Abstract
Introduction
The opioid receptor antagonist naltrexone (NTX) reduces goal-directed alcohol drinking in rats presumably by blunting alcohol reward. However, different operant conditioning behavior can be produced by different reinforcement schedules, with goal-directed operant behavior being more sensitive to changes in reward value than less flexible, habit-associated models.
Objectives
We tested the hypothesis that NTX more effectively reduces alcohol drinking and seeking in a goal-directed than in a habit-associated operant model, and more effectively reduces alcohol versus sucrose self-administration, consistent with diminished alcohol reward.
Materials and Methods
Rats were trained to self-administer 10% alcohol or 1.5% sucrose in a lever-press task and then underwent a within-subject assessment of NTX (0.1–1mg/kg) effects on operant behavior. A fixed-ratio (FR5) reinforcement schedule was used to model goal-directed behavior and a variable-interval (VI30) schedule was used to model habitual behavior.
Results
As predicted, NTX reduced fluid deliveries earned by the FR5-alcohol group significantly more than all other groups. However, NTX reduced lever presses during self-administration sessions in VI30-trained rats without reducing earned deliveries, due to the low contingency between rate of pressing and fluid deliveries under that schedule. Interestingly, when fluid delivery was withheld (extinction), NTX reduced reward-seeking in all rats. Finally, NTX blocked reinstatement of reward-seeking upon presentation of 0.2ml alcohol or sucrose and associated cues in the FR5-trained but not VI30-trained rats.
Discussion
NTX reduced goal-directed alcohol drinking compared to other operant conditions. In addition, NTX blocked reinstatement of reward-seeking in rats trained on the goal-directed FR5 reinforcement schedule but not in rats trained on the habit-like VI30 reinforcement schedule. However, NTX also exerted nonspecific effects on reward-seeking that were revealed under low-effort contingency conditions or absence of reward. Together, the data support the hypothesis that NTX is less effective in conditioning models that are more habit-associated.
Keywords: naltrexone, alcohol, goal directed, habit, extinction
Introduction
The endogenous opioid system has been implicated to mediate reward in alcohol addiction (Herz, 1997). Blockade of μ- or δ-opioid receptors reduces alcohol drinking in rats (Froehlich et al., 1991; Hyytia, 1993), and mice lacking μ-opioid receptors self-administer less alcohol than wildtypes (Roberts et al., 2000). In contrast, morphine, an opioid agonist, increases alcohol drinking in rats (Volpicelli et al., 1991). Consistent with opioid involvement in alcohol drinking, the opioid-receptor antagonist naltrexone (NTX) is an approved pharmacotherapy for alcohol use disorders (AUD; Johnson, 2010).
NTX appears to diminish alcohol reward and motivation in clinical settings and preclinical models (Kreek et al., 2002; Ripley and Stephens, 2011). In rats, NTX reduces alcohol seeking and drinking (Hill and Kiefer, 1997; Le et al., 1999), as well as cue- and drug-induced reinstatement of extinguished alcohol-seeking behavior (Ripley and Stephens, 2011; Shaham et al., 2003). In alcohol-dependent patients, NTX decreases relapse to heavy drinking, with greatest efficacy in non-abstinent subjects (O’Malley et al., 1992; Volpicelli et al., 1992), but these effects are not consistent (Krystal et al., 2001). While genetic factors are undoubtedly important to NTX efficacy (Johnson, 2010; Kimura and Higuchi, 2011), other individual differences may also be important. For instance, clinical evidence has been interpreted to indicate that NTX may be more effective in subjects with less severe AUD (Bogenschutz et al., 2009) and in regular versus less frequent drinkers (Ray et al., 2010). Thus, pharmacotherapy may be differentially beneficial across subjects. One potential difference among alcohol drinkers is the degree to which habit controls alcohol seeking and drinking (Yin and Knowlton, 2006). Habits are actions automatically driven by stimuli and less connected to outcome (Dickinson, 1985). In contrast, goal-directed behaviors are selected to obtain a particular outcome or reward, and the performance of goal-directed behavior is flexible in response to changing reward value. Goal-directed behavior can be modeled by training rats to self-administer alcohol on a reinforcement schedule with a high contingency between the work requirement and alcohol earned, such as a fixed ratio (FR) schedule (Samson et al., 2004; Yin and Knowlton, 2006); this schedule is sensitive to changes in reward, as the animal will vary its response in proportion to the perceived value of reward. Conversely, habitual alcohol-seeking is by definition insensitive to changes in alcohol reward (Dickinson et al., 2002; Mangieri et al., 2012). To model habitual self-administration, researchers have used overtraining of flexible schedules (extending training until goal-directed actions become habits; Adams, 1982; Corbit et al., 2012) or schedules with low work-reward contingency, such as variable interval (VI) reinforcement schedules (Mangieri et al., 2012). It stands to reason that pharmacotherapy targeting alcohol reward would be more effective in goal-directed rather than habitual drinkers. Examination of the literature shows that most preclinical studies of NTX on operant alcohol self-administration have used reinforcement schedules that are presumably goal-directed (i.e., FR schedules), although whether overtraining occurred is unknown.
This study tested the hypothesis that NTX more effectively decreases alcohol seeking and drinking in rats trained on a goal-directed schedule (FR) versus those trained on a habit-promoting schedule (VI). Additionally, we hypothesized that NTX more effectively suppresses cue/prime-induced reinstatement of alcohol seeking in goal-directed versus habit-trained rats. To test these hypotheses, we trained rats to self-administer alcohol on either an FR5 (5 presses = 1 fluid delivery) or a VI30 (a variable interval averaging ~30s must elapse before 1 press = 1 fluid delivery) reinforcement schedule, and then assessed NTX (0.1–1mg/kg) effects on lever pressing in the presence or absence of alcohol deliveries in a within-subject design. To assess the specificity of NTX for alcohol conditioning, we ran parallel experiments in rats self-administering sucrose. Finally, all rats underwent prolonged extinction training before evaluating NTX effects on renewal of lever-press behavior induced by presentation of 0.2ml reward and associated cues.
Materials and Methods
Animals
Adult, male Long-Evans rats were obtained from Harlan Laboratories (Frederick, MD). Rats were housed individually with ad libitum food and water (except as noted) on a 12-hour light/dark cycle with lights on at 07:00; experiments occurred between 09:00 and 14:00 Monday through Friday. These experiments were compliant with the NIH Guide for the Care and Use of Laboratory Animals (2010) and approved by the UNC Institutional Animal Care and Use Committee.
Self-Administration Training
Each behavioral chamber (Med Associates, St Albans, VT) had two retractable levers and two fluid cups between the levers. A cue light was above each lever, and a house light was on the opposite wall. After a 5min wait, training sessions began with the house light illuminating 30s prior to the extension of both levers into the chamber. Presses on the active lever were reinforced with 0.1mL reinforcing fluid dispensed into the cup adjacent to that lever. Fluid delivery was paired with illumination of the cue light and termination of the house light for 5s. In order to slow the press rate of FR5-trained rats to better match VI30-trained rats, both levers retracted for 5s at fluid delivery in the FR5 schedule. After each session, cups were checked to verify fluid consumption. All rats were water-restricted for the first 5 days of training to 60min post-session access; thereafter, home-cage water access was unrestricted.
10% alcohol self-administration
Goal-directed (FR5) rats were initially trained to self-administer 20% w/v sucrose on an FR1 reinforcement schedule in 30–60min sessions (Table 1). Over 20 days, alcohol was increased to 10% w/v while sucrose was eliminated. The response requirement increased to FR5 by Day 8. In this schedule, the active lever switched sides each day (Robinson and Carelli, 2008). From Day 22, sessions ended at 25 fluid deliveries or 30min. This limit was imposed to equate the number of deliveries achieved on the FR5 and VI30 schedules. Rats were maintained on 10% alcohol (7 kcal/g) for 3 weeks before NTX treatment.
