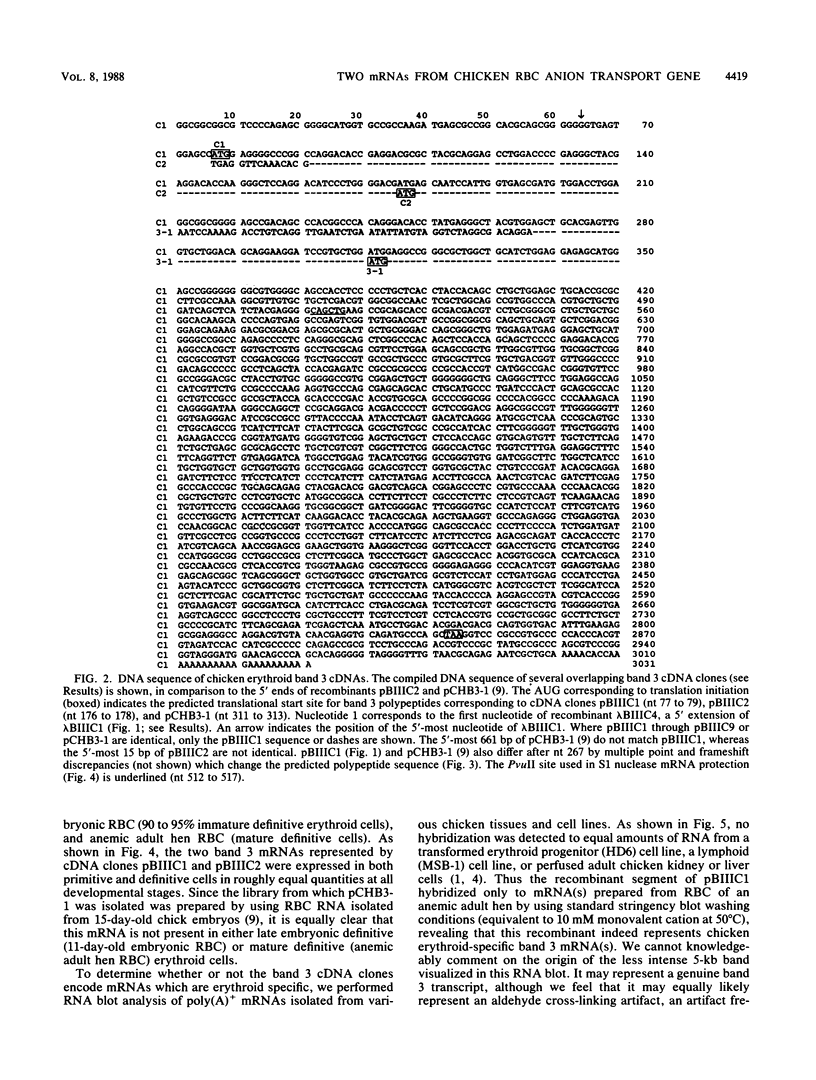
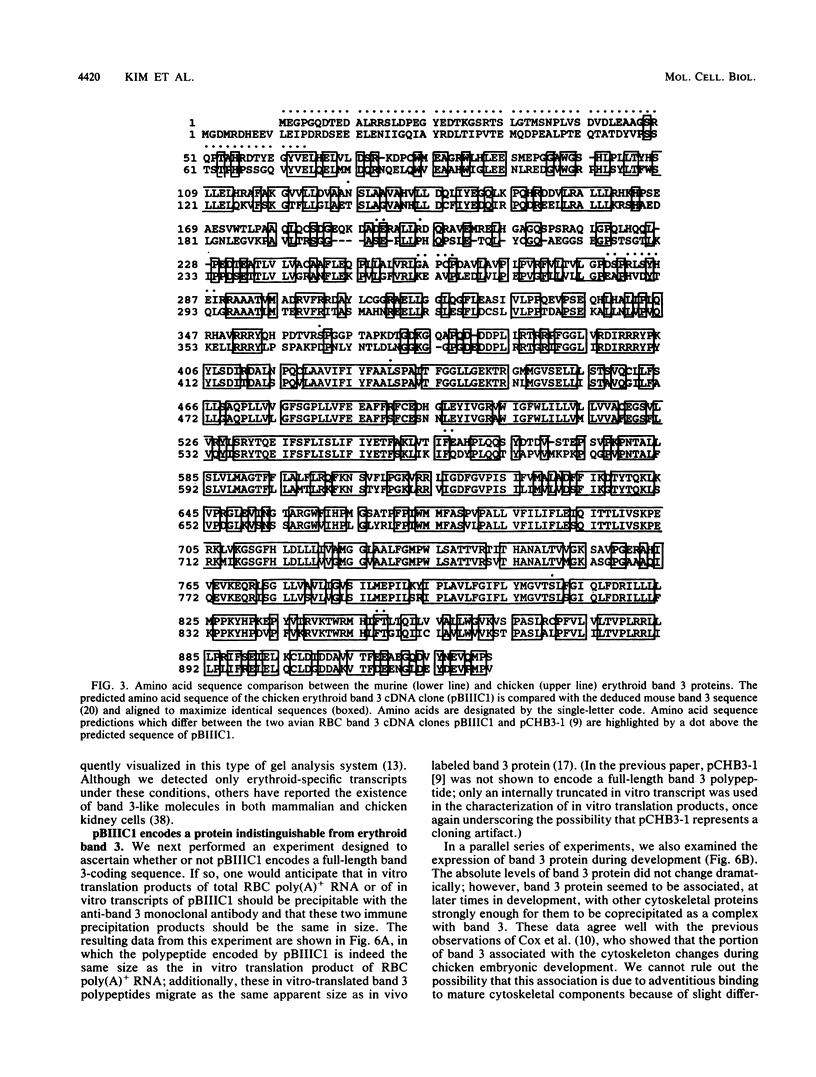
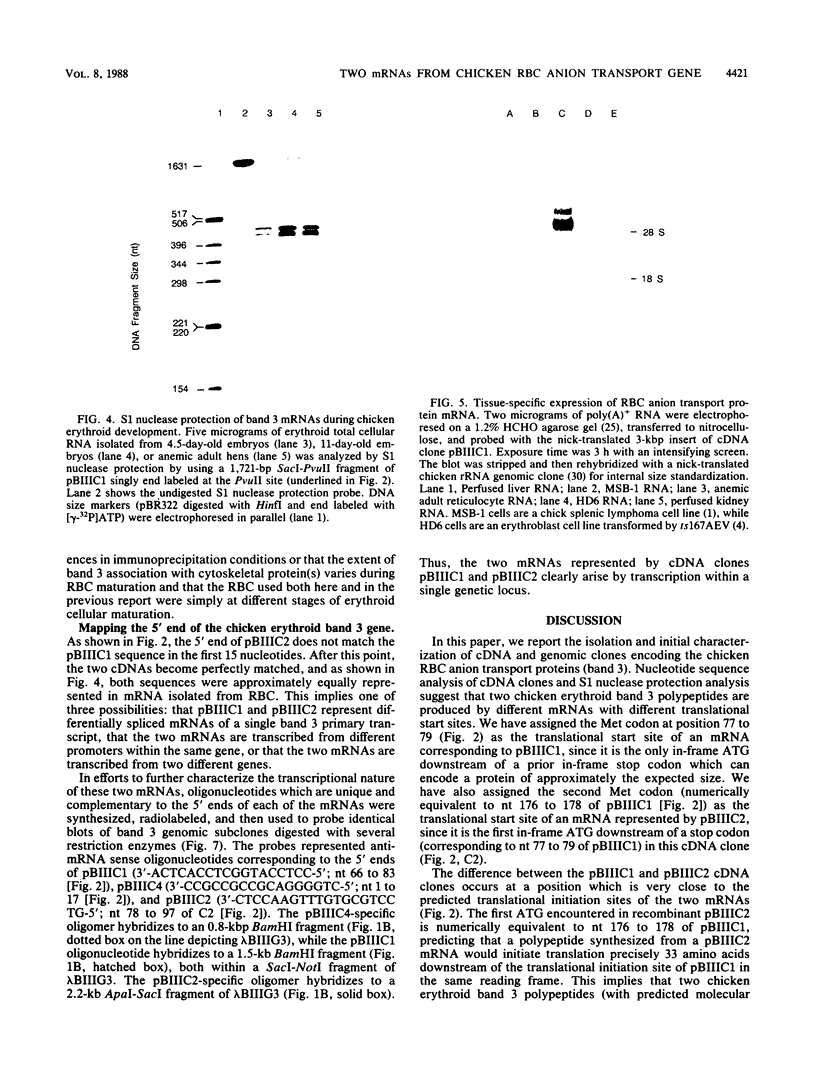
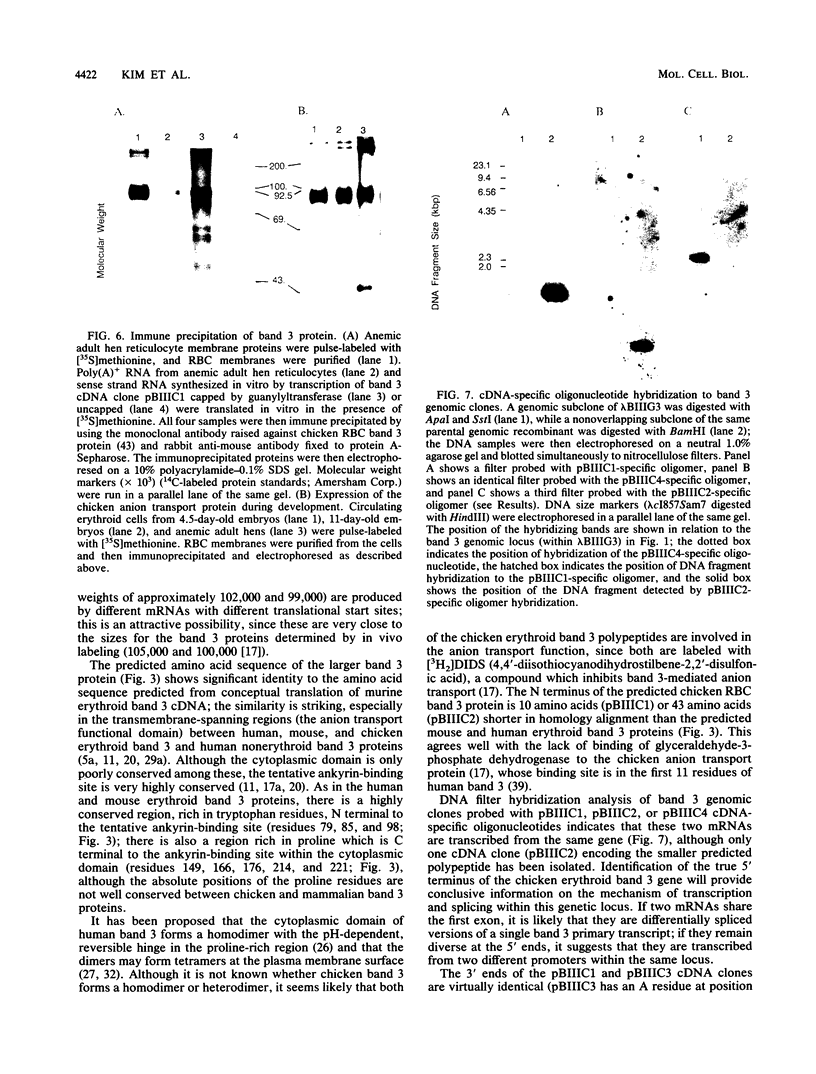
Abstract
The chicken erythrocyte anion transport protein (band 3 of the erythrocyte cytoskeleton) is a central component taking part in two widely divergent functions of erythroid cells; it is a primary determinant of cytoskeletal architecture and responsible for electroneutral Cl-/HCO3- exchange across the plasma membrane. To analyze interesting aspects of the developmental regulation of this gene, we have cloned the cDNA and genomic counterparts of the erythroid-specific anion transport protein. We show that a single genetic locus for band 3 encodes two different erythroid cell-specific mRNAs, with different translational initiation sites, which predict polypeptides of sizes very close to those observed in vivo. In vitro translation and immune precipitation of synthetic mRNA derived from one putative fully encoding cDNA clone demonstrate that this clone gives rise to a protein which is identical in size and antigenicity to bona fide chicken erythroid band 3.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama Y., Kato S. Two cell lines from lymphomas of Marek's disease. Biken J. 1974 Sep;17(3):105–116. [PubMed] [Google Scholar]
- Ansorge W., Sproat B., Stegemann J., Schwager C., Zenke M. Automated DNA sequencing: ultrasensitive detection of fluorescent bands during electrophoresis. Nucleic Acids Res. 1987 Jun 11;15(11):4593–4602. doi: 10.1093/nar/15.11.4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett V., Stenbuck P. J. