Abstract
Over the past 20 years, nucleotide repeat expansion disorders have informed our broader understanding of neurodevelopmental and neurodegenerative disease. One area where this is especially true is the contributions of epigenetic mechanisms to neurological disease pathogenesis. This review describes a few of the myriad ways in which epigenetic processes underlie aspects of repeat expansion disorder pathophysiology and discusses how therapies targeted at epigenetic modulation hold promise for many of these disorders.
Keywords: Fragile X Syndrome, Myotonic Dystrophy, Spinocerebellar Ataxia, Huntington Disease, neurodegeneration, HDAC inhibitors
Introduction
Nucleotide repeat expansion disorders comprise a heterogeneous group of diseases that result from instability and expansion of simple tandem repeats (usually tri-nucleotide repeats). Pathogenic expansions can occur in coding or non-coding regions of genes. In disorders such as Friedreich Ataxia, expansions in non-coding regions cause transcriptional silencing or down-regulation of the associated gene and therefore act as recessively inherited, loss-of-function mutations1–2. In contrast, in disorders such as Huntington disease, tri-nucleotide expansions in the protein coding region introduce an abnormally long stretch of a single amino acid (often glutamine) into the associated protein which leads to a dominantly inherited, gain-of function mutation3. In the nine known polyglutamine diseases, the mutant proteins accumulate in ubiquitin-positive inclusions and interfere with cellular homeostasis through several different mechanisms (for recent reviews, see 3–4). A third set repeat expansion disorders, typified by myotonic dystrophy (DM1), result from dominantly inherited noncoding repeat expansions that elicit toxicity via a gain of function mechanism as RNA5–6.
Intensive research on these relatively rare disorders have often served as stepping stones to greater understanding of basic biological processes, including learning and memory, protein quality control, RNA processing, and neurodegeneration. Among the fields which have informed and been informed by work on these diseases is epigenetics. In almost all repeat expansion disorders described to date, a role for epigenetic alterations in pathogenesis have been proposed (see Tables 1 and 2), either as a mechanism to explain intergenerational or somatic repeat instability, as an explanation for silenced or elevated expression of the mRNA in which the repeat resides, or in the case of the polyglutamine diseases, a direct role for the toxic gain of function protein in epigenetic and transcriptional regulation. This review is designed to give an overview of how epigenetics informs our understanding of these disorders and provides a few specific examples that are covered in detail. By its very nature, it is not comprehensive; we thus refer the reviewer to more specialized reviews on this material for further details1,4–9. We also apologize to those colleagues whose outstanding work we excluded due to space limitations.
Table 1.
Epigenetic alterations surrounding non-coding repeat expansions
| Disease | Gene locus | Repeat type | location | Epigenetic changes | Effects on transcription and nearby genes |
|---|---|---|---|---|---|
| Myotonic Dystrophy type I (DM1) | DMPK | CTG | 3′-UTR | DNA methylation60,87 Altered CTCF binding |
Altered Six5 expression80 |
| DM2 | ZNF9 | CCTG | Intron 1 | Unknown | Decreased ZNF9 expression141 |
| FRA12A.MR | DIP2B | CGG | 5′-UTR | DNA methylation 142 | Decreased DIP2B expression142 |
| FRAXE MR | FMR2 | CCG | 5′-UTR | DNA methylation143–144 | Decreased FMR2 expression143–144 |
| Friedreich’s Ataxia | FXN | GAA | Intron 1 | Hypoacetylation and histone methylation 145 | Decreased FXN transcription145 |
| Fragile X Syndrome | FMR1 | CGG | 5′-UTR | Histone methylation and hypoacetylation DNAmethylation 32,44,46 |
Decreased or absent FMR1 transcription32 Absent ASFMR1 and FMR459 |
| FXTAS | FMR1 | CGG | 5′-UTR | Hyperacetylation of histones68 | Increased FMR1 transcription146 Increased ASFMR1 and FMR459 |
| HDL2 | JPH3 | CTG | 3′-UTR | Unknown | Unknown |
| SCA8 | KLH1 | CTG | 5′-UTR | Unknown | Unknown |
| SCA10 | E46L | ATTCT | 3′-UTR | Unknown | Unknown |
| SCA12 | PPP2R2B | CAG | 5′-UTR and promoter | Unknown | Increased PPP2R2B transcription147 |
| SCA31 | BEAN, TK2 | TGGAA | Intron | Unknown | Unknown |
Table 2.
Polyglutamine proteins in epigenetic and transcriptional regulation
| Disease | polyQ-proteins | Functions related to transcription/epigenetics | Other cellular functions |
|---|---|---|---|
| DRPLA | Atrophin-1 | Transcriptional repressor148 | |
| Huntington Disease | Huntingtin | TF interacting protein120 DNA binding protein122 CBP interacting protein112,114,129 |
Vesicle Transport Signal Transduction |
| SBMA | Androgen receptor | Nuclear receptor149 and transcription factor | |
| SCA1 | Ataxin-1 | Transcriptional regulation, histone acetylation95–97 | |
| SCA2 | Ataxin-2 | Transcriptional co-activator150 | RNA binding151 |
| SCA3 | Ataxin-3 | Transcriptional repressor152 | Deubiquitinase153 |
| SCA6 | CACNA1A | C-terminal PolyQ fragment traffics to nucleus154 | P/Q type Calcium channel155 |
| SCA7 | Ataxin-7 | Coactivator for STAGA histone acetylation complex102,104 | |
| SCA17 | TBP | General transcription factor156 |
Abbreviations: DRPLA- Dentatorubral pallidoluysian atrophy; TF- transcription factor; CBP- CREB binding protein; SBMA- Spinal Bulbar Muscular Atrophy (a.k.a. Kennedy’s Disease); SCA-Spinocerebellar Ataxiaf; PolyQ- polyglutamine; STAGA- SPT3-TAFII31-GCN5L acetylase complex; TBP- TATA binding protein
An Epigenetic Primer
Epigenetics can be broadly defined as any potentially heritable modification that alters gene expression without resulting from direct changes in the primary DNA sequence. Historically, the concept of epigenetics was proposed as a mechanism to explain how a single pluripotent cell with presumably a single genome could give rise to multiple different cell types with different morphologies and behaviors10. Although epigenetic changes by definition are potentially heritable, either through serial cellular divisions or across generations, the definition has morphed over time to include changes which are often transient and modifiable within a given cell. For example, numerous epigenetic alterations occur in terminally differentiated neurons in aging, neurodegeneration and learning and memory consolidation9,11–13.
The majority of epigenetic regulation can be broken down into one of three basic mechanisms: nucleotide modification, such as methylation and hydroxymethylation of cytosine; histone modification, and nucleosome positioning. These mechanisms interact to determine the relative accessibility of a given genetic locus to activating and suppressing transcription factors. However, manipulation of any single one of these mechanisms can have broad and often detrimental effects on genome-wide transcriptional regulation.