Table 1.
Instrumental training schedule.
| Day a | Reinforcer Solution | Session length | Reinforcement schedule | ||
|---|---|---|---|---|---|
| Alcohol (A) | Sucrose (S) | FR5 b | VI30 c | ||
| 1 | 20% S | 20% S | 60 min | FR1 | Random deliveries |
| 2 | 20% S | 20% S | 60 min | FR1 | FR1 |
| 3 | 20% S | 20% S | 60 min | FR1 or FR3 | VI7 |
| 4 | 10% S/2.5% A | 10% S | 30 min | FR3 | VI15 |
| 5 | 10% S/2.5% A | 10% S | 30 min | FR3 | VI30 |
| 6–7 | 10% S/2.5% A | 10% S | 30 min | FR5 | VI30 |
| 8–11 | 10% S/5% A | 10% S | 30 min | FR5 | VI30 |
| 12–15 | 10% S/10% A | 10% S | 30 min | FR5 | VI30 |
| 16–17 | 5% S/10% A | 5% S | 30 min | FR5 | VI30 |
| 18–19 | 2.5% S/10% A | 2.5% S | 30 min | FR5 | VI30 |
| 20+ | 10% A | 1.5% S | 30 min or 25 deliveries | FR5 | VI30 |
Sessions run Monday – Friday
Both levers active on Days 1–3, thereafter a single lever was active, alternating daily
A single lever (left or right) was assigned for the duration of the study
Habit-like (VI30) rats were assigned an active lever (left or right) for the entire experiment (Table 1); pressing the inactive lever had no consequences. Day 1 consisted of noncontingent sucrose deliveries paired to the stimulus cue on the active-lever side. Thereafter, the reinforcement schedule progressed from FR1 to VI7 to VI15 by Day 5. Thereafter, rats received reinforcers on a VI30 schedule during a 30-min session. Alcohol and sucrose fading procedures and imposed fluid limitations matched those for the FR5 training regimen.
1.5% sucrose self-administration
Additional rats were trained to respond for sucrose on either an FR5 or VI30 reinforcement schedule (Table 1). Pilot studies determined that 1.5% w/v sucrose generated pressing rates comparable to 10% ethanol (data not shown). Rats self-administering sucrose followed the same sucrose fading and limited reinforcement schedule as alcohol-reinforced rats, except that alcohol was never introduced. Rats were maintained on 1.5% sucrose (4 kcal/g) for 3 weeks before NTX treatment.
Experimental Procedure
NTX and operant self-administration
In weeks 8 and 9, NTX (Sigma, St. Louis) effects on self-administration of alcohol or sucrose were tested on Tuesdays and Fridays in randomized order for each rat. Given previous data (e.g., Czachowski and Delory, 2009), doses of 0.1, 0.3 and 1mg/kg were compared to saline. NTX was dissolved in saline and injected subcutaneously 30min prior to the session start.
Satiety-specific devaluation
To distinguish rats exhibiting goal-directed versus habitual reward-seeking behavior, a satiety-specific devaluation test (similar to Corbit et al., 2012) was performed following a NTX-free week of operant sessions. Rats received home-cage access to either their training solution (10% ethanol or 1.5% sucrose) or a control solution (2% maltodextrin, 4 kcal/g) for 60min before the session start (counterbalanced order). Maltodextrin is readily consumed by rats (Dwyer, 2005) and controlled for home-cage drinking and stomach filling. Next, lever pressing was recorded in a 10min extinction session (no fluid deliveries or cues). Rats were trained normally for 2–3 days between the two tests.
NTX extinction probe sessions
Rats were given 0, 0.3 or 1mg/kg NTX (within-subject design, balanced order) 30min before a 10min extinction session (no fluid deliveries or cues) during which lever presses were recorded. Rats received normal sessions between test days.
Extinction training and reinstatement
Finally, rats underwent ≥12 extinction sessions (40min, no fluid deliveries or cues) prior to a cue/prime reinstatement session; no lever-press criterion was used to determine the number of extinction sessions. NTX effects on extinction and reinstatement lever-pressing were assessed on the reinstatement day. Rats were randomly assigned to receive either saline or 1mg/kg NTX 10min prior to the start of the test. The first 20min of the test was an extinction period, followed by a 20min reinstatement period (Bienkowski et al., 1999). At the 20min mark, 0.2ml of alcohol or sucrose was delivered into the cup and reinforcer-associated cues were presented every 15s until either the rat pressed the active lever or 15 presentations were made. Cue presentations (3s duration) consisted of turning the cue light on and house light off. Active lever presses during the reinstatement period triggered reward-associated cues but no fluid delivery.
Determination of Blood Ethanol Concentration (BEC)
To compare BEC after alcohol consumed on FR5- and VI30-reinforcement schedules, we trained two additional rats on the FR5-alcohol schedule and collected tail blood samples after four self-administration sessions under either FR5 or VI30 reinforcement (same session parameters as above), several days apart, in the following order: FR5, VI30, VI30, FR5. Blood was collected 30 min after session onset. BEC in each sample was determined as previously described (Stevenson et al., 2008).
Statistical Analysis
Frequency of active lever pressing was used as a behavioral measure of alcohol- and sucrose-seeking, and number of fluid deliveries indicated earned reward. Due to the 25-delivery limit, the number of deliveries earned was not normally distributed, but skewed toward 25. Thus, we calculated the linear slope of deliveries by NTX dose for each rat and ran a 1-sample t-test on data for each group to determine whether NTX significantly affected deliveries (a slope of zero = no effect). Next, to compare slopes across groups, we transformed slopes by rank before analysis with a 2-way ANOVA. The associated lever-press data were assessed with the same analyses. All other data were analyzed using ANOVA with repeated-measures (RM) and post-hoc comparisons as appropriate (Sigma Plot 11.0, Aspire Software, Ashburn, VA; PASW-SPSS Statistics 18, Chicago, IL). Data were transformed by rank before ANOVA when appropriate due to non-normal distribution or unequal variance. 3-way ANOVA were calculated with the General Linear Model with RM in SPSS and, as the assumption of sphericity was not met, multiple comparisons were made with the Greenhouse-Geisser adjustment to degrees of freedom.
Results
For this within-subject study design, group n’s were: FR5-alcohol, n=16; VI30-alcohol, n=16; FR5-sucrose, n=12; VI30-sucrose, n=15. The groups received similar numbers of fluid deliveries during the 20-day acquisition period (before sessions were limited to 25 reinforcements): FR5-Alcohol, 932±78; FR5-Sucrose, 1090±123; VI30-Alcohol, 925±37; VI30-Sucrose, 849±63 (2-way ANOVA, no significant main effects or interaction of schedule and reinforcer). Six rats did not undergo the NTX extinction probe sessions (3 FR5-alcohol, 3 VI30-alcohol) and four rats did not complete the reinstatement sessions (2 FR5-alcohol, 2 VI30-alcohol) due to incorrect extinction procedures.
