Abstract
Micro- and macrovascular complications are commonly seen in diabetic patients and endothelial dysfunction contributes to the development and progression of the complications. Abnormal functions in endothelial cells lead to the increase in vascular tension and atherosclerosis, followed by systemic hypertension as well as increased incident of ischemia and stroke in diabetic patients. Mitochondria are organelles serving as a source of energy production and as regulators of cell survival (e.g., apoptosis and cell development) and ion homeostasis (e.g., H+, Ca2+). Endothelial mitochondria are mainly responsible for generation of reactive oxygen species (ROS) and maintaining the Ca2+ concentration in the cytosol. There is increasing evidence that mitochondrial morphological and functional changes are implicated in vascular endothelial dysfunction. Enhanced mitochondrial fission and/or attenuated fusion lead to mitochondrial fragmentation and disrupt the endothelial physiological function. Abnormal mitochondrial biogenesis and disturbance of mitochondrial autophagy increase the accumulation of damaged mitochondria, such as irreversibly depolarized or leaky mitochondria, and facilitate cell death. Augmented mitochondrial ROS production and Ca2+ overload in mitochondria not only cause the maladaptive effect on the endothelial function, but also are potentially detrimental to cell survival. In this article, we review the physiological and pathophysiological role of mitochondria in endothelial function with special focus on diabetes.
Keywords: fission and fusion, biogenesis, mitophagy, apoptosis, complications
Introduction
Diabetes is a metabolic disorder characterized by glucose intolerance and hyperglycemia due to deficiency of insulin and/or loss of effectiveness to insulin action. There are two main types of diabetes: Type-1 diabetes and Type-2 diabetes. Type-1 diabetes mellitus is mainly caused by autoimmune destruction of the beta cells in the islets of pancreas, where insulin is secreted upon glucose absorption. The patients with Type-1 diabetes exhibit lower or loss of plasma insulin and are characterized by total reliance on exogenous insulin for survival. On the other hand, Type-2 diabetic patients still produce and secrete insulin. However, they develop diabetes because of insufficient production/secretion of insulin and/or improper utilization of insulin (called insulin resistance). Type-1 diabetes usually develops in children or in young adults, whereas Type-2 diabetes mellitus is mainly seen in people over the age of 45 and accounts for nearly 90–95 percent of all diabetes cases. Not surprisingly, the prevalence of Type-2 diabetes in children and adolescents is growing worldwide, which correlates with obesity rate in the population (Rosenbloom, 1999; Broomgarden, 2004).
The common complications of diabetes are heart disease, hypertension, stroke, retinopathy, nephropathy, and neuropathy. Heart disease includes coronary artery disease and cardiac myopathy, which are the risk factors of the heart failure, and it is the leading cause of mortality and morbidity in patients with diabetes. Coronary artery disease is the result from narrowing the diameter of small coronary arteries by atherosclerotic lesion and increased coronary arterial tension. Hypertension is the most common complication in diabetic patients and is induced by increased vascular reactivity in the resistant arteries (e.g., mesenteric artery). Stroke and retinopathy are also caused by abnormal vascular reactivity. Endothelial cells serve as a key player in the development of these diseases. Vascular endothelium, which is a monolayer lining the inner surface of the blood vessels, plays an important role in a) vascular barrier function, which prevents the migration of inflammatory cells and fluid leakage into vascular media (Rao et al., 2007; Dejana et al., 2009), b) regulating vascular tone by releasing vasoconstrictors and vasodilators (Conger, 1994; Esper et al., 2006; Dora, 2010), and c) new vascular formation (Carmeliet, 2000; Madeddu, 2005). Endothelial dysfunction is implicated in many cardiovascular diseases including diabetes. It has been shown that in diabetic patients, as well as in diabetic animal models, 1) vascular tension is increased by attenuated endothelium-dependent relaxation and increased release of vasoconstrictors from endothelium cells (Hink et al., 2001; Vinik and Flemmer, 2002; Farhangkhoee et al., 2006; Hermans, 2007), 2), vascular inflammation is augmented via increased endothelial permeability (Zhang et al., 2003; Spinetti et al., 2008) and surface adhesion molecules (Baumgartner-Parzer et al., 1995; Zou et al., 2002; Savoia and Schiffrin, 2007), and 3) endothelial apoptosis is increased, which is a main cause of the blood-retina barrier breakdown (Kern, 2007; Barber et al., 2011) and the decrease in capillary density in the heart (Yoon et al., 2005).
The mitochondria play a critical role in cell survival and death by regulating ATP synthesis through lipid and glucose metabolism, ROS generation, calcium homeostasis, apoptosis stimulation, and aging (McBride et al., 2006; Contreras et al., 2010). Therefore, the abnormal function of mitochondria leads to various cardiovascular diseases (Duchen, 2004; Ballinger, 2005; Davidson and Duchen, 2007). Endothelial cells produce the energy mainly via the anaerobic glycolytic metabolism of glucose but not through the mitochondrial ATP synthesis (Culic et al., 1997; Quintero et al., 2006), it is thusendothelial mitochondria are more like the sensor and initiator of the cell death. In this article, we will review the mitochondrial functions in the vascular endothelial cells and the pathophysiological role of mitochondria in endothelial dysfunction in diabetes mellitus.
Mitochondrial fusion and fission
1) Mitochondrial fusion- and fission-related proteins
Mitochondria are complex organelles that move, fuse, divide, and constantly change their volume/structure upon physiological stimulus and any stress (Frazier et al., 2006; Bereiter-Hahn et al., 2008). The definition of mitochondrial fission is the division of a mitochondrion within a cell to form two or more separate mitochondrial compartments, whereas mitochondrial fusion is merging two or more mitochondria within a cell to form a single compartment. Increased mitochondrial fission and decreased mitochondrial fusion result in the mitochondrial fragmentation (Detmer and Chan, 2007; Knott et al., 2008).
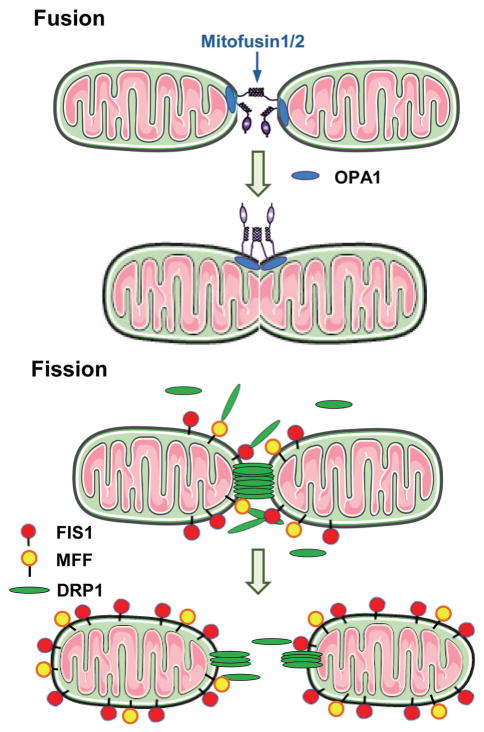
In mammals, mitochondrial fusion is regulated by at least three proteins: optic atrophy 1 (OPA1), mitofusin 1 (MFN1), and mitofusin 2 (MFN2) (Cipolat et al., 2004), whereas mitochondrial fission is controlled by dynamin related protein 1 (DRP1/DLP1/DNM1) and fission 1 (FIS1) (Yoon et al., 2003) (Fig. 1). A recent study identified another tail-anchored mitochondrial outer membrane protein, mitochondrial fission factor (MFF) (Gandre-Babbe and van der Bliek, 2008), and the physiological function of MFF is for the recruitment of DRP1 to the mitochondrial membrane (Otera et al., 2010). Classically, FIS1 was the only one that recruits DRP1 from the cytosol to mitochondria upon the fission reaction (Mozdy et al., 2000; Yoon et al., 2003). OPA1 is located in the inner-mitochondrial membrane, and MFN1 and MFN2 are localized to the outer mitochondrial membrane. During mitochondrial fusion, OPA1 interacts with MFN1 and MFN2 (Cassina et al., 2000; Olichon et al., 2006). These proteins are first found to be related to neuropathy (Delettre et al., 2000; Zuchner et al., 2004; Ferre et al., 2005).
Fig. 1.
Mitochondrial fusion and fission. Mitochondrial fusion is regulated by optic atrophy 1 (OPA1), mitofusin 1 (MFN1), and mitofusin 2 (MFN2), whereas mitochondrial fission is controlled by dynamin related protein 1 (DRP1/DLP1/DNM1), fission1 (FIS1), and mitochondrial fission factor (MFF). OPA1 is located in the inner-mitochondrial membrane, and MFN1 and MFN2 are localized to the outer mitochondrial membrane. During mitochondrial fusion, OPA1 interacts with MFN1 and MFN2. FIS1 and MFF recruit DRP1 from the cytosol to mitochondria upon the fission reaction.
The function of fission/fusion-related proteins is regulated by various regulators, cleavage of the protein, posttranslational modifications, protein-protein interactions, and the lipid environment. GTP hydrolysis is required for fission/fusion-related proteins to be activated (Chan, 2006). DRP1 activity is negatively controlled by cyclic AMP. Phosphorylated DRP1 by cyclic AMP-dependent protein kinase increases the mitochondrial tubular formation, whereas dephosphorylation of DRP1 by calcineurin increases mitochondrial fission (Cribbs and Strack, 2007). OPA1 exhibits both long and short forms for fusion to proceed and the balance of those forms is maintained by constitutive processing. There are two cleavage sites in OPA1, S1 and S2, and YME1L (an intermembrane space AAA protease) cleaves OPA1 at the site of S2 constitutively following mitochondrial import, whereas the loss of mitochondrial membrane potential leads to the cleavage of the S1 site by OMA1 (zinc metalloprotease), which is followed by complete conversion of OPA1 to the short isoform and shutting off mitochondrial fusion (Griparic et al., 2007; Song et al., 2007; Head et al., 2009). Various posttranscriptional modifications of proteins regulate the activity of the fusion and fission machineries. MARCH5, a ubiquitin ligase in the outer membrane, associates with and ubiquitylates MFN1, MFN2, DRP1, and FIS1 (Yonashiro et al., 2006; Park et al., 2010). Although MARCH5 binds to both fission and fusion related proteins, knockdown of MARCH5 induces the mitochondrial elongation via notable accumulation of MFN1 protein (Park et al., 2010). DRP1 is sumoylated by mitochondrial-anchored protein ligase (MAPL, small ubiquitin-like modifier [SUMO] ligase) (Braschi et al., 2009) and desumorylated by sentrin-specific protease 5 (SENP5) (Zunino et al., 2007). Sumoylation of DRP1 stimulates mitochondrial fission (Harder et al., 2004; Zunino et al., 2007; Braschi et al., 2009). We have recently reported that high-glucose treatment leads to O-GlcNAcylation of OPA1 and mitochondrial fragmentation, while an inhibition of O-GlcNAcylation by overexpression of GlcNAcase decreases the mitochondrial fragmentation induced by high glucose (Makino et al., 2011). S-nitrosylation of DRP1 results in mitochondrial fission (Cho et al., 2009).