Nucleotide modification, most commonly cytosine methylation, has been studied extensively in the context of epigenetic silencing associated with tissue differentiation, genetic imprinting, and X-inactivation14–17. This methylation most commonly occurs at CpG dinucleotide rich genomic regions known as CpG islands located in gene promoters18. When unmethylated, these regions typically maintain a more open chromatin structure allowing transcriptional activation18–19. Methylation of CpG islands and neighboring GC rich promoter regions can accompany or trigger heterochromatin formation18–19. DNA methylation induced transcriptional silencing can occur by recruitment of methyl CpG binding domain (MBD) proteins that provide a scaffold for silencing chromatin remodeling complexes9. Alternatively, methylated DNA can directly inhibit interactions between DNA binding proteins and their targets sequences within promoters20.
Histones also play a major role in epigenetics. The core histones H2A, H2B, H3 and H4 together form the nucleosome, the major unit for DNA organization. All histones are subject to post-transcriptional modifications, including acetylation, methylation, phosphorylation, ubiquitination, and SUMOylation and these various modifications have important roles in transcriptional regulation, DNA repair and replication, and chromosome condensation9. The exact effects of all potential combinations of post-translational modification on the activity of DNA associated with a particular histone are complicated and still being worked out, but some general rules apply9. For example, acetylation of certain residues, especially on histone H3 and H4, and trimethylation at H3K4 in particular are associated with euchromatin and transcriptional activation, whereas deacetylation and methylation at H3K9 and H3K27 are often associated with heterochromatin and transcriptional silencing9. Acetylation and deacetylation of histones are mediated by histone acetyltransferases (HATs) and histone deacetylases (HDACs), respectively. Each of these enzyme families has numerous subclasses with differential activities at both specific histone residues and on the regulation of specific genes and processes.
In addition to the modifications discussed above, the position of nucleosomes relative to the start sites of genes is an important regulator of transcription. Most active genes maintain a nucleosome free region just upstream of the transcription start site, allowing access by the transcriptional machinery21. However, for some genes, nucleosomes can act as a barrier to transcription that must be displaced for gene activation to occur. Nucleosome positioning is significantly influenced by both DNA methylation as well as histone modifications9.
Effects of repeat expansion in cis on chromatin structure and transcriptional regulation
Nucleotide repeat expansion can alter local chromatin structure through a number of mechanisms. In vitro, pure CAG/CTG repeats elicit strong nucleosome positioning signals whereas unmethylated CGG/CCG repeats have the opposite effect22–23. In vivo, their effects are more complicated and influenced by sequences surrounding the repeat and the methylation status of cytosines within the repeat itself. In a number of diseases, it is clear that transcription of repeat disorder containing transcripts is negatively influenced by both the repeat and the epigenetic context in which that repeat resides (see table 1). We focus here on the Fragile X spectrum disorders FXTAS and Fragile X Syndrome. We also discuss how effects of repeats in cis might contribute to phenotypic variability in myotonic dystrophy.
Fragile X Spectrum Disorders: a case of epigenetic extremes
Fragile X Syndrome (FXS) is the most common known inherited cause of cognitive impairment and autism24–25. The name “fragile X” itself reflects its long history as a disorder with an aberrant chromatin signature. Originally, the constellation of symptoms and signs associated with this condition was called Martin-Bell syndrome after the clinicians who described it26. However, in the late 1960’s, it was recognized that lymphoblasts derived from these patients demonstrate a predictable “fragile site” on the long arm of Chromosome X at Q27.3, observed as an isochromatid gap in karyotype staining when cells are grown in culture under deoxynucleotide perturbing conditions 27–28. 30 years later, the region coincident with this fragile site was found to contain a polymorphic CGG tri-nucleotide repeat expansion in the 5′UTR of a gene, FMR129–31. In patients with fragile X syndrome, this repeat is greatly expanded, often to thousands of (CGG)s, leading to transcriptional silencing of the FMR1 gene and absent expression of the Fragile X Mental Retardation Protein, FMRP29,32.
Over time, it has become clear that the original Martin Bell Syndrome is but one of many phenotypes associated with expansion of this CGG repeat. Normally, this sequence is less than 45 CGG repeats. A “full mutation” expansion to greater than 200 CGG repeats usually leads to transcriptional silencing and FXS. By contrast, patients with more modest expansions to between 55 and 200 CGG repeats do not develop early cognitive impairment but are instead at risk for the late onset neurodegenerative disorder Fragile X-associated Tremor Ataxia Syndrome (FXTAS)33. This condition usually occurs in male maternal grandfathers of FXS children over the age of 50, with an age dependent penetrance of greater than 50% in men and 15% in women by the time they reach their 80s34–35. Typical features variably include a gait-predominant cerebellar ataxia, intention tremor, dementia, Parkinsonism, peripheral neuropathy and neuropsychiatric symptoms36. In contrast to full mutations, this “premutation” range repeat is transcribed efficiently, but the CGG repeat expansion induces significant translational inefficiency in the FMR1 mRNA, likely by forming a hairpin secondary structure in the 5′UTR that impairs ribosomal scanning 37–38. Thus levels of the Fragile X Mental Retardation Protein (FMRP) are lower in both FXTAS patients and in mouse models of the disease,, despite a 2–8 fold increase in basal FMR1 mRNA levels39–41. In addition to FXTAS, premutation repeats are associated with premature ovarian failure42 and may also lead to an increased incidence of autistic spectrum disorders and neuropsychiatric disease43.
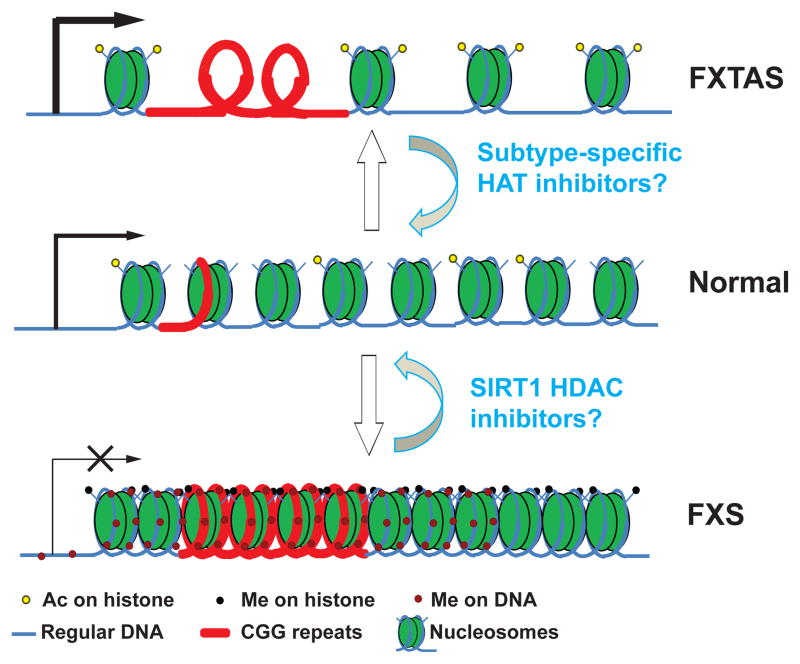
The mechanism by which the FMR1 gene is transcriptionally silenced in Fragile X Syndrome has been an area of significant research over the past 20 years1. The CGG repetitive element as well as an upstream CpG island in the FMR1 promoter is abnormally hyper-methylated in most affected individuals32,44–47. Initially, this methylation was thought to be the primary mediator of epigenetic silencing, with secondary recruitment of histone deacetylases and methyltransferases driving formation of a heterochromatin region over the FMR1 locus (Figure 1). Indeed, this DNA methylation pattern is associated with histone deacetylation and heterochromatin formation across the FMR1 gene in differentiated cells 48–50. Subsequent work has also demonstrated specific histone methylation marks across the FMR1 promoter and first exon, including di- and tri-methylation at Histone H3K9, and trimethylation at H4K20 and H3K2750–53. In rare full mutation patients with no DNA methylation in either the repeat or the upstream promoter, transcription is preserved53–54. In vitro, treatment with the DNA demethylating agent 5-aza-2-deoxycytidine (5-azadC) partially reactivates transcription and leads to a reversal of many but not all chromatin changes associated with transcriptional silencing52,55–56.
