Abstract
Objective: To elucidate the oncologic behavior of Micropapillary Urothelial Bladder Carcinoma (MPBC), a rare aggressive variant histology.
Methods: All MPBC patients in SEER 17 database were compared with those with traditional urothelial carcinoma (UC). Kaplan-Meier curves were used to determine OS and CSS. A Cox proportional hazards model (CPH) was constructed to test the effect of covariates on outcomes.
Results: From 2001-2008, 120 MPBC patients were identified, 0.1% of all bladder cancer. MPBC presented with more high grade (86.1% vs. 38.7%, p<0.0001) and more high stage disease (40.8% NMI vs. 90.4% NMI, p < 0.0001) than UC. Low grade (LG) NMI MPBC had worse OS and CSS compared to LG UC (p=0.0037, p<0.0001 respectively), and did no better than high grade (HG) NMI MPBC. No difference was detected between HG NMI MPBC and HG NMI UC pts. A CPH model controlling for stage, grade, treatment, age, race, and sex detected no significant survival difference in MPBC vs. UC (HR 1.04, p=0.7966). For NMI MPBC (n=49), only 4 patients underwent definitive therapy, of whom none died of disease. However, in those not receiving definitive therapy (n=45), 7 cancer specific deaths occurred (15.6%).
Conclusion: Controlling for stage and grade, no survival difference could be detected between MPBC and UC. Low grade NMI MPBC behaved similarly to both high grade MPBC and high grade UC. We propose that all MPBC (regardless of grade) be managed as high grade disease, and that strong consideration for definitive therapy should be given in all cases.
Keywords: bladder, carcinoma, grade, micropapillary, SEER
INTRODUCTION
Urothelial tumors are very common, with a projected annual incidence of 73,510 bladder tumors alone in the U.S.A.1 Although the predominant histology seen in urothelial cancers is traditional urothelial carcinoma (UC), there are several variant histologic sub-types, including micropapillary bladder carcinoma (MPBC).2,3
Amin first described MPBC in 1994, though fewer than 300 cases have been reported since.2,4-9 Reports confirm that MPBC occurs in only 0.6-6% of bladder cancer cases.5,7 The histology of MPBC resembles that of micropapillary subtypes of breast, lung, stomach and colon, as well as serous ovarian carcinoma.7
MPBC has been uniformly described as aggressive, with higher incidence of locally advanced and metastatic presentation.4,6,8 Standard recommendations for treatment of locally advanced disease involve immediate cystectomy with or without perioperative chemotherapy.5-7,10-12 However, the management of MPBC that is non-muscle invasive (NMI) is more controversial. While bladder-preserving therapies are the standard of care in NMI bladder cancer,13 the limited data currently available suggests that low stage NMI MPBC has markedly worse survival outcomes compared to historical standards for traditional UC.12,14 We reviewed the SEER database in order to better understand the behavior of MPBC and to assess treatment outcomes.
METHODS
Data was collected and reviewed from the SEER 17 database. Patients with MPBC were identified using International Classification of Disease for Oncology, third edition (ICD-O-3) code 8131 (Transitional cell carcinoma, micropapillary). Coding for MPBC commenced in 2001, and review was therefore limited to 2001-2008. The incidence was calculated as the number of cases per 100,000. The contribution of MPBC as a percentage of all bladder cancers was calculated by comparing incidence of MPBC to that of all cases of bladder cancer (ICD-O-3 8120-8139) during the study period.
Age, sex, race, cause of death, histologic grade, cause of death, treatment, and initial stage at presentation was collected for analysis. For cases 2004 and later, AJCC sixth edition staging at presentation is coded for each case. For cases prior to this, AJCC staging was imputed using the extent-of-disease variables, which provided detailed information regarding depth of tumor invasion, nodal spread, and presence of metastatic disease. For univariate and multivariable analysis, identified cases of MPBC were compared to a group with UC with known AJCC stage as identified by ICD-O-3 code 8130 (Papillary transitional cell carcinoma), diagnosed 2004 and later. Data regarding surgical treatment was obtained from the RX summary variable (codes 50, 60-64, 70-74, and 80 were coded as cystectomy; codes 10-16, 20-27, and 30 were coded as local resection only).
