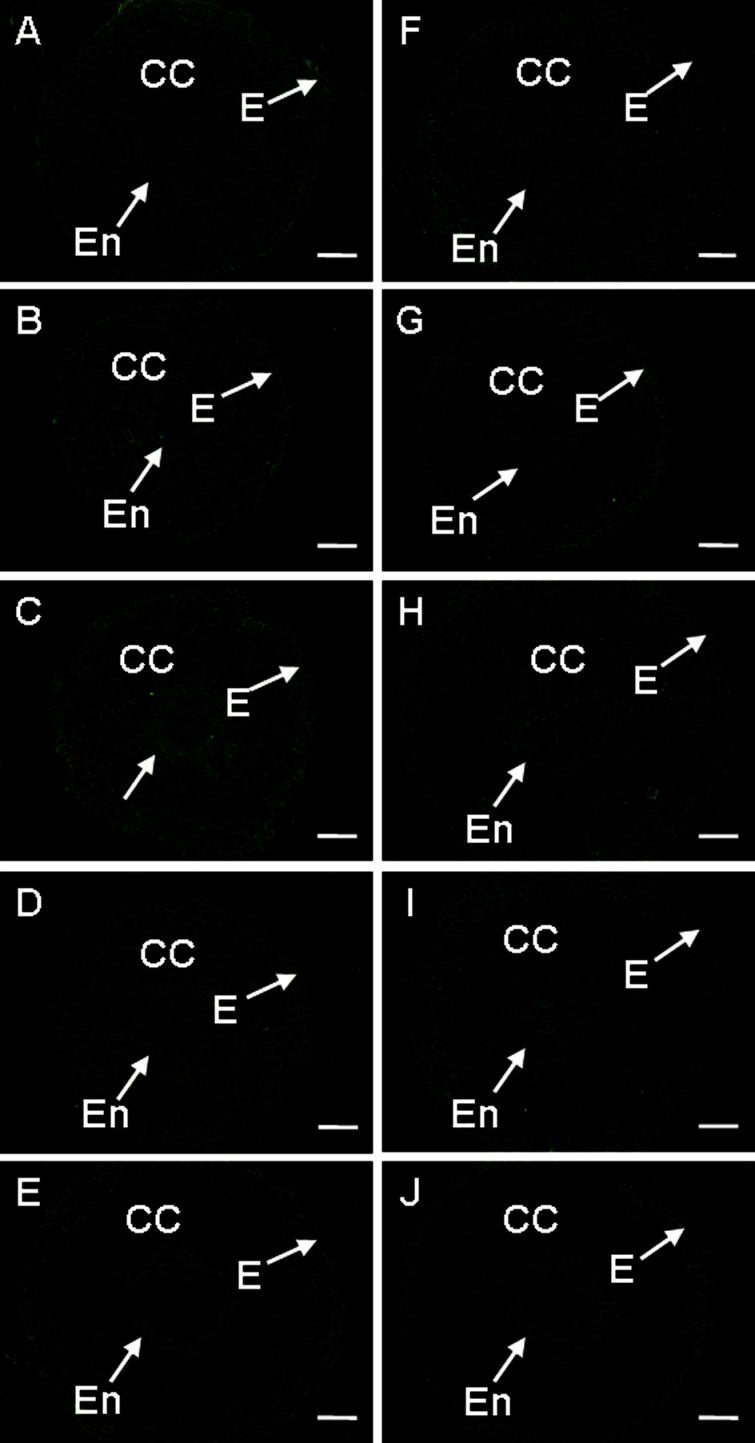
The antigen recognized by 2F4 antibody is HG cross-linked by Ca2+ bridges, the so-called ‘egg-box’ structure (Liners et al., 1989), which is assumed to induce gel formation and thus strengthen the cell wall (Wolf et al., 2009). In banana–Foc interation, no substantial difference in 2F4 labelling was seen in the controls of both cultivars (Fig. 3A, F, Supplementary Fig. S5 at JXB online). In the R cultivar, the 2F4 labelling was stronger after 6h of pathogen treatment, while it was weaker in the S cultivar (Fig. 3B, C, G, H; Supplementary Fig. S5). Longer pathogen treatment resulted in weaker 2F4 labelling in both cultivars (Fig. 3D,E, I, J; Supplementary Fig. S5).
Fig. 3.