An imbalance in the fusion/fission dynamics dramatically changes overall mitochondrial morphology (Bereiter-Hahn and Voth, 1994). Recent evidence from our laboratory, as well as others, has shown that mitochondrial dynamics play important roles in mitochondrial functions, including cell development, apoptosis, ROS generation and functional complementation of mitochondrial DNA (mtDNA) mutations by context mixing (Nakada et al., 2001; Frazier et al., 2006; Makino et al., 2010). Mitochondrial fission is essential for appropriate redistribution of mtDNA during cell division (Scott et al., 2003; Hales, 2004; Taguchi et al., 2007). In addition, damaged mitochondria are removed by mitophagy through mitochondrial fission (Twig et al., 2008a; Twig et al., 2008b). Fusion also influences mitochondrial distribution in neural cells (Chen et al., 2007). Fused mitochondrial networks serve as electrically united systems that transmit the membrane potential generated by the proton pumps of the respiratory chain (Amchenkova et al., 1988; Skulachev, 2001) and also facilitate the propagation of Ca2+ wave and energy transfer in the cells (Szabadkai et al., 2004; Jou, 2008). Mitochondrial fusion is dramatically increased when mitochondrial ATP synthesis is enhanced (Tondera et al., 2009). The damaged/depolarized parts of the mitochondrial membrane are recovered by mitochondrial fusion that facilitates proper mixing of mtDNA and metabolites (Nakada et al., 2001; Twig et al., 2008b).
2) Other factors which induces mitochondrial fragmentation
The endoplasmic reticulum (ER) is an intracellular Ca2+ store and releases Ca2+ via Ca2+ releasing channels upon the stimulation. The mitochondria are located close to the ER to support communication between them such as the transferring lipids, and the exchange of calcium and ATPs (Vance and Shiao, 1996; Mannella et al., 1998; Hayashi et al., 2009; Rizzuto et al., 2009). At the pathological condition, Ca2+ release from the ER causes calcium overload in the mitochondria and leads to mitochondrial fragmentation via facilitating the DRP1 translocation to the mitochondrial outer membrane (Breckenridge et al., 2003).
Mitochondria constantly generate ROS via the electron transport chain reaction. At the physiological condition, majority of molecular oxygen is converted to water and less than 5% of the oxygen is incompletely reduced to O2−. Massive ROS production in the mitochondria is implicated in various cardiovascular diseases (Griendling and FitzGerald, 2003; Sugamura and Keaney, 2011), whereas ROS leads to the mitochondrial fragmentation in many cell types like rat cardiac myocytes (Yu et al., 2008; Fan et al., 2010), and mouse coronary endothelial cells (Makino et al., 2010). These data imply that mitochondrial fragmentation is enhanced by excess ROS production in pathophysiological condition.
3) Mitochondrial morphological change in endothelial cells in diabetes
There is increasing evidence showing that mitochondrial morphology is sensitive to the metabolic properties. The exposure of high glucose to endothelial cells ex vivo increases mitochondrial fragmentation (Makino et al., 2010; Trudeau et al., 2010; Shenouda et al., 2011). We demonstrate that mouse coronary endothelial cells isolated form Type-1 diabetic mice exhibit more fragmented mitochondrial structure and lower DRP1 protein expression levels than endothelial cells from control mice (Makino et al., 2010). The study examining the mitochondrial morphology using venous endothelial cells isolated from patients with Type-2 diabetes shows that FIS1 protein expression level and mitochondrial fragmentation are significantly increased in endothelial cells from diabetic patients compared with cells from control patients (Shenouda et al., 2011). Interestingly, mitochondria in retina endothelial cells are more elongated in the diabetic rat compared to the control rat; although MFN2 protein expression level is significantly decreased and DRP1 expression level is increased in diabetes, implying that the mitochondrial morphological change might be regulated by not fission-fusion related proteins in this case (Zhong and Kowluru, 2011).
4) Altered mitochondrial morphology in other cell types in diabetes
The exposure of free fatty acid to the C2C12 muscle cells augments mitochondrial fragmentation via increased DRP1 and FIS1 protein expression levels andmitochondrial in skeletal muscles in obesity and Type-2 diabetic mice are more fragmented (Jheng et al., 2012). In Type-2 diabetic patients, MFN2 protein expression level is decreased in skeletal muscle compared with control patients (Zorzano et al., 2009). High glucose treatment increases mitochondrial fragmentation (Yu et al., 2008; Makino et al., 2011) via decreased OPA1 protein expression in the neonatal cardiac myocyte (Makino et al., 2011). High glucose or high insulin treatment increases the protein expression of DRP1 and enhances mitochondrial fragmentation in adult dorsal root ganglion neurons, suggesting that mitochondrial morphological change might contribute to the neuropathy in Type 2 diabetes (Vincent et al., 2010). Ex vivo high glucose treatment leads to mitochondrial fragmentation in β-cell via increase in DRP1 protein expression (Men et al., 2009). Mitochondria in β-cell from Type-2 diabetic rat exhibit more fragmented structure than that in control (Dlaskova et al., 2010). Mitochondrial fission- and fusion-related proteins are cloned during the last decade and we expect to see more functional roles of these proteins in diabetic complications in the next decade.
Mitochondrial biogenesis
1) Proteins related with mitochondrial biogenesis
The cells undergo mitochondrial biogenesis process in response to varied physiological stimulus and tissue- or signal-specific modification of mitochondrial gene expression and function. Mitochondrial biogenesis is a complex process that involves the synthesis, import, and incorporation of proteins and lipids to the existing mitochondrial reticulum, as well as replication of the mtDNA (Lopez-Lluch et al., 2008). Upon the stimulation of mitochondrial biogenesis, mitochondrial genes in the nucleus and in mitochondria will be transcribed. Majority of genes required for mitochondrial biogenesis and function are in the nucleus, and the few genes crucial for oxidative phosphorylation, are on the mitochondrial gene. The peroxisome proliferator-activated receptor gamma coactivator-1α (PGC-1α) is the nucleus genome-encoded protein and a transcriptional coactivator of nuclear respiratory factor (NRF)-1, GA-binding protein (GABP) (also known as NRF-2), and peroxisome proliferator-activated receptors (PPARs) (Puigserver et al., 1998; Wu et al., 1999; Duncan et al., 2007). The activation of NRF-1 and 2 by PGC-1α leads to the expression of mitochondrial transporters, mitochondrial ribosomal proteins, oxidative phosphorylation components, and mitochondrial transcription factor (TFAM) (Feige and Auwerx, 2007; Scarpulla, 2008). PPARs regulate a broad set of genes which are required for lipid homeostasis and glucose and lipid oxidation (Djouadi et al., 1998; Burkart et al., 2007; Yang and Li, 2007).
PGC-1α activity is regulated by various kinds of posttranslational modifications. Phosphorylation is required for the activation of PGC-1α and its phosphorylation is regulated by AMP-activated protein kinase (Jager et al., 2007), mitogen-activated protein kinase p38 (Knutti et al., 2001; Akimoto et al., 2005), and glycogen synthase kinase-3 (GSK-3) (Anderson et al., 2008), acetylation by Sirtuin 1 (SIRT1) (Rodgers et al., 2005; Gerhart-Hines et al., 2007), and arginine methylation by protein arginine N-methyltransferase1 (PRMT1) (Teyssier et al., 2005). In the muscle cells, the increase in cytosolic Ca2+ leads to PGC-1α activation through Ca2+/calmodulin-dependent kinases and p38 activation (Ojuka et al., 2003; Wright et al., 2007). Nitric oxide (NO) increases PGC-1α expression via cyclic GMP dependent pathway (Nisoli et al., 2003). On the other hand, PGC-1α is negatively regulated via deacetylation by GCN5 (Lerin et al., 2006) and ubiquitination by SCFcdc4 (Olson et al., 2008). 2) Mitochondrial biogenesis in endothelial cells in diabetes
Since the energy in endothelial cells is mainly generated by glycolytic metabolism instead of via mitochondrial ATP synthesis, mitochondrial biogenesis is not as critical as in muscle cells or in adipocytes. It is, however, important to maintain the good quality and quantity of mitochondria in endothelial cells for cell survival. mtDNA copy number, PGC-1α and NRF-1 protein expression in nuclear extract, PGC-1α activity, and TFAM protein expression level in mitochondria are commonly used to determine the mitochondrial biogenesis. Hyperglycemia significantly decreases PGC-1α protein expression in retinal endothelial cells (Zheng et al., 2010). Santos et al. (2011) demonstrate that retinal mtDNA copy number is decreased in Type-1 diabetic mice and the mitochondrial number is lowered in retina in diabetic patient, and in retinal endothelial cells treated with high glucose.
PPARγ activator, thiazolidinedione (TZD), is approved for use in Type-2 diabetic patients to improve insulin sensitivity by several mechanisms, including increased uptake and metabolism of free fatty acids in adipose tissue (Saltiel and Olefsky, 1996; Spiegelman, 1998; Kalaitzidis et al., 2009). Recent reports demonstrate that TZD induces mitochondrial biogenesis via the activation of PGC-1α in human umbilical vein endothelial cells (Fujisawa et al., 2009) and other cell types (see next paragraph). PGC-1α is the coactivator of PPARγ and the activation of PPARγ by TZD induces PGC-1α expression. Further studies are required to identify the role of TZD on mitochondrial biogenesis and the regulatory mechanisms of the positive feedback by PPARγ activation in endothelial cells.
3) Mitochondrial biogenesis in other cell types in diabetes
mtDNA copy number and mRNA expression of PGC-1α and TFAM are significantly decreased in aorta from Type-2 diabetic mice compared with the control (Csiszar et al., 2009). Lowered mtDNA copy number is observed in skeletal muscle from the patient with Type-2 diabetes (Hsieh et al., 2011) and muscles from Type-2 diabetic rats compared with the control (Shen et al., 2008). On the other hand, it has been reported that there is increased mitochondrial area, mitochondrial number and mtDNA in the heart of Type-1 diabetic mice, but the function of mitochondrial is attenuated (Shen et al., 2004).
