Abstract
Exposure to traffic-related air pollution is associated with risk of cardiovascular disease and mortality. We examined whether exposure to diesel exhaust increased blood pressure in human subjects.
We analyzed data from 45 nonsmoking subjects, age 18–49 in double-blinded, crossover exposure studies, randomized to order. Each subject was exposed to diesel exhaust, maintained at 200 μg/m3 of fine particulate matter, and filtered air for 120 minutes on days separated by at least two weeks. We measured blood pressure pre-exposure, at 30-minute intervals during exposure, and 3, 5, 7 and 24 hours from exposure initiation, and analyzed changes from pre-exposure values.
Compared with filtered air, systolic blood pressure increased at all points measured during and after diesel exhaust exposure; the mean effect peaked between 30 and 60 minutes after exposure initiation (3.8 mmHg [95% CI: −0.4, 8.0] and 5.1 mmHg [95% CI: 0.7, 9.5] respectively). Sex and metabolic syndrome did not modify this effect. Combining readings between 30 and 90 minutes, diesel exhaust exposure resulted in a 4.4 mmHg increase in systolic blood pressure, adjusted for participant characteristics and exposure perception (95% CI: 1.1, 7.7, p=0.0009). There was no significant effect on heart rate or diastolic pressure.
Diesel exhaust inhalation was associated with a rapid, measurable increase in systolic, but not diastolic, blood pressure in young nonsmokers, independent of perception of exposure. This controlled trial in humans confirms findings from observational studies. The effect may be important on a population basis given the worldwide prevalence of exposure to traffic-related air pollution.
Keywords: Air pollution, diesel exhaust, cardiovascular, blood pressure, autonomic nervous system
INTRODUCTION
The relationship between exposure to air pollution, specifically combustion-generated and traffic-related fine particulate matter (PM2.5), and increased risk for cardiovascular disease is increasingly consistent and well supported. Population-based effects have been observed based on increased short-term (e.g. hourly or daily) and long-term (e.g. annual average) exposure levels. The physiological pathway(s) through which exposure induces such effects remain uncertain. Experimental research suggests the process might involve several pathways, including a shift in autonomic balance, systemic inflammatory response mechanisms, and prolonged endothelial dysfunction.1
As diesel exhaust (DE) is the dominant source of urban PM2.52, we use DE inhalation as a model for traffic-based PM2.5 exposure. Based on the hypothesis that alterations in blood pressure are a mode of action through which inhaled pollutants can increase both acute and chronic risk of cardiovascular events, we examined the systemic blood pressure response in volunteers exposed to both DE and filtered air (FA) in an experimental setting.
MATERIALS AND METHODS
A total of 49 adult subjects participated in four exposure studies conducted at the University of Washington DISCOVER Center on Cardiovascular Disease and Traffic-Related Air Pollution. Experiments used the same protocol and equipment, and data were pooled for analysis.3
Subjects were non-smokers, not regularly exposed to second-hand smoke, and not taking anti-hypertensive medication. Thirty-two volunteers were defined as healthy, with no history or evidence of hypertension, asthma, diabetes, hypercholesterolemia, cardiovascular illness, or other chronic medical condition, based on questionnaire, spirometry, fasting glucose and lipid panel, and electrocardiogram. Additional qualifications included body mass index (BMI) <30 kg/m2, fasting blood sugar (glucose) <126 mg/dL, and blood pressure (BP) <130/85 mmHg. Seventeen subjects were adults with Metabolic Syndrome disease (MeS), defined as meeting three of the following conditions: waist circumference ≥102 cm in males and ≥88 cm in females; triglycerides ≥150 mg/dL; HDL cholesterol <40 mg/dL in males and <50 mg/dL in females; systolic blood pressure (SBP) ≥130 mmHg or diastolic blood pressure (DBP) ≥85 mmHg; or fasting glucose ≥100 mg/dL. Age, sex, race/ethnicity, smoking, and medication use were self-reported. Urinary cotinine was measured in samples collected at the first visit to confirm non-smoking status using the CAS-COT kit (Innovacon, Inc.; San Diego, CA). We excluded self-reported smokers or participants with cotinine greater than 200 ng/mL. One volunteer was excused from participating in the study based on cotinine screening, and three were excluded from data analysis after completing all exposures.