The changes in immunolocalization by 2F4 antibody in banana (Musa spp. AAA) roots 6h and 48h after infection with Fusarium oxysporum f. sp. cubense. In all cases, cross-sections through roots are presented. (A, F) The control of the non-treated resistant (A) and the susceptible cultivar (F). (B, G) The resistant (B) and susceptible cultivar (G) 6h after wounding (C, H) The resistant (C) and susceptible cultivar (H) 6h after infection. (D, I) The resistant (D) and susceptible cultivar (I) 48h after wounding. (E, J) The resistant (E) and susceptible cultivar (J) 48h after infection. CC, cortical cells; E, epidermis; En, endodermis. Bars represent 100 µm. (This figure is available in colour at JXB online.)
JIM5
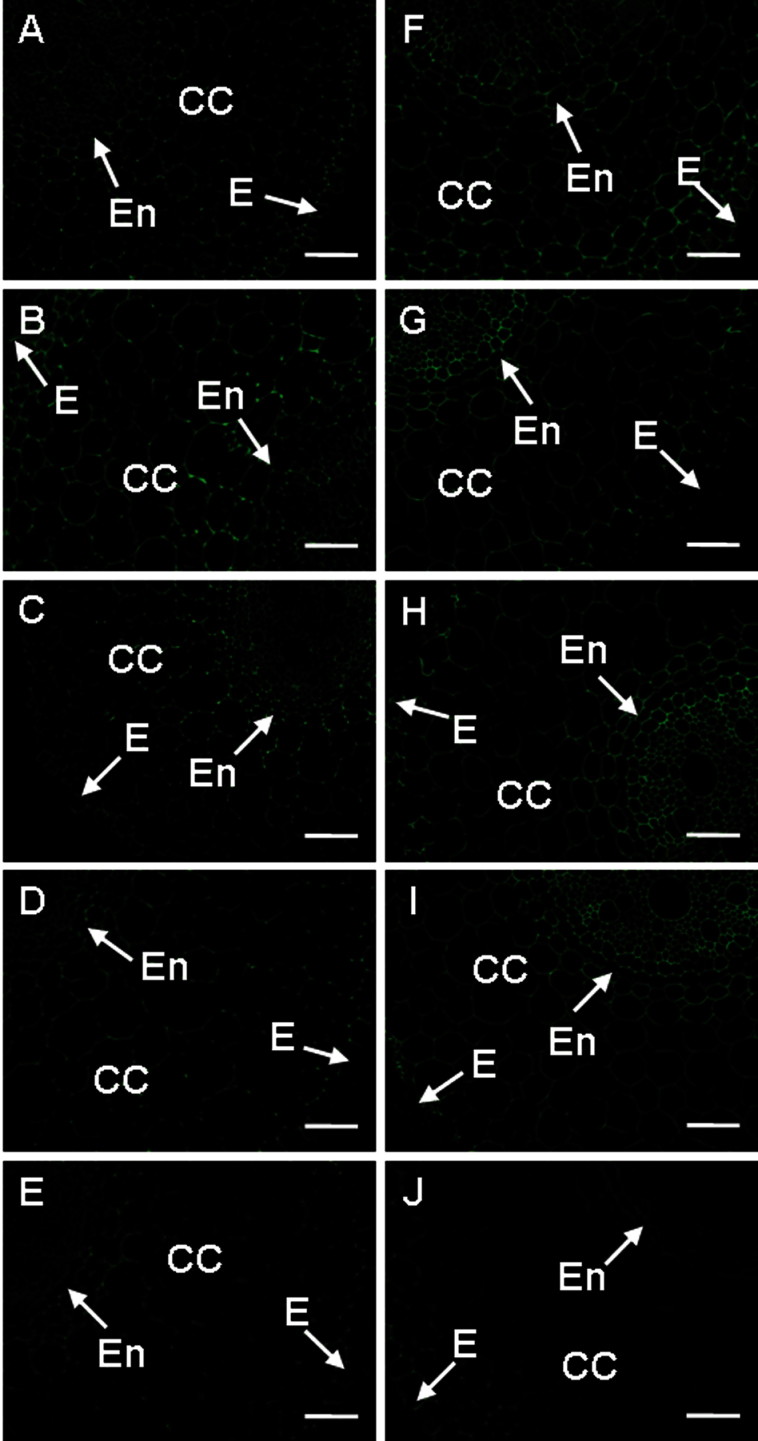
The JIM5 antibody recognizes low methyestrified HGs. Corresponding epitopes were distributed evenly all over the root section, except for weaker immunolabelling in the cortical cells. Before treatment with pathogen, the roots of the R cultivar showed weaker immunolabelling compared with those of the S cultivar (Fig. 4A, F; Supplementary Fig. S5 at JXB online). Increased signal was observed 6h after being cut in both cultivars, especially in the vascular cylinder of the S cultivar (Fig. 4A, B, F, G; Supplementary Fig. S5). When compared with 6h after being cut, JIM5 signal in the S cultivar 48h after cutting was still higher than that in the non-cut control but it was the opposite in the R cultivar (Fig. 4A, D, F, I; Supplementary Fig. S5). After pathogen attack (6h), however, the labelling with this antibody was more intense in the cells of the vascular cylinder but only in the S cultivar (Fig. 4G, H). In contrast, a slight decrease of labelling by JIM5 was found in the R cultivar after 6h of pathogen infection (Fig. 4B, C). The signal decreased in the presence of pathogen after 48h in the S cultivar, while it fell much less in the R cultivar (Fig. 4D, E, I, J; Supplementary Fig. S5). When compared with the R cultivar, the S cultivar had a stronger signal in all cases except 48h after infection, especially in the cells of the vascular cylinder (Fig. 4; Supplementary Fig. S5).