PGC-1α is induced by TZD in white and brown adipocyte cells (Wilson-Fritch et al., 2004; Hondares et al., 2006), and neuronal cells (Miglio et al., 2009). The adipose tissue obtained from the patient with Type-2 diabetes exhibits lower mitochondrial number and TFAM mRNA expression level compared with the control; and TZD treatment restores the mitochondrial abnormality (Bogacka et al., 2005; Hakansson et al., 2011). Mitochondrial biogenesis is significantly attenuated in adipose tissue from Type-2 diabetic mice compared with the control, whereas TZD increases PGC-1α mRNA expression and restores the mitochondrial biogenesis in diabetic mice (Rong et al., 2007). PGC-1β also serves as a key regulator in energy metabolism by promoting mitochondrial biogenesis. There is increasing evidence showing that TZD enhances mitochondrial biogenesis by increase in PGC-1β expression, but not PGC-1α, in adipocyte cells (Deng et al., 2011; Pardo et al., 2011) and osteocytes (Wei et al., 2010).
Mitochondrial autophagy/mitophagy
1) Molecular mechanisms of mitophagy
Autophagy is cellular degradation system through their encapsulation by a double membrane structure called as an autophagosome (Kelekar, 2005). There are two types of autophagy, non-selective autophagy and cargo-specific autophagy. At the low level of energy demand or at the starvation condition, cells undergo non-selective autophagy to supply/re-use the metabolic component and energy by degradation of their organelles, whereas the cargo-specific autophagy could be initiated independent from the nutrient level (Kundu and Thompson, 2005; Komatsu and Ichimura, 2010; Rabinowitz and White, 2010). The selective elimination of mitochondria is called mitophagy (Lemasters, 2005). The purpose of mitophagy is primarily 1) to maintain the mitochondrial integrity in the cells and 2) to eliminate the damaged mitochondria (Narendra et al., 2008; Twig et al., 2008a). The excess amount of mitochondria at the low energy demand is the source of excessive ROS. The damaged mitochondria release various apoptosis-promoting factors and lead to further damage of neighboring mitochondria and entire cell (Crompton et al., 1999). Therefore, mitophagy is the well-designed cytoprotective pathway.
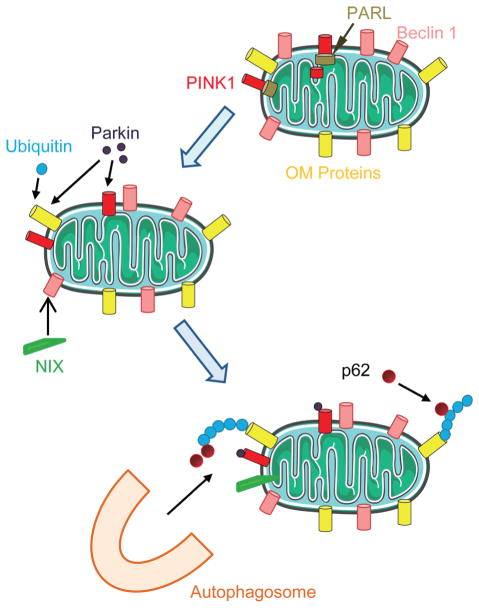
Depolarized mitochondrial membrane is the hallmark of damaged mitochondria and sustains the mitochondrial fission status (Song et al., 2007; Twig et al., 2008a). Mitochondrial fusion is the physiological function to fuse the damaged membrane with intact membrane and minimize the damage. Damaged mitochondria that failed to be fused will be the target of the mitophagy. The PTEN-induced putative kinase protein 1 (PINK1) is a voltage-sensitive kinase and it will be accumulated on the outer membrane in the mitochondrial upon the membrane depolarization (Narendra et al., 2008; Jin et al., 2010; Matsuda et al., 2010). The accumulation of PINK1 facilitates the recruitment of Parkin, an E3 ubiquitin ligase, to the mitochondrial surface (Sha et al., 2010; Vives-Bauza et al., 2010). Ubiquitinated mitochondrial proteins by Parkin interact with the autophagy adaptor p62, and subsequently lead to the autophagosomal degradation of the mitochondria (Geisler et al., 2010; Okatsu et al., 2010) (Fig. 2). Another protein which regulates mitophagy in mammalian cells is NIX (Kanki, 2010), although detailed mechanism is not clear.
Fig. 2.
Mitohpagy. At the healthy condition, the Pten-induced novel kinase 1 (PINK1) is degraded by presenilins-associated rhomboid-like protein (PARL). Upon mitochondrial damage or loss of mitochondrial membrane potential, PINK1 accumulates on the outer mitochondrial membrane (OMM) without degradation and recruits Perkin from the cytosol to the OMM. Perkin ubiquitylates OM proteins and these ubiquitylated proteins are recognized by the adaptor protein p62 and are targeted by autophagosomes and eventually are degraded in lysosomes.
Extensive damage by sustained membrane depolarization facilitates the opening of the mitochondrial permeability transition pore (mPTP), increases mitochondrial membrane permeability and releases of pro-apoptotic molecules, and results in cell apoptosis. This will be described in the following section (4. Mitochondria-induced cell apoptosis).
The autophagy induced by starvation (non-selective autophagy) is mediated by mammalian target of rapamycin (mTOR)/AMP-activated protein kinase (AMPK) pathway. At the high nutrient, mTOR phosphorylates UNC-51-like kinase (ULK) that has inhibitory effects on the kinase activity of ULK. Starvation increases AMPK activation, which promotes mTOR inhibition and activates ULK, and subsequently leads to autophagy (Lee et al., 2010; Egan et al., 2011; Kim et al., 2011). It has to be noted that AMPK increases SIRT1 activity, which deacetylates PGC-1, and results in mitochondrial biogenesis as described above (2. Mitochondrial biogenesis). Therefore, mitochondrial autophagy and biogenesis are coordinately regulated.
There are other factors which possibly regulate the autophagy, including ROS and Bcl2. During autophagic process, autophagy-regulating protein (ATG) 8 conjugates to the autophagosomal membrane through an ubiquitin-like conjugation system. ATG4 negatively regulates ATG8 function by cleavage of ATG8, which releases ATG8 from the autophagosomal membrane and inhibits autophagy (Kaminskyy and Zhivotovsky, 2012). ATG4is redox sensitive and oxidation of ATG4 inhibits the cleavage activity of ATG4 and stabilizes the ATG8-mediated autophagosomal expansion (Scherz-Shouval et al., 2007). Beclin1, the mammalian ortholog of yeast ATG6, was identified as a Bcl-2-interacting protein (Kabeya et al., 2000) and it induces the formation of autophagosomes and promotes autophagy (Sinha and Levine, 2008). Anti-apoptotic protein Bcl-2 binds to Beclin1 and inhibits autophagy (Pattingre et al., 2005; Kang et al., 2011).
2) Mitophagy in diabetes
Interestingly, there is no report which demonstrates the change in mitochondrial autophagy/mitophagy in endothelial cells in diabetes. The exposure of oxidized LDL (ox-LDL) leads to autophagic pathway in HUVECs and HMECs (Zhang et al., 2010; Muller et al., 2011). High-glucose treatment augments autophagy in H9c2 cardiomyoblasts via increase in Beclin1 and LC3 protein expression level (Younce et al., 2010). Cardiac myocytes in Type-1 diabetic mice exhibits decreased autophagy determined by the number of autophagic vacuoles number in the cells (Xie et al., 2011), whereas skeletal muscles from Type-2 diabetic rat show increased autophagy assessed by the protein expression level of LC3 and Beclin1 (Yan et al., 2011). Mitochondrial autophagy is increased in pancreatic cell of Type-2 diabetic mice (Lo et al., 2010) and in adipose tissue in Type-2 diabetic patients (Ost et al., 2010). The reason for these inconsistent results might be due to the differences of the diabetic model, tissues used for the experiments, and methods to determine the mitochondrial autophagy.
Mitochondria-induced cell apoptosis
Mitochondria serve as the key organelles which maintain the cell function via generating ATP, regulating cellular Ca2+ homeostasis and heme biosynthesis, whereas mitochondria also determine the cell fate, as well as terminate the cell life, through several mitochondria-induced cell apoptosis pathways. In this section, we discuss the cell apoptosis pathway induced by mitochondria (e.g., regulation of Bcl-2 protein family, mitochondrial ROS generation and mitochondrial Ca2+ overload).
1) Mitochondria-mediated apoptosis
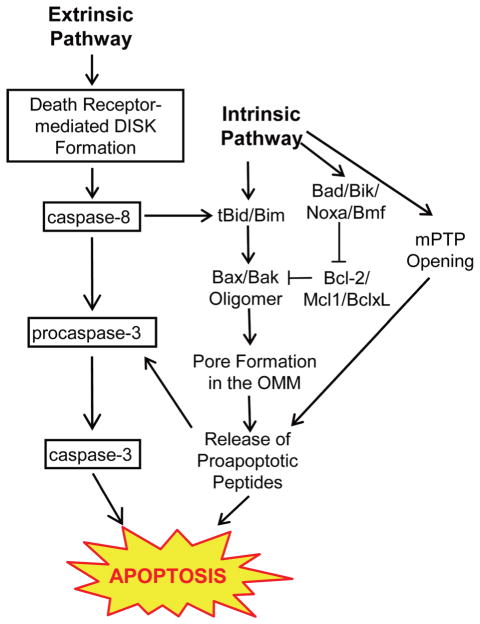
There are two main apoptotic pathways: the extrinsic pathway and intrinsic pathway (Fig. 3). The extrinsic pathway is initiated by the binding of the ligands to the cell surface-specific receptors, called “death receptors”, whereas the intrinsic pathway is initiated by mitochondria. Both pathways are overlap at the point of mitochondrial outer membrane permeabilization (MOMP). MOMP is triggered by the formation of pores in the outer mitochondrial membrane (OMM) and the Bcl-2 protein family regulates the pore formation. Bcl-2 protein families can be classified into four groups based on their functions: 1) effectors whose oligomerization creates pores [Bax and Bak], 2) inhibitors of Bax and Bak [Bcl-2, Mcl1 and BclxL], 3) activators of Bax and Bak [Bid and Bim], and 4) sensitizers which antagonize antiapoptotic Bcl-2 like proteins [Bad, Bik, Noxa and Bmf]. The extrinsic pathway involves formation of a death-inducing signal complex (DISC) in the plasma membrane. DISCs contain multiple adaptor proteins that recruit and promote the activation of initiator procaspases, including procaspase 8. Activated caspase 8 induces cell apoptosis through two pathways; 1) direct cleavage of procaspase 3 followed by the cleavage of a variety of substrates and the cell apoptosis, 2) truncation of Bid to tBid, which leads to Bax/Bak oligomers, creates pores in OMM, and releases proapoptotic peptides such as cytochrome c (Liu et al., 1996), apoptosis inducing factor(AIF) (Susin et al., 1999), endonuclease G (EndoG) (Li et al., 2001), Smac/Diablo (Du et al., 2000), Omi/HtrA2 (Hedge et al., 2002). These molecules activate both caspase dependent and independent cell death pathways (Donovan and Cotter, 2004).