Continuous variables were evaluated with T-test, while chi-square and Fisher's exact test were used for nominal variables. Kaplan-Meier curves and log-rank tests were used to determine effects on cancer specific (CSS) and overall survival (OS). A p-value less than 0.05 was considered statistically significant. All tests were two-tailed. Potential prognostic variables were evaluated with Cox proportional hazards models to test the effect of covariates on OS and CSS. Variables with a univariate p-value <0.3 were included in the multivariable model, and only patients who had known values for the variables of interest were included.
RESULTS
A total of 120 patients were identified with MPBC. The incidence of MPBC in comparison to all bladder cancers was 0.1% (incidence curves are available in supplemental data). The comparison group of all UC patients of known AJCC stage from 2004-2008 included a total of 32,140 patients. Mean age at diagnosis for MPBC was 70.3 years, with 76.7% of male sex, and 90.8% of Caucasian race. Comparison of baseline characteristics between patients with MPBC and UC is presented in Table 1. No differences in age, sex, or race were detected between the groups. MPBC patients presented with significantly more high grade disease than UC (86.1% vs. 38.7%, p<0.0001). Compared to UC, MPBC presented with significantly less NMI disease (40.8% vs. 90.4%, p < 0.0001), more muscle invasive disease (T2 N0 M0, 23.3% vs. 5.70%, p < 0.0001), more locally advanced disease (T3/4 N0 M0, 13.3% vs. 1.85%, p < 0.0001), more nodal disease (N+ M0, 17.5% vs. 0.97%, p < 0.0001) and more metastatic disease (5% vs. 1.1%, p = 0.0023).
Table 1.
Demographics and baseline characteristics comparing micropapillary bladder cancer (MPBC) and conventional urothelial carcinoma (UC) groups.
| MPBC | UC | p value | ||
|---|---|---|---|---|
| N | 120 | 32140 | ||
| Age | Mean | 70.31 | 69.01 | 0.265 |
| Sex | Female | 23.33% | 24.40% | 0.8322 |
| Male | 76.67% | 75.60% | ||
| Race | Asian | 4.02% | 3.92% | 0.8158 |
| Black | 5.00% | 4.06% | 0.4911 | |
| White | 90.83% | 91.47% | 0.7436 | |
| Grade | High Grade | 86.11% | 38.70% | <0.0001 |
| Low Grade | 13.89% | 61.30% | <0.0001 | |
| Stage | Superficial (Ta/1/is) | 40.83% | 90.39% | <0.0001 |
| Ta | 10.83% | 65.61% | ||
| T1 | 26.67% | 23.14% | ||
| Tis | 3.33% | 1.65% | ||
| Muscle invasive (T2) | 23.33% | 5.70% | <0.0001 | |
| Locally advanced (T3/4) | 13.33% | 1.85% | <0.0001 | |
| T3 | 4.17% | 0.76% | ||
| T4 | 9.17% | 1.09% | ||
