Abstract
Background
Cancer survivors represent a growing population, heterogeneous in their need for medical care, psychosocial support, and practical assistance. To inform survivorship research and practice, this manuscript will describe the prevalent population of cancer survivors in terms of overall numbers and prevalence by cancer site and time since diagnosis.
Methods
Incidence and survival data from 1975–2007 were obtained from the Surveillance, Epidemiology, and End Results Program and population projections from the United States (US) Census Bureau. Cancer prevalence for 2012 and beyond was estimated using the Prevalence Incidence Approach Model, assuming constant future incidence and survival trends but dynamic projections of the US population.
Results
As of January 1, 2012, approximately 13.7 million cancer survivors were living in the US with prevalence projected to approach 18 million by 2022. Sixty-four percent of this population have survived ≥ 5 years; 40% have survived ≥ 10 years; and 15% have survived ≥ 20 years after diagnosis. Over the next decade, the number of people who have lived ≥ 5 years after their cancer diagnosis is projected to increase approximately 37% to 11.9 million.
Conclusions
A coordinated agenda for research and practice is needed to address cancer survivors’ long-term medical, psychosocial, and practical needs across the survivorship trajectory.
Impact
Prevalence estimates for cancer survivors across the survivorship trajectory will inform the national research agenda as well as future projections about the health service needs of this population.
Introduction
The number of people diagnosed with cancer during their lifetime has been steadily increasing. This trend is due to two complementary phenomena. The first is an improvement in cancer survival rates, driven by advances in early detection and improvements in cancer treatment (1). The second, and most significant, is the aging of the population (2). Because cancer incidence rates tend to increase with age, an aging population results in progressively more people being diagnosed with cancer. It is estimated that two thirds of all cancer survivors will be age 65 or older by 2020 (3). The growing population of cancer survivors will put pressure on a healthcare system in which cancer drug shortages are increasingly common and the demand for oncology services is poised to outpace the supply of oncologists (4–6). The growing number of older survivors also presents a unique challenge to the healthcare system because older cancer survivors are more likely to have multiple chronic diseases and tend to experience poorer physical functioning than younger survivors (7–8).
As the number of cancer survivors increases so does the cost of cancer care. By 2020, it is estimated that population growth alone will escalate annual cost of cancer care by 27% (9). Health care costs in the first year first year after a cancer diagnosis tend to be higher than annual costs thereafter for survivors who are not in their last year of life (9–10). Nevertheless, among survivors who are more than one year post-diagnosis, annual healthcare expenditures are double that of the general population, suggesting that the economic burden of cancer in terms of medical expeditures is both considerable and persistent (11).
The Survivorship Trajectory: Treatment Pathways and Patients' Experiences
Cancer survivors' need for medical and supportive care services evolve from the point of diagnosis forward. In general, primary treatment ensues shortly after diagnosis with therapy typically consisting of surgery, radiation and/or chemotherapy. Cancer and its treatment can precipitate a range of physical and psychosocial sequelae that may require additional care; most commonly, functional limitations, fatigue, pain, cognitive changes, body image concerns, sexual dysfunction, psychosocial issues and socioeconomic problems (12–16). The period immediately following the end of treatment presents another set of challenges as cancer survivors experience changes in the frequency of contact with their healthcare team, manage the lingering side effects of treatment, and resume important social roles and activities---all of which can precipitate feelings of distress (17–19). Following primary treatment, survivors' health service needs will vary depending on their clinical trajectory and treatment toxicities, which can vary substantially. Although some survivors will require no additional treatment others will undergo maintenance therapy (e.g., hormonal therapy) to lower their risk of recurrence. Survivors diagnosed with advanced or metastatic disease may receive ongoing treatment in an effort to slow or halt the progression of their disease or to palliate symptoms. In many cases, the acute effects of treatment begin to subside as time since diagnosis increases (15, 20–21); however, a proportion of survivors will experience lingering side effects of their treatment that can persist for many years, negatively affecting quality of life (14–16, 21–23). Cancer treatment can also precipitate health problems that arise months or years after the end of active treatment. These late effects include second malignancies, disorders of the cardiovascular, genito-urinary or gastrointestinal systems, gonadal toxicity and endocrine disorders, compromised pulmonary function, and neurological and neurophysiological sequelae (24–25). Data from the National Health Interview Survey, in which over 60% of cancer survivors were five or more years post-diagnosis, suggested that approximately 30% of survivors were in fair or poor health, 17% were unable to work due to health problems, and 58% had one or more functional limitation (26). Across each of these outcomes, cancer survivors had poorer health and functioning than people without a cancer history (26).
Survivors at the end of life report worse self-reported health, more activity limitations and lower values on the Health Activities and Limitations Index compared to other survivors (27). At the end of life, contact with the healthcare system becomes more frequent as survivors pursue salvage therapy, palliative care, or hospice. That over one quarter of total Medicare payments go to beneficiaries in their last year of life reflects the intensive medical care received during this period (28). In fact, healthcare costs for survivors in the last year of life are higher than costs incurred at other times following diagnosis (9).
To inform cancer survivorship research and health care practice and policy, this manuscript will describe the prevalent population of cancer survivors in terms of overall numbers and prevalence by cancer site and time since diagnosis. To estimate the extent to which the number of cancer survivors is estimated to increase over time, prevalence estimates will be projected through the year 2022.