NTX and Operant Self-Administration
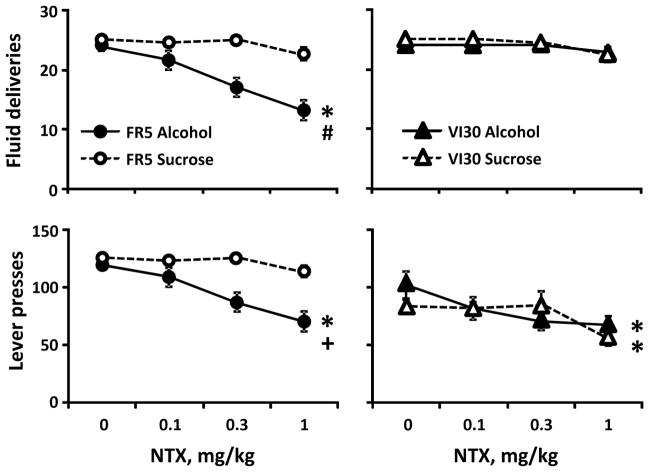
We tested NTX effects on lever pressing and fluid deliveries during alcohol and sucrose self-administration (Figure 1). We calculated the slope of deliveries by dose; a zero slope would indicate no effect of NTX. Only the FR5-alcohol slopes significantly differed from zero (FR5-alcohol: t15=−6.5, p<0.001; other group t’s=−1.1 to −2.0, p’s>0.05). Comparing slope across groups, a 2-way ANOVA yielded main effects of schedule (F1,55=10.4, p<0.01) and reinforcer solution (F1,55=5.6, p<0.05) and a significant interaction of schedule by solution (F1,55=11.5, p<0.01). Post-hoc comparisons showed that slopes of the FR5-alcohol group were steeper than those of other groups (Dunn’s test, Q=3.1 versus FR5-sucrose, Q=3.8 versus VI30-alcohol, Q=3.2 versus VI30-sucrose; all p’s<0.05), while other groups did not differ from each other. Indeed, 1mg/kg NTX reduced FR5-alcohol deliveries by 40%, while reducing FR5-sucrose or VI30 deliveries by <10%. This difference was reflected in the alcohol dose consumed in the alcohol groups (Table 2). Note that one VI30-alcohol rat left 0.9 and 0.2ml in the cup after 0.3 and 1mg/kg NTX, respectively; another VI30-alcohol rat left 0.2ml after 1mg/kg; doses were adjusted for these volumes. A 2-way ANOVA revealed main effects of reinforcement schedule (F1,120=27.54, p<0.001) and NTX dose (F3,120=7.3, p<0.001) and a significant interaction of schedule by NTX on alcohol dose (F3,120=4.3, p<0.01). NTX reduced alcohol dose only in the FR5-alcohol group (see Table 2 for posthoc comparisons).
Figure 1.
NTX effects on operant self-administration of alcohol and sucrose. Top: NTX effects on reinforcements earned in FR5-trained (left) and VI30-trained (right) rats. The slope of the line for deliveries by dose for the FR5-alcohol group was significantly lower than slopes of the other groups. Bottom: NTX effects on lever presses in FR5-trained (left) and VI30-trained (right) rats. NTX decreased the rate of pressing significantly more in the FR5-alcohol than FR5-sucrose rats, as indicated by a steeper slope. NTX also decreased responding in both VI30-trained groups. * slope different from zero, p<0.05); # slope different from all other groups, p<0.05; + slope different from FR5-sucrose, p<0.05.
Table 2.
Alcohol dose consumed during operant session following NTX treatment.
| NTXmg/kg | Alcohol consumed (g/kg) | |
|---|---|---|
| FR5 | VI30 | |
| 0 | 0.50 ± 0.02 | 0.51 ± 0.03 |
| 0.1 | 0.45 ± 0.03 | 0.51 ± 0.02 |
| 0.3 | 0.35 ± 0.03 a,b | 0.50 ± 0.02 |
| 1.0 | 0.28 ± 0.04 a,b,c | 0.49 ± 0.03 |
All values are mean ± SE.
Different from saline, p<0.05;
different from VI30, p<0.05;
different from 0.1mg/kg NTX, p<0.05.
NTX effects on lever presses (Figure 1) were also converted to slope; a zero slope would indicate no effect of NTX. Slopes of the FR5-alcohol and VI30, but not FR5-sucrose, groups significantly differed from zero (FR5-alcohol: t15=−5.6, p<0.001; FR5-sucrose: t11=−2.0, p>0.05; VI30-alcohol: t15=−2.8, p<0.05; VI30-sucrose: t14=−4.6, p<0.001). Comparing across groups, a 2-way ANOVA yielded no main effects of reinforcer schedule or solution, but a significant interaction of schedule by solution (F1,55=10.7, p<0.01). Post-hoc comparisons showed that only slopes of the FR5-alcohol and FR5-sucrose groups significantly differed (Dunn’s test, Q=3.3, p<0.05). Together, these data show that VI30-trained rats were able to earn more fluid deliveries than FR5-alcohol rats after NTX even though total responding was reduced similarly to FR5-alcohol rats.
Because we imposed a limit of 25 fluid deliveries, we examined NTX effects on patterns of operant responding (Table 3), including latency to press, median inter-reinforcement interval (IRI), first-bout length and lever-press rates. When analyzed by 3-way RM ANOVA, latency to first press did not vary by reinforcement schedule, reinforcer fluid, or NTX dose (no significant main effects or interactions).
Table 3.
Effect of NTX on timing parameters of operant self-administration of alcohol and sucrose.
| NTX mg/kg | FR5
|
VI30
|
|||
|---|---|---|---|---|---|
| Alcohol | Sucrose | Alcohol | Sucrose | ||
|
|
|
||||
| Latency to Press (s) | 0 | 6.4 ± 1.8 | 3.4 ± 1.0 | 6.0 ± 1.2 | 8.5 ±1.9 |
| 0.1 | 6.6 ± 3.3 | 5.4 ± 1.4 | 14.8 ± 7.3 | 6.3 ± 2.1 | |
| 0.3 | 4.5 ± 1.2 | 4.3 ± 1.4 | 11.5 ± 3.7 | 7.2 ± 2.3 | |
| 1.0 | 7.9 ± 2.8 | 5.2 ± 2.3 | 17.0 ± 6.8 | 10.6 ± 2.4 | |
| Median IRI (s) | 0 | 11.0 ± 0.8 | 8.6 ± 0.3 | 42.0 ± 8.4 | 35.8 ± 2.0 |
| 0.1 | 14.1 ± 1.1 | 16.5 ± 4.6 a | 45.5 ± 3.0 | 39.0 ± 1.9 | |
| 0.3 | 17.1 ± 2.0 a | 12.8 ± 1.9 | 47.4 ± 4.0 | 41.5 ± 2.8 | |
| 1.0 | 45.5 ± 13.0 a | 17.8 ± 3.4 a | 48.2 ± 4.0 | 48.1 ± 4.8 a | |
| FR5 First Bout Length (m) | 0 | 3.7 ± 0.5 b | 4.4 ± 0.3 | ||
| 0.1 | 3.5 ± 0.4 b | 4.3 ± 0.6 | |||
| 0.3 | 2.9 ± 0.4 b | 3.8 ± 0.6 | |||
| 1.0 | 2.2 ± 0.4 b, c | 3.6 ± 0.5 c | |||
| FR5 First Bout Presses | 0 | 95.1 ± 9.1 b | 121.9 ± 4.5 | ||
| 0.1 | 79.3 ± 7.6 b, c | 102.1 ± 11.8 c | |||
| 0.3 | 60.7 ± 7.8 b, c | 96.1 ± 9.7 c | |||
| 1.0 | 46.0 ± 8.8 b, c | 76.8 ± 11.2 c | |||
| VI30 Cumulative Response Slope | 0 | 0.10 ± 0.02 | 0.08 ± 0.01 | ||
| 0.1 | 0.07 ± 0.01 c | 0.07 ± 0.01 c | |||
| 0.3 | 0.06 ± 0.01 c | 0.07 ± 0.02 c | |||
| 1.0 | 0.05 ± 0.01 c | 0.05 ± 0.01 c | |||
All values are mean ± SE.