Figure 1. Fragile X Spectrum Disorders: a case of epigenetic extremes.
Multiple disorders result from CGG expansions in the 5′UTR of FMR1. Middle panel: Normally, there are up to 45 CGG repeats (red segment) in the FMR1 5′UTR. These are unmethylated and associated with moderately acetylated histones (yellow dots) and active gene transcription (black arrow). Top panel: in FXTAS, premutation CGG repeat lengths (55–200) are accompanied by hyperacetylation of histones and a more open chromatin state, leading to elevated FMR1 transcription. Bottom panel: in FXS, large (>200 CGG) repeat expansions trigger heterochromatin formation and DNA methylation (dark red dots) across the promoter and through the repeat. Recent studies suggest that histone deacetylation and trimethylation at critical residues such as H3K9 and H3K27 (black dots) precede DNA methylation. However, the mechanism by which this silencing is triggered remains unknown. The combination of heterochromatin formation and DNA methylation silences FMR1 transcription. Potential therapeutic strategies (shown in blue) include use of SIRT1 HDAC inhibitors targeted at reactivation of transcription in FXS and use of subtype-specific HAT inhibitors to suppress excess transcription in FXTAS.
Until recently, evaluating the temporal processes involved in transcriptional silencing of full mutation FMR1 loci has not been feasible. A significant advance in this area occurred with the use of human stem cells derived from a full mutation pre-implantation embryo. As undifferentiated human embryonic stem cells (hESCs), these cells demonstrate elevated FMR1 mRNA transcription compared to controls57. The FMR1 locus is unmethylated and associated with acetylated histones, consistent with a euchromatin state despite the presence of between 200 and 1000 CGG repeats. However, upon differentiation, FMR1 transcription decreases significantly. Surprisingly, this transcriptional suppression precedes DNA methylation at the FMR1 locus, but histone acetylation at H3K9 is lost and replaced by histone methylation at that same lysine. Subsequent differentiation into fibroblasts triggers methylation and transcriptional silencing at this locus, suggesting that methylation is not the initial event driving heterochromatin formation 57–58. Interestingly, re-derivation of induced pluripotent stem cells from these same fibroblasts fails to reactivate transcription or demethylation of the DNA at the repeat, suggesting that gene inactivation, once achieved, is a stable state58.
An alternative model for FMR1 gene silencing in FXS proposes a role for RNA induced transcriptional silencing (RITS)1,59. This process involves the formation of double stranded RNA and components of the RNA interference pathway60–61. RITS most often arises in the setting of bidirectional transcription, which occurs at the FMR1 5′UTR and promoter59,62.. However, evidence to date for a role or RITS in FXS pathogenesis remains largely circumstantial and it is unclear why this process would not be triggered by shorter repeat sizes as occur in FXTAS. Thus, work in emerging model systems like hESCs is still needed to determine the exact mechanism by which transcriptional silencing occurs.
In contrast to FXS, premutation carriers express too much FMR1 mRNA, often demonstrating a 2–5 fold increase in expression with repeat sizes between 55 and 200. The repeat containing mRNA itself is thought to elicit neurodegeneration through a toxic gain of function mechanism5,63–66, implying that increased FMR1 mRNA accumulation is a proximal step in disease pathogenesis. The increase in FMR1 mRNA is not the result of increased RNA stability induced by the repeat but instead reflects increased transcription67. Our group evaluated the hypothesis that the elevation in FMR1 mRNA expression might reflect an intrinsic epigenetic response related to the CGG repeat expansion. In a drosophila model of FXTAS where the 5′UTR of the FMR1 gene is placed upstream of a reporter gene (eGFP), co-expression of any of three different classes of HDACs led to suppression of CGG repeat induced neurodegeneration and transcriptional silencing of the transgene68. This suppression requires the presence of the CGG repeat expansion. In FXTAS patient derived cell lines, there is an increase in acetylated histones at the FMR1 locus around the repeat that correlates with both CGG repeat length and FMR1 mRNA expression. Interestingly, both the elevated FMR1 mRNA expression and the histone acetylation could be reversed by treatment with histone acetyltransferase inhibitors, suggesting that FMR1 transcriptional upregulation may be dynamic and modifiable. Taken together with in vitro data that CGG repeats actively exclude nucleosome assembly22, these findings suggest that unmethylated CGG repeat expansions act in cis to create a more open chromatin structure that favors increased transcription.
However, not all data to date is consistent with this hypothesis. Studies in Xenopus oocytes demonstrate transcriptional repression with moderate sized CGG repeat elements introduced into the 5′UTR of a heterologous gene that were partially reversed by treatment with HDAC inhibitors69. More recently, a group generated stable integrated cell lines with interrupted CGG repeats of different sizes70. These cell lines showed decreased transcription with increasing repeat size above 30 CGGs that were partially corrected by demethylating agents70. It is possible that the interruptions (a CTAGG every 20 CGGs) explain this observation as AGG interruptions in vitro alter the ability of CGG repeats to incorporate into nucleosomes22. Nevertheless, given data demonstrating transcriptional upregulation with expanded repeats in two different knock-in mouse models40,66,71 and in human patient derived fibroblasts, lymphoblasts and brains, it appears likely that in the context of the native FMR1 locus, larger uninterrupted and unmethylated CGG repeats favor an open chromatin state, as is often seen with large CpG islands through the genome.