Materials and Methods
Cancer prevalence was modeled using the Prevalence Incidence Approach Model (PIAMOD), which calculates prevalence from cancer survival, cancer incidence all-cause mortality (29–30). The method first fits parametric models to incidence and survival data. Complete prevalence is then calculated and projected as the sum of the incidence and survival models product. In this study we used the first malignant cancer by cancer site diagnosed between 1975 and 2007 from the nine oldest registries in the Surveillance, Epidemiology, and End Results (SEER) program. The sample is comprised of children and adults who have been diagnosed with a broad range of cancers. However, cases of in situ disease and basal or squamous cell skin cancers were excluded from the sample. The most recent year of available data (2008) was excluded due to anticipated undercounts because of reporting delay. Incidence counts were estimated by applying SEER incidence proportions by cancer site, sex, year, age and race to the respective United States (US) populations. US populations (1975–2009) and projections from 2010 through 2022 were obtained from the US Census Bureau. The National Center for Health Statistics US mortality data from 1969–2008 were obtained from the SEER*Stat software and projected mortality rates for 2009 to 2022 were calculated by applying the 2006–2008 mortality rates by age and sex to the respective US populations. Complete cancer prevalence by years since diagnosis, for years beyond 2007 (i.e., the last year of data) was projected assuming constant future mortality, incidence, and survival trends, but dynamic projections of the US population. The modeled prevalence estimates and projections were adjusted to match the 1/1/2009 cancer prevalence reported in SEER Cancer Statistics Review, 1975–2009 (1) that uses the prevalence data more directly and with fewer assumptions. For each cancer site and sex we calculated an adjustment by comparing the 1/1/2009 modeled prevalence with the 1/1/2009 observed prevalence (1). The cancer specific adjustments were applied to the whole series of modeled prevalence from 1975 through 2022. The PIAMOD method also provides estimates by time since diagnosis in years. We have used 0 to <1 years from diagnosis to represent survivors receiving more intensive care in the first year after diagnosis, 1 to <5 years survivors in a more intensive monitoring phase, 5 to <10 years, 10 to <15 years and 15 + years to represent long term survivors in the survivorship trajectory.
Results
The number of people currently living who have been diagnosed with cancer during their lifetime has been steadily increasing since the 1970s (Figure 1). As of January 1, 2012, there were an estimated 13.7 million people in the US who had been diagnosed with cancer (Table 1), which is approximately a 3.4 fold increase compared to 1975. Over the next decade, the number of cancer survivors is projected to increase by 31% to almost 18 million1, which represents an increase of more than 4 million survivors in 10 years.
Figure 1.
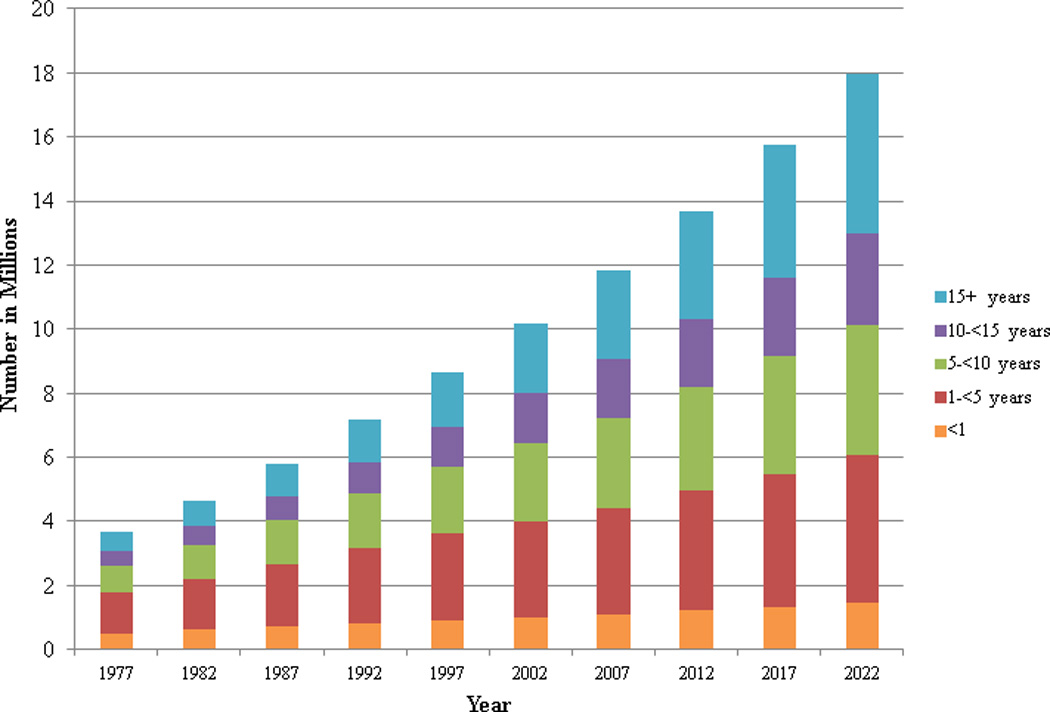
Estimated and projected number of cancer survivors in the United States from 1977–2022 by years since diagnosis.
Table 1.
Estimated number of cancer survivors by age, gender and cancer site as of January 1, 2012 (invasive cancers and 1st primary cases only)
| All ages | 0–9 | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | 70–79 | 80+ | |
|---|---|---|---|---|---|---|---|---|---|---|
| All sites | ||||||||||
| Male | 6,442,282 | 18,499 | 43,137 | 74,785 | 134,634 | 350,354 | 930,143 | 1,705,730 | 1,858,259 | 1,326,741 |
| Female | 7,241,571 | 8,636 | 36,912 | 105,114 | 250,921 | 647,838 | 1,365,042 | 1,801,428 | 1,607,632 | 1,418,047 |
| Total | 13,683,853 | 2,7135 | 80,049 | 179,899 | 385,555 | 998,192 | 2,295,185 | 3,507,158 | 3,465,891 | 2,744,788 |
| Bladder | ||||||||||
| Male | 437,179 | 15 | 112 | 590 | 2,700 | 13,220 | 49,481 | 109,759 | 141,414 | 119,888 |
| Female | 148,208 | 22 | 102 | 350 | 1,131 | 4,450 | 15,161 | 32,660 | 44,590 | 49,743 |
| Total | 585,387 | 37 | 214 | 940 | 3,831 | 17,670 | 64,642 | 142,419 | 186,004 | 169,631 |
| Breast | ||||||||||
| Male | * | * | * | * | * | * | * | * | * | * |
| Female | 2,971,606 | 0 | 27 | 2,658 | 40,168 | 226,604 | 599,361 | 808,281 | 696,389 | 598,118 |