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979 Aug 9;280(5722):468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Tanner M. J., Kempf C. The human erythrocyte anion-transport protein. Partial amino acid sequence, conformation and a possible molecular mechanism for anion exchange. Biochem J. 1983 Sep 1;213(3):577–586. doi: 10.1042/bj2130577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brush D., Dodgson J. B., Choi O. R., Stevens P. W., Engel J. D. Replacement variant histone genes contain intervening sequences. Mol Cell Biol. 1985 Jun;5(6):1307–1317. doi: 10.1128/mcb.5.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Engel J. D. A 3' enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult beta-globin gene. Nature. 1986 Oct 23;323(6090):731–734. doi: 10.1038/323731a0. [DOI] [PubMed] [Google Scholar]
- Cox J. V., Lazarides E. Alternative primary structures in the transmembrane domain of the chicken erythroid anion transporter. Mol Cell Biol. 1988 Mar;8(3):1327–1335. doi: 10.1128/mcb.8.3.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J. V., Stack J. H., Lazarides E. Erythroid anion transporter assembly is mediated by a developmentally regulated recruitment onto a preassembled membrane cytoskeleton. J Cell Biol. 1987 Sep;105(3):1405–1416. doi: 10.1083/jcb.105.3.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demuth D. R., Showe L. C., Ballantine M., Palumbo A., Fraser P. J., Cioe L., Rovera G., Curtis P. J. Cloning and structural characterization of a human non-erythroid band 3-like protein. EMBO J. 1986 Jun;5(6):1205–1214. doi: 10.1002/j.1460-2075.1986.tb04348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Dolan M., Dodgson J. B., Engel J. D. Analysis of the adult chicken beta-globin gene. Nucleotide sequence of the locus, microheterogeneity