Abstract
Galectins, a family of glycan-binding proteins, can control tumor progression by promoting transformation, angiogenesis and immune escape. We identified a dynamically regulated ‘galectin signature’, which delineates the progression of prostate cancer, highlighting galectin-1 as an attractive target for anti-angiogenic therapy in advanced stages of the disease.
Keywords: angiogenesis, galectins, gene signature, metastasis, prostate cancer
Galectins, a family of evolutionarily-conserved glycan-binding proteins, can influence tumor progression by mediating communication among tumor, stromal, endothelial and immune cells. These lectins are defined by a consensus sequence of approximately 130 amino acids within the carbohydrate-recognition domain (CRD), which mediates their specific interaction with N-acetyllactosamine [Galβ(1–4)-GlcNAc]-enriched glycoconjugates.1 Within the intracellular compartment, galectins can modulate a variety of signaling processes.1 However, these endogenous lectins can also be secreted in the extracellular milieu through a non-classical pathway, where they cross-link specific glycoconjugates and modulate a variety of cellular processes including proliferation, differentiation and apoptosis.1 A number of studies in preclinical models and cancer patients have documented a significant association between the expression of galectins and the aggressiveness of multiple tumor types. In most settings, galectin expression is associated with poor clinical outcome.1 Of note, whereas 15 galectins have been identified so far in a diversity of tissues and species, most studies have focused on galectin-1 and galectin-3.1
We and others have identified a critical role for galectin-1 in the evasion of tumor cells from immune responses in different types of cancer, including melanoma, Hodgkin’s lymphoma, lung carcinoma and neuroblastoma.1-5 The analysis of the mechanisms underlying these effects revealed the ability of galectin-1 to modulate T-cell survival, dendritic-cell immunogenicity and regulatory T-cell function.1-5 In addition, galectin-1 is strongly upregulated by hypoxia and has been shown to promote angiogenesis in different tumor types, including melanoma and Kaposi’s sarcoma.6-8 However, despite considerable progress in the understanding of the roles of individual galectins in tumor biology, an integrated view of the galectin network in the tumor microenvironment, including their regulation and coordinated function is still lacking. In an attempt to fill this gap, we conducted a study to delineate the ‘galectin signature’ of the human prostate cancer (PCa) microenvironment with the overarching goal of selecting novel molecular targets for prognostic and therapeutic purposes.9
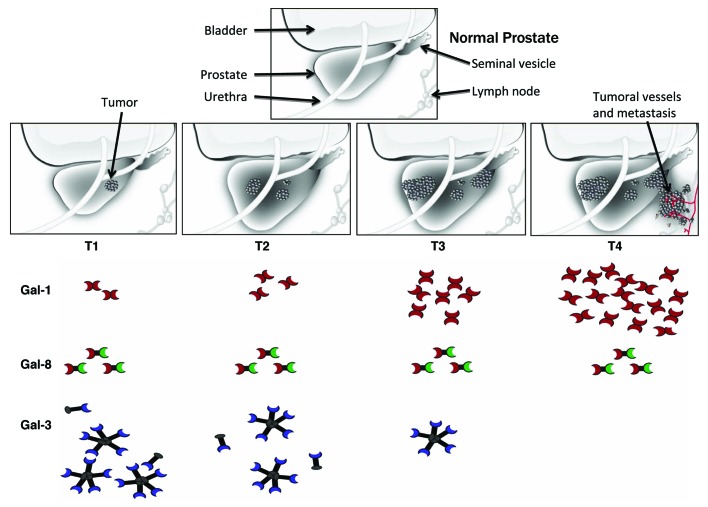
The analysis of the galectin profile in prostatectomies from a cohort of therapy-naïve patients demonstrated that galectin-1 is the most abundantly expressed galectin in this setting and is the only member of the family that is substantially upregulated during PCa progression. A similar profile was observed in representative PCa cell lines, at both the mRNA and protein levels. All other galectin family members are expressed at comparatively lower levels. While galectin-3, -4, -9 and -12 are downregulated in the course of the disease, the expression of galectin-8 does remain unaltered during disease progression. The selective upregulation of galectin-1 prompted us to investigate the function of this lectin in the PCa microenvironment. As galectin-1-N-glycan interactions can link tumor hypoxia to angiogenesis in Kaposi’s sarcoma,8 we examined whether this lectin plays any role in PCa angiogenesis. In tissue arrays from PCa patients, elevated expression levels of galectin-1 correlated with increased number of blood vessels. This positive correlation was even more pronounced during advanced stages of the disease. However, no significant correlation was found in human breast cancer tissue arrays, suggesting a tissue-specific pro-angiogenic effect of this lectin.9
Given the promising therapeutic value of anti-angiogenic regimens for advanced castration-resistant PCa,10 we examined the effects of targeting galectin-1 during PCa angiogenesis. In vitro, the blockade of galectin-1 using a newly-developed neutralizing monoclonal antibody prevented the morphogenesis of endothelial cells as induced by human PCa cell lines. Furthermore, the inhibition of galectin-1 expression in vivo by means of lentiviral-transduced short-hairpin RNAs (shRNAs) or upon injection of the galectin-1 neutralizing antibody prevented angiogenesis as induced by Matrigel-embedded PCa cells. These results demonstrate that silencing galectin-1 in PCa cells is sufficient to prevent angiogenesis, as tumor cells transduced with a galectin-1-specific shRNA almost completely failed to form blood vessels even in the presence of the host-derived lectin. Interestingly, the pro-angiogenic effects of galectin-1 were independent of the expression of other angiogenesis-related factors, suggesting a pivotal role for this lectin in tumor neovascularization.