| Nodal disease (N+M0) | 17.50% | 0.97% | <0.0001 | |
| Metastatic (M1) | 5% | 1.10% | 0.0023 |
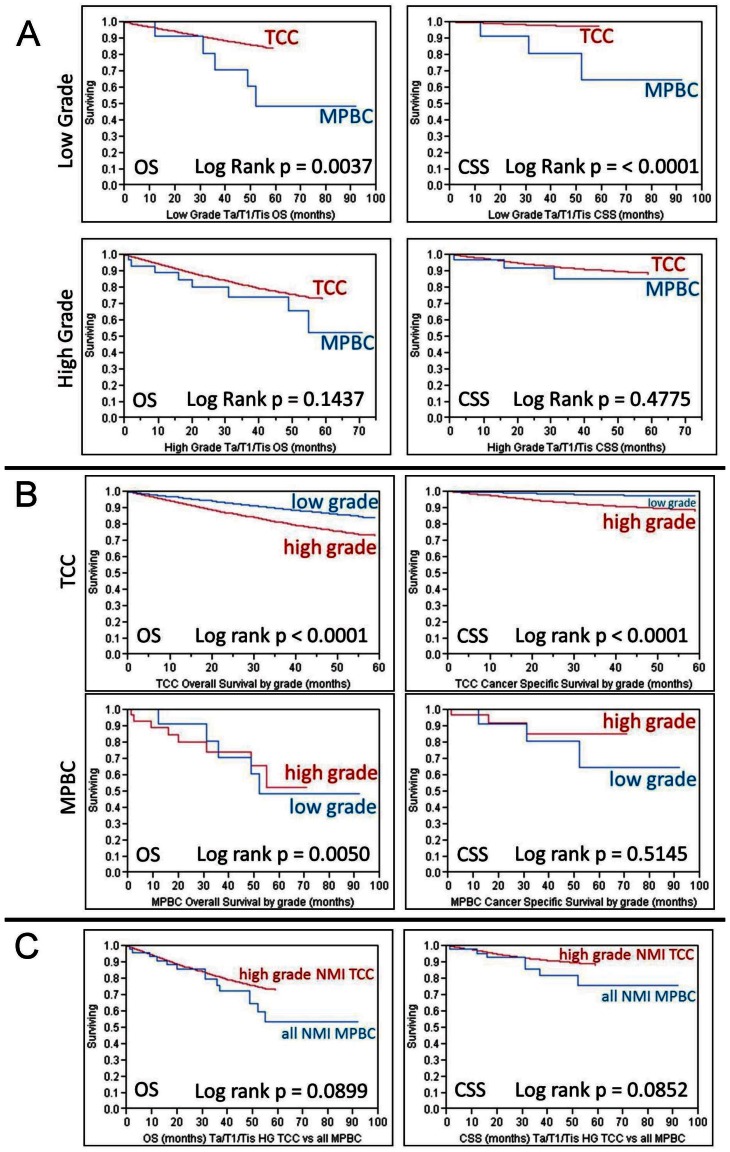
Kaplan-Meier survival curves for CSS and OS for all cases of MPBC are presented in the Additional File 1: supplemental figures. As expected, poorer survival was associated with more advanced stage (p < 0.0001). Comparison of both CSS and OS between MPBC and UC, as calculated by Kaplan-Meier survival analysis is presented in Table 2. On this univariate analysis (not controlling for grade) NMI MPBC had significantly worse survival than similar stage UC. This difference in survival was not detected in individuals with higher stage disease. In order to control for the higher grade presentation of MPBC, survival was assessed stratifying patients with NMI disease (Ta/T1/Tis) by disease grade (Figure 1A). Low grade MPBC had poorer OS and CSS than low grade UC (p = 0.0037 and p < 0.0001 respectively), but such a difference could not be detected when comparing those with high grade disease (Figure 1A). As expected, low grade NMI UC survived longer than high grade NMI UC, however this difference was not seen in MPBC. Interestingly, when patients with low grade NMI MPBC were compared to those with high grade NMI MPBC, CSS was similar and, in fact, OS was worse in those with low grade MPBC (p = 0.0050) (Figure 1B). When compared to high grade NMI UC, MPBC of similar stage had worse mean OS (45.1 vs. 50.1 months) and mean CSS (46.9 vs. 55.0 months), but this did not reach statistical significance (p = 0.0899 and p = 0.0852, respectively) (Figure 1C).
Table 2.
Overall survival (OS) and cancer specific survival (CSS) of micropapillary bladder cancer (MPBC) vs conventional urothelial carcinoma (UC) as stratified by stage. (DNR denotes “did not reach”).
| OS (months) | CSS (months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stage | MPBC | UC | Log rank | MPBC | UC | Log rank | ||||
| mean | median | mean | median | p value | mean | median | mean | median | p value | |
| Nonmuscle invasive (Ta/T1/Tis) | 45.14 | DNR | 52.69 | DNR | 0.0014 | 46.91 | DNR | 57.02 | DNR | < 0.0001 |
| Muscle invasive (T2) | 26.8 | 37 | 33.27 | 41 | 0.9675 | 14.1 | DNR | 36.4 | DNR | 0.1019 |
| Locally advanced (T3/T4) | 30.96 | 23 | 30.96 | 29 | 0.2966 | 42.38 | 17 | 30.51 | DNR | 0.5371 |
| Nodal disease (N+) | 29.37 | 28 | 23.88 | 19 | 0.1565 | 29.37 | 28 | 25.24 | 22 | 0.2564 |
| Metastatic (M1) | 15.28 | 15 | 9.97 | 7 | 0.5216 | 18.06 | 25 | 10.88 | 7 | 0.2074 |
Figure 1.