| Total | 2,971,606 | 0 | 27 | 2,658 | 40,168 | 226,604 | 599,361 | 808,281 | 696,389 | 598,118 |
| Cervix | ||||||||||
| Male | * | * | * | * | * | * | * | * | * | * |
| Female | 245,022 | 0 | 61 | 2,333 | 13,823 | 36,239 | 56,852 | 57,009 | 42,848 | 35,856 |
| Total | 245,022 | 0 | 61 | 2,333 | 13,823 | 36,239 | 56,852 | 57,009 | 42,848 | 35,856 |
| Colorectal | ||||||||||
| Male | 595,213 | 9 | 105 | 846 | 4,744 | 23,681 | 82,493 | 158,470 | 180,450 | 144,415 |
| Female | 603,527 | 11 | 138 | 1,034 | 5,174 | 22,995 | 73,927 | 137,208 | 170,380 | 192,659 |
| Total | 1,198,740 | 20 | 243 | 1,880 | 9,918 | 46,676 | 156,421 | 295,678 | 350,830 | 337,074 |
| Oral cavity | ||||||||||
| Male | 185,242 | 54 | 274 | 1,089 | 3,874 | 15,150 | 42,487 | 57,738 | 40,628 | 23,948 |
| Female | 95,521 | 102 | 467 | 1,343 | 3,155 | 8,275 | 18,320 | 24,770 | 20,958 | 18,131 |
| Total | 280,763 | 156 | 741 | 2,432 | 7,030 | 23,425 | 60,807 | 82,508 | 61,585 | 42,079 |
| Kidney | ||||||||||
| Male | 212,996 | 1,602 | 2,344 | 2,419 | 4,149 | 15,494 | 42,469 | 62,523 | 51,422 | 30,573 |
| Female | 147,882 | 1,767 | 2,955 | 3,035 | 4,202 | 11,509 | 26,474 | 36,683 | 33,386 | 27,871 |
| Total | 360,878 | 3,368 | 5,299 | 5,455 | 8,351 | 27,003 | 68,943 | 99,206 | 84,808 | 58,444 |
| Leukemia | ||||||||||
| Male | 167,737 | 5,704 | 11,242 | 13,467 | 13,842 | 18,282 | 27,358 | 32,441 | 27,210 | 18,192 |
| Female | 130,428 | 4,977 | 9,244 | 10,910 | 110,58 | 14,044 | 19,819 | 22,489 | 19,912 | 17,975 |
| Total | 298,165 | 10,680 | 20,486 | 24,376 | 24,900 | 32,326 | 47,177 | 54,929 | 47,122 | 36,167 |
| Lung | ||||||||||
| Male | 189,077 | 0 | 6 | 76 | 746 | 5,799 | 25,758 | 57,097 | 60,794 | 38,800 |
| Female | 223,149 | 1 | 15 | 154 | 1,141 | 7,332 | 29,405 | 61,049 | 67,547 | 56,503 |
| Total | 412,226 | 2 | 21 | 230 | 1,887 | 13,131 | 55,163 | 11,8147 | 128,342 | 95,303 |
| Melanoma | ||||||||||
| Male | 481,040 | 48 | 799 | 5,367 | 18,944 | 52,796 | 105,289 | 129,682 | 102,888 | 65,227 |
| Female | 496,210 | 52 | 1,443 | 11,177 | 35,356 | 77,324 | 117,430 | 112,466 | 78,783 | 62,179 |
| Total | 977,250 | 100 | 2,242 | 16,544 | 54,300 | 130,121 | 222,719 | 242,147 | 181,672 | 127,407 |
| Ovary | ||||||||||
| Male | * | * | * | * | * | * | * | * | * | * |
| Female | 192,753 | 46 | 891 | 3,016 | 6,819 | 18,913 | 41,155 | 50,762 | 39,499 | 31,652 |
| Total | 192,753 | 46 | 891 | 3,016 | 6,819 | 18,913 | 41,155 | 50,762 | 39,499 | 31,652 |
| Prostate | ||||||||||
| Male | 2,778,626 | 0 | 0 | 11 | 770 | 23,149 | 236,096 | 783,687 | 1,005,993 | 728,921 |
| Female | * | * | * | * | * | * | * | * | * | * |
| Total | 2,778,626 | 0 | 0 | 11 | 770 | 23,149 | 236,096 | 783,687 | 1,005,993 | 728,921 |
| Corpus | ||||||||||
| Male | * | * | * | * | * | * | * | * | * | * |
| Female | 606,913 | 0 | 21 | 586 | 4,558 | 24,947 | 89,380 | 163,951 | 167,142 | 156,328 |
| Total | 606,913 | 0 | 21 | 586 | 4,558 | 24,947 | 89,380 | 163,951 | 167,142 | 156,328 |
| Hodgkin lymphoma | ||||||||||
| Male | 96,841 | 150 | 2,186 | 9,458 | 16,391 | 21,726 | 22,589 | 15,114 | 6,546 | 2,681 |
| Female | 91,751 | 65 | 1,835 | 10,195 | 17,763 | 21,359 | 19,743 | 12,081 | 5,533 | 3,177 |
| Total | 188,591 | 214 | 4,021 | 19,653 | 34,154 | 43,084 | 42,333 | 27,195 | 12,079 | 5,858 |
| Non-Hodgkin lymphoma | ||||||||||
| Male | 279495 | 648 | 2,709 | 6,117 | 12,484 | 29,062 | 58,052 | 73,431 | 59,868 | 37,125 |
| Female | 255454 | 416 | 1,927 | 4,722 | 9,068 | 20,765 | 44,767 | 62,484 | 58,686 | 52,620 |
| Total | 534949 | 1,063 | 4,636 | 10,839 | 21,552 | 49,827 | 102,819 | 135,915 | 118,553 | 89,744 |
| Thyroid | ||||||||||
| Male | 121,670 | 21 | 431 | 2,913 | 8,942 | 19,912 | 30,854 | 29,542 | 18,717 | 10,340 |
| Female | 436,590 | 39 | 1,657 | 14,742 | 44,872 | 84,921 | 108,822 | 89,072 | 54,022 | 38,443 |
| Total | 558,261 | 60 | 2,087 | 17,655 | 53,814 | 104,832 | 139,676 | 118,614 | 72,739 | 48,783 |
| Testis | ||||||||||
| Male | 230,913 | 73 | 2,210 | 16,124 | 40,917 | 61,946 | 60,052 | 33,768 | 11,767 | 4055 |
| Female | * | * | * | * | * | * | * | * | * | * |
| Total | 230,913 | 73 | 2,210 | 16,124 | 40,917 | 61,946 | 60,052 | 33,768 | 11,767 | 4,055 |
Note: Table one includes prevalence data for all cancer sites combined and data for the 16 most prevalent cancer sites.