Fig. 4.
The changes in immunolocalization by JIM5 antibody in banana (Musa spp. AAA) roots after infection with Fusarium oxysporum f. sp. cubense. In all cases, cross-sections through roots are presented. (A–E) Resistant cultivar. (F–J) Susceptible cultivar. The immunolabelling observed before the treatment (A, F), 6h after wounding (B, G,), 6h after infection (C, H), 48h after wounding (D, I), and 48 after infection (E, J) is presented. CC, cortical cells; E, epidermis; En, endodermis; Pe, pericycle. Bars represent 50 µm. (This figure is available in colour at JXB online.)
LM18 and LM19
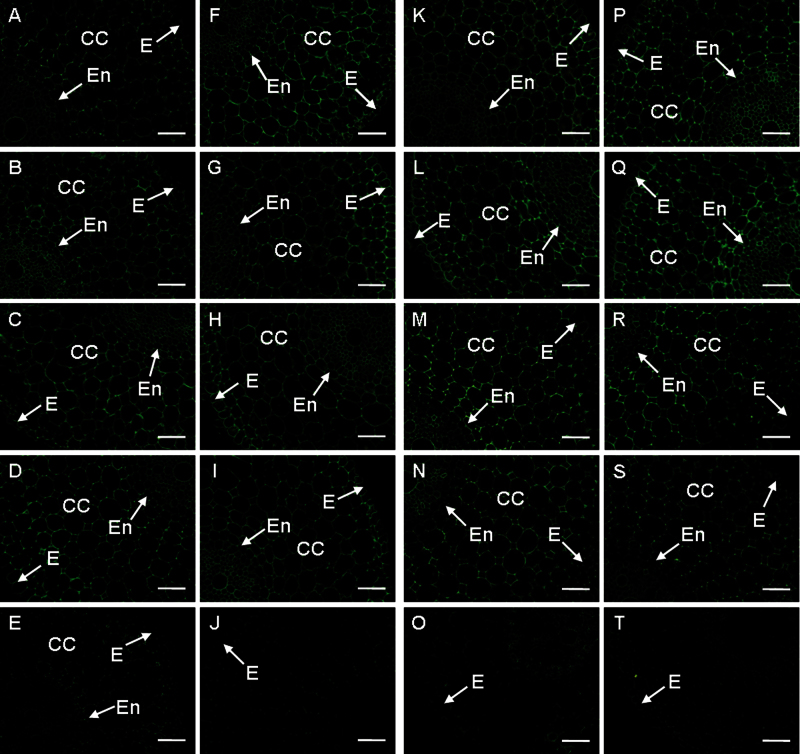
LM18 antibody recognizes low methyles terified HGs. Labelling by this antibody was stronger in the S cultivar before treatment (Fig. 5A, F; Supplementary Fig. S5 at JXB online). In the R cultivar, the abundance of the LM18 antigen increased after cutting (Fig. 5A, B, D; Supplementary Fig. S5) but decreased in the S cultivar (Fig. 5F, G, I; Supplementary Fig. S5). Pathogen infection decreased the antigen abundance in the R cultivar (Fig. 5B–E; Supplementary Fig. S5). However, a decrease in signal was only observed in the roots 48h after pathogen infection in the S cultivar (Fig. 5G–J; Supplementary Fig. S5). These results suggested that the LM18 antigen possibly contributed to the resistance.