Insulation, DNA methylation, and congenital Myotonic Dystrophy
Myotonic Dystrophy type I (DM1) is the third most common cause of muscular dystrophy6. Patients with the classical adult onset form of DM1 present with distal muscle weakness, myotonia and complications related to cardiac dysfunction, cataracts, and mild neuropsychiatric symptoms6,72. However, the range of potential phenotypes in DM1 is quite broad, from mildly affected patients who have only cataracts and mild myotonia to a congenital form of DM1 characterized by diffuse hypotonia, respiratory compromise, and cognitive impairment in over 50% of patients6–7. DM1 results from an expanded CTG repeat in the 3′UTR of the DMPK gene73–75. Normally, the repeat is up to 35 CTGs. Repeat sizes from 50–150 lead to only mild symptoms, while repeats between 100 and 1000 usually cause the classical phenotype. In patients with congenital DM1 (cDM1), the repeats are usually larger than 2000 CTGs, but can be in a range more typically associated with the classical phenotype. After initial evaluations ruled out a significant role for insufficiency of the DMPK protein or neighboring proteins in disease pathogenesis for at least the adult onset form of the disease76–80, attention turned to how the CUG repeat as RNA might elicit toxicity via sequestration of specific RNA binding proteins81–83. Specifically, CUG repeats can bind to and sequester the RNA splicing factor MBNL, leading to altered splicing in trans of mRNAs important for muscle function and myotonia.
Perhaps the most compelling evidence for this hypothesis came with the identification of the gene responsible for myotonic dystrophy type 2 (DM2)84. DM2 has a clinical phenotype that is similar to DM1, but results from a CCUG expansion in an intronic region of an unrelated gene, zinc finger nuclease 984. These CCUG repeats act very much like their CUG counterparts in DM1, forming nuclear RNA foci that sequester MBNL proteins and are associated with similar splicing defects84–85. However, in DM2, there is no congenital form of the disease and only minimal central nervous system involvement despite very large (>10,000) CCUG repeat expansions. This discrepancy has led multiple investigators to re-analyze the DM1 locus and the epigenetic effects of large CTG repeat expansions.
The DMPK locus lies in a gene-enriched region of chromosome 19, where the 3′UTR of DMPK is contiguous with the promoter of a neighboring gene, Six5. The CTG repeat itself is surrounded by two CTCF binding sites80. CTCF is a multifunctional zinc finger protein that binds to DNA and influences gene expression, nucleosome positioning, and chromatin organization86. CTCF binding can serve as a genetic insulator, preventing co-activation or suppression of one gene with that of close neighbors86. At the DM1 locus, these CTCF sites prevent the co-activation of DMPK transcription with Six5, which encodes a homeodomain protein expressed during early development80. An additional layer of complexity arises with production of an antisense transcript that initiates within the Six5 promoter and extends back through the DM1 repeat, producing a CAG-containing noncoding RNA60. This antisense transcript can trigger RNA induced transcriptional silencing (RITS) and heterochromatin formation around the repeat60. However, antisense transcription through the repeat and subsequent RITS is impeded when CTCF binding is present60.
CTCF binding to DNA is methylation state sensitive, with decreased binding at methylated DNA. In DM1 the CTG repeat induces DNA methylation throughout the DMPK 3′UTR that can exclude CTCF binding60,87. In fetal derived patient samples, this methylation is most pronounced proximal to the repeat87. However, the correlation of these events to gene expression changes from the DMPK and Six5 loci is imperfect and may vary through development7,87. Thus, large CTG expansions and their accompanying chromatin changes can potentially trigger three alternative pathogenic pathways7. First, they may lead to decreased Six5 transcription during early development due to RITS. Alternatively, the loss of CTCF binding could allow temporally aberrant expression of large CUG containing mRNAs during early development when the Six5 gene is most active7. Lastly, the transcription of long CAG containing RNA may be toxic independent of DMPK expression88. To date, there is no direct evidence of these events during early development. However, new models such as hESC or iPS cells may soon reveal answers to these important questions89–90.
Epigenetic and transcriptional dysregulation in polyglutamine disorders
An emerging concept in polyglutamine (PolyQ) disorder research is the role of alterations in native protein function in disease pathogenicity91–92. Although polyglutamine tracts expressed in isolation elicit neurodegeneration, the normal function of the host protein greatly influences the specificity and mechanism by which this neurodegeneration occurs. In this context, it is intriguing that all of the known polyglutamine proteins have direct or indirect roles in transcriptional and epigenetic regulation in their native contexts (Table 2)8. Moreover, in most if not all of these disorders, there is evidence for broad spectrum alterations in transcription that accompany and sometimes precede neurodegeneration8. We focus here on three examples where these effects have been extensively studied: SCA1, SCA7, and Huntington disease.
Spinocerebellar Ataxia Type I: Altered Ataxin I interactions drive disease pathogenesis
Ataxin-1 is the protein mutated in Spinocerebellar Ataxia type I, a common cause of dominantly inherited cerebellar degeneration. Ataxin-1 is normally a predominantly nuclear protein widely expressed in the central nervous system 93. Studies of transgenic animal models of SCA1 reveal down regulation of numerous genes during early development of cerebellar Purkinje cells 94–95. Two transcription factors, the retinoid acid receptor-related orphan receptor alpha (RORα) and Capicua, play prominent roles in SCA1 pathogenesis 96–97. Capicua (CIC), is a transcriptional repressor containing a sox-like high mobility group (HMG) box. Human Capicua forms complexes with Ataxin-1 and co-expression of Ataxin-1 synergistically enhances CIC based transcriptional suppression. However, this enhancement is absent with a mutant Axaxin-1 containing 82 polyQ repeats96. Interestingly, both the polyglutamine dependent impairment of transcriptional repression and neurodegeneration are abolished with a S776D point mutation distant from the repeat. This point mutation also alters the ability of Ataxin-1to interact with Capicua. Perhaps more impressive is that cerebellar neurodegeneration can be triggered in the absence of polyglutamine expansions by a phospho-mimetic mutation at this same residue, suggesting that the interactions influenced by the phosphorylation state of this residue are critical to disease pathogenesis92,96.
Although SCA1 is an adult onset neurodegenerative disorder, abnormalities occur during cerebellar development in SCA1[82Q] transgenic mice. When the SCA1 mutant transgene is expressed from early developmental stages of the cerebellum (3 days postnatal), the mice are profoundly ataxic. In contrast, when the mutant transgene is turned on after cerebellar development is complete (12 weeks postnatal), no ataxic phenotype is observed97. The transcription factor RORα plays a critical role in the SCA1[82Q] mediated early cerebellar developmental defects. Ataxin-1 interacts with RORα and the Tat-interactive protein 60 kDa (Tip60), a histone acetyltransferase and a nuclear receptor coactivator98. Tip60 mediates the interaction between Ataxin-1 and RORα99 and it directly interacts with Ataxin-197. Under partial loss of Tip60 (Tip60+/− background), RORα expression is increased in SCA1[82Q] transgenic mice 100 and these mice show delayed disease progression, indicating Tip60 interaction with the mutant SCA1[82Q] accelerates pathogenesis, likely through epigenetic modifications at Tip60 targeted genes99.