Different from saline (p<0.05).
Collapsed across NTX dose, different from FR5-sucrose group (p<0.05).
Collapsed across alcohol and sucrose groups, different from saline (p<0.05).
The median IRI reflects lever-press rate in FR5-trained rats but reflects interval contingency in VI30-trained rats, leading to longer IRIs in VI30-trained groups (Table 3). A 3-way RM ANOVA yielded a main effect of dose (F1.7,53=9.1, p<0.001) and a significant interaction of dose by reinforcer fluid by training schedule (F1.7,53=9.1, p<0.05). Subsequent analysis by group showed that all groups exhibited slower IRIs after NTX except the VI30-alcohol group (Friedman RM ANOVA on ranks, FR5-alcohol: χ23 =24.2, p<0.001; FR5-sucrose: χ23 =17.3, p<0.001; VI30-alcohol: χ23 =5.2, p>0.05; VI30-sucrose: χ23 =8.0, p<0.05). Posthoc comparisons are depicted on Table 3 (all q’s≥4, p’s≤0.05).
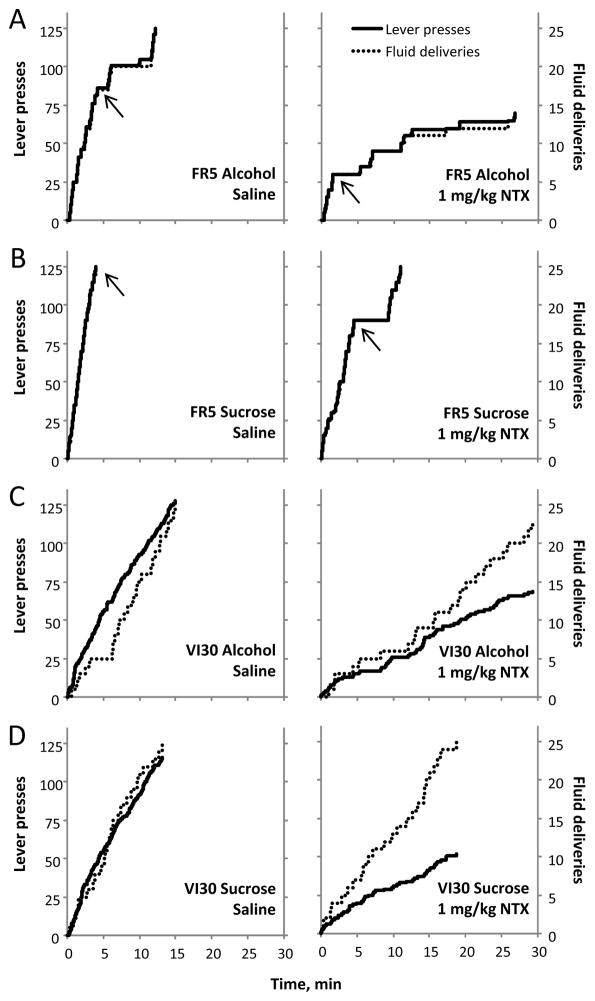
We next evaluated drinking bouts in FR5 groups and pressing rates in VI30 groups. Drinking bouts were defined as successive presses with <60s between presses. Consistent with previous reports (Samson and Hodge, 1996), FR5-trained rats exhibited initial bouts that either ended with the 25th reinforcer delivery or were followed by subsequent, smaller bouts. In contrast, VI30-trained rats typically pressed at a slower but persistent rate throughout the session. These different lever-press patterns are illustrated in representative cumulative-response plots (Figure 2). Thus, we analyzed initial-bout length in FR5-trained rats and assessed the cumulative-response slope by time in VI30-trained rats.
Figure 2.
Representative cumulative response plots from alcohol and sucrose self-administration sessions. Under control conditions, FR5-trained rats (Panels A and B left) show a pronounced initial bout of pressing which is marked by an arrow in the diagram. Note that the fluid delivery (dotted) and lever press (solid) lines largely overlap on the FR5 group plots. Pressing bouts were typically absent in VI30-trained rats (Panels C and D left), whose pattern of lever presses was steady and persistent throughout the session. NTX (right) reduced the size of first bout in FR5-trained rats and slowed the rate of pressing in the VI30-trained rats.
NTX shortened initial bouts in FR5-trained rats, as examined by bout length (min) and number of presses (Table 3). Analysis yielded main effects of NTX dose (F3,78=5.3, p<0.05) and reinforcer solution (F1,78=3.1, p<0.05) on bout length, but no dose by solution interaction. Specifically, FR5-alcohol rats exhibited shorter initial bouts than FR5-sucrose rats (t=2.3, p<0.05). Moreover, when collapsed across group, bouts after 1mg/kg NTX were shorter than after saline (t=2.7, p<0.05). Similarly, lever presses in initial bouts were modulated by NTX dose (F3,78=15.2, p<0.001) and reinforcer solution (F1,78=9.8, p<0.01), with no dose by solution interaction. FR5-alcohol rats pressed less during initial bouts than FR5-sucrose rats (t=3.1, p<0.01). Furthermore, when collapsed across group, all NTX doses reduced initial bout presses versus saline (Bonferroni-corrected, t’s>2.4, p’s<0.05). Thus, while FR5-alcohol rats exhibited shorter drinking bouts than FR5-sucrose rats, NTX reduced bout size similarly between groups.
In VI30-trained rats, NTX slowed lever-press rates across the session (Table 3), as revealed by a main effect of NTX dose (F3,87=13.5, p<0.001), but no effect of reinforcer solution or dose by solution interaction. Specifically, all NTX doses decreased the slope of cumulative presses over time compared to saline (Bonferroni-corrected, t’s>3.1, p’s<0.05), indicating a non-specific effect of NTX to slow pressing under the VI30-reinforcement schedule.
Satiety-Specific Devaluation
We tested whether reward-seeking was altered by satiety-induced devaluation of alcohol or sucrose. Reward-seeking was assessed in an extinction session following home-cage access to the reinforcing fluid or maltodextrin. Volumes consumed during pre-exposure are shown in Table 4. We used the standard method to account for any differences in response rate between the VI and FR schedules (Dews, 1955) and calculated a devaluation ratio of lever presses after reinforcer satiety to presses after the non-devaluation control maltodextrin (Table 4); ratios close to 1 indicated insensitivity to devaluation and habit-like behavior. A 2-way ANOVA on devaluation ratios yielded a main effect of training schedule (F1,55=6.7, p<0.05), but no effect of reinforcing fluid or interaction. Collapsed across reinforcing fluid, FR5-trained rats exhibited lower devaluation ratios than VI30-trained rats (t=2.6, p<0.05), consistent with more goal-directed behavior in the FR5 groups and more habitual reward-seeking in the VI30 groups.
Table 4.
Satiety-specific devaluation: reinforcer consumption and subsequent seeking behavior.
| FR5-Alcohol | FR5-Sucrose | VI30-Alcohol | VI30-Sucrose | |
|---|---|---|---|---|
| Maltodextrin consumed, ml | 12.5 ± 0.8 | 13.9 ± 1.1 | 11.3 ± 0.7 | 12.8 ± 1.1 |
| Reinforcer fluid consumed, ml | 7.3 ± 0.5 a | 13.9 ± 1.3 | 7.9 ± 0.7 a | 13.2 ± 1.3 |
| Alcohol dose, g/kg | 1.4 ± 0.1 | 1.6 ± 0.1 | ||
| Avgcaloric load after maltodextrin, kcal | 1.0 | 1.1 | 0.9 | 1.0 |
| Avg caloric load after reinforcer, kcal | 5.1 | 0.8 | 5.5 | 0.8 |
| Lever presses after maltodextrin | 119.3 ± 13.5 | 117.1 ± 18.4 | 47.3 ± 5.2 | 45.5 ± 5.9 |
| Lever presses after reinforcer | 60.1 ± 13.0 | 92.6 ± 18.0 | 40.1 ± 3.9 | 42.7 ± 5.0 |
| Devaluation ratio b | 0.56 ± 0.13 c | 0.82 ± 0.12 c | 0.91± 0.09 | 1.09 ± 0.15 |
All values are mean ± SE.