1A - Kaplan-Meier analysis demonstrating overall survival (OS) and cancer specific survival (CSS) for patients with non-muscle invasive bladder cancer (Ta/T1/Tis) comparing micropapillary bladder cancer (MPBC) to urothelial carcinoma (UC) as stratified by tumor grade (high grade vs low grade). 1B - Kaplan-Meier analysis demonstrating OS and CSS for patients with non-muscle invasive bladder cancer (Ta/T1/Tis) comparing high grade disease to low grade disease as stratified by tumor histology. 1C - Kaplan-Meier analysis demonstrating OS and CSS comparing patients with non-muscle invasive (Ta/T1/Tis) high grade UC to all similarly staged patients with MPBC.
Treatment modality for MPBC was tabulated and is presented in Table 3. For NMI MPBC (n=49), the vast majority (88%, n=43) were managed with local treatment only (TURBT or partial cystectomy). Among the remaining patients, 2 (4%) underwent cystectomy and 2 (4%) received external beam radiation therapy. All patients receiving definitive therapy (defined here as either cystectomy or external beam radiation) had high grade disease. Two patients (4%) received no additional therapy subsequent to diagnosis. During the median follow-up period of 31 months, none of the 4 patients receiving definitive therapy had cancer specific mortality. In those who did not receive definitive therapy (n=45) there were a total of 7 cancer specific deaths (15.6%, Kaplan-Meier curve available in supplemental data).
Table 3.
Treatment modality of patients with micropapillary bladder cancer (MPBC) as stratified by stage.
| Stage | None | local tx | cystectomy | XRT | cystectomy + XRT |
Total |
|---|---|---|---|---|---|---|
| Ta/T1/Tis | 2 | 43 | 2 | 2 | 0 | 49 |
| 4.08% | 87.76% | 4.08% | 4.08% | 0% | ||
| T2 | 1 | 19 | 8 | 0 | 0 | 28 |
| 3.57% | 67.86% | 28.57% | 0% | 0% | ||
| T3/T4 | 2 | 7 | 5 | 1 | 1 | 16 |
| 12.50% | 43.75% | 31.25% | 6.25% | 6.25% | ||
| N+ | 1 | 2 | 17 | 0 | 0 | 20 |
| 5% | 10% | 85% | 0% | 0% | ||
| M1 | 1 | 2 | 1 | 2 | 0 | 6 |
| 16.67% | 33.33% | 16.67% | 33.33% | 0% |
Results of the multivariate Cox proportional hazards model are presented in Table 4. Covariates included were stage, grade, histology (MPBC vs. UC), treatment strategy (definitive therapy vs. not), age, race (African-American vs. other), and sex. When including both MPBC and UC patients in the multivariable model, stage, grade, age, race and sex remained significant predictors of OS and CSS on multivariate analysis. When factoring for the above covariates, no statistically significant survival difference could be detected in those with MPBC in comparison to those with UC (HR 1.04, p = 0.7966). When including only patients with MPBC in the model, only stage remained a significant predictor of OS and CSS. Consistent with previous univariate analysis, grade was not a significant predictor of OS or CSS in the analysis limited to MPBC patients (HR 0.59, p = 0.3169). In this model, definitive therapy for those with MPBC was associated with improved survival (HR 0.36), though this did not quite reach statistical significance (p = 0.0584).
Table 4.