Fig. 3.
Extrinsic Pathway. The binding of the death ligands to the death receptors forms a death-inducing signal complex (DISK). Procaspase 8 is autoactivated at the DISK and converted to the active form, caspase 8. Caspase 8 leads to cell apoptosis via caspase 3 activation and by truncation of Bid to tBid. IntrinsicPathway. Intrinsic pathway is activated by intracellular stimuli (e.g., ROS) and involves the formation of the pore in the OMM (Bax/Bak oligomer) and the opening of the mitochondrial permeability transition pore (mPTP). These pores release proapoptotic peptides and induce cell apoptosis.
The intrinsic pathway involves both MOMP and the opening of the mPTP. The mPTP is a transmembrane channel formed between the inner and outer mitochondrial membranes and composed of voltage-dependent anion channel (VDAC) in OMM, adenine nucleotide translocator (ANT) in the inner mitochondrial membrane, and cyclophilin D in the mitochondrial matrix (Crompton et al., 1999; Halestrap and Pasdois, 2009). VDAC is a bidirectional transporter and permeable to solutes of up to 5 kDa. At the physiological condition, VDAC serves as a shuttle of respiratory chain substrates such as ATP. On the other hand, ANT is impermeable under normal conditions. During apoptosis, excess Ca2+ influx triggers the increase of ANT conductivity, followed by an inward flux of protons and ions through ANT. The increase in matrix osmolality leads to water influx, mitochondrial swelling, and apoptogenic protein release from the mitochondrial storage to the cytosol though the mPTP opening, BAX/BAK-VDAC channel, and or ruptured OMM (Ott et al., 2002; Tsujimoto and Shimizu, 2002; Baines, 2011). It has been reported that the opening of mPTP is regulated by Bcl-2 and pH change in inner mitochondrial membrane (Matsuyama and Reed, 2000).
2) Mitochondrial O2− generation and apoptosis
Mitochondria continuously generate superoxide anion (O2−) through reduction of molecular oxygen by the electron transport chain to produce superoxide. The electron transport chain (ETC) is composed of four multiple subunit complexes; complex I (NADH-ubiquinone oxidoreductase), II (succinate-dehydrogenease), III (ubiquinol-cytochrome c oxidoreductase), and IV (cytochrome c oxidase), and the main function is to oxidize NADH and FADH2 to NAD+ and FAD+, that will be used in the tricarboxylic acid cycle (TCA cycle) to generate ATPs. The protons transported across the membrane in the ETC will serve as a motive force in complex V to synthesize ATPs. O2− is primarily generated at complexes I and III; complex I releases O2− predominately into the matrix, while complex III releases O2− to both sides of the mitochondrial inner membrane (Han et al., 2001; Muller et al., 2004; Lenaz et al., 2006). O2− is dismutated to hydrogen peroxide by CuZn-superoxide dismutase (SOD, in the inter-membrane space and cytosol) and Mn-SOD (in the matrix) (Faraci and Didon, 2004), and subsequently reduced to water by catalase (in the cytosol) and glutathione peroxidase (in mitochondria and cytosol) (Chance et al., 1979; Phung et al., 1994). Majority of O2− is reduced to water and very few O2− is leaked out from the ETC in normal cells, whereas excess O2− production in mitochondria is implicated in the pathogenesis of cardiovascular diseases (Li and Shah, 2004; Ballinger, 2005).
mtDNA is more sensitive than genomic DNA to ROS-induced damage, as it is not protected by histones and its repair capabilities are limited (Wei and Lee, 2002). Damaged mtDNA promotes outer membrane permeabilization and the release of cytochrome c, AIF, or Smac/Diablo from mitochondria to the cytosol and leads to cell apoptosis (Ryter et al., 2007). ROS also stimulates the extrinsic or intrinsic apoptotic signaling via activation of JNK (Dhanasekaran and Reddy, 2008). The translocation of activated JNK to nuclear initiates activator protein 1-mediated expression of proapoptotic factors, such as TNFα, FasL, and Bak (Fan et al., 2001), while the translocation to mitochondria promotes to release cytochrome c (Kharbanda et al., 2000). Furthermore, the interaction of ROS with NO regulates the cell apoptotic pathway (Cassina et al., 2000; Jang and Han, 2006; Nakagawa et al., 2007; Wang et al., 2008). 3) Mitochondrial Ca2+ homeostasis and apoptosis
There are several Ca2+-sensitive intramitochondrial enzymes that regulate physiological cell functions (e.g., pyruvate dehydrogenase phosphate phosphatase, NAD+-isocitrate dehydrogenase) (McCormack and Denton, 1984). It is thus important to maintain appropriate level of Ca2+ in the mitochondrial for their routine work, while Ca2+ overload in mitochondria causes the maladaptive effect on mitochondrial function, as well as cell function, and it is implicated in variety of different disease processing (Esper et al., 2006; Halestrap and Pasdois, 2009). Mitochondrial Ca2+ overload leads to an opening of mPTP and it is followed by cell apoptosis and necrosis (see details in the section 4.1.). Excess mitochondrial [Ca2+] causes an increase in mitochondrial O2− via several mechanisms including by stimulation of the ETC to increase electron leak, by facilitating cytochrome c dislocation and by enhancing NO• generation which blocks complex IV and causes electron leak from complex III (Peng and Jou, 2010), and subsequently leads tomitochondria-mediated cell apoptosis (see details in the section 4.2.). Ca2+ overload in mitochondrial decreases mitochondrial membrane potential, which leads to mitochondrial fission. The fragmented/damaged mitochondria will be the target of the mitophagy, but too much fission will lead to more caspase release and cause cell apoptosis (Jeong and Seol, 2008; Suen et al., 2008; Liesa et al., 2009; Jahani-Asl et al., 2010; Westermann, 2011).
Where is the source of Ca2+ which accumulates in mitochondria? Mitochondria increase their [Ca2+] in response to elevated cytosolic [Ca2+] (Szabadkai et al., 2001; Pitter et al., 2002), and this Ca2+ transfer might be required for the physiological mitochondrial function such as ATP synthesis. There is increasing evidence showing that Ca2+ released from the ER is the main source of mitochondrial Ca2+ overload in pathophysiological condition (reviewed in Contreras et al., 2010; de Brito and Scorrano, 2010; Patergnani et al., 2011). Mitochondrial Ca2+ uptake is achieved by VDAC in the OMM and mitochondrial Ca2+ uniporter (MCU) in the IMM. VDAC is a channel permeable to both anions and cations, and the selectivity of the channel depends on the mitochondrial membrane potential; low potential is more preferable to anion transfer and high potential to cation. It has been demonstrated that VDAC is more permeable to Ca2+ in the closed states of the channel, and thus VDAC closure is a proapoptotic signal (Rostovtseva et al., 2005; Tan and Colombini, 2007). MCU is the highly selective ion channel and Ca2+ uptake by MCU is also driven by the membrane potential (Gunter and Gunter, 1994). It has been shown that Ca2+ has a biphasic effect on the MCU activity. Before reaching a certain level, cytosolic Ca2+ inactivates the uniporter and prevents further Ca2+ uptake. This mechanism allows the mitochondrial Ca2+ oscillation, but it prevents an excessive mitochondrial Ca2+ accumulation. Above the certain range of [Ca2+]cyt, Ca2+ activates MCU by the Ca2+-dependent calmodulin activation (Moreau et al., 2006).
4) Mitochondria-induced endothelial cell apoptosis in diabetes
Pathophysiological changes of metabolic parameters in diabetes are related with or lead to the increase in endothelial apoptosis (Nakagami et al., 2005; Piconi et al., 2006; Leduc et al., 2010; van den Oever et al., 2010; Barber et al., 2011). As described above, cell apoptosis could be induced in a mitochondria-dependent or mitochondria-independent manner, and the mitochondria-dependent cell apoptosis is modulated by mitochondrial functional and morphological changes including the increase in mitochondrial ROS formation, mitochondrial fission, mitochondrial Ca2+ overload, and the opening of mPTP. In addition, these mitochondrial pathophysiological changes interact and regulate each other. Although the initiation of mitochondria-mediated apoptosis could be varied and complex, it seems to be one common downstream, which is the opening of mPTP and the release of the proapoptotic factors from mitochondrial to the cytosol. In diabetes, increased mitochondrial O2− is well documented in endothelial cells (Nishikawa and Araki, 2007; Di Lisa et al., 2009; Giacco and Brownlee, 2010; Cheng et al., 2011). Hyperglycemia leads to increased BAX expression (Meng et al., 2008; Yang et al., 2008; Guan et al., 2011), mitochondrial Ca2+ overload (Paltauf-Doburzynska et al., 2004), opening of mPTP in endothelial cells (Detaille et al., 2005; Huang et al., 2010) and releasing the proapoptotic proteins from the mitochondria (Kowluru and Abbas, 2003; Detaille et al., 2005; Kowluru, 2005; Leal et al., 2009; Li et al., 2009; Trudeau et al., 2010; Chong et al., 2011; Li et al., 2011), and subsequently increases endothelial apoptosis. Type-2 diabetic mellitus is usually accompanied by hyperlipidemia and ox-LDL accumulation (Shimada et al., 2004). Increased ox-LDL also induces mitochondria-mediated apoptosis in endothelial cells (Zhang et al., 2003; Chen et al., 2004; Vindis et al., 2005; Takabe et al., 2010; Chang et al., 2011). These data suggest that the changes of metabolic parameter in diabetes lead to endothelial cell apoptosis via mitochondrial dysfunction that may regulate the vascular permeability and capillary density as well as the vascular tone in diabetes.