Significantly less than maltodextrin volume in same group and sucrose volumes in sucrose groups (p<0.05).
Lever presses after reinforcer exposure/presses after maltodextrin reinforcer.
Collapsed across reinforcer, FR5-trained groups had significantly lower devaluation scores than VI30-trained groups.
NTX Extinction Probe Sessions
To determine NTX effects on reward-seeking behavior in the absence of fluid delivery, we gave NTX prior to a brief extinction session. Compared to saline, NTX reduced reward-seeking by 40–55% in all groups (Figure 3). A 3-way RM ANOVA yielded a main effect of dose (F1.7,48=32.3, p<0.001) and a significant interaction of dose by reinforcement schedule (F1.7,48=4.3, p<0.05). Collapsed across reinforcer, a posthoc, 2-way ANOVA resulted in significant main effects of dose (F2,51=33.4, p<0.001) and schedule (F1,51=21.7, p<0.001), but no interaction. Specifically, while the FR-trained rats pressed more overall versus the VI30-trained rats, 0.3 and 1mg/kg NTX decreased presses versus saline in similar proportions across rats (Bonferonni-corrected, t’s>6.3, p’s<0.001). Furthermore, a 3-way ANOVA of the proportional ratios revealed no main effects or interactions (all F’s<1.4, p’s>0.05) (see Supplemental Table 1). Thus, NTX non-selectively diminished reward-seeking under brief extinction conditions.
Figure 3.
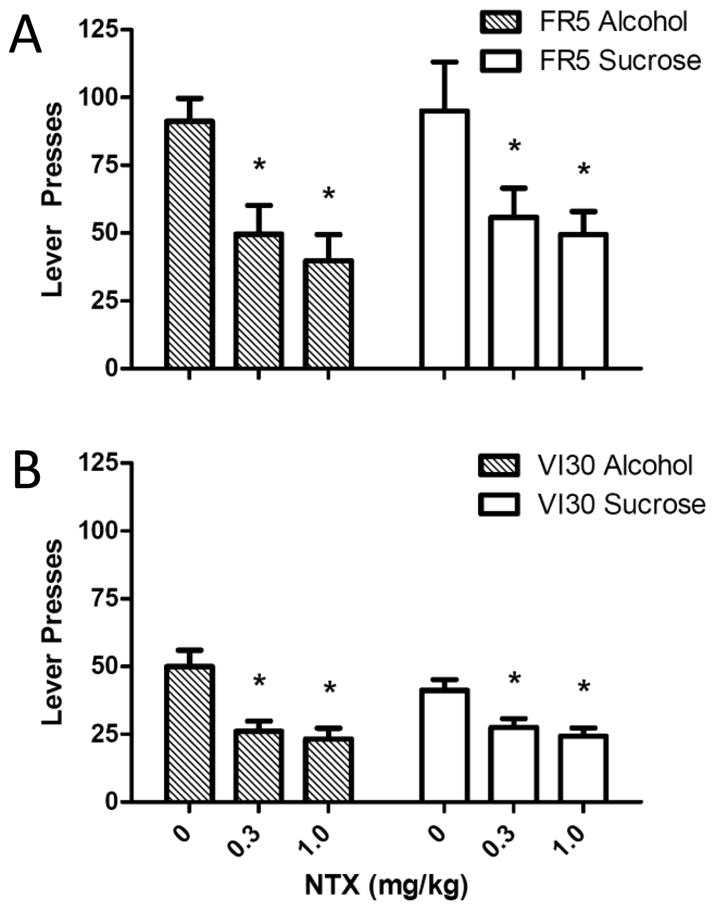
NTX effects on reward-seeking during brief extinction sessions. FR5-trained rats (top) pressed twice as much as VI30-trained rats (bottom) in a 10-min extinction session following saline injection, consistent with the slower response rates engendered by the VI30-reinforcement schedule. However, NTX at 0.3 and 1mg/kg reduced seeking for both alcohol and sucrose by 40–55%, indicating that NTX nonselectively decreases reward-seeking behavior (*collapsed across groups, different from saline, p<0.05).
Extinction Training and Reinstatement
We tested the ability of NTX to blunt renewed lever pressing upon presentation of 0.2ml reinforcing fluid and reward-associated cues (Bienkowski et al., 1999). All rats underwent ≥12 days of extinction training (Table 5). On the reinstatement day, the first 20min of the session was extinction, and the second 20min was reinstatement. For this stage of the study, experimental groups were split, with half receiving saline and half receiving NTX. The resulting group n’s (n=5–8/group; Table 5) were underpowered for between-group comparisons; instead, we calculated within-subject analyses of pressing across days (average of the prior three extinction days versus test day) and period (first versus second 20min). Comparison of presses during the first period on test day versus extinction days revealed NTX effects on reward-seeking during extinction. Reinstatement of reward-seeking was indicated by a significant increase in presses during the second period on test day versus extinction days. Note that the within-subject analysis of these data limits the comparison of reinstatement magnitude between groups (Nieuwenhuis et al., 2011). Table 5 shows the number of cue presentations delivered by group.
Table 5.
Cue presentations during reinstatement session.
| Group | Extinction training days | Condition | Group n | Cue presentations |
|---|---|---|---|---|
| FR5-Alcohol | 14.6 ± 0.3 | NTX | 6 | 8 ± 2 |
| Saline | 8 | 5 ± 2 | ||
|
| ||||
| FR5-Sucrose | 14.5 ± 0.5 | NTX | 5 | 6 ± 2 |
| Saline | 7 | 5 ± 2 | ||
|
| ||||
| VI30-Alcohol | 14.4 ± 0.3 | NTX | 7 | 5 ± 1 |
| Saline | 7 | 5 ± 1 | ||
|
| ||||
| VI30-Sucrose | 15.7 ± 0.6 | NTX | 7 | 6± 1 |
| Saline | 8 | 7 ± 2 | ||
FR5-alcohol rats given saline showed renewed pressing upon presentation of the alcohol prime and cues, while rats given 1mg/kg NTX exhibited blunted pressing across the session (Figure 4A). Specifically, we found a significant interaction of day by period on presses in saline-treated rats (F1,7=15.4, p<0.01); they pressed 50% less during the first period but >5-fold more in the second period on test day versus extinction days (Bonferroni-corrected t’s>2.5, p’s<0.05). In contrast, we found a main effect of day on presses (F1,5=9.7, p<0.05) in NTX-treated rats, but only a marginal effect of period (F1,5=6.3, p<0.055) and no interaction of day by period, indicating that NTX reduced alcohol seeking across the session and blocked cue/prime-induced reinstatement.