Summary results of Cox proportional hazards (CPH) model evaluating the effect of covariates on overall survival (OS) and cancer specific survival (CSS). (HR is Hazard ratio, MPBC is micropapillary bladder cancer, and UC is traditional urothelial carcinoma).
| CPH Model including those with MPBC and UC | CPH Model including only those with MPBC | |||||||
|---|---|---|---|---|---|---|---|---|
| OS | CSS | OS | CSS | |||||
| Covariate | HR | p value | HR | p value | HR | p value | HR | p value |
| Stage | 3.30 - T2 vs Ta/T1/Tis 1.38 - T3/T4 vs T2 1.76 - N+ vs T3/T4 2.23 - M+ vs N+ |
<0.0001 | 6.05 - T2 vs Ta/T1/Tis 1.50 - T3/T4 vs T2 1.93 - N+ vs T3/T4 2.34 - M+ vs N+ |
<0.0001 | 3.05 - T2 vs Ta/T1/Tis 2.78 - T3/T4 vs T2 0.97 - N+ vs T3/T4 1.07 - M+ vs N+ |
0.0007 | 2.54 - T2 vs Ta/T1/Tis 5.97 - T3/T4 vs T2 1.08 - N+ vs T3/T4 1.15 - M+ vs N+ |
0.0008 |
|
Grade (High vs Low) |
1.56 | <0.0001 | 2.93 | <0.0001 | 0.59 | 0.3169 | 0.35 | 0.1878 |
|
Age (per yr) |
1.07 | <0.0001 | 1.05 | <0.0001 | 1.06 | 0.0001 | 1.03 | 0.1290 |
|
Race (Black vs not) |
1.43 | <0.0001 | 1.61 | <0.0001 | 0.65 | 0.4990 | 0.42 | 0.3978 |
|
Sex (Female vs Male) |
0.93 | 0.0322 | 1.14 | 0.0099 | 1.07 | 0.8653 | 1.15 | 0.7965 |
|
Definitive tx vs not |
0.94 | 0.2709 | 0.90 | 0.0933 | 0.36 | 0.0584 | 0.43 | 0.1947 |
| MPBC vs TCC | 1.04 | 0.7966 | 0.80 | 0.2417 | ||||
DISCUSSION
MPBC is a rare entity, comprising less than 1% of all urothelial carcinoma. It presents with high grade and stage, and has been reported as having a worse prognosis when compared to traditional UC. Perepletchikov attributed this aggressiveness to a reversal of cell polarity seen in MPBC. They noted that basal, stromal-facing sides of epithelial cells acquire some secretory properties of the apical component, possibly facilitating tumor release into the stromal space.15 Maranchie noted MPBC's ability to invade stromal spaces without robust reaction from the neighboring microenvironment. They hypothesized that this could lead to rapid spread along tissue planes.16
Possibly due to these characteristics, many have noted high rates of advanced disease at diagnosis. In their original report, Amin noted lymph node invasion and adjacent organ involvement in 8 and 3 of 18 patients, respectively. In additional, all had high grade nuclear characteristics and evidence of lymphatic invasion.2 Johansson reported on 20 cases of MPBC with only 3 of 20 patients with T1 stage disease. Lymphovascular invasion was present in 15 of 20 cases.4 Similarly, Heudel reported on 11 patients, all of whom presented with high grade invasive disease with 35% having associated carcinoma in situ (CIS).11
In our study, we report the largest number of patients with MPBC in a retrospective analysis. We found 120 patients with MPBC representing 0.1% of all bladder tumors. The proportion of muscle invasive (T2), locally advanced (T3/T4), nodal (N+), and metastatic (M+) disease was significantly higher in the MPBC group compared to UC (see Table 1). High grade carcinoma was significantly more common in the MPBC group (86.1% vs. 38.7%, p < 0.0001). These findings are consistent with the previous reports supporting the aggressive presentation of MPBC.