5) Mitochondria-mediated apoptosis in other cell types in diabetes
Although there are many reports describing the mitochondria-mediated cell apoptosis in diabetes (reviewed in Duchen, 2004; Allen et al., 2005; Joza et al., 2009 Szabadkai and Duchen, 2009), it is limited number of reports in which the actual assessment of the mPTP activity is carried out. Most prominent cell types which were examined for mPTP opening in diabetes are the cardiac myocyte. Cardiac myocytes isolated from diabetic patients (Anderson et al., 2010) and diabetic animal models (Oliveira, 2005; Bhamra et al., 2008; Williamson et al., 2010; Lumini-Oliveira et al., 2011) exhibit augmented mPTP activity compared with the control. On the other hand, the increase in proapoptotic protein in the cytosol has been demonstrated in many cell types in diabetes, which is the downstream cascade of the mPTP opening (reviewed in (Adeghate, 2004; Duchen, 2004).
Apoptosis plays an important role in biological processes and various pathophysiological events. An alteration of mitochondrial function is heavily involved in the death pathway, which results in the cardiovascular dysfunction in many diseases. The molecular mechanisms of mitochondria-mediated cell apoptosis has been extensively studied during past 10 years and it greatly helps understanding the cell fate determined by mitochondria in diabetes.
Conclusion
Mitochondria are small organelles in the cytosol and have been known as an ATP producing organelle. During past decades, their role has been expanded not only in the physiological cell function, but also in the development and progression of many diseases including in diabetes. Main function of mitochondrial morphological changes is to ensure proper inheritance and distribution of mitochondria and to maintain them in a healthy state. Mitochondrial autophagy takes care of damaged mitochondria to minimize the maladaptive effect on cell functions and mitochondrial biogenesis keeps energy supply to the cell demands. Any abnormal alteration in these steps affects cell fate and tissue functions.
Endothelial dysfunction is the key risk factor of complications seen in diabetes, and here we demonstrate that mitochondrial dysfunction in endothelial cells represent a crucial step in the development of endothelial dysfunction. There are still many things to be examined to define mitochondrial pathophysiological role in endothelial function in diabetes, such as the relation between mitochondrial autophagy and endothelial dysfunction in diabetes and the contribution of mitochondrial abnormality to decreased quantity and quality of circulating endothelial progenitor cells in diabetes. Mitochondrial morphological and functional alteration in endothelial cells will remain an exciting field of diabetic research in another decade.
Acknowledgments
This work was supported by the grant of DK083506 (A. Makino) from the National Institutes of Health.
References
- Adeghate E. Molecular and cellular basis of the aetiology and management of diabetic cardiomyopathy: a short review. Mol Cell Biochem. 2004;261:187–191. doi: 10.1023/b:mcbi.0000028755.86521.11. [DOI] [PubMed] [Google Scholar]
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, Williams RS, Yan Z. Exercise stimulates Pgc-1alpha transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Allen DA, Yaqoob MM, Harwood SM. Mechanisms of high glucose-induced apoptosis and its relationship to diabetic complications. J Nutr Biochem. 2005;16:705–713. doi: 10.1016/j.jnutbio.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Amchenkova AA, Bakeeva LE, Chentsov YS, Skulachev VP, Zorov DB. Coupling membranes as energy-transmitting cables. I. Filamentous mitochondria in fibroblasts and mitochondrial clusters in cardiomyocytes. J Cell Biol. 1988;107:481–495. doi: 10.1083/jcb.107.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Rodriguez E, Anderson CA, Thayne K, Chitwood WR, Kypson AP. Increased propensity for cell death in diabetic human heart is mediated by mitochondrial-dependent pathways. Am J Physiol. 2010;300:H118–H124. doi: 10.1152/ajpheart.00932.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Barger JL, Edwards MG, Braun KH, O’Connor CE, Prolla TA, Weindruch R. Dynamic regulation of PGC-1alpha localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell. 2008;7:101–111. doi: 10.1111/j.1474-9726.2007.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines CP. The mitochondrial permeability transition pore and the cardiac necrotic program. Pediatr Cardiol. 2011;32:258–262. doi: 10.1007/s00246-010-9880-9. [DOI] [PubMed] [Google Scholar]
- Ballinger SW. Mitochondrial dysfunction in cardiovascular disease. Free Radic Biol Med. 2005;38:1278–1295. doi: 10.1016/j.freeradbiomed.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, Abcouwer SF. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2011;52:1156–1163. doi: 10.1167/iovs.10-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner-Parzer SM, Wagner L, Pettermann M, Gessl A, Waldhausl W. Modulation by high glucose of adhesion molecule expression in cultured endothelial cells. Diabetologia. 1995;38:1367– 1370. doi: 10.1007/BF00401771. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Voth M. Dynamics of mitochondria in living cells: shape changes, dislocations, fusion, and fission of mitochondria. Microsc Res Tech. 2008;27:198–219. doi: 10.1002/jemt.1070270303. [DOI] [PubMed] [Google Scholar]
- Bereiter-Hahn J, Voth M, Mai S, Jendrach M. Structural implications of mitochondrial dynamics. Biotechnol J. 2008;3:765–780. doi: 10.1002/biot.200800024. [DOI] [PubMed] [Google Scholar]
- Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- Bloomgarden ZT. Type 2 diabetes in the young: the evolving epidemic. Diabetes Care. 2004;27:998–1010. doi: 10.2337/diacare.27.4.998. [DOI] [PubMed] [Google Scholar]
- Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- Braschi E, Zunino R, McBride HM. MAPL is a new mitochondrial SUMO E3 ligase that regulates mitochondrial fission. EMBO Rep. 2009;10:748–754. doi: 10.1038/embor.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC. Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol. 2003;160:1115–1127. doi: 10.1083/jcb.200212059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–3939. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- Cassina AM, Hodara R, Souza JM, Thomson L, Castro L, Ischiropoulos H, Freeman BA, Radi R. Cytochrome c nitration by peroxynitrite. J Biol Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Chang HC, Chen TG, Tai YT, Chen TL, Chiu WT, Chen RM. Resveratrol attenuates oxidized LDL-evoked Lox-1 signaling and consequently protects against apoptotic insults to cerebrovascular endothelial cells. J Cereb Blood Flow Metab. 2011;31:842–854. doi: 10.1038/jcbfm.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Chan DC. Emerging functions of mammalian mitochondrial fusion and fission. Hum Mol Genet. 2005;14(Spec No. 2):R283–R289. doi: 10.1093/hmg/ddi270. [DOI] [PubMed] [Google Scholar]
- Chen H, McCaffery JM, Chan DC. Mitochondrial fusion protects against neurodegeneration in the cerebellum. Cell. 2007;130:548–562. doi: 10.1016/j.cell.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Chen J, Mehta JL, Haider N, Zhang X, Narula J, Li D. Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res. 2004;94:370–376. doi: 10.1161/01.RES.0000113782.07824.BE. [DOI] [PubMed] [Google Scholar]
- Cheng X, Siow RC, Mann GE. Impaired redox signaling and antioxidant gene expression in endothelial cells in diabetes: a role for mitochondria and the nuclear factor-E2-related factor 2-Kelch-like ECH-associated protein 1 defense pathway. Antioxid Redox Signal. 2011;14:469–487. doi: 10.1089/ars.2010.3283. [DOI] [PubMed] [Google Scholar]
- Cho DH, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-nitrosylation of Drp1 mediates beta-amyloid-related mitochondrial fission and neuronal injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Hou J, Shang YC, Wang S, Maiese K. EPO relies upon novel signaling of Wnt1 that requires Akt1, FoxO3a, GSK-3beta, and beta-catenin to foster vascular integrity during experimental diabetes. Curr Neurovasc Res. 2011;8:103–120. doi: 10.2174/156720211795495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101:15927–15932. doi: 10.1073/pnas.0407043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger JD. Endothelial regulation of vascular tone. Hosp Pract (Off Ed) 1994;29:117–122. 125–116. doi: 10.1080/21548331.1994.11443095. [DOI] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Cribbs JT, Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M, Virji S, Doyle V, Johnson N, Ward JM. The mitochondrial permeability transition pore. Biochem Soc Symp. 1999;66:167–179. doi: 10.1042/bss0660167. [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, Ungvari Z. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culic O, Gruwel ML, Schrader J. Energy turnover of vascular endothelial cells. Am J Physiol. 1997;273:C205–C213. doi: 10.1152/ajpcell.1997.273.1.C205. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Duchen MR. Endothelial mitochondria: contributing to vascular function and disease. Circ Res. 2007;100:1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 2010;29:2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E, Tournier-Lasserve E, Weinstein BM. The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev Cell. 2009;16:209–221. doi: 10.1016/j.devcel.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- Deng T, Sieglaff DH, Zhang A, Lyon CJ, Ayers SD, Cvoro A, Gupte AA, Xia X, Baxter JD, Webb P, Hsueh WA. A peroxisome proliferator-activated receptor gamma (PPARgamma)/PPARgamma coactivator 1beta autoregulatory loop in adipocyte mitochondrial function. J Biol Chem. 2011;286:30723–30731. doi: 10.1074/jbc.M111.251926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC. Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol. 2007;8:870–879. doi: 10.1038/nrm2275. [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lisa F, Kaludercic N, Carpi A, Menabo R, Giorgio M. Mitochondria and vascular pathology. Pharmacol Rep. 2009;61:123–130. doi: 10.1016/s1734-1140(09)70014-3. [DOI] [PubMed] [Google Scholar]