Exposures were double-blind, crossover, and randomized to order, with sessions separated by a minimum two-week washout period to eliminate carry-over effects. All exposures and assessments were conducted following the same daily schedule to limit variation between sessions. Women were only exposed during the first two weeks of a menstrual cycle, and pregnancy was ruled out by a urine pregnancy test prior to each exposure. Subjects were instructed to fast for a minimum of eight hours prior to the exposure session. Baseline BP and vital signs were taken shortly after subject arrival, at approximately 7:30 am.
Each subject was exposed on separate days to each condition, DE or FA, for 120 minutes. Each 120-minute exposure began at approximately 8:30 am. Resting BP and heart rate (HR) measurements were taken during exposure (at 5, 30, 60, 90 and 110 minutes from exposure start), and 3, 5, 7 and 24 hours post exposure, using an automated digital oscillometric monitor (Welch-Allyn Atlas 300 or 52000, Skaneateles Falls, NY; Omron HEM-705CP, Vernon Hills, IL), with cuff placed on the left upper arm. Subjects were resting and seated during the exposure period and post-exposure. For each participant, a consistent position and measurement device was used for all measurements, with a single measurement taken at each time point. Following exposure, subjects rested at the Clinical Research Center (CRC) at the University of Washington Medical Center until release, at least 8 hours post exposure start. Each subject received an identical defined composition meal between the 3 and 5 hour post-exposure measurements. Most subjects left the CRC 8 hours after exposure commencement and returned for follow-up measurements approximately 24 hours post exposure. Ten subjects with MeS participated in a more closely monitored protocol and remained at the CRC overnight and through the next day follow-up period.4
All researchers, nurses and technicians participating in the study were blinded to exposure type, with the exception of the exposure engineer. To evaluate blinding adequacy, subjects were asked during exposure to estimate the level of DE in the chamber (as high, medium, or none). We considered this perception of exposure in our analysis. The Human Subjects Division of the University of Washington approved subject consent forms and the study protocol.
Exposure System
As previously described,5 DE was generated using a 2002 model turbocharged direct-injection 5.9-L Cummins B-series engine in a 100 kW generator set, running at steady state prior to subject arrival (6BT5.9G6; Cummins, Inc., Columbus, IN). Load was maintained at 75% of rated capacity, using a load-adjusting load bank (Simplex, Springfield, IL), no. 2 undyed on-highway low sulfur diesel fuel and Valvoline 15W-40 crankcase oil. Emission dilution was completed in two phases, with final PM2.5 concentration maintained at 200 μg/m3 in the breathing zone (average 205.4 μg/m3; standard deviation 5.4 μg/m3). To ensure exposure levels are stable, PM2.5 concentrations were assessed in real-time using a tapered element oscillating microbalance (1400a PM2.5, Rupprecht & Patashnick Co., Albany, NY) and adjusted continuously with a feedback control system based on nephelometry measurements. The 116 m3 exposure room was maintained at a temperature of 20 to 21°C with 50% relative humidity. Based on multistage impactor-collected samples, the facility’s DE particle mass median diameter was 0.080 μm. Typical particle count per cm3 for our exposure scenario was 2.8 × 103 for FA and 5.3 × 104 for DE exposures, based on one-minute averages. Nephelometers positioned within the room are used to confirm spatial uniformity of particle concentrations. For DE exposures, average concentrations of nitrogen dioxide were 35 ppb (approximately 1.5% of total NOx). Concentrations of carbon monoxide averaged 0.30 ppm for FA and 0.80 ppm for DE. FA was ambient air passed through a carbon matrix filter and HEPA filter (99.99% efficient) and was used to dilute the exhaust in DE exposures.
Gene-Environment Interactions
To further understand potential mechanisms, we also examined whether response to DE varied by a common nucleotide variant (A1166C, rs5186) in the angiotensin II type 1 receptor (AGTR1) gene, a component of the renin-angiotensin system (RAS) intricately involved in blood pressure regulation.6,7 The analysis, conducted on this one single nucleotide polymorphism selected on an a priori basis, can be found in the online supplement (please see http://hyper.ahajournals.org).