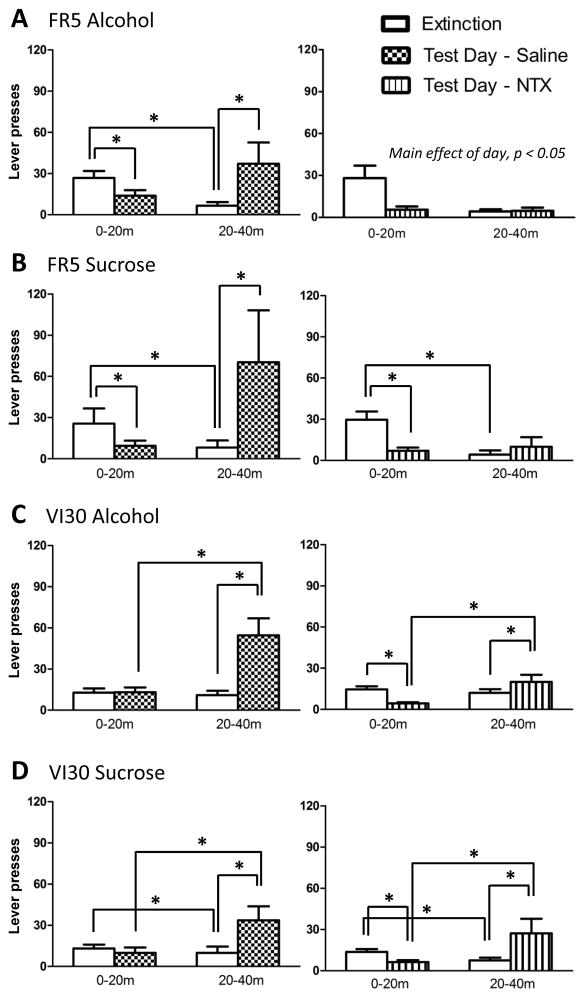
Figure 4.
NTX effects on reward-seeking in FR5-trained rats (Panels A and B) and VI30-trained rats (Panels C and D) during extinction and after presentation of 0.2ml reinforcing fluid and associated cues. Lever presses in the first (0–20min) and second (20–40min) periods of the test session (patterned bars) were compared to those of the average of the previous three extinction days (open bars). Following saline injections (left), all groups showed significant reinstatement of pressing behavior after presentation of cues and 0.2ml of reinforcement solution versus the previous extinction days (second period, p<0.05). Following 1mg/kg NTX (right), lever pressing by the FR5-trained rats during the first period was reduced (p<0.05), and cue/prime-induced lever pressing in the second period was blocked. In VI30-trained rats, NTX reduced lever presses during the first period (extinction), similar to FR5-trained rats (p<0.05). However, NTX did not prevent a significant increase in lever presses in response to the fluid prime and cue presentations (second period, p<0.05).
Similar effects were observed in FR5-sucrose rats (Figure 4B): saline-treated rats exhibited renewed lever pressing at the sucrose prime and cues, while NTX-treated rats did not reinstate pressing. Both saline and NTX injections reduced lever pressing during the initial 20min on test day. In saline-treated rats, we found a significant interaction of day by period on presses (F1,6=11.6, p<0.05); rats pressed 35% less during the first period but 8-fold more in the second period on test day versus extinction days (Bonferroni-corrected, t’s>2.7, p’s<0.05). Data from NTX-treated rats also yielded a significant interaction of day by period on presses (F1,4=50.9, p<0.01); while these rats pressed 25% less during the initial period (Bonferroni-corrected, t=5.3, p<0.05), pressing in the reinstatement period was not different from the first period on test day or the second period on extinction days.
In contrast, VI30-trained rats exhibited renewed pressing at the presentation of cues and reward prime after saline or NTX administration (Figure 4C–4D). We found significant interactions of day by period on presses in saline-treated VI30-alcohol (F1,6=14.4, p<0.01) and VI30-sucrose rats (F1,7=16.3, p<0.01); in both groups, more presses occurred in the reinstatement period on test day versus both the initial period of test day and the second period of extinction days (Bonferroni-corrected, t’s>3.6, p’s<0.05). Similarly, lever-press data from NTX-treated VI30-alcohol and VI30-sucrose rats yielded significant interactions of day by period (F1,6=40.3, p<0.001 and F1,6=43.7, p<0.001, respectively). NTX reduced pressing by 30–45% during the initial 20-min period of test day when compared to extinction days, but did not prevent renewed pressing during the reinstatement period, both compared to the initial period on test day and to the second period of extinction days (Bonferroni-corrected, t’s>2.5, p’s<0.05). Specifically, the increase in pressing during the second period on reinstatement versus extinction days was: VI30-alcohol-saline, 500%; VI30-alcohol-NTX, 165%; VI30-sucrose-saline, 335%; VI30-sucrose-NTX, 360%.
In summary, all groups exhibited renewed lever pressing at presentation of the combined cue/prime under saline conditions. NTX reduced reward-seeking during extinction across all groups, but blocked cue/prime-induced reinstatement of reward-seeking only in FR5-trained rats.
BEC after FR5- and VI30-reinforcement schedules
BEC was determined in two rats after FR5 operant sessions and in the same rats after VI30 sessions. These rats performed similarly in the operant task and reached equivalent ethanol doses in comparison to the other groups (Supplemental Table 2). The BEC after the FR5 sessions was 30.7±3.8mg/dl (range 20.1–37.3) and after the VI30 sessions was 27.3±4.9mg/dl (range 14.9–38.6). Thus, BEC under the two schedules did not differ 30 min after session onset (paired t=0.540, p>0.05).
Discussion
The transition from casual to compulsive drug use may involve a shift from goal-directed to habitual behaviors (Everitt et al., 2008; Stewart et al., 1984; Tiffany, 1990), which are less flexible to changes in reward. Animal studies have suggested that alcohol, including self-administered alcohol, promotes habit development (Corbit et al., 2012; Dickinson et al., 2002; Mangieri et al., 2012), although not all studies support this view (Samson et al., 2004). A transition toward habitual alcohol use has implications for treatment, as pharmacological strategies that reduce drug reward may effectively blunt goal-directed drinking without reducing habit-associated use. The present study explored this possibility by testing the efficacy of NTX, an FDA-approved drug used to treat AUD that is thought to reduce alcohol reward, in animal models of alcohol self-administration that differ in the persistence and flexibility of the resulting operant behavior. Specifically, we trained rats to self-administer alcohol or sucrose on one of two reinforcement schedules – FR5 to model goal-directed behavior or VI30 to model habitual behavior – and examined NTX effects in various self-administration and extinction sessions. Consistent with previous reports, the FR5-trained rats exhibited goal-directed behavior in the satiety-specific devaluation test while the VI30-trained rats exhibited habit-like behavior (Corbit et al., 2012; Dickinson et al., 2002; Mangieri et al., 2012; Samson et al., 2004). In contrast to studies using variable ratio schedules (Corbit et al., 2012; Mangieri et al., 2012), we did not observe habit-like behavior from overtraining in the FR5 groups, possibly because the active lever alternated daily or because the ratio was fixed. We hypothesized that NTX would more effectively reduce goal-directed versus habitual reward-seeking and more effectively reduce alcohol versus sucrose drinking.
Consistent with our hypothesis, NTX selectively reduced drinking in self-administration sessions in FR5-alcohol rats versus other groups, indicated by fewer fluid deliveries and less alcohol consumed. Moreover, NTX blocked cue/prime-induced reinstatement of lever pressing in FR5-trained but not VI30-trained rats, indicating reduced efficacy for “relapse”-like reward-seeking in rats trained on a habit-promoting schedule. However, NTX also produced nonspecific effects on reward-seeking in the absence of immediate feedback. Specifically, NTX reduced extinction pressing across all groups: in brief, isolated trials and after extensive extinction training. Moreover, NTX slowed self-administration of alcohol and sucrose in all groups, as indicated by smaller initial bouts in FR5-trained rats and reduced and slower pressing in VI30-trained rats. Notably, slower press-rates did not impact the fluid deliveries achieved or alcohol consumed under the VI30 schedule (although two VI30-alcohol rats did not consume all alcohol after NTX). An important caveat of the present study is the difference in rate of alcohol drinking between the FR5 and VI30 schedules and resultant BECs. While BECs were similar following 30-min sessions of alcohol self-administration under FR5 and VI30 schedules, they may differ in the rate of initial rise of BECs. Lower initial BECs in VI30-alcohol rats due to slower intake may limit the ability of NTX to pharmacologically blunt alcohol reward; future studies can address this possibility by manipulation of reinforcement intervals to closely match the time of drinking between groups. Nevertheless, under the present conditions NTX more effectively reduced alcohol versus sucrose self-administration and more effectively blocked cue/prime-induced reinstatement in rats trained on goal-directed versus less flexible operant schedules, but NTX also had nonspecific effects on motivation that were especially evident when the reinforcing fluid was absent or when required effort was low.
