Abstract
Melanoma is a highly chemoresistant and metastatic tumor that, in the absence of BRAF mutations, is generally treated with the alkylating agent dacarbazine (DTIC). We discovered that DTIC upregulates the expression of NKG2D ligands on tumor cells, leading to the activation of natural killer (NK) and CD8+ T cells. These observations underscore the immunogenic properties of DTIC and provide a rationale to combine DTIC with immunotherapeutic agents.
Keywords: B16, NK cells, NKG2D, dacarbazine, melanoma
Melanoma is a highly metastatic and chemoresistant tumor. Recent progress in tumor genetics led to the discovery that BRAF—coding for a mitogenic serine/threonine kinase—is mutated in approximately 40% of melanoma patients, which hence can benefit from BRAF-targeted agents. When such targeted therapies cannot be employed, most patients bearing metastatic melanoma receive chemotherapeutic regimens, most often based on the alkylating agent dacarbazine (DTIC). This drug has been administrated as a first line therapy against metastatic melanoma since the 1970s, yet it promotes relatively poor response rates.1 While the cytotoxic activity of DTIC is well characterized, little information is currently available on its immunogenic properties. In this setting, we have recently evaluated the relative contribution of innate and adaptive immunity to the antineoplastic effects of DTIC in a mouse model of melanoma.2 We observed that DTIC neither inhibits immunosuppressive cells including regulatory T cells and myeloid derived suppressor cells, neither promotes the maturation of dendritic cells (DCs), nor triggers the immunogenic cell death and/or the exposure of calreticulin on the surface of tumor cells. However, in B16F10 melanoma model, the antineoplastic effects of DTIC in vivo are completely dependent on the immune system, as DTIC loses its efficacy in nude mice as well as in mice selectively depleted of CD8+ T cells, natural killer (NK) cells, or interferon γ (IFNγ).
Recent evidence demonstrates that the ligands for NKG2D, an NK-cell activating receptor, are poorly expressed on the surface of normal cells but consistently upregulated by tumor cells or virus-infected cells.3 In particular, NKG2D ligands were found to be highly expressed on the surface of some melanoma cell lines and primary lesions.4 Morevoer, both radiation5 and DNA-damaging agents6 can increase by several fold the expression of NKG2D ligands. This process is regulated by major DNA-damage checkpoint pathways such as those initiated by ataxia telangiectasia, mutated (ATM) and ATM- and Rad3-related (ATR) protein kinases.7
We discovered that DTIC is capable of triggering or enhancing the expression of NKG2D ligands on both murine and human tumor cells, thus promoting NK-cell cytotoxicity and IFNγ secretion (Fig. 1). In turn, IFNγ augments the expression of MHC class I/peptide complexes at the tumor cell surface, de facto increasing antigen presentation and facilitating the elicitation of cytotoxic T-lymphocyte responses. Thus, DTIC can be added to the expanding list of chemotherapeutic agents that exert antitumor effects as they promote or enhance anticancer immune responses.8
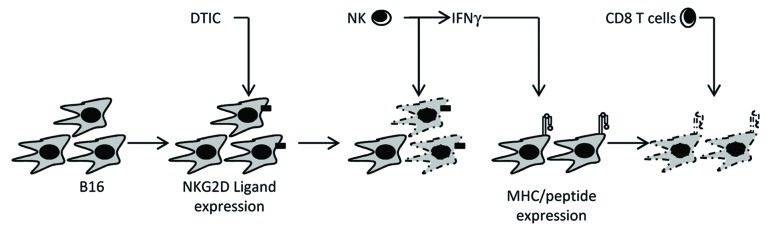
Figure 1. Dacarbazine-mediated immune antitumor effects. Based on results obtained in a subcutaneous B16F10 mouse model, we concluded that: (1) after dacarbazine (DTIC) administration, tumor cells express NKG2D ligands; (2) these ligands promote the activation of natural killer (NK) cells, resulting in cytotoxic functions and interferon γ (IFNγ) secretion; (3) IFNγ stimulates the expression of MHC class I/peptide complexes on the surface of DTIC-resistant tumor cells, whereupon tumor-specific CD8+ T cells can proceed to antigen-dependent lysis.