Statistical Analysis
Statistical analysis was performed using Stata 10.0 statistical software (Stata Corp, College Station, TX) and R (www.r-project.org). Descriptive analyses, paired t-tests, nonparametric median testswere used in initial analyses, with the χ2-test used for categorical variables. BP at each time point was first baseline-corrected by subtracting the same session’s pre-exposure BP (time (t)=−60 minutes), giving change in BP measured at each recorded interval. The baseline-corrected value for the FA session was then compared to the analogous value in the DE session for a time-specific, participant-specific measure of DE effect. To estimate the effect at specific time points (and for ease of incorporation into multivariate modeling), the BP difference-indifferences (DD) was calculated for each participant i and time point t in minutes (t = 0 is the initiation of the exposure period):
in which y represents the BP measurement. At each time point, DD values from all 45 participants were used for a raw estimate of the mean DE effect, with significance evaluated via a one-sample t-test against the null hypothesis of no effect.
To pool data from different time points and allow for evaluation of potential confounders and the considerable individual variability in BP, SBP readings were analyzed in a hierarchical regression model. The DE effect was modeled as the interaction between exposure and an indicator function for mid-exposure measurements (30 – 90 minutes), and a second for post-exposure readings (180 minutes - next day) – each contrasted with the pre-exposure measurement. The model was adjusted for gender, MeS, AGTR1 genotype, perception of exposure, and an indicator for the participant’s first session exposure type. Subgroup sizes were not sufficient to examine effect modification for these variables, which in our specification is equivalent to a three-way interaction. The model was fit via linear mixed effects in the R language ‘lme4’ package, and allowed for an estimation of the mean effect while accounting for random variations in session-specific individual baselines. Seven subjects were excluded from the regression analysis, due to missing AGTR1 genotype, or missing or incompatible exposure-perception data.
RESULTS
Subject characteristics are summarized in Table 1. All baseline characteristics of healthy and MeS subjects were significantly different with the exception of sex and race/ethnicity. One MeS subject was excluded based on data collection difficulties due to body dimension, permitting complete data analysis for 45 participants.
Table 1.
Characteristics of study participants.
All values reported are mean (± SD) unless otherwise noted.
| Characteristic | Healthy | Metabolic |
|---|---|---|
| No. | 31 | 14 |
| Male, n (%) | 22 (71) | 8 (57) |
| Age, yrs | 28 ± 8.6 | 39 ± 7.3 |
| Caucasian, n (%) | 24 (77) | 10 (71) |
| Body mass index | 23 ± 2.1 | 39 ± 6.9 |
| SBP, mmHg | 112 ± 16.1 | 130 ± 14.5 |
| DBP, mmHg | 67 ± 10.6 | 77 ± 8.3 |
| Heart Rate, bpm | 64 ± 9.6 | 77 ± 8.5 |
| Total cholesterol, mg/dL | 158 ± 30.1 | 197 ± 33.7 |
| LDL | 96 ± 23.6 | 126 ± 31.4 |
| HDL | 48 ± 11.3 | 37 ± 5.0 |
| Triglycerides | 73 ± 42.0 | 182 ± 118 |
| Glucose, mg/dL | 90 ± 5.1 | 98 ± 9.3 |
Differences between healthy and metabolic syndrome subjects were statistically significant, with the exception of race and gender. DBP and trigylcerides p ≤ 0.01; all others p ≤ 0.001.