Spinocerebellar ataxia type 7: a direct role in epigenetic modulation
Spinocerebellar Ataxia type 7 (SCA7) is unique in the polyQ neurodegenerative diseases in that it elicits retinal degeneration101. Ataxin-7 interacts with the retina specific transcription activator Cone-rod homeobox protein (CRX)102. Both wildtype and mutant ataxin-7 bind to CRX, but mutant ataxin-7 sequesters CRX into nuclear aggregates, triggering disruption of CRX-mediated expression of retina specific genes102. However, in a knock-in mouse model of SCA7, decreases of other rod specific genes like Rom1 precede CRX mediated effects, indicating that the sequestration of CRX via mutant ataxin-7 is not the only pathway involved in retinal degeneration in this model103.
How normal and mutant ataxin-7 influence gene transcription became clearer after the observation that ataxin-7 serves as the subunit of histone acetyltransferase GCN5 complex TFTC (the TATA-binding protein-free TBP associated factor-containing complex) and the STAGA complex (the SPT3/TAF GCN5 complex) 104. STAGA/TFTC complexes are the mammalian homologue of yeast SAGA (Spt/Ada/Gcn5) complexes which acetylate nucleosomal histones and serve as a transcription coactivators105. The expansion of polyQ in ataxin-7 does not stop its incorporation into such complexes 104,106, but it does affect the composition of the STAGA complex in that some components such as ADA2b and Spt3 of STAGA are diminished 107–108. Such alterations compromise their HAT activity. Although free histone acetylation in vitro is not altered by mutant ataxin-7, polyglutamine expansion of ataxin-7 substantially disrupts the acetylation of histones in nucleosomes107. In mouse retina, CRX recruits the STAGA complex via binding to ataxin-7 to the promoters of CRX-responsive genes, leading these promoters to have higher levels of H3 acetylation and gene transcription. In contrast, mutant ataxin-7 inhibits STAGA complex HAT activity in the retina at these same genes, leading to hypoacetylation of H3 histones associated with promoters of CRX responsive genes108. Thus, in SCA7, the polyglutamine expansion leads to direct dysfunction in an epigenetic maintenance complex that plays a critical role in retinal degeneration in SCA-7.
Huntington Disease: Global transcriptional suppression and an altered epigenetic profile
Huntington disease (HD) is an autosomal dominant neurodegenerative disease due to polyQ expansion in the N-terminus of the huntingtin (HTT) protein3. HTT is a large (348Kd) predominantly cytoplasmic protein which contains multiple protein-protein interaction motifs and which interacts with a large number of neuronal proteins. While the mechanisms of disease pathogenesis in HD are myriad3–4,109, one important pathway involves dysregulated gene expression through altered interactions of mutant HTT with specific transcriptional and epigenetic coactivators8. These interacting proteins include Sp1, REST, and CBP.
Sp1 is a transcriptional activator that binds to upstream GC-rich elements of target genes and recruits the general transcription factor TFIID110. The N-terminal fragment and full-length HTT both interact directly with Sp1111–112. Both in vitro experiments and in mouse models of HD, the expanded polyglutamine stretch in mutant HTT increases binding to Sp1112. This interaction inhibits binding of Sp1 to important target gene promoters, including the promoter of nerve growth factor receptor (NGFR)112. Huntingtin can also affect SP1 targets by binding to the TBP associated factor TAFII130, a coactivator of Sp1 and a component of TFIID111. Mutant huntingtin inhibits dopamine D2 receptor gene expression in cultured striatal cells and this inhibition is reversed by co-expression of either Sp1 or TAFII130111. Transcription dysregulation by mutant huntingtin via Sp1 was further validated through chromatin immunoprecipitation analysis of Sp1 target genes. Although Sp1 protein levels are normal in HD mouse and HD patient brains, Sp1 promoter binding activity at susceptible genes is decreased113.
HTT also interacts directly with the repressor element 1 (RE1) silencing transcription factor/neuron restrictive silencer factor (REST/NRSF)114. REST/NRSF is a transcription factor that binds to neuron restrictive silencer elements (NRSEs). Initially it was thought that REST acted primarily to repress target gene expression in non-neural tissues115–117. However, a number of observations over the past decade now suggest it also plays prominent roles in neurons, where it can act as a regulator of chromatin organization at numerous genes via its interactions with numerous corepressor complexes118. Normally, HTT binds to and sequesters REST in the cytoplasm, allowing de-repression of NRSE containing genes in neurons. However, mutant huntingtin fails to adequately sequester REST, leading to aberrant nuclear accumulation of REST and repression of numerous target genes, such as brain-derived neurotrophic factor (BDNF)114. Consistent with this, higher REST occupancy is seen at NRSE containing promoters in both homozygotic HD knock-in mice and HD transgenic mice119. REST interacts with numerous epigenetic modifiers to achieve gene expression repression. Specifically, REST can associate with histone demethylases such as SMCX, which is involved in X linked mental retardation 120, Histone Deacetylases such as HDAC1 and 2, histone methyltransferases such as G9a 121, and the chromatin remodeling factor SWI/SNF 122. Further study of the how those epigenetic modifiers participate in REST-associated transcriptional changes in HD may well produce specific therapeutic targets109.
A more direct connection to dysregulation of the epigenetic landscape in HD comes from work on the CREB-binding protein (CBP). CBP, a histone actetyltransferase and transcription co-activator in CREB-mediated transcription 123–124, is found in polyQ aggregates of HD 125. HTT interacts directly with CBP through a short PolyQ tract that is present normally in CBP, and mutant HTT sequesters CBP into aggregates, effectively depleting it in HD neurons and triggering alterations in CREB mediated gene transcription126–128. Consistent with this observation, overexpression of CBP rescues the mutant HTT toxicity in cells and in drosophila126–127. The polyQ tracts in HTT can bind directly to the histone acetyltransferase domains of both CBP and p300/CEB-associated factor (P/CAF), interfering with their ability to add acetyl groups to histones129. Expression of the mutant N terminal of HTT in cultured cells decreases histone acetylation at H3 and H4, while HDAC inhibitors can reverse this reduction129. Hypoacetylation of H3 at down regulated genes is also observed in HD transgenic mice 130, and treatment of wildtype cells with specific histone acetyltransferase inhibitors decreases expression of these same target genes. These studies make a strong argument that altered histone acetylation, especially at CREB target genes, may play an important role in the progression of HD.
Epigenetic therapeutic targets in Repeat disorders
Given the epigenetic perturbations associated with repeat expansion disorders, it is perhaps not surprising that epigenetic therapeutic targets have been proposed for a number of these diseases. In Fragile X Syndrome, a number of techniques have been tried to achieve transcriptional reactivation. DNA demethylating agents such as 5-azadC partially reactivate transcription from the FMR1 locus in FXS patient derived cells, but the agents have proven too toxic to consider using in patients and do not work in non-dividing cells55. In contrast, broad spectrum HDAC inhibitors such as Trichostatin A fail to significantly reactivate FMR1 transcription48,55–56. However, a more recent study suggests that significant reactivation might be achieved by utilizing sirtuin subtype HDAC inhibitors56. Treatment with the SIRT1 specific inhibitor splitomycin was able to achieve gene reactivation and partial reversal of the heterochromatin state of the FMR1 locus in patient derived cell lymphoblasts. Interestingly, this re-expression was achieved without a significant change in DNA methylation over the repeat or the upstream CpG island in the FMR1 promoter56. However, in patients with very large CGG expansions (>500), this increase in FMR1 mRNA was not accompanied by any production of FMRP, likely because of translational block induced by the CGG repeat as mRNA54. Although the safety profile of this class of agents in humans remains to be determined, in FXS patients with shorter repeats that do not show complete translational inefficiency, such therapeutic approaches remain promising56.