at the 5'-end of beta-globin mRNA, and aberrant nuclear RNA species. J Biol Chem. 1983 Mar 25;258(6):3983–3990. [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Histone genes are clustered but not tandemly repeated in the chicken genome. Proc Natl Acad Sci U S A. 1981 May;78(5):2856–2860. doi: 10.1073/pnas.78.5.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Hargreaves W. R., Giedd K. N., Verkleij A., Branton D. Reassociation of ankyrin with band 3 in erythrocyte membranes and in lipid vesicles. J Biol Chem. 1980 Dec 25;255(24):11965–11972. [PubMed] [Google Scholar]
- Jay D. G. Characterization of the chicken erythrocyte anion exchange protein. J Biol Chem. 1983 Aug 10;258(15):9431–9436. [PubMed] [Google Scholar]
- Kaul R. K., Murthy S. N., Reddy A. G., Steck T. L., Kohler H. Amino acid sequence of the N alpha-terminal 201 residues of human erythrocyte membrane band 3. J Biol Chem. 1983 Jul 10;258(13):7981–7990. [PubMed] [Google Scholar]
- Kay M. M. Mechanism of removal of senescent cells by human macrophages in situ. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3521–3525. doi: 10.1073/pnas.72.9.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopito R. R., Lodish H. F. Primary structure and transmembrane orientation of the murine anion exchange protein. Nature. 1985 Jul 18;316(6025):234–238. doi: 10.1038/316234a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen T., Voss H., Ansorge W. A simple and rapid preparation of M13 sequencing templates for manual and automated dideoxy sequencing. Nucleic Acids Res. 1987 Jul 24;15(14):5507–5516. doi: 10.1093/nar/15.14.