Fig. 5.
The changes in immunolocalization by LM18 (A–J) and LM19 (K–T) antibodies in banana (Musa spp. AAA) roots after infection with Fusarium oxysporum f. sp. cubense. In all cases, cross-sections through roots are presented. (A–E, K–O) Resistant cultivar. (F–J, P–T) Susceptible cultivar. The immunolabelling observed before the treatment (A, F, K, P), 6h after wounding (B, G, L, Q), 6h after infection (C, H, M, R), 48h after wounding (D, I, N, S), and 48 after infection (E, J, O, T) is presented. CC, cortical cells; E, epidermis; En, endodermis; Ph, phloem. Bars represent 50 µm. (This figure is available in colour at JXB online.)
LM19 antibody also recognizes low methylesterified HGs. Stronger labelling by this antibody was found in the S cultivar when compared with the R cultivar in control roots (Fig. 5K, P; Supplementary Fig. S5 at JXB online). The root cut caused an increased LM19 signal in both cultivars, except in the R cultivar 48h after cutting (Fig. 5K, L, N, P, Q, S; Supplementary Fig. S5). Labelling by LM19 increased only slightly in response to pathogen attack after 6h in the R cultivar, while it decreased slightly in the S cultivar (Fig. 5L, M, Q, R; Supplementary Fig. S5). Notably, this labelling was very weak in both cultivars 48h after pathogen infection (Fig. 5N, O, S, T; Supplementary Fig. S5).
JIM7 and LM20
JIM7 antibody binds partially methyles terified HGs with up to 80% methylesterified residues. The corresponding antigen was evenly distributed in the section of banana roots before pathogen treatment and generally was stronger in the S cultivar (Fig. 6A, F; Supplementary Fig. S5 at JXB online). Cutting caused a continuous increase in signal in the R cultivar, while this was not the case with the S cultivar (Fig. 6A, B, D, F, G, I; Supplementary Fig. S5). At 6h after pathogen attack, the labelling by JIM7 in the R cultivar became stronger when compared with the cut control, but no obvious changes were observed in the S cultivar (Fig. 6B, C, G, H; Supplementary Fig. S5). At 48h after pathogen attack, the signal in both cultivars became weaker (Fig. 6D, E, I, J; Supplementary Fig. S5). The R cultivar had a stronger signal in all cases, except in the non-cut control (Fig. 6; Supplementary Fig. S5).
Fig. 6.
The changes in immunolocalization by JIM7 (A–J) and LM20 (K–T) antibodies in banana (Musa spp. AAA) roots after infection with Fusarium oxysporum f. sp. cubense. In all cases, cross-sections through roots are presented. (A–E, K–O) Resistant cultivar. (F–J, P–T) Susceptible cultivar. The immunolabelling observed before the treatment (A, F, K, P), 6h after wounding (B, G, L, Q), 6h after infection (C, H, M, R), 48h after wounding (D, I, N, S), and 48 after infection (E, J, O, T) is presented. CC, cortical cells; E, epidermis; En, endodermis. Bars represent 50 µm. (This figure is available in colour at JXB online.)
Further, LM20 antibody binds highly methylesterified HGs. The signal was weaker in the R cultivar than in the S cultivar in the non-cut controls (Fig. 6K, P; Supplementary Fig. S5 at JXB online). The antigen levels in the cut controls were higher than in the non-cut controls in both cultivars, except for the S cultivar 48h after cutting (Fig. 6K, L, N, P, Q, S; Supplementary Fig. S5). The co-incubation of the roots with the pathogen resulted in reduction of labelling by LM20 in both cultivars, especially 48h after infection (Fig. 6L–O, Q–T; Supplementary Fig. S5). Notably, the abundance of antigen in the R cultivar at 48h, after both cutting and pathogen attack, was higher than that in the S cultivar (Fig. 6; Supplementary Fig. S5).
The signal of CCRC-M38 antibody (recognizing fully de-esterified HG) was the strongest when compared with the other nine antibodies. However, a rapid decrease of CCRC-M38 labelling was only observed after 48h of pathogen attack in both cultivars (Supplementary Fig. S6 at JXB online). No substantial signal and differences were observed by using CCRC-M34 and CCRC-M130 antibodies in banana–Foc interactions (Supplementary Fig. S7 at JXB online). No signal was detectable with LM7 antibody in banana roots (data not shown). The changes in the abundance of HGs with different DMs revealed by immunodot analysis are shown in Supplementary Fig. S8 at JXB online.