Women with breast cancer account for 22% of survivors while men with prostate cancer account for 20% of survivors (Figure 2). Although lung cancer is the second most common cancer diagnosed in men and women, only 3% of survivors were diagnosed with lung cancer. It is estimated that 64% of cancer survivors have survived ≥ 5 years; 40% have survived ≥10 years; and 15% have survived ≥ 20 years after their diagnosis (Figure 3). Although the proportion of long term survivors is quite high overall, there is considerable heterogeneity in the proportion of long-term survivors by cancer site (Figure 4). For example, of the 245,022 cervical cancer survivors currently living in the US, 83% have lived ≥ 5 years after diagnosis. In contrast, of the 412,226 lung cancer survivors currently living in the US, only 42% have survived ≥ 5 years after diagnosis. Generally speaking, the proportion of long-term survivors for a particular cancer is both a function of the 5-year survival rates for that cancer as well as the median age at diagnosis such that the proportion of long terms survivors will be relatively high for cancers with a high 5-year survival rate but a low median age at diagnosis.
Figure 2.
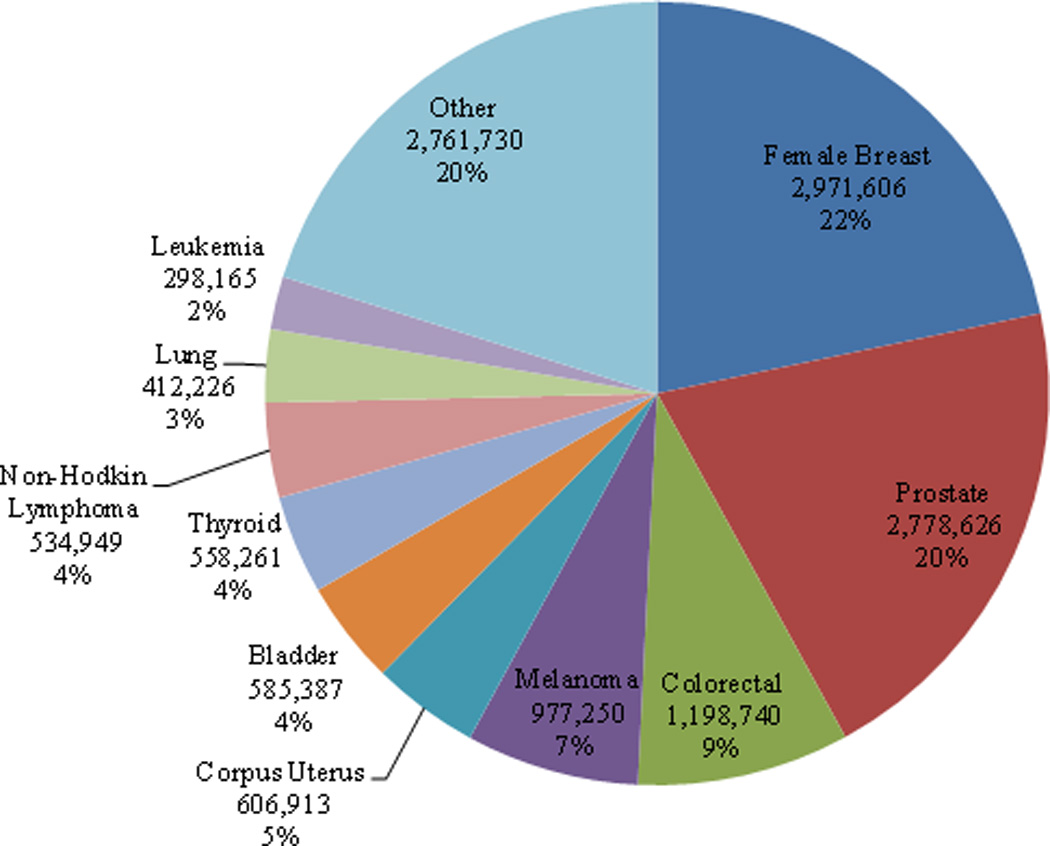
Estimated number of cancer survivors in the United States as of January 1, 2012 by cancer site.
Figure 3.
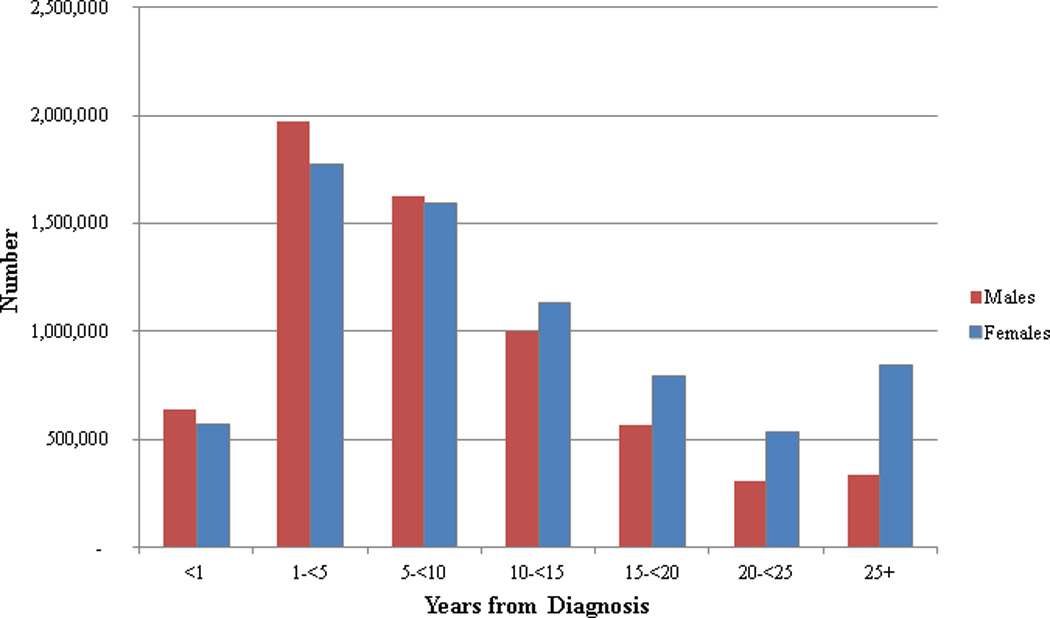
Esimated number of cancer survivors in the United States as of January 1, 2012 by time since diagnosis and sex.
Figure 4.