Several large series reporting clinical outcomes in MPBC have been published in recent years. Comperat reported on 72 patients with MPBC diagnosed on transurethral resection (TUR). Of the 12 patients diagnosed with pTa disease, 8 eventually underwent cystectomy and were upstaged to pT2-T4 on final pathology. These authors found that 38% of patients died of disease, with mean survival of only 17.8 months. There was a correlation between the amount of MPBC found on TUR specimen and disease-specific survival, though this was significant on univariate analysis but not on multivariate analysis. Nevertheless, the pathologic stage of MPBC remained significant in predicting DSS. 7
The largest reported series of 100 patients comes from the MD Anderson Cancer Center. They report 5 and 10-year OS of 54% and 27%, respectively, despite a higher proportion of Ta/T1 patients than most series (n = 44). The authors stratified survival by clinical stage, pathologic stage and mode of therapy. They found a similar 5-year OS between immediate cystectomy and neoadjuvant chemotherapy groups (71% and 63%, respectively). When examining only patients with NMI disease, there was a trend towards improved OS with immediate cystectomy (87% vs. 49%, p = 0.06). The authors posed the question whether neoadjuvant chemotherapy should be avoided due to delay in primary treatment.6 Ghoneim attempted to determine the role of neoadjuvant chemotherapy in 38 MPBC patients. They reported median OS of 40 months. Median OS of patients who had received neoadjuvant chemotherapy and those who had not was 23 and 46 months, respectively. Interestingly, 26 of the 28 (93%) patients with ≤cT2 disease were found to have either a higher pathological stage or node positive disease at radical cystectomy. Furthermore, 32 of 37 patients who were clinically N0 had positive lymph nodes at cystectomy. This high rate of upstaging led the authors to the conclusion that MPBC should be considered micrometastatic at the time of diagnosis. The authors supported the use of perioperative chemotherapy given the high risk of occult metastatic disease, but acknowledged that their data does not support its use.12
Given its aggressive behavior, the management of MPBC is most challenging with NMI presentation. We reviewed survival after the diagnosis of MPBC as stratified by stage. We demonstrate that all MPBC, regardless of grade, behaves similarly (if not slightly worse) than high grade UC in regards to survival. In addition, we demonstrate that low grade MPBC behaves more aggressively than low grade traditional UC (OS and CSS). Although the low grade tumors could reflect significant misgrading of truly high grade tumors, we hypothesize that this may be due to the fact that disease classified as low grade MPBC may not exhibit any improved oncologic behavior in comparison to high grade disease. In addition, we believe that this diagnosis of low grade contributed to under-treatment of the disease, evidenced by the fact that no patient with low grade MPBC was treated with definitive therapy. Furthermore, we noted no cancer specific mortality in those MPBC patients treated with definitive therapy, in contrast to those managed with local therapy alone, which had 15% cancer specific mortality. Based upon these data, we propose that strong consideration be given for definitive therapy in all patients with NMI MPBC, including those classified as low grade. In addition, an argument can be made for re-classifying all MPBC tumors as high grade, thus removing any potential confusion in management that may result from retaining the low grade classification for this group of tumors.
These finding are echoed by previous reports. Shapur performed a retrospective review of 144 patients with high grade traditional UC and 22 patients with variant histology undergoing intravesical therapy with BCG. All patients had pTa or pT1 disease diagnosed on staging transurethral resection. They found a significantly decreased time to progression in the variant histology group (19.8 vs. 56 months, p < 0.001), but no significant difference in progression free survival (PFS). Of note, they did not separate each individual variant histology, thus MPBC was grouped with squamous, glandular, nested and sarcomatoid histologies.14
The report by Kamat6 represents the largest series of MPBC patients treated with intravesical therapy. They reviewed 27 patients with T1/Ta disease and 2 with T2 disease (who refused cystectomy) who were treated with BCG. At a median follow up of 8 months, 18 of 27 patients (67%) had disease progression (to muscle invasion). At a median of 30 months, only 17% had no evidence of disease, leading these authors to recommend against bladder-sparing therapy for MPBC. These findings are consistent with those of the present study, in which mean OS and CSS were found to be worse in Ta/T1 patients with MPBC when compared to traditional UC (45.14 months vs. 52.69 months, p = 0.0014 and 46.9 months vs. 57.02 months, p < 0.0001, respectively). Given this finding, it may be preferable to counsel patients against bladder-sparing therapy for any MPBC.
The limitations of this current study include its retrospective nature, the lack of centralized pathologic review, and limited information regarding treatment of these patients, especially about chemotherapy. While a prospective trial would be ideal, the extremely low prevalence of this histology makes adequate accrual to complete a meaningful trial exceedingly difficult. Future research should include centralized pathologic review and seek to address whether chemotherapy is beneficial for these patients and, if so, whether it should be administered in the neoadjuvant or adjuvant setting. In addition, given the rarity of this disease, multiinstitutional consortium efforts should be supported.