Discussion
Wound-induced PMEs enhanced banana susceptibility to Foc
PMEs catalyse the demethylation of pectin within plant cell walls and modify the composition and structure of the wall polysaccharides as part of regular plant developmental programmes (Cantu et al., 2008). Recently, PMEs were proposed to be involved in plant resistance to pathogens (Lionetti et al., 2007; An et al., 2008; Raiola et al., 2011; Volpi et al., 2011; Wojtasik et al., 2011). In the present study, PME activities were found to be significantly higher in a banana R cultivar than in an S cultivar before root wounding and infection. This indicates that higher pectin demethylesterification is probably required for banana resistance against Foc race 4 infection. Albersheim and Valent (1978) argued that PMEs produce, directly or indirectly, three types of substances that are involved in plant defence, namely methanol, negatively charged carboxylic groups, and protons in the pectic matrix. Determination of which types of substances are involved in banana resistance to Fusarium in the R cultivar needs further investigation. Moreover, pathogen attack resulted in decreased PME activities, PME expression levels, and transcription level of one PME isoform, namely MaPME1, in both R and S cultivars. Decreased levels of PME mRNAs were also observed in flax (Linum usitatissimum L. Nike) upon infection with Fusarium (Wojtasik et al., 2011). These results suggest that a decrease in PME activity and/or expression levels are among the usual responses of bananas to pathogen attack and that PMEs are directly involved in the banana resistance/susceptibility to this pathogen.
No obvious changes in the PME activities were seen in the R cultivar, while they were highly increased in the S cultivar after injury, which was generally considered necessary for successful Fusarium penetration (Sequeira et al., 1958). As a result, the PME activities in the cut controls of the R cultivar were lower than those of the S cultivar, although the opposite was the case in the non-cut controls. These results were further confirmed by immunofluorescence labelling and qPCR analysis of the MaPME1 gene. Together, these data indicate that increased PME activity and expression level after wounding are probably responsible for the susceptibility of banana to Fusarium.
It is not clear what factors are responsible for the difference in PME activities and expression between R and S cultivars in response to injury and pathogen attack. An additional PME isoform was found in potato cultivars susceptible to the bacterium Erwinia carotovora (Marty et al., 1997). It is known that PME activity could be finely regulated by the presence of multiple enzyme isoforms and by the action of PMEI (Pelloux et al., 2007). In banana–Foc interaction, further work is needed to address the specific roles of given isoforms of the PME family in both banana cultivars and PME–PEMI interactions.
How do PMEs modify pectins and individual HGs involved in banana–Foc interaction?
Plant PMEs have major roles in pectin remodelling by controlling the methylesterification status of the most abundant pectin polysaccharide HGs (Pelloux et al., 2007). De-estification of HGs by PMEs and subsequent cleavage by other pectinases such as PGs may lead to reduced physical strength and rigidity of thr cell wall barrier, and thus pathogens can easily penetrate into the cells.
A key purpose of the present study was to understand better how PMEs modify pectins and individual HGs involved in banana–Foc interactions. Therefore, a systematic immunodot and immunofluorescence mapping of HGs with diverse DMs was performed with 10 monoclonal antibodies to monitor the changes of individual HGs during banana–Foc interaction. At 6h after wounding, an increased signal of PMEs was observed mainly in the cortical cells and cells close to the endodermis (e.g. phloem) in both cultivars. Subsequently, control roots of the R cultivar displayed lower PME signal intensity 48h after being cut, while a stronger signal was still observed in S control plants at this time point, especially in the cells close to the endodermis. The different response in PME abundance to wounding between R and S banana cultivars can partially explain why the S cultivar was more susceptible to the penetration of the pathogen hyphae in the endodermis (Supplementary Fig. S2 at JXB online). Meanwhile, enhanced PME in the wounded S cultivar correlated well with decreased DM and stronger labelling by JIM5 antibody that recognizes low methyestrified HGs, especially in the vascular cylinder. However, a continuous increase of JIM7 labelling (partially methyesterified HGs, up to 80%) was observed in the R cultivar. These findings indicated that PMEs are likely to be involved in the modification of pectins, especially those recognized by JIM5 and JIM7 antibodies, which ultimately determine the susceptibility of banana to Foc.