A caveat to the present study is that the FR5 groups exhibited more presses overall than VI30 groups, potentially translating into a rate-dependency effect after satiety or NTX, in that changes were easier to detect simply because the baseline response rates were higher. To address this, we included analyses on the ratio of responding after satiety or NTX versus responding under control conditions (Dews, 1955). The finding that NTX reduced lever presses proportionally across groups during extinction argues that the VI30 response rates were high enough to be effectively modulated.
NTX may diminish alcohol reward by modulating opioid and dopamine systems (Cowen and Lawrence, 1999; Gianoulakis, 2009; Herz, 1997). Support for this comes from the specificity of opioid-receptor antagonists to decrease alcohol consumption at doses lower than those required to reduce sucrose, saccharin or water consumption (June et al., 1999; Shoemaker et al., 2002; Slawecki et al., 1997). In the present study, NTX specifically lowered alcohol versus sucrose self-administration in goal-directed rats, indicated by fewer operant responses and fluid deliveries earned. In contrast, alcohol-specific effects of NTX were not observed in rats trained on the habit-promoting VI30-reinforcement schedule. In these groups, NTX only slightly reduced fluid deliveries earned, while more substantially decreasing the amount of work expended to obtain those deliveries. This nonselective effect of NTX to reduce pressing in both VI30-alcohol and VI30-sucrose groups leads to two conclusions: (1) any NTX-induced pharmacological reduction in alcohol reward was ineffective to change drinking in VI30-trained rats, and (2) NTX may have another, nonselective effect on motivation to seek reward that is revealed under low contingency settings that do not require high rates of responding.
If the exclusive effect of NTX is to devalue alcohol reward, then NTX would not change reward-seeking in the absence of alcohol. Consistent with this, NTX did not significantly reduce latency to initially respond in any group during self-administration sessions. In contrast, when administered prior to either a brief extinction session or after prolonged extinction training, NTX reduced reward-seeking behavior comparably across all groups (an effect seen both in lever press and proportional ratio data). When combined with self-administration, these data suggest that NTX nonspecifically reduced motivation to seek reinforcement, but the delivery of the reinforcer was able to overcome that effect. However, as NTX more effectively diminished alcohol versus sucrose reward, alcohol was less able than sucrose to overcome nonspecific reductions in motivation. The discrepancy in reward value was particularly apparent when reinforcing effortful FR5 responding, but less apparent when reinforcing VI30 responding that did not require high rates of operant behavior.
Another possibility is that NTX blunts the invigorating effect of conditioned cues. Several rodent studies demonstrate that NTX reduces alcohol-seeking triggered by contextual, discrete, or discriminative cues (Burattini et al., 2006; Katner et al., 1999; Liu and Weiss, 2002). Naloxone, another opioid-receptor antagonist, facilitates extinction to ethanol-conditioned place preference in mice (Cunningham et al., 1998). These findings are consistent with reports that NTX reduces craving (O’Malley et al., 2002) and diminishes impulsive actions (Mitchell et al., 2007) in people with AUD. In the present study, NTX blocked cue/prime-induced renewal of lever pressing in goal-directed, FR5-trained rats but not in habit-like, VI30-trained rats. More studies are required to parse out the contribution of reward-associated cues to this discrepant effect of NTX on reinstatement, but one interpretation is that NTX blunted the ability of conditioned cues to reinitiate pressing in goal-directed but not habitual animal models. Another explanation is that NTX differentially impeded the ability of reward-associated cues to sustain lever pressing in the absence of further fluid deliveries; indeed, the finding that NTX reduced reward-seeking during extinction sessions could be due to NTX facilitation of extinction learning (but see Williams and Schimmel, 2008).
The present study examined effects of acute NTX, and while clinical trials have found that “targeted” dosing (taking NTX when needed) is effective (Heinala et al., 2001; Kranzler et al., 1997), most therapy consists of chronic dosing (Johnson, 2010). Chronic NTX may affect habitual versus goal-directed animal models differently. Chronic NTX decreased both alcohol and sucrose consumption after an operant requirement of 2 presses (Czachowski and Delory, 2009), suggesting that NTX did not affect low press-rates but more effectively diminished reinforcer drinking than we observed in the present study. Moreover, chronic NTX blunted alcohol-seeking more than sucrose-seeking in non-dependent rats, an effect not observed in rats with a history of alcohol dependence. In another study, repeated naloxone shifted behavior from goal-directed to habitual when administered during the acquisition of an instrumental task (Wassum et al., 2009), suggesting that opioids are necessary for goal-directed but not habitual operant conditioning. Thus, multiple factors – individual history of alcohol use, goal-directed versus habit-associated patterns of behavior, and NTX dosing – likely contribute to the efficacy of NTX to reduce alcohol use.
In summary, we examined acute NTX as an agent that reduces alcohol reward and found that it most effectively reduced drinking and cue/prime-induced reinstatement of alcohol seeking in a goal-directed operant model. On the other hand, NTX uniformly decreased reward-seeking in the absence of reinforcer. This latter finding is consistent with clinical reports of diminished alcohol craving while on NTX therapy (O’Malley et al., 1992; Volpicelli et al., 1992), but may be undesirable if reduced motivation extends to non-drug rewards. Therapeutic goals for AUD include reduction of both alcohol seeking and drinking, but pharmacotherapeutic efficacy may differ by individual differences in goal-directed versus habitual motivations. Further studies that tease apart the multiple mechanisms and motivations, including habit, contributing to alcohol use may lead to novel therapeutic targets.
Supplementary Material
Acknowledgments
The authors thank Randall Ung, Joel Shillinglaw, Vahid Sanii, and Margo Williams for assistance in training rats. We thank David Marshall and Dr. Jeffrey Klein for writing programs to assist in data analysis. Completion of this project partially fulfilled honors thesis requirements for R.A.H. in the UNC Department of Psychology. This research was funded by NIH (D.L.R.: AA018008 with ARRA revisions 01S1 and 02S1, NADIA Project grants AA019972 and AA020024; C.W.H.: AA11605, AA016629, and AA014983), the Foundation of Hope, the UNC Bowles Center for Alcohol Studies, and the UNC Office of Undergraduate Research.
Footnotes
The authors have no potential conflicts of interest to disclose.