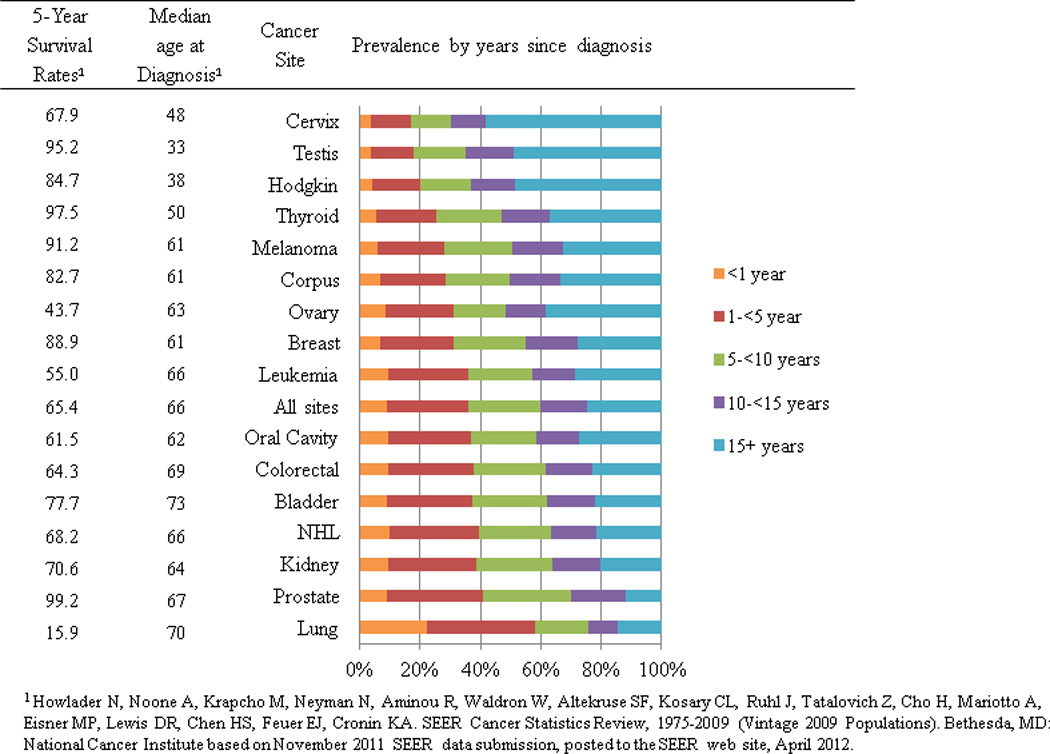
Estimated number of cancer survivors in the United States as of January 1, 2012 by cancer site and years from diagnosis.
The number of new cancer cases and the number of long-term survivors is projected to increase over the next decade (Figure 1). From 2012 to 2022 the number of survivors who are < 5 years from diagnosis will increase from 4.9 million to 6.0 million or 22%; whereas, the number of survivors who are ≥ 5 years from diagnosis will increase from 8.7 million to 11.9 million or 37%. Over the next decade, the relative proportion of survivors who are ≥5 years from diagnosis will increase from 64% to 67% with a corresponding decrease in the relative proportion of survivors who are <5 years from diagnosis. Of note, the largest relative increase in the number of cancer survivors by time since diagnosis will be among people who are 15+ years post-diagnosis where prevalence will increase from 3.4 million or 25% of all survivors in 2012 to 5.0 million or 28% of all survivors in 2022.
Discussion
Over the next decade, prevalence across the survivorship trajectory is projected to increase as a result of increasing cancer incidence rates associated with the aging of the population and improvements in long-term survival rates. Women with breast cancer and men with prostate cancer represent the two largest groups of cancer survivors due to the relatively high incidence and low mortality rates associated with these diseases. Women are over-represented among survivors who are 10+ years post diagnosis, because of the large proportion of women diagnosed with breast cancer, of which 90% will survive for five years or longer (31), as well as the longer life expectancy of women (32). Although prevalence is expected to increase across the survivorship trajectory, the largest group of survivors, both currently and moving forward, are those who are ≥ 5 years from diagnosis.
The burgeoning cohort of survivors where the absolute number of survivors is projected to increase by approximately 4.3 million survivors over the next 10 years presents a significant challenge to the health care system. This is especially true given that most survivors can now expect to live longer after diagnosis and often have complex needs stemming from chronic and late effects of treatment as well as comorbid diseases. The growth of the cancer survivor population will drive up the overall cost of cancer care an estimated 27% by the year 2020 from 2010 levels with more acute escalations in costs projected for certain subgroups of suvivors (9). Costs for prostate and breast cancer survivors in the continuing phase of care (i.e., ≥1 year post-diagnosis but >1 year from death) are projected to increase 42% and 32% respectively (9). Although annual costs for survivors in the continuing phase of care tend to be low compared to survivors who are either < 1 year post-diagnosis or in the last year of life, the large number of survivors in the continuing phase of care is a significant driver of healthcare costs.
Over the next decade, a coordinated agenda for research and practice is needed to better understand and address the medical, psychosocial, and practical needs of cancer survivor across the survivorship trajectory. To this end, we propose the following recommendations.
1. Identify effective and efficient models for delivering long-term follow up care
Clinical guidelines for survivorship care have been issued by professional societies and the Institute of Medicine (33). However, to date, guidelines pertaining to survivorship care have been largely based on consensus rather than empirical data. The formal evaluation of follow-up care models is in its infancy, and most comparative studies in this area have focused on survivors at low risk for adverse health outcomes (33). Few studies have evaluated the cost effectiveness of different approaches (33). Risk-based follow up care where the intensity of follow up care is matched to survivors’ risk for long-term and late-onset treatment effects is increasingly being explored. For example, risk-based follow up care in the context of a shared care model has been proposed for the long-term follow up of childhood cancer survivors (34–35). In addition, risk stratification of follow up care is a central component of the United Kingdom’s National Cancer Survivorship Initiative (36). However, as with other models of care, evaluation is needed to assess whether a risk-based model results in improved clinical, cost, quality of life, and satisfaction with care outcomes.