In addition, when compared with the R cultivar, the S cultivar always contained more low methylesterified HGs recognized by the antibodies CCRC-M38, JIM5, LM18, and LM19, and high methylesterified HGs recognized by antibodies JIM7, LM20, and CCRC-M130 in the non-cut control. However, 48h after wounding and pathogen attack, the R cultivar contained more highly methylesterified HGs, although both cultivars lost nearly all tested HGs at this time point. All these results suggest that a higher abundance of fully de-esterified, low, and partially methylesterified HGs, resulting from de-esterification by PMEs, was related to pathogen susceptibility. Thus, maintaining higher levels of highly methylesterified HGs and relatively higher pectin DMs is another strategy of the resistant banana cultivar in defence against Fusarium.
Degeneration of HGs recognized by the antibodies JIM5 and JIM7 was the usual response of most plants to the pathogen (Salerno et al., 2004; Boudjeko et al., 2006; Curvers et al., 2010; Digonnet et al., 2012); however, an increase in the labeling by JIM7 was also reported (Simon et al., 2005). In the present study, PMEs, pectin DMs, and individual HG levels all showed dynamic changes. This indicated that the sampling timing was possibly crucial for the result and it could partially explain why contradictory results were observed in different plants.
Importance of dynamic changes in PMEs and pectins
Pectin is secreted into the cell wall in a highly methylesterified form and is de-methylesterified by PMEs. Thus, changes in activities of PMEs could be closely related to the DM. Volpi et al. (2011) argued that cell wall pectin methylesterification can influence plant resistance because highly methylesterified pectin can be less susceptible to the hydrolysis by fungal pectic enzymes. This argument was supported by some other observations. For example, a tomato (Solanum lycopersicum) mutant with increased resistance to the necrotrophic fungus Botrytis cinerea showed a higher DM of pectins as compared with the wild type (Curvers et al., 2010). Similar results were reported for wheat (Triticum aestivum L.) (Volpi et al., 2011) and Arabidopsis thaliana (Lionetti et al., 2007). In the present study, however, lower PME activities and expression levels (immunolabelling quantitation) were identified in the S banana cultivar before injury and Fusarium treatment when compared with the R cultivar. Moreover, the activities of PGs and PMGs also showed a similar tendency. Consistent with these enzymatic data, the DM of pectins was higher in the S cultivar. Furthermore, a higher pectin DM was also observed in the S cultivar before pathogen treatment in some other plant species (Wiethölter et al., 2003; Osorio et al., 2008). The present data appear to contradict some of these observations. However, as described above, no obvious changes were observed in the PME activities and expression levels with the R cultivar after injury, while PME activities and expression levels increased a lot in the S cultivar. After wounding, pectin DM of the S cultivar decreased, while that of the R cultivar did not. Finally, the PME activities and expression levels of the S cultivar after injury were higher, while the pectin DM was lower than those of the R cultivar. Similar results were also observed after infection. This, in combination with the dynamic changes in individual HGs described above, indicate that interdependent changes in PMEs and pectins are probably more important than their constitutive levels when the susceptibility of banana to Foc is determined.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. A transection through a banana (Musa spp. AAA) root in the elongation zone.
Figure S2. The tissue-specific pathogen diffusion and quantification of hyphae in the host roots.
Figure S3. The changes in PG and PMG activities of banana (Musa spp. AAA) in banana–Foc interaction.
Figure S4. The quantification of the PME fluorescence signal from immunolocalization studies.
Figure S5. The quantification of the fluorescence signal of individual HGs from immunolocalization studies.
Figure S6. The changes in immunolocalization by CCRC-M38 antibody in banana–Foc interaction.
Figure S7. The changes in immunolocalization by CCRC-M130 and CCRC-M34 antibodies in banana–Foc interaction.
Figure S8. Immunodot analysis of changes in the abundance of HGs.
Table S1. Primers for qPCR.
Table S2. Antibodies recognizing different HGs and their antigens.
Methods S1. Enzyme assay (PG and PMG).
Methods S2. Immunodot assay.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31272117), Key Technologies Rese arch and Development Program of Guangdong Province (2010B020305004), the earmarked fund for Modern Agro-industry Technology Research System (nycytx-33), Key Laboratory of Innovation and Utilization for Germplasm Resources in Horticultural Crops in Southern China of Guangdong Higher Education Institutes, South China Agricultural University (KBL11008), and by grant no. ED0007/01/01 from the Centre of the Region Haná for Biotechnological and Agricultural Research. Generation of the CCRC series of monoclonal antibodies used in this work was supported by a grant from the NSF Plant Genome Program (DBI-0421683). PME monoclonal antibody was generated by Abmart (Shanghai, China). JIM antibodies and the other antibodies used in this work were from PlantProbe (Leeds University, UK).