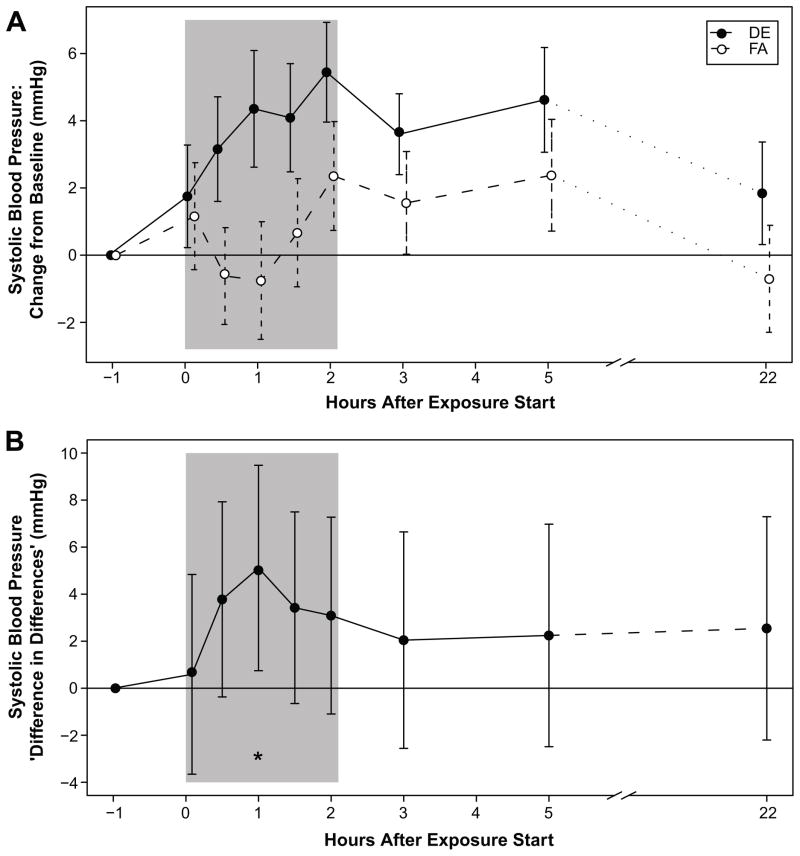
Mean effect of exposure on SBP is shown in Figure 1A, and effect attributable to DE in Figure 1B. In both healthy and MeS subjects, SBP increase (change from session baseline) with DE exposure was greater than that with exposure to FA (Figure 1A). The largest effects were 30 and 60 minutes after DE exposure commenced (3.8 mmHg [95% CI: −0.4, 8.0; p=0.08] and 5.1 mmHg [95% CI: 0.7, 9.5; p=0.02] respectively). Increases in SBP persisted following termination of DE exposure, with mean differences ranging from 2.0 mmHg to 3.5 mmHg over baseline through the following morning, approximately 24 hours later.
Figure 1.
Figure 1A. Mean Change in Systolic Blood Pressure from Baseline.
Mean difference between systolic blood pressure (SBP) at each time point and SBP pre-exposure, for diesel exhaust (DE) and filtered air (FA). Error bars represent 95% confidence intervals for the mean. The shaded area is the exposure period.
Figure 1B. Mean Diesel Exhaust Effect on Systolic Blood Pressure. Mean difference between change (from pre-exposure) in SBP with DE exposure and change in SBP with FA exposure: a measure of the DE effect on SBP. The mean effect is positive at all time points, with peak difference (5.1 mmHg [95% CI: 0.7, 9.5], p=0.02) occurring approximately 60 minutes after exposure start. Error bars represent 95% confidence intervals for the paired t-test. The shaded area is the exposure period.
In order to further investigate the strength of evidence for a DE effect, we developed the hierarchical regression model described in Methods. The mid-exposure differential DE effect (30–90 minutes, pooled) was estimated as +4.4 mmHg (95% CI: 1.1, 7.7; p=0.0009). Effect estimates for subgroups are reported in the online supplement (please see http://hyper.ahajournals.org).