2. Develop the infrastructure to collect long-term clinical, psychosocial, and behavioral data from adult cancer survivors
To develop and evaluate models of follow up care, longitudinal data on current and emerging long-term and late effects associated with different cancer treatments are needed to characterize survivors’ level of risk across a range of psychosocial and clinical risk factors (37). Extant clinical trials, epidemiology cohorts and consortia, data from integrated Health Maintenance Organizations, and population-based studies provide a wealth of data that can be leveraged for outcomes research with cancer survivors. A report summarizing the activities of the 2011 NCI-sponsored workshop, “Utilizing Data from Cancer Survivor Cohorts: Understanding the Current State of Knowledge and Developing Future Research Priorities,” highlights examples of how each of these data sources can be used for cancer survivorship research and recommends priority directions for future research, data collection considerations, and a summary of resources needed to advance survivorship research (38). A recognized challenge of using existing data for survivorship research is that research questions are constrained by the data available, and many existing cohort studies lack detailed treatment exposure data and data on tumor pathology. Thus, the need persists for a longitudinal cohort that captures both detailed information about the tumor and treatment history, biospecimens for future molecular, genetic, and genomic research, as well as patient reported outcomes, characterizing survivors’ health behaviors and their physical, psychosocial, and practical needs after the conclusion of cancer treatment.
3. Optimize health IT and other technologies that facilitate care coordination and improve survivors’ long-term health outcomes
Over the last decade, technological advances have given rise to electronic medical records (EMRs), personal health records, real-time capture of biological and self-report data, and platforms to deliver interventions via mobile devices. Health IT in particular has the potential to improve care coordination by giving all members of the health care team access to a survivor’s health information. Recent research found that 75% of cancer survivors felt it was very important for their health providers to have access their medical information electronically—suggesting a desire for communication and coordination among their providers (39). Unfortunately, many existing electronic record systems are proprietary and only accessible to providers practicing in a given healthcare setting (40). Lack of interoperability among different EMR systems is an important barrier to delivering appropriate long-term follow up care to cancer survivors because providers outside of the cancer center (e.g., PCPs and other specialists) do not have direct access to cancer and treatment information and must rely on the survivors themselves to both possess and communicate a summary of their cancer and treatment. With the growing population of survivors, it is critical to enhance the capacity for health information exchange across health care settings. Current efforts to address this problem include the Health Information Technology for Economic and Clinical Health (HITECH) Act that was passed to incentivize the "meaningful use" of electronic health records and electronic health information exchange (41) and an alternative proposal by the President’s Council of Advisors on Science and Technology who advocate creating a universal exchange language to enable the sharing of health information across electronic record systems (40). However, the impact of these approaches is yet unknown.
Advances in mHealth represent another vehicle for connecting survivors with extensive information about their disease and function as a resource to support survivors in managing their own health (42–43). mHealth technology can also support effective symptom management by providing a way to monitor symptoms and other health problems and communicate this information to a healthcare provider (44–45). As the use of mHealth technology increases, targeted outreach may be needed to overcome the “digital divide” and avoid social disparities in access to these promising new tools (46–47). Futher, because most cancer survivors are over the age of 65 (3), it is important to improve the acceptability and usability of mHealth technology among older adults. As of 2012, only 21% of older adults used a mobile device to access the Internet and 13% owned a smart phone (48). It is possible that a combination of functional limitations (e.g., limited manual dexterity, impaired vision) and low perceived utility of mobile devices accounts for the relatively low uptake among older adults. However, efforts are needed to fully characterize and address the range of factors affecting the use of mHealth technology among this population. Efforts to evaluate the reliability and validity of new technology for all segments of the population needs to keep pace with the development of novel tools, and evaluation methods are needed to provide timely data about this rapidly evolving field (49). Ongoing efforts and partnerships especially between academic researchers, information technology companies, and cancer care specialists in diverse communities are needed to ensure that the devices, new platforms, and associated applications are based on cutting-edge biomedical and behavioral research (49).
4. Address important knowledge gaps about long-term survivors
Research is needed to better understand the needs of adult survivors who are more than 5 years post diagnosis—a group representing 64% of all cancer survivors. Most of what is known about cancer survivorship has stemmed from research conducted among breast cancer survivors. For the field of cancer survivorship to move forward, it is critical to gain a better understanding of the needs of survivors with other diagnoses and across the post-treatment trajectory. Finally, there is little research on survivors with recurrent disease or second cancers. Survivors with recurrent disease report distress, lack of energy, difficulty sleeping, pain, worry, and sexual dysfunction (50–52). However, there are few studies and fewer interventions dedicated to this clinical population.
5. Improve integrative palliative care
Research in palliative medicine has shown evidence that palliative care interventions are associated with lower costs of care, as well as increased quality of life and improved symptom management. Benefits of palliative care interventions also extend to grieving family members. In addition, recent studies provide preliminary data that early palliative care interventions increase not only quality of life, but also quantity of life (53–54). Expanding research in this area to evaluate the optimal timing of palliative care interventions, concurrent palliative and oncologic treatments, and symptom management to those living significant periods of time with advance disease (e.g., Stage IV breast cancer) could lead to both improved patient experiences in the last year of life, as well as decreased overall health care costs to patients, payors, and health systems.
Strengths and limitations
There were limitations to our analysis. Our estimates of cancer prevalence were based on cancer incidence and survival from the SEER-9 areas, which do not cover the entire US. The SEER areas had lower incidence rates than most other states and have been found to have higher socioeconomic status, greater urban population, and more specialty care than the rest of the US population. Our prevalence estimates differ somewhat from estimates used in the calculations of costs of cancer care reported elsewhere (9). In both studies, the prevalence projections were based on constant incidence and survival trends but dynamic population trends. Thus, they reflect the impact of population dynamics, especially the aging effect, on the overall burden of cancer, under currently disseminated cancer control policies and interventions. Different future assumptions of survival and incidence trends have shown to have a smaller impact on projections of the number of cancer survivors compared with the aging of the US population (9). A study of costs of cancer care [18] shows that using constant future incidence and survival we underestimate the number of cancer survivors for kidney and melanoma cancers, for which incidence has been increasing and slightly overestimate survival for cancer sites that have decreasing incidence in the most recent years, e.g., cervix, ovary, breast, prostate, colorectal and lung cancers.
Conclusions and Impact
The proportion of cancer survivors in the United States is projected to increase 31% by the year 2022. Owing to advances in early detection and cancer treatment, people are living longer after a cancer diagnosis, giving rise to a growing proportion of long term survivors. A multi-pronged approach is essential to assess the needs of cancer survivors across the survivorship trajectory. Efforts are needed to identify effective and efficient models for delivering long-term follow up care; develop infrastructure to collect long-term clinical and patient-reported outcome data from survivors; harness health IT and other technologies that facilitate care coordination and improvement in survivors’ long-term health outcomes; address important knowledge gaps about long-term survivors; and improve integrative palliative care. Progress in these areas is critical to achieving optimal clinical, cost, quality of life, and satisfaction with care outcomes.