Melanoma is a highly immunogenic tumor and many immunotherapeutic approaches have been developed to circumvent disease progression. These strategies have obtained little success in the clinic because of their complexity, costs, toxicity and often inconsistent clinical efficacy. The recent introduction into the clinics of monoclonal antibodies that specifically target immunosuppressive T-cell receptors such as CTLA4 and PD1 has largely expanded the battery of immunotherapeutic tools that are available for cancer therapy. Indeed, blocking immunosuppression with the anti-CTLA4 antibody ipilimumab has recently been shown to improve the overall survival of patients affected metastatic melanoma who had failed first-line chemotherapy.9 Moreover, Robert et al. have recently shown that a chemoimmunotherapy protocol based on the combination of ipilimumab and DTIC is superior in terms of overall survival as compared with DTIC administered as a standalone intervention.10 While this combinatorial regimen improved response rates and survival, the clinical survival advantage was observed among a small percentage of patients. In addition, such therapeutic approach is frequently associated with immune system-related adverse events, which can be relatively severe and long lasting. Finally, ipilimumab is an expensive drug, costing around $100,000 per patient. Therefore, there is an emerging need of predictive biomarkers allowing for the identification of melanoma patients who would actually benefit from the combination ipilimumab and DTIC. In this regard, our results may be of particular importance. Indeed, we demonstrated that the combination of DTIC and anti-CTLA4 antibodies exerts synergistic antineoplastic effect against B16F10 melanomas, yet only when NK cells can be activated upon the administration of DTIC. One may wonder that some tumors may be insensitive to the DTIC mediated-upregulation of NKG2D ligands, a setting that would compromise the immunogenic effects of DTIC. Our results predict that the administration of anti-CTLA4 antibodies in such patients results in poor, if any, antitumor effects. Overall, our findings provide a rationale for determining whether NKG2D ligands can be upregulated by tumor cells and/or whether NK cells become activated upon DTIC administration, both of which may constitute predictive biomarkers of the clinical efficacy of ipilimumab.
Disclosure of Potential Conflicts of Interest
No conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/23714
References
- 1.Lui P, Cashin R, Machado M, Hemels M, Corey-Lisle PK, Einarson TR. Treatments for metastatic melanoma: synthesis of evidence from randomized trials. Cancer Treat Rev. 2007;33:665–80. doi: 10.1016/j.ctrv.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 2.Hervieu A, Rébé C, Végran F, Chalmin F, Bruchard M, Vabres P, et al. Dacarbazine-Mediated Upregulation of NKG2D Ligands on Tumor Cells Activates NK and CD8 T Cells and Restrains Melanoma Growth. J Invest Dermatol. 2013;133:499–508. doi: 10.1038/jid.2012.273. [DOI] [PubMed] [Google Scholar]
- 3.Mistry AR, O’Callaghan CA. Regulation of ligands for the activating receptor NKG2D. Immunology. 2007;121:439–47. doi: 10.1111/j.1365-2567.2007.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, et al. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res. 2009;15:5208–15. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Son YO, Park SW, Bae JH, Chung JS, Kim HH, et al. Increase of NKG2D ligands and sensitivity to NK cell-mediated cytotoxicity of tumor cells by heat shock and ionizing radiation. Exp Mol Med. 2006;38:474–84. doi: 10.1038/emm.2006.56. [DOI] [PubMed] [Google Scholar]
- 6.Fine JH, Chen P, Mesci A, Allan DS, Gasser S, Raulet DH, et al. Chemotherapy-induced genotoxic stress promotes sensitivity to natural killer cell cytotoxicity by enabling missing-self recognition. Cancer Res. 2010;70:7102–13. doi: 10.1158/0008-5472.CAN-10-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, et al. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113:3503–11. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 8.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 9.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robert C, Thomas L, Bondarenko I, O’Day S, M D JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]