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Low P. S., Westfall M. A., Allen D. P., Appell K. C. Characterization of the reversible conformational equilibrium of the cytoplasmic domain of erythrocyte membrane band 3. J Biol Chem. 1984 Nov 10;259(21):13070–13076. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Martin S. A., Paoletti E., Moss B. Purification of mRNA guanylyltransferase and mRNA (guanine-7-) methyltransferase from vaccinia virions. J Biol Chem. 1975 Dec 25;250(24):9322–9329. [PubMed] [Google Scholar]
- Mawby W. J., Findlay J. B. Characterization and partial sequence of di-iodosulphophenyl isothiocyanate-binding peptide from human erythrocyte anion-transport protein. Biochem J. 1982 Sep 1;205(3):465–475. doi: 10.1042/bj2050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W., Skalka A. M. Analysis of chicken ribosomal RNA genes and construction of lambda hybrids containing gene fragments. Science. 1977 Apr 8;196(4286):195–197. doi: 10.1126/science.557836. [DOI] [PubMed] [Google Scholar]
- Murthy S. N., Liu T., Kaul R. K., Köhler H., Steck T. L. The aldolase-binding site of the human erythrocyte membrane is at the NH2 terminus of band 3. J Biol Chem. 1981 Nov 10;256(21):11203–11208. [PubMed] [Google Scholar]
- Pappert G., Schubert D. The state of association of band 3 protein of the human erythrocyte membrane in solutions of nonionic detergents. Biochim Biophys Acta. 1983 Apr 21;730(1):32–40. doi: 10.1016/0005-2736(83)90313-9. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlüter K., Drenckhahn D. Co-clustering of denatured hemoglobin with band 3: its role in binding of autoantibodies against band 3 to abnormal and aged erythrocytes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6137–6141. doi: 10.1073/pnas.83.16.