Collectively, our findings identify a dynamically regulated ‘galectin signature’, which accompanies the evolution of PCa, highlighting a major role for galectin-1 as a target for anti-angiogenic therapy in castration-resistant advanced stages of the disease. Similar to recent findings in melanoma6,7 and Kaposi’s sarcoma,8 we found that galectin-1 is a pivotal stimulator of PCa neovascularization independently of other angiogenesis-related molecules,9 and demonstrated that tumor cells are the primary source of this pro-angiogenic lectin. As many cancers are refractory to conventional chemotherapeutic, anti-angiogenic and immunotherapeutic agents, our results suggest an alternative strategy to suppress tumor growth and prevent tumor metastasis. Importantly, the blockade of galectin-1 may serve not only to prevent tumor angiogenesis, but also to stimulate T cell-mediated immunity and abrogate tumor-cell invasiveness and migration, as previously demonstrated in other tumor types2 (Fig. 1).
Figure 1. A unique ‘galectin signature’ delineates human prostate cancer progression. The analysis of the galectin profile in a cohort of therapy-naïve prostatectomies demonstrated that galectin (Gal)-1 is the most abundant galectin in this setting and the only member of the family that is substantially upregulated during prostate cancer (PCa) progression. While Gal-3, -4, -9 and -12 are downregulated in the course of the disease, Gal-8 expression levels remain virtually unaltered. Targeting Gal-1 suppresses PCa angiogenesis, suggesting a novel molecular target for the therapy of castration-resistant advanced stages of the disease. T1, T2, T3 and T4 indicate different stages of PCa evolution.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23565
References
- 1.Rabinovich GA, Croci DO. Regulatory circuits mediated by lectin-glycan interactions in autoimmunity and cancer. Immunity. 2012;36:322–35. doi: 10.1016/j.immuni.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Rubinstein N, Alvarez M, Zwirner NW, Toscano MA, Ilarregui JM, Bravo A, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5:241–51. doi: 10.1016/S1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 3.Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, et al. The AP1-dependent secretion of galectin-1 by Reed Sternberg cells fosters immune privilege in classical Hodgkin lymphoma. Proc Natl Acad Sci U S A. 2007;104:13134–9. doi: 10.1073/pnas.0706017104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soldati R, Berger E, Zenclussen AC, Jorch G, Lode HN, Salatino M, et al. Neuroblastoma triggers an immunoevasive program involving galectin-1-dependent modulation of T cell and dendritic cell compartments. Int J Cancer. 2012;131:1131–41. doi: 10.1002/ijc.26498. [DOI] [PubMed] [Google Scholar]
- 5.Banh A, Zhang J, Cao H, Bouley DM, Kwok S, Kong C, et al. Tumor galectin-1 mediates tumor growth and metastasis through regulation of T-cell apoptosis. Cancer Res. 2011;71:4423–31. doi: 10.1158/0008-5472.CAN-10-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thijssen VL, Barkan B, Shoji H, Aries IM, Mathieu V, Deltour L, et al. Tumor cells secrete galectin-1 to enhance endothelial cell activity. Cancer Res. 2010;70:6216–24. doi: 10.1158/0008-5472.CAN-09-4150. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu V, de Lassalle EM, Toelen J, Mohr T, Bellahcène A, Van Goietsenoven G, et al. Galectin-1 in melanoma biology and related neo-angiogenesis processes. J Invest Dermatol. 2012;132:2245–54. doi: 10.1038/jid.2012.142. [DOI] [PubMed] [Google Scholar]
- 8.Croci DO, Salatino M, Rubinstein N, Cerliani JP, Cavallin LE, Leung HJ, et al. Disrupting galectin-1 interactions with N-glycans suppresses hypoxia-driven angiogenesis and tumorigenesis in Kaposi’s sarcoma. J Exp Med. 2012;209:1985–2000. doi: 10.1084/jem.20111665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laderach DJ, Gentilini LD, Giribaldi L, Delgado VC, Nugnes L, Croci DO, et al. A unique galectin signature in human prostate cancer progression suggests galectin-1 as a key target for treatment of advanced disease. Cancer Res. 2013;73:86–96. doi: 10.1158/0008-5472.CAN-12-1260. [DOI] [PubMed] [Google Scholar]
- 10.Karlou M, Tzelepi V, Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat Rev Urol. 2010;7:494–509. doi: 10.1038/nrurol.2010.134. [DOI] [PubMed] [Google Scholar]