- Djouadi F, Weinheimer CJ, Saffitz JE, Pitchford C, Bastin J, Gonzalez FJ, Kelly DP. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator- activated receptor alpha- deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlaskova A, Spacek T, Santorova J, Plecita-Hlavata L, Berkova Z, Saudek F, Lessard M, Bewersdorf J, Jezek P. 4Pi microscopy reveals an impaired three-dimensional mitochondrial network of pancreatic islet beta-cells, an experimental model of type-2 diabetes. Biochim Biophys Acta. 2010;1797:1327–1341. doi: 10.1016/j.bbabio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Donovan M, Cotter TG. Control of mitochondrial integrity by Bcl-2 family members and caspase-independent cell death. Biochim Biophys Acta. 2010;1644:133–147. doi: 10.1016/j.bbamcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Dora KA. Coordination of vasomotor responses by the endothelium. Circ J. 2010;74:226–232. doi: 10.1253/circj.cj-09-0879. [DOI] [PubMed] [Google Scholar]
- Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- Duchen MR. Roles of mitochondria in health and disease. Diabetes. 2004;53(Suppl 1):S96–S102. doi: 10.2337/diabetes.53.2007.s96. [DOI] [PubMed] [Google Scholar]
- Duncan JG, Fong JL, Medeiros DM, Finck BN, Kelly DP. Insulin-resistant heart exhibits a mitochondrial biogenic response driven by the peroxisome proliferator-activated receptor-alpha/PGC-1alpha gene regulatory pathway. Circulation. 2007;115:909–917. doi: 10.1161/CIRCULATIONAHA.106.662296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan M, Goodwin ME, Birrer MJ, Chambers TC. The c-Jun NH(2)-terminal protein kinase/AP-1 pathway is required for efficient apoptosis induced by vinblastine. Cancer Res. 2001;61:4450–4458. [PubMed] [Google Scholar]
- Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med. 2010;49:1646–1654. doi: 10.1016/j.freeradbiomed.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Didion SP. Vascular protection: superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1373. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- Farhangkhoee H, Khan ZA, Kaur H, Xin X, Chen S, Chakrabarti S. Vascular endothelial dysfunction in diabetic cardiomyopathy: pathogenesis and potential treatment targets. Pharmacol Ther. 2006;111:384–399. doi: 10.1016/j.pharmthera.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Feige JN, Auwerx J. Transcriptional coregulators in the control of energy homeostasis. Trends Cell Biol. 2007;17:292–301. doi: 10.1016/j.tcb.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Ferre M, Amati-Bonneau P, Tourmen Y, Malthiery Y, Reynier P. eOPA1: an online database for OPA1 mutations. Hum Mutat. 2005;25:423–428. doi: 10.1002/humu.20161. [DOI] [PubMed] [Google Scholar]
- Frazier AE, Kiu C, Stojanovski D, Hoogenraad NJ, Ryan MT. Mitochondrial morphology and distribution in mammalian cells. Biol Chem. 2006;387:1551–1558. doi: 10.1515/BC.2006.193. [DOI] [PubMed] [Google Scholar]
- Fujisawa K, Nishikawa T, Kukidome D, Imoto K, Yamashiro T, Motoshima H, Matsumura T, Araki E. TZDs reduce mitochondrial ROS production and enhance mitochondrial biogenesis. Biochem Biophys Res Commun. 2009;379:43–48. doi: 10.1016/j.bbrc.2008.11.141. [DOI] [PubMed] [Google Scholar]
- Gandre-Babbe S, van der Bliek AM. The novel tail-anchored membrane protein Mff controls mitochondrial and peroxisomal fission in mammalian cells. Mol Biol Cell. 2008;19:2402–2412. doi: 10.1091/mbc.E07-12-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griendling KK, FitzGerald GA. Oxidative stress and cardiovascular injury: Part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM. Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol. 2007;178:757–764. doi: 10.1083/jcb.200704112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Q, Zhang Y, Yu C, Liu Y, Gao L, Zhao J. Hydrogen sulfide protects against high glucose-induced apoptosis in endothelial cells. J Cardiovasc Pharmacol. 2011;2011 doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- Hakansson J, Eliasson B, Smith U, Enerback S. Adipocyte mitochondrial genes and the forkhead factor FOXC2 are decreased in type 2 diabetes patients and normalized in response to rosiglitazone. Diabetol Metab Syndr. 2011;3:32. doi: 10.1186/1758-5996-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales KG. The machinery of mitochondrial fusion, division, and distribution, and emerging connections to apoptosis. Mitochondrion. 2004;4:285–308. doi: 10.1016/j.mito.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Halestrap AP, Pasdois P. The role of the mitochondrial permeability transition pore in heart disease. Biochim Biophys Acta. 2009;1787:1402–1415. doi: 10.1016/j.bbabio.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Han D, Williams E, Cadenas E. Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem J. 2001;353:411–416. doi: 10.1042/0264-6021:3530411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder Z, Zunino R, McBride H. Sumo1 conjugates mitochondrial substrates and participates in mitochondrial fission. Curr Biol. 2004;14:340–345. doi: 10.1016/j.cub.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Rizzuto R, Hajnoczky G, Su TP. MAM: more than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187:959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R, Srinivasula SM, Zhang Z, Wassell R, Mukattash R, Cilenti L, DuBois G, Lazebnik Y, Zervos AS, Fernandes-Alnemri T, Alnemri ES. Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem. 2002;277:432–438. doi: 10.1074/jbc.M109721200. [DOI] [PubMed] [Google Scholar]
- Hermans MP. Diabetes and the endothelium. Acta Clin Belg. 2007;62:97–101. doi: 10.1179/acb.2007.017. [DOI] [PubMed] [Google Scholar]
- Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- Hondares E, Mora O, Yubero P, Rodriguez de la Concepcion M, Iglesias R, Giralt M, Villarroya F. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-coactivator (PGC)-1alpha gene transcription: an autoregulatory loop controls PGC-1alpha expression in adipocytes via peroxisome proliferator-activated receptor-gamma coactivation. Endocrinology. 2006;147:2829–2838. doi: 10.1210/en.2006-0070. [DOI] [PubMed] [Google Scholar]
- Hsieh CJ, Weng SW, Liou CW, Lin TK, Chen JB, Tiao MM, Hung YT, Chen IY, Huang WT, Wang PW. Tissue-specific differences in mitochondrial DNA content in type 2 diabetes. Diabetes Res Clin Pract. 2011;92:106–110. doi: 10.1016/j.diabres.2011.01.010. [DOI] [PubMed] [Google Scholar]
- Huang QR, Li Q, Chen YH, Li L, Liu LL, Lei SH, Chen HP, Peng WJ, He M. Involvement of anion exchanger-2 in apoptosis of endothelial cells induced by high glucose through an mPTP-ROS-Caspase-3 dependent pathway. Apoptosis. 2010;15:693–704. doi: 10.1007/s10495-010-0477-9. [DOI] [PubMed] [Google Scholar]
- Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahani-Asl A, Germain M, Slack RS. Mitochondria: joining forces to thwart cell death. Biochim Biophys Acta. 2010;1802:162–166. doi: 10.1016/j.bbadis.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Jang B, Han S. Biochemical properties of cytochrome c nitrated by peroxynitrite. Biochimie. 2006;88:53–58. doi: 10.1016/j.biochi.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Jeong SY, Seol DW. The role of mitochondria in apoptosis. BMB Rep. 2008;41:11–22. doi: 10.5483/bmbrep.2008.41.1.011. [DOI] [PubMed] [Google Scholar]
- Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou MJ. Pathophysiological and pharmacological implications of mitochondria-targeted reactive oxygen species generation in astrocytes. Adv Drug Deliv Rev. 2008;60:1512–1526. doi: 10.1016/j.addr.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Joza N, Pospisilik JA, Hangen E, Hanada T, Modjtahedi N, Penninger JM, Kroemer G. AIF: not just an apoptosis-inducing factor. Ann N Y Acad Sci. 2009;1171:2–11. doi: 10.1111/j.1749-6632.2009.04681.x. [DOI] [PubMed] [Google Scholar]
- Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis RG, Sarafidis PA, Bakris GL. Effects of thiazolidinediones beyond glycaemic control. Curr Pharm Des. 2009;15:529–536. doi: 10.2174/138161209787315693. [DOI] [PubMed] [Google Scholar]
- Kaminskyy V, Zhivotovsky B. Proteases in autophagy. Biochim Biophys Acta. 2012;1824:44–50. doi: 10.1016/j.bbapap.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki T. Nix, a receptor protein for mitophagy in mammals. Autophagy. 2010;6:433–435. doi: 10.4161/auto.6.3.11420. [DOI] [PubMed] [Google Scholar]
- Kelekar A. Autophagy. Ann N Y Acad Sci. 2005;1066:259–271. doi: 10.1196/annals.1363.015. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95–103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott AB, Perkins G, Schwarzenbacher R, Bossy-Wetzel E. Mitochondrial fragmentation in neurodegeneration. Nat Rev Neurosci. 2008;9:505–518. doi: 10.1038/nrn2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci U S A. 2001;98:9713–9718. doi: 10.1073/pnas.171184698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ichimura Y. Selective autophagy regulates various cellular functions. Genes Cells. 2010;15:923–933. doi: 10.1111/j.1365-2443.2010.01433.x. [DOI] [PubMed] [Google Scholar]
- Kowluru RA. Diabetic retinopathy: mitochondrial dysfunction and retinal capillary cell death. Antioxid Redox Signal. 2005;7:1581–1587. doi: 10.1089/ars.2005.7.1581. [DOI] [PubMed] [Google Scholar]
- Kowluru RA, Abbas SN. Diabetes-induced mitochondrial dysfunction in the retina. Invest Ophthalmol Vis Sci. 2003;44:5327–5334. doi: 10.1167/iovs.03-0353. [DOI] [PubMed] [Google Scholar]
- Kundu M, Thompson CB. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005;12(Suppl 2):1484–1489. doi: 10.1038/sj.cdd.4401780. [DOI] [PubMed] [Google Scholar]
- Leal EC, Aveleira CA, Castilho AF, Serra AM, Baptista FI, Hosoya K, Forrester JV, Ambrosio AF. High glucose and oxidative/nitrosative stress conditions induce apoptosis in retinal endothelial cells by a caspase-independent pathway. Exp Eye Res. 2009;88:983–991. doi: 10.1016/j.exer.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: possible role of the mitochondria. Eur J Obstet Gynecol Reprod Biol. 2010;149:127–130. doi: 10.1016/j.ejogrb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Lee JW, Park S, Takahashi Y, Wang HG. The association of AMPK with ULK1 regulates autophagy. PLoS One. 2010;5:e15394. doi: 10.1371/journal.pone.0015394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- Lenaz G, Fato R, Genova ML, Bergamini C, Bianchi C, Biondi A. Mitochondrial Complex I: structural and functional aspects. Biochim Biophys Acta. 2006;1757:1406–1420. doi: 10.1016/j.bbabio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Lerin C, Rodgers JT, Kalume DE, Kim SH, Pandey A, Puigserver P. GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 2006;3:429–438. doi: 10.1016/j.cmet.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Li J, Chen X, Xiao W, Ma W, Li T, Huang J, Liu X, Liang X, Tang S, Luo Y. Mitochondria- targeted antioxidant peptide SS31 attenuates high glucose-induced injury on human retinal endothelial cells. Biochem Biophys Res Commun. 2011;404:349–356. doi: 10.1016/j.bbrc.2010.11.122. [DOI] [PubMed] [Google Scholar]
- Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412:95–99. doi: 10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu H, Khardori R, Song YH, Lu YW, Geng YJ. Insulin-like growth factor-1 receptor activation prevents high glucose-induced mitochondrial dysfunction, cytochrome-c release and apoptosis. Biochem Biophys Res Commun. 2009;384:259–264. doi: 10.1016/j.bbrc.2009.04.113. [DOI] [PubMed] [Google Scholar]
- Liesa M, Palacin M, Zorzano A. Mitochondrial dynamics in mammalian health and disease. Physiol Rev. 2009;89:799–845. doi: 10.1152/physrev.00030.2008. [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Lo MC, Lu CI, Chen MH, Chen CD, Lee HM, Kao SH. Glycoxidative stress-induced mitophagy modulates mitochondrial fates. Ann N Y Acad Sci. 2010;1201:1–7. doi: 10.1111/j.1749-6632.2010.05630.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumini-Oliveira J, Magalhaes J, Pereira CV, Moreira AC, Oliveira PJ, Ascensao A. Endurance training reverts heart mitochondrial dysfunction, permeability transition and apoptotic signaling in long-term severe hyperglycemia. Mitochondrion. 2011;11:54–63. doi: 10.1016/j.mito.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Madeddu P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp Physiol. 2005;90:315–326. doi: 10.1113/expphysiol.2004.028571. [DOI] [PubMed] [Google Scholar]
- Makino A, Scott BT, Dillmann WH. Mitochondrial fragmentation and superoxide anion production in coronary endothelial cells from a mouse model of type 1 diabetes. Diabetologia. 2010;53:1783–1794. doi: 10.1007/s00125-010-1770-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol. 2011;300:R1296–R1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannella CA, Buttle K, Rath BK, Marko M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors. 1998;8:225–228. doi: 10.1002/biof.5520080309. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S, Reed JC. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000;7:1155–1165. doi: 10.1038/sj.cdd.4400779. [DOI] [PubMed] [Google Scholar]
- McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- McCormack JG, Denton RM. Role of Ca2+ ions in the regulation of intramitochondrial metabolism in rat heart. Evidence from studies with isolated mitochondria that adrenaline activates the pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase complexes by increasing the intramitochondrial concentration of Ca2+ Biochem J. 1984;218:235–247. doi: 10.1042/bj2180235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men X, Wang H, Li M, Cai H, Xu S, Zhang W, Xu Y, Ye L, Yang W, Wollheim CB, Lou J. Dynamin-related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol. 2009;41:879–890. doi: 10.1016/j.biocel.2008.08.031. [DOI] [PubMed] [Google Scholar]
- Meng X, Li ZM, Zhou YJ, Cao YL, Zhang J. Effect of the antioxidant alpha-lipoic acid on apoptosis in human umbilical vein endothelial cells induced by high glucose. Clin Exp Med. 2008;8:43–49. doi: 10.1007/s10238-008-0155-1. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Collino M, Lombardi G, Fantozzi R. PPARgamma stimulation promotes mitochondrial biogenesis and prevents glucose deprivation-induced neuronal cell loss. Neurochem Int. 2009;55:496–504. doi: 10.1016/j.neuint.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Moreau B, Nelson C, Parekh AB. Biphasic regulation of mitochondrial Ca2+ uptake by cytosolic Ca2+ concentration. Curr Biol. 2006;16:1672–1677. doi: 10.1016/j.cub.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multi-step process requiring the novel integral membrane component Fis1p. J Cell Biol. 2000;151:367– 380. doi: 10.1083/jcb.151.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Salvayre R, Negre-Salvayre A, Vindis C. HDLs inhibit endoplasmic reticulum stress and autophagic response induced by oxidized LDLs. Cell Death Differ. 2011;18:817–828. doi: 10.1038/cdd.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller FL, Liu Y, Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- Nakada K, Inoue K, Ono T, Isobe K, Ogura A, Goto YI, Nonaka I, Hayashi JI. Inter-mitochondrial complementation: Mitochondria-specific system preventing mice from expression of disease phenotypes by mutant mtDNA. Nat Med. 2001;7:934–940. doi: 10.1038/90976. [DOI] [PubMed] [Google Scholar]
- Nakagami H, Kaneda Y, Ogihara T, Morishita R. Endothelial dysfunction in hyperglycemia as a trigger of atherosclerosis. Curr Diabetes Rev. 2005;1:59–63. doi: 10.2174/1573399052952550. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Komai N, Takusagawa M, Miura Y, Toda T, Miyata N, Ozawa T, Ikota N. Nitration of specific tyrosine residues of cytochrome C is associated with caspase-cascade inactivation. Biol Pharm Bull. 2007;30:15–20. doi: 10.1248/bpb.30.15. [DOI] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa T, Araki E. Impact of mitochondrial ROS production in the pathogenesis of diabetes mellitus and its complications. Antioxid Redox Signal. 2007;9:343–353. doi: 10.1089/ars.2006.1458. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Ojuka EO, Jones TE, Han DH, Chen M, Holloszy JO. Raising Ca2+ in L6 myotubes mimics effects of exercise on mitochondrial biogenesis in muscle. FASEB J. 2003;17:675–681. doi: 10.1096/fj.02-0951com. [DOI] [PubMed] [Google Scholar]
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, Tanaka K, Matsuda N. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichon A, Guillou E, Delettre C, Landes T, Arnaune-Pelloquin L, Emorine LJ, Mils V, Daloyau M, Hamel C, Amati-Bonneau P, Bonneau D, Reynier P, Lenaers G, Belenguer P. Mitochondrial dynamics and disease, OPA1. Biochim Biophys Acta. 2006;1763:500–509. doi: 10.1016/j.bbamcr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Oliveira PJ. Cardiac mitochondrial alterations observed in hyperglycaemic rats—what can we learn from cell biology? Curr Diabetes Rev. 2005;1:11–21. doi: 10.2174/1573399052952578. [DOI] [PubMed] [Google Scholar]
- Olson BL, Hock MB, Ekholm-Reed S, Wohlschlegel JA, Dev KK, Kralli A, Reed SI. SCFCdc4 acts antagonistically to the PGC-1alpha transcriptional coactivator by targeting it for ubiquitin- mediated proteolysis. Genes Dev. 2008;22:252–264. doi: 10.1101/gad.1624208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ost A, Svensson K, Ruishalme I, Brannmark C, Franck N, Krook H, Sandstrom P, Kjolhede P, Stralfors P. Attenuated mTOR signaling and enhanced autophagy in adipocytes from obese patients with type 2 diabetes. Mol Med. 2010;16:235–246. doi: 10.2119/molmed.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otera H, Wang C, Cleland MM, Setoguchi K, Yokota S, Youle RJ, Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paltauf-Doburzynska J, Malli R, Graier WF. Hyperglycemic conditions affect shape and Ca2+ homeostasis of mitochondria in endothelial cells. J Cardiovasc Pharmacol. 2004;44:423–436. doi: 10.1097/01.fjc.0000139449.64337.1b. [DOI] [PubMed] [Google Scholar]
- Pardo R, Enguix N, Lasheras J, Feliu JE, Kralli A, Villena JA. Rosiglitazone-Induced Mitochondrial Biogenesis in White Adipose Tissue Is Independent of Peroxisome Proliferator-Activated Receptor gamma Coactivator-1alpha. PLoS One. 2011;6:e26989. doi: 10.1371/journal.pone.0026989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YY, Lee S, Karbowski M, Neutzner A, Youle RJ, Cho H. Loss of MARCH5 mitochondrial E3 ubiquitin ligase induces cellular senescence through dynamin-related protein 1 and mitofusin 1. J Cell Sci. 2010;123:619–626. doi: 10.1242/jcs.061481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patergnani S, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Giorgi C, Marchi S, Missiroli S, Poletti F, Rimessi A, Duszynski J, Wieckowski MR, Pinton P. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927– 939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Peng TI, Jou MJ. Oxidative stress caused by mitochondrial calcium overload. Ann N Y Acad Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- Phung CD, Ezieme JA, Turrens JF. Hydrogen peroxide metabolism in skeletal muscle mitochondria. Arch Biochem Biophys. 1994;315:479–482. doi: 10.1006/abbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- Piconi L, Quagliaro L, Assaloni R, Da Ros R, Maier A, Zuodar G, Ceriello A. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006;22:198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- Pitter JG, Maechler P, Wollheim CB, Spat A. Mitochondria respond to Ca2+ already in the submicromolar range: correlation with redox state. Cell Calcium. 2002;31:97–104. doi: 10.1054/ceca.2001.0264. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Quintero M, Colombo SL, Godfrey A, Moncada S. Mitochondria as signaling organelles in the vascular endothelium. Proc Natl Acad Sci U S A. 2006;103:5379–5384. doi: 10.1073/pnas.0601026103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JD, White E. Autophagy and metabolism. Science. 2010;330:1344–1348. doi: 10.1126/science.1193497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RM, Yang L, Garcia-Cardena G, Luscinskas FW. Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ Res. 2007;101:234–247. doi: 10.1161/CIRCRESAHA.107.151860b. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, Giorgi C, Leo S, Rimessi A, Siviero R, Zecchini E, Pinton P. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- Rosenbloom AL, Joe JR, Young RS, Winter WE. Emerging epidemic of type 2 diabetes in youth. Diabetes Care. 1999;22:345–354. doi: 10.2337/diacare.22.2.345. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J Bioenerg Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes. 1996;45:1661–1669. doi: 10.2337/diab.45.12.1661. [DOI] [PubMed] [Google Scholar]
- Santos JM, Tewari S, Goldberg AF, Kowluru RA. Mitochondrial biogenesis and the development of diabetic retinopathy. Free Radic Biol Med. 2011;51:1849–1860. doi: 10.1016/j.freeradbiomed.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Vascular inflammation in hypertension and diabetes: molecular mechanisms and therapeutic interventions. Clin Sci (Lond) 2007;112:375–384. doi: 10.1042/CS20060247. [DOI] [PubMed] [Google Scholar]
- Scarpulla RC. Transcriptional paradigms in mammalian mitochondrial biogenesis and function. Physiol Rev. 2008;88:611–638. doi: 10.1152/physrev.00025.2007. [DOI] [PubMed] [Google Scholar]
- Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007;26:1749–1760. doi: 10.1038/sj.emboj.7601623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Cassidy-Stone A, Meeusen SL, Nunnari J. Staying in aerobic shape: how the structural integrity of mitochondria and mitochondrial DNA is maintained. Curr Opin Cell Biol. 2003;15:482–488. doi: 10.1016/s0955-0674(03)00070-x. [DOI] [PubMed] [Google Scholar]
- Sha D, Chin LS, Li L. Phosphorylation of parkin by Parkinson disease-linked kinase PINK1 activates parkin E3 ligase function and NF-kappaB signaling. Hum Mol Genet. 2010;19:352–363. doi: 10.1093/hmg/ddp501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hao J, Tian C, Ren J, Yang L, Li X, Luo C, Cotma CW, Liu J. A combination of nutriments improves mitochondrial biogenesis and function in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. PLoS One. 2008;3:e2328. doi: 10.1371/journal.pone.0002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Jr, Klein JB, Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab. 2004;287:E896–905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Mokuno H, Matsunaga E, Miyazaki T, Sumiyoshi K, Kume A, Miyauchi K, Daida H. Predictive value of circulating oxidized LDL for cardiac events in type 2 diabetic patients with coronary artery disease. Diabetes Care. 2004;27:843–844. doi: 10.2337/diacare.27.3.843. [DOI] [PubMed] [Google Scholar]
- Sinha S, Levine B. The autophagy effector Beclin 1: a novel BH3-only protein. Oncogene. 2008;27(Suppl 1):S137–148. doi: 10.1038/onc.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev VP. Mitochondrial filaments and clusters as intracellular power-transmitting cables. Trends Biochem Sci. 2001;26:23–29. doi: 10.1016/s0968-0004(00)01735-7. [DOI] [PubMed] [Google Scholar]
- Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- Spinetti G, Kraenkel N, Emanueli C, Madeddu P. Diabetes and vessel wall remodelling: from mechanistic insights to regenerative therapies. Cardiovasc Res. 2008;78:265–273. doi: 10.1093/cvr/cvn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22:1577–1590. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51:978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR. Mitochondria mediated cell death in diabetes. Apoptosis. 2009;14:1405–1423. doi: 10.1007/s10495-009-0363-5. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Pitter JG, Spat A. Cytoplasmic Ca2+ at low submicromolar concentration stimulates mitochondrial metabolism in rat luteal cells. Pflugers Arch. 2001;441:678–685. doi: 10.1007/s004240000466. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell. 2004;16:59–68. doi: 10.1016/j.molcel.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- Takabe W, Li R, Ai L, Yu F, Berliner JA, Hsiai TK. Oxidized low-density lipoprotein-activated c-Jun NH2-terminal kinase regulates manganese superoxide dismutase ubiquitination: implication for mitochondrial redox status and apoptosis. Arterioscler Thromb Vasc Biol. 2010;30:436–441. doi: 10.1161/ATVBAHA.109.202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W, Colombini M. VDAC closure increases calcium ion flux. Biochim Biophys Acta. 2007;1768:2510–2515. doi: 10.1016/j.bbamem.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier C, Ma H, Emter R, Kralli A, Stallcup MR. Activation of nuclear receptor coactivator PGC-1alpha by arginine methylation. Genes Dev. 2005;19:1466–1473. doi: 10.1101/gad.1295005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Molina AJ, Guo W, Roy S. High glucose disrupts mitochondrial morphology in retinal endothelial cells: implications for diabetic retinopathy. Am J Pathol. 2010;177:447–455. doi: 10.2353/ajpath.2010.091029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE, Shiao YJ. Intracellular trafficking of phospholipids: import of phosphatidylserine into mitochondria. Anticancer Res. 1996;16:1333–1339. [PubMed] [Google Scholar]
- Vincent AM, Edwards JL, McLean LL, Hong Y, Cerri F, Lopez I, Quattrini A, Feldman EL. Mi24 tochondrial biogenesis and fission in axons in cell culture and animal models of diabetic neuropathy. Acta Neuropathol. 2010;120:477–489. doi: 10.1007/s00401-010-0697-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindis C, Elbaz M, Escargueil-Blanc I, Auge N, Heniquez A, Thiers JC, Negre-Salvayre A, Salvayre R. Two distinct calcium-dependent mitochondrial pathways are involved in oxidized LDL-induced apoptosis. Arterioscler Thromb Vasc Biol. 2005;25:639–645. doi: 10.1161/01.ATV.0000154359.60886.33. [DOI] [PubMed] [Google Scholar]
- Vinik A, Flemmer M. Diabetes and macrovascular disease. J Diabetes Complications. 2002;16:235–245. doi: 10.1016/s1056-8727(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RL, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrane J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc Natl Acad Sci U S A. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Azad N, Kongkaneramit L, Chen F, Lu Y, Jiang BH, Rojanasakul Y. The Fas death signaling pathway connecting reactive oxygen species generation and FLICE inhibitory protein down-regulation. J Immunol. 2008;180:3072–3080. doi: 10.4049/jimmunol.180.5.3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Wang X, Yang M, Smith LC, Dechow PC, Sonoda J, Evans RM, Wan Y. PGC1beta mediates PPARgamma activation of osteoclastogenesis and rosiglitazone-induced bone loss. Cell Metab. 2010;11:503–516. doi: 10.1016/j.cmet.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YH, Lee HC. Oxidative stress, mitochondrial DNA mutation, and impairment of antioxidant enzymes in aging. Exp Biol Med (Maywood) 2002;227:671–682. doi: 10.1177/153537020222700901. [DOI] [PubMed] [Google Scholar]
- Westermann B. Organelle Dynamics: ER Embraces Mitochondria for Fission. Curr Biol. 2011;21:R922–924. doi: 10.1016/j.cub.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Williamson CL, Dabkowski ER, Baseler WA, Croston TL, Alway SE, Hollander JM. Enhanced apoptotic propensity in diabetic cardiac mitochondria: influence of subcellular spatial location. Am J Physiol Heart Circ Physiol. 2010;298:H633–642. doi: 10.1152/ajpheart.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1alpha and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, Zou MH. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Feng Z, Liu J, Shen W, Wang Y, Wertz K, Weber P, Long J. Enhanced autophagy plays a cardinal role in mitochondrial dysfunction in type 2 diabetic Goto-Kakizaki (GK) rats: ameliorating effects of (–)-epigallocatechin-3-gallate. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Yang Q, Li Y. Roles of PPARs on regulating myocardial energy and lipid homeostasis. J Mol Med (Berl) 2007;85:697–706. doi: 10.1007/s00109-007-0170-9. [DOI] [PubMed] [Google Scholar]
- Yang Z, Mo X, Gong Q, Pan Q, Yang X, Cai W, Li C, Ma JX, He Y, Gao G. Critical effect of VEGF in the process of endothelial cell apoptosis induced by high glucose. Apoptosis. 2008;13:1331–1343. doi: 10.1007/s10495-008-0257-y. [DOI] [PubMed] [Google Scholar]
- Yonashiro R, Ishido S, Kyo S, Fukuda T, Goto E, Matsuki Y, Ohmura-Hoshino M, Sada K, Hotta H, Yamamura H, Inatome R, Yanagi S. A novel mitochondrial ubiquitin ligase plays a critical role in mitochondrial dynamics. EMBO J. 2006;25:3618–3626. doi: 10.1038/sj.emboj.7601249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Krueger EW, Oswald BJ, McNiven MA. The mitochondrial protein hFis1 regulates mitochondrial fission in mammalian cells through an interaction with the dynamin-like protein DLP1. Mol Cell Biol. 2003;23:5409–5420. doi: 10.1128/MCB.23.15.5409-5420.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon YS, Uchida S, Masuo O, Cejna M, Park JS, Gwon HC, Kirchmair R, Bahlman F, Walter D, Curry C, Hanley A, Isner JM, Losordo DW. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation. 2005;111:2073–2085. doi: 10.1161/01.CIR.0000162472.52990.36. [DOI] [PubMed] [Google Scholar]
- Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87:665–674. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SY, Breslin JW, Perrin R, Gaudreault N, Guo M, Kargozaran H, Wu MH. Microvascular permeability in diabetes and insulin resistance. Microcirculation. 2007;14:363–373. doi: 10.1080/10739680701283091. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shokeen M, Li D, Mehta JL. Identification of apoptosis-inducing factor in human coronary artery endothelial cells. Biochem Biophys Res Commun. 2003;301:147–151. doi: 10.1016/s0006-291x(02)02981-9. [DOI] [PubMed] [Google Scholar]
- Zhang YL, Cao YJ, Zhang X, Liu HH, Tong T, Xiao GD, Yang YP, Liu CF. The autophagy-lysosome pathway: a novel mechanism involved in the processing of oxidized LDL in human vascular endothelial cells. Biochem Biophys Res Commun. 2010;394:377–382. doi: 10.1016/j.bbrc.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li Q, Li P, Su L, Gu Q, Xu X. Improvement of retinal vascular injury in diabetic rats by statins is associated with the inhibition of mitochondrial reactive oxygen species pathway mediated by peroxisome proliferator-activated receptor gamma coactivator 1alpha. Diabetes. 2010;59:2315–2325. doi: 10.2337/db10-0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Kowluru RA. Diabetic retinopathy and damage to mitochondrial structure and transport machinery. Invest Ophthalmol Vis Sci. 2011;52:8739–8746. doi: 10.1167/iovs.11-8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–1854. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Zou MH, Shi C, Cohen RA. High glucose via peroxynitrite causes tyrosine nitration and inactivation of prostacyclin synthase that is associated with thromboxane/prostaglandin H(2) receptor-mediated apoptosis and adhesion molecule expression in cultured human aortic endothelial cells. Diabetes. 2002;51:198–203. doi: 10.2337/diabetes.51.1.198. [DOI] [PubMed] [Google Scholar]
- Zuchner S, Mersiyanova IV, Muglia M, Bissar-Tadmouri N, Rochelle J, Dadali EL, Zappia M, Nelis E, Patitucci A, Senderek J, Parman Y, Evgrafov O, Jonghe PD, Takahashi Y, Tsuji S, Pericak-Vance MA, Quattrone A, Battaloglu E, Polyakov AV, Timmerman V, Schroder JM, Vance JM. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat Genet. 2004;36:449–451. doi: 10.1038/ng1341. [DOI] [PubMed] [Google Scholar]
- Zunino R, Schauss A, Rippstein P, Andrade-Navarro M, McBride HM. The SUMO protease SENP5 is required to maintain mitochondrial morphology and function. J Cell Sci. 2007;120:1178–1188. doi: 10.1242/jcs.03418. [DOI] [PubMed] [Google Scholar]