Footnotes
The authors have no conflicts of interest to disclose.
Disclaimer: Findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the National Cancer Institute or the Patient-Centered Outcomes Research Institute.
Projected prevalence=17,981,391
References
- 1.Howlader N, Noone A, Krapcho M, Neyman N, Aminou R, Waldron W, et al. Bethesda, MD: National Cancer Institute; 2012. Apr, SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations) based on November 2011 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Vincent GK, Velkoff VA U.S. Census Bureau. The next four decades : the older population in the United States : 010 to 2050. Washington, D.C.: U.S. Dept. of Commerce, Economics and Statistics Administration, U.S. Census Bureau; 2010. [Google Scholar]
- 3.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erikson C, Salsberg E, Forte G, Bruinooge S, Goldstein M. Future supply and demand for oncologists : challenges to assuring access to oncology services. J Oncol Pract. 2007;3:79–86. doi: 10.1200/JOP.0723601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabner BA. Drug shortages--a critical challenge for the generic-drug market. N Engl J Med. 2011;365:2147–2149. doi: 10.1056/NEJMp1112633. [DOI] [PubMed] [Google Scholar]
- 6.Gatesman ML, Smith TJ. The shortage of essential chemotherapy drugs in the United States. N Engl J Med. 2011;365:1653–1655. doi: 10.1056/NEJMp1109772. [DOI] [PubMed] [Google Scholar]
- 7.Bellizzi KM, Mustian KM, Palesh OG, Diefenbach M. Cancer survivorship and aging : moving the science forward. Cancer. 2008;113:3530–3539. doi: 10.1002/cncr.23942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Avis NE, Deimling GT. Cancer survivorship and aging. Cancer. 2008;113:3519–3529. doi: 10.1002/cncr.23941. [DOI] [PubMed] [Google Scholar]
- 9.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States : 2010–2020. J Natl Cancer Inst. 2011;103:117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, et al. Cost of care for elderly cancer patients in the United States. J Natl Cancer Inst. 2008;100:630–641. doi: 10.1093/jnci/djn103. [DOI] [PubMed] [Google Scholar]
- 11.Short PF, Moran JR, Punekar R. Medical expenditures of adult cancer survivors aged <65 years in the United States. Cancer. 2011;117:2791–2800. doi: 10.1002/cncr.25835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeve BB, Potosky AL, Smith AW, Han PK, Hays RD, Davis WW, et al. Impact of cancer on health-related quality of life of older Americans. J Natl Cancer Inst. 2009;101:860–868. doi: 10.1093/jnci/djp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alfano CM, Rowland JH. Recovery issues in cancer survivorship: a new challenge for supportive care. Cancer J. 2006;12:432–443. doi: 10.1097/00130404-200609000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Polsky D, Doshi JA, Marcus S, Oslin D, Rothbard A, Thomas N, et al. Long-term risk for depressive symptoms after a medical diagnosis. Arch Intern Med. 2005;165:1260–1266. doi: 10.1001/archinte.165.11.1260. [DOI] [PubMed] [Google Scholar]
- 15.Berger AM, Visovsky C, Hertzog M, Holtz S, Loberiza FR., Jr Usual and worst symptom severity and interference with function in breast cancer survivors. J Support Oncol. 2012;10:112–118. doi: 10.1016/j.suponc.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue: implications for breast cancer survivors. Cancer. 2012;118:2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 17.Stanton AL. What happens now? Psychosocial care for cancer survivors after medical treatment completion. J Clin Oncol. 2012;30:1215–1220. doi: 10.1200/JCO.2011.39.7406. [DOI] [PubMed] [Google Scholar]
- 18.Hewitt M, Greenfield S, Stovall E. From Cancer Patient to Cancer Survivor, Lost in Transition. Washington DC: Institute of Medicine and National Research Council. The National Academies Press; 2006. [Google Scholar]
- 19.Henselmans I, Helgeson VS, Seltman H, de Vries J, Sanderman R, Ranchor AV. Identification and prediction of distress trajectories in the first year after a breast cancer diagnosis. Health Psychol. 2010;29:160–168. doi: 10.1037/a0017806. [DOI] [PubMed] [Google Scholar]
- 20.Costanzo ES, Lutgendorf SK, Mattes ML, Trehan S, Robinson CB, Tewfik F, et al. Adjusting to life after treatment: distress and quality of life following treatment for breast cancer. Br J Cancer. 2007;97:1625–1631. doi: 10.1038/sj.bjc.6604091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michael YL, Kawachi I, Berkman LF, Holmes MD, Colditz GA. The persistent impact of breast carcinoma on functional health status: prospective evidence from the Nurses' Health Study. Cancer. 2000;89:2176–2186. doi: 10.1002/1097-0142(20001201)89:11<2176::aid-cncr5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 22.Lazovich D, Robien K, Cutler G, Virnig B, Sweeney C. Quality of life in a prospective cohort of elderly women with and without cancer. Cancer. 2009;115:4283–4297. doi: 10.1002/cncr.24580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker F, Denniston M, Haffer SC, Liberatos P. Change in health-related quality of life of newly diagnosed cancer patients, cancer survivors, and controls. Cancer. 2009;115:3024–3033. doi: 10.1002/cncr.24330. [DOI] [PubMed] [Google Scholar]
- 24.Shad A, Myers SN, Hennessy K. Late effects in cancer survivors: "the shared care model". Curr Oncol Rep. 2012;14:182–190. doi: 10.1007/s11912-012-0224-1. [DOI] [PubMed] [Google Scholar]
- 25.Fossa SD, Vassilopoulou-Sellin R, Dahl AA. Long term physical sequelae after adult-onset cancer. J Cancer Surviv. 2008;2:3–11. doi: 10.1007/s11764-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 26.Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. Journal of Gerontology. 2003;58:82–91. doi: 10.1093/gerona/58.1.m82. [DOI] [PubMed] [Google Scholar]
- 27.Yabroff KR, McNeel TS, Waldron WR, Davis WW, Brown ML, Clauser S, et al. Health limitations and quality of life associated with cancer and other chronic diseases by phase of care. Med Care. 2007;45:629–637. doi: 10.1097/MLR.0b013e318045576a. [DOI] [PubMed] [Google Scholar]