Glossary
Abbreviations:
- AIR
alcohol-insoluble residue
- CWDE
cell wall-degrading enzyme
- DM
degree of methylesterification
- Foc
Fusarium oxysporum f. sp. cubense
- GalA
galacturonic acid
- HG
homogalacturonan
- PG
polygalacturonase
- PME
pectin methylesterase
- PMEI
PME inhibitor
- PMG
polymethylgalacturonase
- R
resistant
- S
susceptible.
References
- Albersheim P, Valent BS. 1978. Host–pathogen interactions in plants. Plants when exposed to oligosaccharides of fungal origin defend themselves by accumulating antibiotics. Journal of Cell Biology 78, 627–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An SH, Sohn KH, Choi HW, Hwang IS, Lee SC, Hwang BK. 2008. Pepper pectin methylesterase inhibitor protein CaPMEI1 is required for antifungal activity, basal disease resistance and abiotic stress tolerance. Planta 228, 61–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudjeko T, Andème-Onzighi C, Vicré M, Balangé AP, Ndoumou DO, Driouich A. 2006. Loss of pectin is an early event during infection of cocoyam roots by Pythium myriotylum . Planta 223, 271–282. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry 72, 248–254. [DOI] [PubMed] [Google Scholar]
- Cantu D, Vicente AR, Labavitch JM, Bennett A, Powell ALT. 2008. Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends in Plant Science 13, 610–617. [DOI] [PubMed] [Google Scholar]
- Chen HB, Feng QR, Xu CX, Huo RX, Li JG, Wang ZH. 2006. Screening of banana clones for resistance to fusarium wilt (Fusarium oxysporum f. sp. cubense). Journal of South China Agricultural University 27, 9–12. [Google Scholar]
- Curvers K, Seifi H, Mouille G, et al. 2010. Abscisic acid deficiency causes changes in cuticle permeability and pectin composition that influence tomato resistance to Botrytis cinerea . Plant Physiology 154, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’ Hont A, Denoeud F, Aury J, et al. 2012. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 488, 213–217. [DOI] [PubMed] [Google Scholar]
- Digonnet C, Martinez Y, Denancé N, Chasseray M, Dabos P, Ranocha P, Marco Y, Jauneau A, Goffner D. 2012. Deciphering the route of Ralstonia solanacearum colonization in Arabidopsis thaliana roots during a compatible interaction: focus at the plant cell wall. Planta 236, 1419–1431. [DOI] [PubMed] [Google Scholar]
- Gothoskar SS, Scheffer RP, Walker JC, Stahman MA. 1953. A phytopathological note. The role of pectic enzymes in Fusarium wilt of tomato. Phytopathology 79, 1095–1100. [Google Scholar]
- Liners LF, Letesson JJ, Didembourg C, van, Cutsem P. 1989. Monoclonal antibodies against pectin: recognition of a conformation induced by calcium. Plant Physiology 91, 1419–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionetti V, Cervone F, Bellincampi D. 2012. Methyl esterification of pectin plays a role during plant–pathogen interactions and affects plant resistance to diseases. Journal of Plant Physiology 169, 1623–1630. [DOI] [PubMed] [Google Scholar]
- Lionetti V, Raiola A, Camardella L, Giovane A, Obel N, Pauly M, Favaron F, Cervone F, Bellincampi D. 2007. Overexpression of pectin methylesterase inhibitors in Arabidopsis restricts fungal infection by Botrytis cinerea . Plant Physiology 143, 1871–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2 [Delta] [Delta] CT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Louvet R, Rayon C, Domon JM, et al. 2011. Major changes in the cell wall during silique development in Arabidopsis thaliana . Phytochemistry 72, 59–67. [DOI] [PubMed] [Google Scholar]
- Marcus L, Schejter A. 1983. Single step chromatographic purification and characterization of the endopolygalacturonases and pectinesterases of the fungus Botrytis cinerea Pers. Physiological Plant Pathology 23, 1–13. [Google Scholar]
- Marty P, Jouan B, Bertheau Y, Vian B, Goldberg R. 1997. Charge density in stem cell walls of Solanum tuberosum genotypes and susceptibility to blackleg. Phytochemistry 44, 1435–1441. [Google Scholar]