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw G. M., Aminoff D., Balcerzak S. P., LoBuglio A. F. Clustered IgG on human red blood cell membranes may promote human lymphocyte antibody-dependent cell-mediated cytotoxicity. J Immunol. 1980 Aug;125(2):501–507. [PubMed] [Google Scholar]
- Tsai I. H., Murthy S. N., Steck T. L. Effect of red cell membrane binding on the catalytic activity of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1982 Feb 10;257(3):1438–1442. [PubMed] [Google Scholar]
- Wagner S., Vogel R., Lietzke R., Koob R., Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol. 1987 Aug;253(2 Pt 2):F213–F221. doi: 10.1152/ajprenal.1987.253.2.F213. [DOI] [PubMed] [Google Scholar]
- Walder J. A., Chatterjee R., Steck T. L., Low P. S., Musso G. F., Kaiser E. T., Rogers P. H., Arnone A. The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. J Biol Chem. 1984 Aug 25;259(16):10238–10246. [PubMed] [Google Scholar]
- Waugh S. M., Low P. S. Hemichrome binding to band 3: nucleation of Heinz bodies on the erythrocyte membrane. Biochemistry. 1985 Jan 1;24(1):34–39. doi: 10.1021/bi00322a006. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Yew N. S., Federspiel M., Dodgson J. B., Hayashi N., Engel J. D. Isolation of recombinant cDNAs encoding chicken erythroid delta-aminolevulinate synthase. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3702–3706. doi: 10.1073/pnas.82.11.3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew N. S., Choi H. R., Gallarda J. L., Engel J. D. Expression of cytoskeletal protein 4.1 during avian erythroid cellular maturation. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1035–1039. doi: 10.1073/pnas.84.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]