Friedreich Ataxia (FA) is an autosomal recessive disorder characterized by slowly progressive ataxia, dysarthria, weakness and cardiomyopathy2. FA typically results from GAA expansions in the first intron of FXN, a gene which encodes the mitochondrial protein Frataxin2. As with FXS, this repetitive element elicits heterochromatin formation over the FXN locus and transcriptional silencing in a length dependent manner. However, in contrast to FXS, some low level of FXN transcription and functional Frataxin is present in all FA patients, as its complete loss is lethal during early embryogenesis and small differences in mRNA expression impact both the age of onset and the severity of the disease2,131–132. Further, the GAA repeat remains unmethylated and is spliced out of the mature mRNA, meaning it has no impact on translational efficiency. Thus, in many ways, it represents an ideal target for epigenetic therapies. Indeed, treatment of FA patient derived cell lines with broad spectrum HDAC inhibitors lead to significant transcriptional reactivation133. Subsequent work has identified a specific class of pimelic o-aminobenzamide HDAC inhibitors that achieve gene reactivation in mouse models of the disease and demonstrate reversal of key pathological findings134–136. Phase one clinical trials with these agents are planned.
In Huntington disease, large scale transcriptional down regulation of numerous genes is an early event in pathogenesis130,137. Many of these transcriptional abnormalities are associated with epigenetic alterations that are broadly distributed across the genome.130 Consistent with this, in both Drosophila and mouse models of HD, broad spectrum HDAC inhibitors suppress neuronal degeneration and reduce lethality129,138. Attempts to narrow the spectrum of such agents in a drosophila disease model suggest that inhibition of sir2 (akin to the mammalian HDAC SIRT1) and RPD3 (akin to class I HDACs in mammals) are synergistically beneficial in suppressing toxicity139. In mice, the sirtuin inhibitor nicotinamide slows the onset of motor phenotypes and corrects some aspects of transcriptional dysregulation in HD model mice, despite not affecting HTT aggregate formation140. A clinical trial in HD patients has been conducted with the broad spectrum HDAC inhibitor phenylbutyrate but results have not yet been reported (Steven Hersch, personal communication). Future trials with more specific agents are planned.
In summary, nucleotide repeat elements that cause human disease elicit epigenetic alterations in cis and in trans that directly impact on the molecular pathogenesis of these disorders. With an ever greater understanding of these alterations and more specific pharmacological interventions possible, epigenetic targets hold great promise for therapeutic development in this patient population.
Acknowledgments
This work is supported by NIH grant K08069809.
References
- 1.Kumari D, Usdin K. Chromatin remodeling in the noncoding repeat expansion diseases. J Biol Chem. 2009;284(12):7413–7417. doi: 10.1074/jbc.R800026200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campuzano V, Montermini L, Molto MD, et al. Friedreich’s ataxia: autosomal recessive disease caused by an intronic GAA triplet repeat expansion. Science. 1996;271(5254):1423–1427. doi: 10.1126/science.271.5254.1423. [DOI] [PubMed] [Google Scholar]
- 3.Orr HT, Zoghbi HY. Trinucleotide repeat disorders. Annu Rev Neurosci. 2007;30:575–621. doi: 10.1146/annurev.neuro.29.051605.113042. [DOI] [PubMed] [Google Scholar]
- 4.Williams AJ, Paulson HL. Polyglutamine neurodegeneration: protein misfolding revisited. Trends Neurosci. 2008;31(10):521–528. doi: 10.1016/j.tins.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Todd PK, Paulson HL. RNA-mediated neurodegeneration in repeat expansion disorders. Ann Neurol. 2010;67(3):291–300. doi: 10.1002/ana.21948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheeler TM, Thornton CA. Myotonic dystrophy: RNA-mediated muscle disease. Curr Opin Neurol. 2007;20(5):572–576. doi: 10.1097/WCO.0b013e3282ef6064. [DOI] [PubMed] [Google Scholar]
- 7.Cho DH, Tapscott SJ. Myotonic dystrophy: emerging mechanisms for DM1 and DM2. Biochim Biophys Acta. 2007;1772(2):195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Riley BE, Orr HT. Polyglutamine neurodegenerative diseases and regulation of transcription: assembling the puzzle. Genes Dev. 2006;20(16):2183–2192. doi: 10.1101/gad.1436506. [DOI] [PubMed] [Google Scholar]
- 9.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28(10):1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 10.Choudhuri S. From Waddington’s epigenetic landscape to small noncoding RNA: some important milestones in the history of epigenetics research. Toxicol Mech Methods. 2011;21(4):252–274. doi: 10.3109/15376516.2011.559695. [DOI] [PubMed] [Google Scholar]
- 11.Nelson ED, Monteggia LM. Epigenetics in the mature mammalian brain: Effects on behavior and synaptic transmission. Neurobiol Learn Mem. 2011;96(1):53–60. doi: 10.1016/j.nlm.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan JS, Haggarty SJ, Giacometti E, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459(7243):55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marques SC, Oliveira CR, Pereira CM, Outeiro TF. Epigenetics in neurodegeneration: a new layer of complexity. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(2):348–355. doi: 10.1016/j.pnpbp.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Bird AP. CpG-rich islands and the function of DNA methylation. Nature. 1986;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- 15.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187(4173):226–232. [PubMed] [Google Scholar]
- 16.Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 17.Kalantry S. Recent advances in X-chromosome inactivation. J Cell Physiol. 2011;226(7):1714–1718. doi: 10.1002/jcp.22673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Straussman R, Nejman D, Roberts D, et al. Developmental programming of CpG island methylation profiles in the human genome. Nat Struct Mol Biol. 2009;16(5):564–571. doi: 10.1038/nsmb.1594. [DOI] [PubMed] [Google Scholar]
- 19.Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462(7271):315–322. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esteller M. Epigenetic gene silencing in cancer: the DNA hypermethylome. Hum Mol Genet. 2007;16(Spec No 1):R50–59. doi: 10.1093/hmg/ddm018. [DOI] [PubMed] [Google Scholar]