- Mbéguié-A-Mbéguié D, Hubert O, Baurens FC, Matsumoto T, Chillet M, Fils-Lycaon B, Sidibé-Bocs S. 2009. Expression patterns of cell wall-modifying genes from banana during fruit ripening and in relationship with finger drop. Journal of Experimental Botany 60, 2021–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Castillejo C, Quesada MA, Medina-Escobar N, Brownsey GJ, Suau R, Heredia A, Botella MA, Valpuesta V. 2008. Partial demethylation of oligogalacturonides by pectin methyl esterase 1 is required for eliciting defence responses in wild strawberry (Fragaria vesca). The Plant Journal 54, 43–55. [DOI] [PubMed] [Google Scholar]
- Pelloux J, Rustérucci C, Mellerowicz EJ. 2007. New insight into pectin methylesterase structure and function. Trends in Plant Science 12, 267–277. [DOI] [PubMed] [Google Scholar]
- Raiola A, Lionetti V, Elmaghraby I, Immerzeel P, Mellerowicz EJ, Salvi G, Cervone F, Bellincampi D. 2011. Pectin methylesterase is induced in Arabidopsis upon infection and is necessary for a successful colonization by necrotrophic pathogens. Molecular Plant-Microbe Interactions 24, 432–440. [DOI] [PubMed] [Google Scholar]
- Salerno MI, Gianinazzi S, Arnould C, Gianinazzi-Pearson V. 2004. Ultrastructural and cell wall modifications during infection of Eucalyptus viminalis roots by a pathogenic Fusarium oxysporum strain. Journal of General Plant Pathology 70, 145–152. [Google Scholar]
- Saravanan T, Muthusamy M, Marimuthu T. 2003. Development of integrated approach to manage the fusarial wilt of banana. Crop Protection 22, 1117–1123. [Google Scholar]
- Sequeira L, Steeves TA, Steeves MW, Riedhart JM. 1958. Role of root injury in Panama disease infections. Nature 182, 309–311. [Google Scholar]
- Simon UK, Bauer R, Rioux D, Simard M, Oberwinkler F. 2005. The intercellular biotrophic leaf pathogen Cymadothea trifolii locally degrades pectins, but not cellulose or xyloglucan in cell walls of Trifolium repens . New Phytologist 165, 243–260. [DOI] [PubMed] [Google Scholar]
- van den Berg N, Berger DK, Hein I, Birch PRJ, Wingfield MJ, Viljoen A. 2007. Tolerance in banana to Fusarium wilt is associated with early up-regulation of cell wall-strengthening genes in the roots. Molecular Plant Pathology 8, 333–341. [DOI] [PubMed] [Google Scholar]
- Volpi C, Janni M, Lionetti V, Bellincampi D, Favaron F, D’Ovidio R. 2011. The ectopic expression of a pectin methyl esterase inhibitor increases pectin methyl esterification and limits fungal diseases in wheat. Molecular Plant-Microbe Interactions 24, 1012–1019. [DOI] [PubMed] [Google Scholar]
- Vorwerk S, Somerville S, Somerville C. 2004. The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science 9, 203–209. [DOI] [PubMed] [Google Scholar]
- Wiethölter N, Graeßner B, Mierau M, Mort AJ, Moerschbacher BM. 2003. Differences in the methyl ester distribution of homogalacturonans from near-isogenic wheat lines resistant and susceptible to the wheat stem rust fungus. Molecular Plant-Microbe Interactions 16, 945–952. [DOI] [PubMed] [Google Scholar]
- Wojtasik W, Kulma A, Kostyn K, Szopa J. 2011. The changes in pectin metabolism in flax infected with Fusarium . Plant Physiology and Biochemistry 49, 862–872. [DOI] [PubMed] [Google Scholar]
- Wolf S, Mouille G, Pelloux J. 2009. Homogalacturonan methyl-esterification and plant development. Molecular Plant 2, 851–860. [DOI] [PubMed] [Google Scholar]
- Xu CX, Takáč BC, Menzel D, Šamaj J. 2011. a Developmental localization and the role of hydroxyproline rich glycoproteins during somatic embryogenesis of banana (Musa spp. AAA). BMC Plant Biology 11, 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CX, Zhao L, Pan X, Šamaj J. 2011. b Developmental localization and methylesterification of pectin epitopes during somatic embryogenesis of banana (Musa spp. AAA). PLoS One 6, e22992 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.