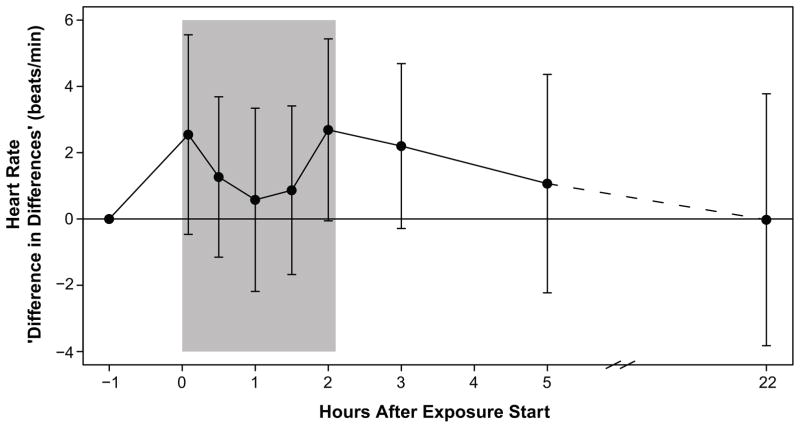
Changes in DBP were not associated with DE and did not vary widely between FA and DE exposures (Figure 2). Baseline-corrected HR was higher on average with DE than with FA exposure, however, the effect was not significant and no trend was apparent (Figure 3). Regression analysis revealed similar trends for DBP and HR: both were elevated during the exposure session, regardless of arm assignment, however, unlike SBP, the differential mid-exposure DE effect between arms was not significant for either endpoint.
Figure 2. Mean Diesel Exhaust Effect on Diastolic Blood Pressure.
Mean difference between change (from pre-exposure) in DBP with DE exposure and with FA exposure. Error bars represent 95% confidence intervals for the paired t-test. The shaded area is the exposure period.
Figure 3. Mean Diesel Exhaust Effect on Heart Rate.
Mean difference between change (from pre-exposure) in HR with DE exposure and with FA exposure. Error bars represent 95% confidence intervals for the paired t-test. The shaded area is the exposure period.
Participants correctly identified the exposure in 61% of exposure sessions, though only 34% correctly identified the exposure for both sessions, and 53% chose the same exposure level at both sessions.
DISCUSSION
The systolic blood pressure increase we detected during and after experimental DE inhalation supports earlier observational research linking air pollution, especially traffic-related air pollution, with increased blood pressure. This evidence provides insight into the key sequences underlying the acute effects of pollution-related risk for cardiovascular events. Our findings in healthy and MeS subjects suggest that the hemodynamic response to DE is rapid, moderately sustained, not related to perception of exposure, and limited to SBP.
Previous controlled human inhalation studies on air pollutants have produced somewhat inconsistent results in terms of BP outcomes, which may be due to methodological differences. Controlled exposures to concentrated ambient particles (CAPs) and ozone have been associated with acute arterial vasoconstriction and increased DBP (3 – 6 mmHg), but small or no increases in SBP.8,9 In a two-city study, Brook et al found both SBP and DBP increased with exposure to urban-sourced CAPs (likely rich in traffic-related pollutants), but only DBP increased with exposure to CAPs sourced from a suburban/rural setting.8 CAPs studies are often challenged by inconsistent exposure composition and concentrations, which can complicate physiological interpretation, unlike our DE exposures.
Congruent with our findings, Mills and colleagues reported a non-significant increase in SBP and DBP (8 mmHg and 6 mmHg, respectively) in young men two hours after controlled exposure to 300 μg/m3 DE.10 In a study of healthy adults completing a two-hour walk along a Beijing roadway, Gong et al. found exposure to ambient PM2.5 (86–140 μg/m3) amplified exercise-induced increases in SBP; on average, SBP was 7 mmHg lower when the participants wore PM-reducing masks.11 It is important to note that the BP measurements reported in both studies were taken only after, and not during, the exposure. Our results indicate that the most significant BP response to DE occurs more rapidly, within the first hour of exposure. The recording of this response during inhalation, and hence the ability to more accurately describe the time course of effects, constitutes a major strength of this study.