CONCLUSION
MPBC has a significantly worse stage and grade presentation in comparison to traditional UC. Furthermore, low grade MPBC behaves similarly to both high grade MPBC and high grade traditional UC, thus strong consideration should be given to treating NMI MPBC aggressively regardless of grade. Given the similar outcomes for high grade and low grade MPBC, abolishing the classification of low grade MPBC may be warranted. Future research should include centralized pathologic analysis and support multiinstitutional consortium efforts to overcome the challenges of studying this rare aggressive disease.
Supplementary Material
Supplemental Figure 1-3.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Amin MB, Ro JY, el-Sharkawy T. et al. Micropapillary variant of transitional cell carcinoma of the urinary bladder. Histologic pattern resembling ovarian papillary serous carcinoma. Am J Surg Pathol. 1994;18:1224–32. doi: 10.1097/00000478-199412000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22(Suppl 2):S96–S118. doi: 10.1038/modpathol.2009.26. [DOI] [PubMed] [Google Scholar]
- 4.Johansson SL, Borghede G, Holmang S. Micropapillary bladder carcinoma: a clinicopathological study of 20 cases. J Urol. 1999;161:1798–802. doi: 10.1016/s0022-5347(05)68807-6. [DOI] [PubMed] [Google Scholar]
- 5.Alvarado-Cabrero I, Sierra-Santiesteban FI, Mantilla-Morales A. et al. Micropapillary carcinoma of the urothelial tract. A clinicopathologic study of 38 cases. Ann Diagn Pathol. 2005;9:1–5. doi: 10.1053/j.anndiagpath.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Kamat AM, Dinney CP, Gee JR. et al. Micropapillary bladder cancer: a review of the University of Texas M. D. Anderson Cancer Center experience with 100 consecutive patients. Cancer. 2007;110:62–7. doi: 10.1002/cncr.22756. [DOI] [PubMed] [Google Scholar]
- 7.Comperat E, Roupret M, Yaxley J. et al. Micropapillary urothelial carcinoma of the urinary bladder: a clinicopathological analysis of 72 cases. Pathology. 2010;42:650–4. doi: 10.3109/00313025.2010.522173. [DOI] [PubMed] [Google Scholar]
- 8.Samaratunga H, Khoo K. Micropapillary variant of urothelial carcinoma of the urinary bladder; a clinicopathological and immunohistochemical study. Histopathology. 2004;45:55–64. doi: 10.1111/j.1365-2559.2004.01895.x. [DOI] [PubMed] [Google Scholar]
- 9.Oh YL, Kim KR. Micropapillary variant of transitional cell carcinoma of the ureter. Pathol Int. 2000;50:52–6. doi: 10.1046/j.1440-1827.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Beltran A, Montironi R, Blanca A. et al. Invasive micropapillary urothelial carcinoma of the bladder. Hum Pathol. 2010;41:1159–64. doi: 10.1016/j.humpath.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Heudel P, El Karak F, Ismaili N. et al. Micropapillary bladder cancer: a review of Leon Berard Cancer Center experience. BMC Urol. 2009;9:5. doi: 10.1186/1471-2490-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoneim IA, Miocinovic R, Stephenson AJ. et al. Neoadjuvant systemic therapy or early cystectomy? Single-center analysis of outcomes after therapy for patients with clinically localized micropapillary urothelial carcinoma of the bladder. Urology. 2011;77:867–70. doi: 10.1016/j.urology.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 13.Montie JE, Bahnson RR, Cohen SM. et al. Bladder cancer. Clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2005;3:4–5. 19-34. [PubMed] [Google Scholar]
- 14.Shapur NK, Katz R, Pode D. et al. Is radical cystectomy mandatory in every patient with variant histology of bladder cancer. Rare Tumors. 2011;3:e22. doi: 10.4081/rt.2011.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perepletchikov AM, Parwani AV. Micropapillary urothelial carcinoma: clinico-pathologic review. Pathol Res Pract. 2009;205:807–10. doi: 10.1016/j.prp.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Maranchie JK, Bouyounes BT, Zhang PL. et al. Clinical and pathological characteristics of micropapillary transitional cell carcinoma: a highly aggressive variant. J Urol. 2000;163:748–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1-3.