- 28.Riley GF, Lubitz JD. Long-term trends in Medicare payments in the last year of life. Health Serv Res. 2010;45:565–576. doi: 10.1111/j.1475-6773.2010.01082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verdecchia A, De Angelis G, Capocaccia R. Estimation and projections of cancer prevalence from cancer registry data. Statistics in Medicine. 2002;21:3511–3526. doi: 10.1002/sim.1304. [DOI] [PubMed] [Google Scholar]
- 30.Mariotto AB, Yabroff KR, Feuer EJ, De Angelis R, Brown M. Projecting the number of patients with colorectal carcinoma by phases of care in the US: 2000–2020. Cancer Causes Control. 2006;17:1215–1226. doi: 10.1007/s10552-006-0072-0. [DOI] [PubMed] [Google Scholar]
- 31.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 32.Minino AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 33.Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, et al. Models of care for post-treatment follow-up of adult cancer survivors: a systematic review and quality appraisal of the evidence. J Cancer Surviv. 2012 doi: 10.1007/s11764-012-0232-z. [DOI] [PubMed] [Google Scholar]
- 34.Oeffinger KC, McCabe MS. Models for delivering survivorship care. J Clin Oncol. 2006;24:5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- 35.Hudson MM, Landier W, Ganz PA. Impact of survivorship-based research on defining clinical care guidelines. Cancer Epidemiol Biomarkers Prev. 2011;20:2085–2092. doi: 10.1158/1055-9965.EPI-11-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards M, Corner J, Maher J. The National Cancer Survivorship Initiative: new and emerging evidence on the ongoing needs of cancer survivors. Br J Cancer. 2011;105(Suppl 1):S1–S4. doi: 10.1038/bjc.2011.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson EK, Rose PW, Neal RD, Hulbert-Williams N, Donnelly P, Hubbard G, et al. Personalised cancer follow-up: risk stratification, needs assessment or both? Br J Cancer. 2012;106:1–5. doi: 10.1038/bjc.2011.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elena JW, Travis LB, Simonds NI, Ambrosone CB, Ballard-Barbash R, Bhatia S, et al. Leveraging Epidemiology and Clinical Studies of Cancer Outcomes: Recommendations and Opportunities for Translational Research. JNCI. doi: 10.1093/jnci/djs473. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Beckjord EB, Rechis R, Nutt S, Shulman L, Hesse BW. What Do People Affected by Cancer Think About Electronic Health Information Exchange? Results From the 2010 LIVESTRONG Electronic Health Information Exchange Survey and the 2008 Health Information National Trends Survey. J Oncol Pract. 2011;7:237–241. doi: 10.1200/JOP.2011.000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Report to the President Realizing the Ful Potential of Health Information Technology to Improve Healthcare for Americans: The Path Forward: Executive Offfice of the President, President's Council of Advisors on Science and Technology. 2010 [Google Scholar]
- 41.Glaser J. HITECH lays the foundation for more ambitious outcomes-based reimbursement. Am J Manag Care. 2010;16:SP19–SP23. [PubMed] [Google Scholar]
- 42.Gustafson DH, Hawkins R, McTavish F, Pingree S, Chen WC, Volrathongchai K, et al. Internet-Based Interactive Support for Cancer Patients: Are Integrated Systems Better? J Commun. 2008;58:238–257. doi: 10.1111/j.1460-2466.2008.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker TB, Hawkins R, Pingree S, Roberts LJ, McDowell HE, Shaw BR, et al. Optimizing eHealth breast cancer interventions: which types of eHealth services are effective? Transl Behav Med. 2011;1:134–145. doi: 10.1007/s13142-010-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol. 2011;29:994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruland CM, Holte HH, Roislien J, Heaven C, Hamilton GA, Kristiansen J, et al. Effects of a computer-supported interactive tailored patient assessment tool on patient care, symptom distress, and patients' need for symptom management support: a randomized clinical trial. J Am Med Inform Assoc. 2010;17:403–410. doi: 10.1136/jamia.2010.005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun GH. The Digital Divide in Internet-Based Patient Education Materials. Otolaryngol Head Neck Surg. 2012 doi: 10.1177/0194599812456153. [DOI] [PubMed] [Google Scholar]
- 47.Lustria ML, Smith SA, Hinnant CC. Exploring digital divides: an examination of eHealth technology use in health information seeking, communication and personal health information management in the USA. Health Informatics J. 2011;17:224–243. doi: 10.1177/1460458211414843. [DOI] [PubMed] [Google Scholar]
- 48.Zickuhr K, Smith AB. Digital differences. Washington, D.C.: Pew Research Center; 2012. [Google Scholar]
- 49.Nilsen W, Kumar S, Shar A, Varoquiers C, Wiley T, Riley WT, et al. Advancing the science of mHealth. J Health Commun. 2012;17(Suppl 1):5–10. doi: 10.1080/10810730.2012.677394. [DOI] [PubMed] [Google Scholar]
- 50.Kenne Sarenmalm E, Ohlen J, Oden A, Gaston-Johansson F. Experience and predictors of symptoms, distress and health-related quality of life over time in postmenopausal women with recurrent breast cancer. Psychooncology. 2008;17:497–505. doi: 10.1002/pon.1258. [DOI] [PubMed] [Google Scholar]
- 51.Andrykowski MA. Physical and mental health status of survivors of multiple cancer diagnoses: Findings from the National Health Interview Survey. Cancer. 2012;118:3645–3653. doi: 10.1002/cncr.26678. [DOI] [PubMed] [Google Scholar]
- 52.Vivar CG, Canga N, Canga AD, Arantzamendi M. The psychosocial impact of recurrence on cancer survivors and family members: a narrative review. J Adv Nurs. 2009;65:724–736. doi: 10.1111/j.1365-2648.2008.04939.x. [DOI] [PubMed] [Google Scholar]
- 53.Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 54.Greer JA, Pirl WF, Jackson VA, Muzikansky A, Lennes IT, Heist RS, et al. Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non-small-cell lung cancer. J Clin Oncol. 2012;30:394–400. doi: 10.1200/JCO.2011.35.7996. [DOI] [PubMed] [Google Scholar]