Physiological responses in animal models have been inconsistent, appearing to vary based on exposure method, species and genotype/phenotype. PM2.5 inhalation exposures, likely most germane to the human response to air pollutants, have been shown to synergistically enhance SBP and mean arterial pressure in both rats and mice when combined with infusion of angiotensin II (AngII).12,13 Possibly indicative of a PM2.5 – RAS interaction, a separate study reported significant increases in plasma AngII concentrations in ratsfollowing short-term inhalation of PM2.5.14
Epidemiological research has reported the most consistent associations between short-term PM2.5 exposures and SBP increase. A study of 347 adults in three Detroit neighborhoods found consistent and linear escalations in SBP associated with each 10 μg/m3 rise in ambient PM2.5, with an overall average 3.2 mmHg increase; among those not on anti-hypertensives living within the most polluted community, the corresponding increase reached 10.3 mmHg.15 Delfino and colleagues reported similar increases in both SBP (mean 8.2 mmHg) and DBP (mean 5.8 mmHg) associated with a 5.2 μg/m3 increase in ambient organic carbon, a combustion-generated component of PM2.5.16 Our results provide needed experimental confirmation to these and other observations by demonstrating in a well-controlled study that DE inhalation alone can increase SBP in young adults.
The time course of exposure-related effects has been challenging for population-based investigations. Most studies average pollutant concentrations over 24-hour periods, however actual ambient conditions often include transient spikes in pollutant levels. Peters and colleagues found that risk of onset of myocardial infarction increased 2.6–3.9-fold within one hour of exposure to urban traffic17, suggesting a more acute response than might be predicted with longer-term modeling. While we detected DE-related changes in SBP up to one day after exposure, peak increases occurred only 30 to 60 minutes from commencement of DE inhalation. This rapid change is a novel finding, supporting the hypothesis that acute exposure to PM2.5 induces dysregulation of the autonomic nervous system (ANS).18
It is plausible that PM2.5 interaction with nociceptive or noradrenergic receptors stimulate the sympathetic nervous system (SNS), either directly via vasoconstrictive effects of norepinephrine, or indirectly via RAS, raising circulating levels of the vasoconstrictor AngII. In an earlier publication we reported higher plasma concentrations of endothelin-1 (ET-1) with vasoconstriction following DE exposure; as discussed endothelium-derived ET-1 synthesis might be a secondary response to ANS activation, via catecholamine stimulation or alpha-1 vascular smooth muscle receptors.19 In addition to elevating BP, DE effects on ET-1 may further decrease blood flow to the heart, contributing to cardiacevents.20 We did not find a significant increase in HR, as might be expected with beta-adrenergic activation. We have also previously reported that we did not find evidence of changes in HRV in this experimental setting.19 This may suggest a vasoconstrictive alpha-adrenergic response without any corresponding beta-adrenergic modulation of HR and vasodilation.21
Though SBP decreased following DE exposure termination, levels remained above baseline throughout the follow-up period, up to 24 hours post-exposure. The pattern presented suggests a return to pre-exposure levels, however, we do not have data beyond our protocol’s timeframe to confirm this.
Unlike other controlled exposure studies, we did not see any change in DBP. This discrepancy may be due to dissimilarity in exposure pollutants. It is possible that distinct constituents induce particular physiologic responses; most of the studies reporting DBP increase used CAP inhalation, CAP + ozone, or ambient air monitoring. A population-based study in Taiwan found that while ambient PM was related to increases in SBP, a rise in DBP was associated with elevated ozone levels.22 Unfortunately, the wide variation in pollutant proportions and components of locally generated CAPs make it challenging to directly compare study results, or parse out from where discrepancies may stem.
Though our exposures cannot replicate the full mix and variability of actual pollutants in urban air, our DE facility allows for consistent exposures to a standardized, replicable pollutant composition and concentration levels, using a valid model traffic-related air pollutant. The crossover design and adjustment for pre-exposure BP enabled us to detect a pressure response to DE, accounting for inter- and intra-individual variability. We cannot account for all activities and exposures subjects may have had prior to exposure and between the 8 and 24-hour post-exposure measurements for those participants who left the hospital. We have no reason to believe that such activities and exposure would be systematically different between days of DE exposure or FA, and hence it is unlikely that these uncontrolled influences could bias results toward the results observed. The more sophisticated statistical modeling of our outcome also permitted us to consider a variety of potential confounding factors and take account of within-individual correlations and the risk of multiple comparisons.
We did not find a gene-environment interaction with the AGTR1 SNP selected a priori. Though subgroup sizes were not sufficient to evaluate effect modification, there was a suggestion of variation by sex, MeS, and perception. All these subgroups exhibited a positive mid-exposure DE effect estimate, in accordance with our overall findings.