- 21.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128(4):707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Mulvihill DJ, Nichol Edamura K, Hagerman KA, Pearson CE, Wang YH. Effect of CAT or AGG interruptions and CpG methylation on nucleosome assembly upon trinucleotide repeats on spinocerebellar ataxia, type 1 and fragile X syndrome. J Biol Chem. 2005;280(6):4498–4503. doi: 10.1074/jbc.M413239200. [DOI] [PubMed] [Google Scholar]
- 23.Wang YH, Gellibolian R, Shimizu M, Wells RD, Griffith J. Long CCG triplet repeat blocks exclude nucleosomes: a possible mechanism for the nature of fragile sites in chromosomes. J Mol Biol. 1996;263(4):511–516. doi: 10.1006/jmbi.1996.0593. [DOI] [PubMed] [Google Scholar]
- 24.Turner G, Webb T, Wake S, Robinson H. Prevalence of fragile X syndrome. Am J Med Genet. 1996;64(1):196–197. doi: 10.1002/(SICI)1096-8628(19960712)64:1<196::AID-AJMG35>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Hantash FM, Goos DM, Crossley B, et al. FMR1 premutation carrier frequency in patients undergoing routine population-based carrier screening: insights into the prevalence of fragile X syndrome, fragile X-associated tremor/ataxia syndrome, and fragile X-associated primary ovarian insufficiency in the United States. Genet Med. 2011;13(1):39–45. doi: 10.1097/GIM.0b013e3181fa9fad. [DOI] [PubMed] [Google Scholar]
- 26.Martin JP, Bell J. A Pedigree of Mental Defect Showing Sex-Linkage. J Neurol Psychiatry. 1943;6(3–4):154–157. doi: 10.1136/jnnp.6.3-4.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lubs HA. A marker X chromosome. Am J Hum Genet. 1969;21(3):231–244. [PMC free article] [PubMed] [Google Scholar]
- 28.Sutherland GR. Fragile sites on human chromosomes: demonstration of their dependence on the type of tissue culture medium. Science. 1977;197(4300):265–266. doi: 10.1126/science.877551. [DOI] [PubMed] [Google Scholar]
- 29.Fu YH, Kuhl DP, Pizzuti A, et al. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- 30.Kremer EJ, Pritchard M, Lynch M, et al. Mapping of DNA instability at the fragile X to a trinucleotide repeat sequence p(CCG)n. Science. 1991;252(5013):1711–1714. doi: 10.1126/science.1675488. [DOI] [PubMed] [Google Scholar]
- 31.Verkerk AJ, Pieretti M, Sutcliffe JS, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 32.Pieretti M, Zhang FP, Fu YH, et al. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- 33.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology. 2001;57(1):127–130. doi: 10.1212/wnl.57.1.127. [DOI] [PubMed] [Google Scholar]
- 34.Jacquemont S, Hagerman RJ, Leehey MA, et al. Penetrance of the fragile X-associated tremor/ataxia syndrome in a premutation carrier population. JAMA. 2004;291(4):460–469. doi: 10.1001/jama.291.4.460. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, et al. Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet. 2009 doi: 10.1038/ejhg.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berry-Kravis E, Abrams L, Coffey SM, et al. Fragile X-associated tremor/ataxia syndrome: clinical features, genetics, and testing guidelines. Mov Disord. 2007;22(14):2018–2030. doi: 10.1002/mds.21493. quiz 2140. [DOI] [PubMed] [Google Scholar]
- 37.Kaufmann WE, Abrams MT, Chen W, Reiss AL. Genotype, molecular phenotype, and cognitive phenotype: correlations in fragile X syndrome. Am J Med Genet. 1999;83(4):286–295. [PubMed] [Google Scholar]
- 38.Primerano B, Tassone F, Hagerman RJ, et al. Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. RNA. 2002;8(12):1482–1488. [PMC free article] [PubMed] [Google Scholar]
- 39.Tassone F, Hagerman RJ, Garcia-Arocena D, et al. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. 2004;41(4):e43. doi: 10.1136/jmg.2003.012518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Entezam A, Biacsi R, Orrison B, et al. Regional FMRP deficits and large repeat expansions into the full mutation range in a new Fragile X premutation mouse model. Gene. 2007;395(1–2):125–134. doi: 10.1016/j.gene.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brouwer JR, Mientjes EJ, Bakker CE, et al. Elevated Fmr1 mRNA levels and reduced protein expression in a mouse model with an unmethylated Fragile X full mutation. Exp Cell Res. 2007;313(2):244–253. doi: 10.1016/j.yexcr.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman SL. Premature ovarian failure in the fragile X syndrome. Am J Med Genet. 2000;97(3):189–194. doi: 10.1002/1096-8628(200023)97:3<189::AID-AJMG1036>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Farzin F, Perry H, Hessl D, et al. Autism spectrum disorders and attention-deficit/hyperactivity disorder in boys with the fragile X premutation. J Dev Behav Pediatr. 2006;27(2 Suppl):S137–144. doi: 10.1097/00004703-200604002-00012. [DOI] [PubMed] [Google Scholar]
- 44.Sutcliffe JS, Nelson DL, Zhang F, et al. DNA methylation represses FMR-1 transcription in fragile X syndrome. Hum Mol Genet. 1992;1(6):397–400. doi: 10.1093/hmg/1.6.397. [DOI] [PubMed] [Google Scholar]
- 45.Oberle I, Vincent A, Abbadi N, et al. New polymorphism and a new chromosome breakpoint establish the physical and genetic mapping of DXS369 in the DXS98-FRAXA interval. Am J Med Genet. 1991;38(2–3):336–342. doi: 10.1002/ajmg.1320380234. [DOI] [PubMed] [Google Scholar]
- 46.Bell MV, Hirst MC, Nakahori Y, et al. Physical mapping across the fragile X: hypermethylation and clinical expression of the fragile X syndrome. Cell. 1991;64(4):861–866. doi: 10.1016/0092-8674(91)90514-y. [DOI] [PubMed] [Google Scholar]
- 47.Vincent A, Heitz D, Petit C, et al. Abnormal pattern detected in fragile-X patients by pulsed-field gel electrophoresis. Nature. 1991;349(6310):624–626. doi: 10.1038/349624a0. [DOI] [PubMed] [Google Scholar]
- 48.Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet. 1999;22(1):98–101. doi: 10.1038/8807. [DOI] [PubMed] [Google Scholar]
- 49.Coffee B, Zhang F, Ceman S, Warren ST, Reines D. Histone modifications depict an aberrantly heterochromatinized FMR1 gene in fragile x syndrome. Am J Hum Genet. 2002;71(4):923–932. doi: 10.1086/342931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pietrobono R, Tabolacci E, Zalfa F, et al. Molecular dissection of the events leading to inactivation of the FMR1 gene. Hum Mol Genet. 2005;14(2):267–277. doi: 10.1093/hmg/ddi024. [DOI] [PubMed] [Google Scholar]
- 51.Kumari D, Usdin K. The distribution of repressive histone modifications on silenced FMR1 alleles provides clues to the mechanism of gene silencing in fragile X syndrome. Hum Mol Genet. 2010;19(23):4634–4642. doi: 10.1093/hmg/ddq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tabolacci E, Pietrobono R, Moscato U, et al. Differential epigenetic modifications in the FMR1 gene of the fragile X syndrome after reactivating pharmacological treatments. Eur J Hum Genet. 2005;13(5):641–648. doi: 10.1038/sj.ejhg.5201393. [DOI] [PubMed] [Google Scholar]