References
- Adams CD. Variations in the sensitivity of instrumental responding to reinforcer devaluation. Q J Exp Psychol. 1982;33:109–122. [Google Scholar]
- Bienkowski P, Kostowski W, Koros E. Ethanol-reinforced behaviour in the rat: effects of naltrexone. Eur J Pharmacol. 1999;374:321–327. doi: 10.1016/s0014-2999(99)00245-9. [DOI] [PubMed] [Google Scholar]
- Bogenschutz MP, Scott Tonigan J, Pettinati HM. Effects of alcoholism typology on response to naltrexone in the COMBINE study. Alcohol Clin Exp Res. 2009;33:10–18. doi: 10.1111/j.1530-0277.2008.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burattini C, Gill TM, Aicardi G, Janak PH. The ethanol self-administration context as a reinstatement cue: acute effects of naltrexone. Neuroscience. 2006;139:877–887. doi: 10.1016/j.neuroscience.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Corbit LH, Nie H, Janak PH. Habitual Alcohol Seeking: Time Course and the Contribution of Subregions of the Dorsal Striatum. Biol Psychiatry. 2012;72:389–395. doi: 10.1016/j.biopsych.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen MS, Lawrence AJ. The role of opioid-dopamine interactions in the induction and maintenance of ethanol consumption. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23:1171–1212. doi: 10.1016/s0278-5846(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Henderson CM, Bormann NM. Extinction of ethanol-induced conditioned place preference and conditioned place aversion: effects of naloxone. Psychopharmacology. 1998;139:62–70. doi: 10.1007/s002130050690. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Delory MJ. Acamprosate and naltrexone treatment effects on ethanol and sucrose seeking and intake in ethanol-dependent and nondependent rats. Psychopharmacology. 2009;204:335–348. doi: 10.1007/s00213-009-1465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB. Studies on behavior. I. Differential sensitivity to pentobarbital of pecking performance in pigeons depending on the schedule of reward. J Pharmacol Exp Ther. 1955;113:393–401. [PubMed] [Google Scholar]
- Dickinson A. Actions and Habits: The development of behavioural autonomy. Phil Trans R Soc Lond. 1985;308:67–78. [Google Scholar]
- Dickinson A, Wood N, Smith JW. Alcohol seeking by rats: action or habit? Q J Exp Psychol. 2002;55:331–348. doi: 10.1080/0272499024400016. [DOI] [PubMed] [Google Scholar]
- Dwyer DM. Reinforcer devaluation in palatability-based learned flavor preferences. J Exp Psychol. 2005;31:487–492. doi: 10.1037/0097-7403.31.4.487. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehlich JC, Zweifel M, Harts J, Lumeng L, Li TK. Importance of delta opioid receptors in maintaining high alcohol drinking. Psychopharmacology (Berl) 1991;103:467–472. doi: 10.1007/BF02244246. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C. Endogenous opioids and addiction to alcohol and other drugs of abuse. Curr Top Med Chem. 2009;9:999–1015. doi: 10.2174/156802609789630956. [DOI] [PubMed] [Google Scholar]
- Heinala P, Alho H, Kiianmaa K, Lonnqvist J, Kuoppasalmi K, Sinclair JD. Targeted use of naltrexone without prior detoxification in the treatment of alcohol dependence: a factorial double-blind, placebo-controlled trial. J Clin Psychopharmacol. 2001;21:287–292. doi: 10.1097/00004714-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- Hill KG, Kiefer SW. Naltrexone treatment increases the aversiveness of alcohol for outbred rats. Alcohol Clin Exp Res. 1997;21:637–641. [PubMed] [Google Scholar]
- Hyytia P. Involvement of mu-opioid receptors in alcohol drinking by alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;45:697–701. doi: 10.1016/0091-3057(93)90527-z. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Medication treatment of different types of alcoholism. Am J Psychiatry. 2010;167:630–639. doi: 10.1176/appi.ajp.2010.08101500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June HL, McCane SR, Zink RW, Portoghese PS, Li TK, Froehlich JC. The delta 2-opioid receptor antagonist naltriben reduces motivated responding for ethanol. Psychopharmacology. 1999;147:81–89. doi: 10.1007/s002130051145. [DOI] [PubMed] [Google Scholar]
- Katner SN, Magalong JG, Weiss F. Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1999;20:471–479. doi: 10.1016/S0893-133X(98)00084-0. [DOI] [PubMed] [Google Scholar]
- Kimura M, Higuchi S. Genetics of alcohol dependence. Psychiatry Clin Neurosci. 2011;65:213–225. doi: 10.1111/j.1440-1819.2011.02190.x. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Tennen H, Penta C, Bohn MJ. Targeted naltrexone treatment of early problem drinkers. Addict Behav. 1997;22:431–436. doi: 10.1016/s0306-4603(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–726. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA. Naltrexone in the treatment of alcohol dependence. N Engl J Med. 2001;345:1734–1739. doi: 10.1056/NEJMoa011127. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri RA, Cofresi RU, Gonzales RA. Ethanol seeking by long evans rats is not always a goal-directed behavior. PLoS One. 2012;7:e42886. doi: 10.1371/journal.pone.0042886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JM, Tavares VC, Fields HL, D’Esposito M, Boettiger CA. Endogenous opioid blockade and impulsive responding in alcoholics and healthy controls. Neuropsychopharmacology. 2007;32:439–449. doi: 10.1038/sj.npp.1301226. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers EJ. Erroneous analyses of interactions in neuroscience: a problem of significance. Nature neuroscience. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ray LA, Krull JL, Leggio L. The Effects of Naltrexone Among Alcohol Non-Abstainers: Results from the COMBINE Study. Front Psychiatry. 2010;1:26. doi: 10.3389/fpsyt.2010.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Stephens DN. Critical thoughts on current rodent models for evaluating potential treatments of alcohol addiction and withdrawal. Br J Pharmacol. 2011;164:1335–1356. doi: 10.1111/j.1476-5381.2011.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, McDonald JS, Heyser CJ, Kieffer BL, Matthes HW, Koob GF, Gold LH. mu-Opioid receptor knockout mice do not self-administer alcohol. J Pharmacol Exp Ther. 2000;293:1002–1008. [PubMed] [Google Scholar]
- Robinson DL, Carelli RM. Distinct subsets of nucleus accumbens neurons encode operant responding for ethanol versus water. Eur J Neurosci. 2008;28:1887–1894. doi: 10.1111/j.1460-9568.2008.06464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson HH, Cunningham CL, Czachowski CL, Chappell A, Legg B, Shannon E. Devaluation of ethanol reinforcement. Alcohol. 2004;32:203–212. doi: 10.1016/j.alcohol.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Samson HH, Hodge C. Neurobehavioral regulation of ethanol intake. In: Deitrich RA, Erwin G, editors. Pharmacological Effects of Ethanol on the Nervous System. Boca Raton: CRC Press, Inc; 1996. pp. 203–226. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shoemaker WJ, Vavrousek-Jakuba E, Arons CD, Kwok FC. The acquisition and maintenance of voluntary ethanol drinking in the rat: effects of dopaminergic lesions and naloxone. Behav Brain Res. 2002;137:139–148. doi: 10.1016/s0166-4328(02)00290-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Hodge CW, Samson HH. Dopaminergic and opiate agonists and antagonists differentially decrease multiple schedule responding maintained by sucrose/ethanol and sucrose. Alcohol. 1997;14:281–294. doi: 10.1016/s0741-8329(96)00153-x. [DOI] [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology. 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J, de Wit H, Eikelboom R. Role of unconditioned and conditioned drug effects in the self-administration of opiates and stimulants. Psychol Rev. 1984;91:251–268. [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–168. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR, Hopson N. Alcohol drinking in rats during and following morphine injections. Alcohol. 1991;8:289–292. doi: 10.1016/0741-8329(91)90401-h. [DOI] [PubMed] [Google Scholar]
- Wassum KM, Cely IC, Maidment NT, Balleine BW. Disruption of endogenous opioid activity during instrumental learning enhances habit acquisition. Neuroscience. 2009;163:770–780. doi: 10.1016/j.neuroscience.2009.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KL, Schimmel JS. Effect of naltrexone during extinction of alcohol-reinforced responding and during repeated cue-conditioned reinstatement sessions in a cue exposure style treatment. Alcohol. 2008;42:553–563. doi: 10.1016/j.alcohol.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.