Subject blinding can be of concern in this research approach. Subjects correctly identified the exposure slightly better than expected by random chance, however we did find blinding to be moderately effective; just slightly over one-third of participants correctly identified the exposure for both sessions while nearly half selected the same exposure type in both DE and FA sessions. Due to concern that perception of exposure could influence the sympathetic response and BP we adjusted for perception in our statistical modeling approach.
Our experimental model is specifically designed to reflect the effects of exposure to urban air pollution. Exposures to DE are rapidly increasing worldwide with greater urbanization and industrialization; mean 24-hour ambient and personal PM2.5 levels in Beijing were found to be 128.5 and 102.5 μg/m3 respectively, closer to our controlled exposure level than to recommended air quality standards.23 Though PM2.5 concentrations in most U.S. cities are below 35 μg/m3 on average, transient spikes in pollutant levels, and therefore exposure levels, are not uncommon. An acute rise in BP resulting from such an exposure has the potential to induce a variety of precursors to myocardial infarction including atherosclerotic plaque disruption and myocardial ischemia. In this context it is important to note that the findings we present reflect the mean SBP response to DE young, generally healthy subjects. Individual responses varied considerably, and our findings likely underrepresent the risk in susceptible populations.
This controlled study demonstrates that DE inhalation can increase BP in young adults. Transient effects detected during brief exposures may be important in conceptualizing how long-term exposures can induce proin ammatory, hypertrophic, and pro brotic phenomena leading to clinical cardiovascular disease. The findings we present may also help clarify how exposure to air pollutants can elevate risk for both acute and chronic cardiovascular outcomes.
PERSPECTIVES
DE inhalation can trigger a rapid and moderately sustained increase in SBP without accompanying changes in DBP or HR. Using a model of urban traffic-related air pollution exposure, our findings provide important insight into the timing associated with pollution-attributed vascular events. In isolation, the magnitude of the average DE effect on BP may not be clinically significant to an individual healthy adult, however, considering the prevalence of exposure, and the growing base of susceptible individuals, pollutant-related effects on population risk of cardiovascular disease may be substantial.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
This study was supported by funding from the National Institute of Environmental Health Sciences grants K24ES013195, P30ES07033, P50ES015915 and Environmental Protection Agency grants R830954 and R827355.
Footnotes
ClinicalTrials.gov identifier: NCT00434005
CONFLICT OF INTEREST / DISCLOSURE
None
References
- 1.Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong YL, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate Matter Air Pollution and Cardiovascular Disease: An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Environmental Protection Agency. Diesel Exhaust in the United States. U.S. Environmental Protection Agency, Office of Research and Development, National Center for Environmental Assessment, Washington Office; Washington, DC: 2003. EPA/420f03022. [Google Scholar]
- 3.In some experiments there were additional sessions at different exposure levels, or with additional treatments in conjunction with DE and FA; those sessions were excluded from the analysis.
- 4.Allen J, Trenga CA, Peretz A, Sullivan JH, Carlsten CC, Kaufman JD. Effect of diesel exhaust inhalation on antioxidant and oxidative stress responses in adults with metabolic syndrome. Inhal Toxicol. 2009;21:1061–1067. doi: 10.3109/08958370902721424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gould T, Stewart J, Slater D, McEwen N, Kaufman JD, Larson T. A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal Toxicol. 2008;19:49–52. doi: 10.1080/08958370701758478. [DOI] [PubMed] [Google Scholar]
- 6.Mottl AK, Shoham DA, North KE. Angiotensin II type I receptor polymorphisms and susceptibility to hypertension: A HuGE review. Genetics in Medicine. 2008;8:560–574. doi: 10.1097/gim.0b013e3181809613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchesi C, Paradis P, Schiffrin EL. Role of the renin–angiotensin system in vascular inflammation. Trends in Pharmacological Sciences. 2008;29:367–74. doi: 10.1016/j.tips.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 8.Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, Harkema J, Corey P, Silverman F, Gold DR, Wellenius G, Mittleman MA, Rajagopalan S, Brook JR. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Urch B, Silverman F, Corey P, Brook JR, Lukic KZ, Rajagopalan S, Brook RD. Acute blood pressure responses in healthy adults during controlled air pollution exposures. Environmental Health Perspect. 2005;113:1052–1055. doi: 10.1289/ehp.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills NL, Tornquvist H, Robinson SD, Gonzales M, Darnley K, MacNee W, Boon NA, Donaldson K, Bloomberg A, Sandstrom T, Newby DE. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous brinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 11.Langrish JP, Mills NL, Chan JKK, Leseman DLAC, Aitken RJ, Fokkens PHB, Cassee FR, Li J, Donaldson K, Newby DE, Jiang LX. Beneficial cardiovascular effects of reducing exposure to particulate air pollution with a simple facemask. Particle Fibre Toxicol. 2009;6:8. doi: 10.1186/1743-8977-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ying Z, Yue P, Xu X, Zhong M, Sun Q, Mikolaj M, Wang A, Brook RD, Chen CY, Rajagopalan S. Air pollution and cardiac remodeling: a role for RhoA/Rho-kinase. Am J Physiol. 2009;296:H1540–1550. doi: 10.1152/ajpheart.01270.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun Q, Yue P, Ying Z, Cardounel AJ, Brook RD, Devlin R, Hwang JS, Zweier JL, Chen LC, Rajagopalan S. Air pollution exposure potentiates hypertension through reactive oxygen species mediated activation of Rho/ROCK. Arterioscler Thromb Vasc Biol. 2008;28:1760–1766. doi: 10.1161/ATVBAHA.108.166967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghelfi E, Wellenius GA, Lawrence J, Millet E, Gonzalez-Flecha B. Cardiac oxidative stress and dysfunction by fine concentrated ambient particles (CAPs) are mediated by angiotensin-II. Inhalation Toxicology. 2010;22:963–972. doi: 10.3109/08958378.2010.503322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dvonch JT, Kannan S, Schulz AJ, Keeler GJ, Mentz G, House J, Benjamin A, Max P, Bard RL, Brook RD. Acute Effects of Ambient Particulate Matter on Blood Pressure. Hypertension. 2009;53:853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delfino RJ, Tjoa T, Gillen DL, Staimer N, Polidori A, Arhami M, Jamner L, Sioutas C, Longhurst J. Traffic-related Air Pollution and Blood Pressure in Elderly Subjects With Coronary Artery Disease. Epidemiology. 2010;21:396–404. doi: 10.1097/EDE.0b013e3181d5e19b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peters A, von Klot S, Heier M, Trentinaglia I, Hormann A, Wichmann HE, Lowel H. Exposure to Traffic and the Onset of Myocardial Infarction. N Engl J Med. 2004;351:1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 18.Ibald-Mulli A, Stieber J, Wichmann HE, Koenig W, Peters A. Effects of air pollution on blood pressure: a population-based approach. Am J Public Health. 2001;91:571–577. doi: 10.2105/ajph.91.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peretz A, Sullivan JH, Leotta DF, Trenga CA, Sands FN, Allen J, Carlsten C, Wilkinson CW, Gill EA, Kaufman JD. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116:937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherng TW, Campen MJ, Knuckles TL, Gonzalez Bosc L, Kanagy NL. Impairment of coronary endothelial cell endothelin β receptor function after short-term inhalation exposure to whole diesel emissions. Am J Physiol Regul Integr Comp Physiol. 2009;297:R640–R64. doi: 10.1152/ajpregu.90899.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. Journal of the American College of Cardiology. 2008;51:1725–1733. doi: 10.1016/j.jacc.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Chuang K, Yan Y, Cheng T. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. Journal of Occupational & Environmental Medicine. 2010;52:258–262. doi: 10.1097/JOM.0b013e3181ceff7a. [DOI] [PubMed] [Google Scholar]
- 23.Du XA, Kong QA, Ge WH, Zhang SJ, Fu LX. Characterization of personal exposure concentration of fine particles for adults and children exposed to high ambient concentrations in Beijing, China. Journal of Environmental Sciences –China. 2010;22:1757–1764. doi: 10.1016/s1001-0742(09)60316-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.