- 53.Tabolacci E, Moscato U, Zalfa F, et al. Epigenetic analysis reveals a euchromatic configuration in the FMR1 unmethylated full mutations. Eur J Hum Genet. 2008;16(12):1487–1498. doi: 10.1038/ejhg.2008.130. [DOI] [PubMed] [Google Scholar]
- 54.Feng Y, Zhang F, Lokey LK, et al. Translational suppression by trinucleotide repeat expansion at FMR1. Science. 1995;268(5211):731–734. doi: 10.1126/science.7732383. [DOI] [PubMed] [Google Scholar]
- 55.Chiurazzi P, Pomponi MG, Willemsen R, Oostra BA, Neri G. In vitro reactivation of the FMR1 gene involved in fragile X syndrome. Hum Mol Genet. 1998;7(1):109–113. doi: 10.1093/hmg/7.1.109. [DOI] [PubMed] [Google Scholar]
- 56.Biacsi R, Kumari D, Usdin K. SIRT1 inhibition alleviates gene silencing in Fragile X mental retardation syndrome. PLoS Genet. 2008;4(3):e1000017. doi: 10.1371/journal.pgen.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Eiges R, Urbach A, Malcov M, et al. Developmental study of fragile X syndrome using human embryonic stem cells derived from preimplantation genetically diagnosed embryos. Cell Stem Cell. 2007;1(5):568–577. doi: 10.1016/j.stem.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 58.Urbach A, Bar-Nur O, Daley GQ, Benvenisty N. Differential modeling of fragile X syndrome by human embryonic stem cells and induced pluripotent stem cells. Cell Stem Cell. 2010;6(5):407–411. doi: 10.1016/j.stem.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ladd PD, Smith LE, Rabaia NA, et al. An antisense transcript spanning the CGG repeat region of FMR1 is upregulated in premutation carriers but silenced in full mutation individuals. Hum Mol Genet. 2007;16(24):3174–3187. doi: 10.1093/hmg/ddm293. [DOI] [PubMed] [Google Scholar]
- 60.Cho DH, Thienes CP, Mahoney SE, et al. Antisense transcription and heterochromatin at the DM1 CTG repeats are constrained by CTCF. Mol Cell. 2005;20(3):483–489. doi: 10.1016/j.molcel.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 61.Morris KV, Chan SW, Jacobsen SE, Looney DJ. Small interfering RNA-induced transcriptional gene silencing in human cells. Science. 2004;305(5688):1289–1292. doi: 10.1126/science.1101372. [DOI] [PubMed] [Google Scholar]
- 62.Khalil AM, Faghihi MA, Modarresi F, Brothers SP, Wahlestedt C. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS ONE. 2008;3(1):e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jin P, Zarnescu DC, Zhang F, et al. RNA-mediated neurodegeneration caused by the fragile X premutation rCGG repeats in Drosophila. Neuron. 2003;39(5):739–747. doi: 10.1016/s0896-6273(03)00533-6. [DOI] [PubMed] [Google Scholar]
- 64.Arocena DG, Iwahashi CK, Won N, et al. Induction of inclusion formation and disruption of lamin A/C structure by premutation CGG-repeat RNA in human cultured neural cells. Hum Mol Genet. 2005;14(23):3661–3671. doi: 10.1093/hmg/ddi394. [DOI] [PubMed] [Google Scholar]
- 65.Handa V, Goldwater D, Stiles D, et al. Long CGG-repeat tracts are toxic to human cells: implications for carriers of Fragile X premutation alleles. FEBS Lett. 2005;579(12):2702–2708. doi: 10.1016/j.febslet.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Hashem V, Galloway JN, Mori M, et al. Ectopic expression of CGG containing mRNA is neurotoxic in mammals. Hum Mol Genet. 2009 doi: 10.1093/hmg/ddp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tassone F, Beilina A, Carosi C, et al. Elevated FMR1 mRNA in premutation carriers is due to increased transcription. RNA. 2007;13(4):555–562. doi: 10.1261/rna.280807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Todd PK, Oh SY, Krans A, et al. Histone deacetylases suppress CGG repeat-induced neurodegeneration via transcriptional silencing in models of fragile X tremor ataxia syndrome. PLoS Genet. 2010;6(12):e1001240. doi: 10.1371/journal.pgen.1001240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chandler SP, Kansagra P, Hirst MC. Fragile X (CGG)n repeats induce a transcriptional repression in cis upon a linked promoter: evidence for a chromatin mediated effect. BMC Mol Biol. 2003;4:3. doi: 10.1186/1471-2199-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Solvsten C, Nielsen AL. FMR1 CGG repeat lengths mediate different regulation of reporter gene expression in comparative transient and locus specific integration assays. Gene. 2011 doi: 10.1016/j.gene.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 71.Brouwer JR, Huizer K, Severijnen LA, et al. CGG-repeat length and neuropathological and molecular correlates in a mouse model for fragile X-associated tremor/ataxia syndrome. J Neurochem. 2008;107(6):1671–1682. doi: 10.1111/j.1471-4159.2008.05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Modoni A, Silvestri G, Pomponi MG, et al. Characterization of the pattern of cognitive impairment in myotonic dystrophy type 1. Arch Neurol. 2004;61(12):1943–1947. doi: 10.1001/archneur.61.12.1943. [DOI] [PubMed] [Google Scholar]
- 73.Brook JD, McCurrach ME, Harley HG, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;69(2):385. doi: 10.1016/0092-8674(92)90418-c. [DOI] [PubMed] [Google Scholar]
- 74.Fu YH, Pizzuti A, Fenwick RG, Jr, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255(5049):1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- 75.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255(5049):1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 76.Jansen G, Groenen PJ, Bachner D, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13(3):316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- 77.Reddy S, Smith DB, Rich MM, et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat Genet. 1996;13(3):325–335. doi: 10.1038/ng0796-325. [DOI] [PubMed] [Google Scholar]
- 78.Klesert TR, Cho DH, Clark JI, et al. Mice deficient in Six5 develop cataracts: implications for myotonic dystrophy. Nat Genet. 2000;25(1):105–109. doi: 10.1038/75490. [DOI] [PubMed] [Google Scholar]
- 79.Sarkar PS, Appukuttan B, Han J, et al. Heterozygous loss of Six5 in mice is sufficient to cause ocular cataracts. Nat Genet. 2000;25(1):110–114. doi: 10.1038/75500. [DOI] [PubMed] [Google Scholar]
- 80.Filippova GN, Thienes CP, Penn BH, et al. CTCF-binding sites flank CTG/CAG repeats and form a methylation-sensitive insulator at the DM1 locus. Nat Genet. 2001;28(4):335–343. doi: 10.1038/ng570. [DOI] [PubMed] [Google Scholar]
- 81.Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128(6):995–1002. doi: 10.1083/jcb.128.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mankodi A, Logigian E, Callahan L, et al. Myotonic dystrophy in transgenic mice expressing an expanded CUG repeat. Science. 2000;289(5485):1769–1773. doi: 10.1126/science.289.5485.1769. [DOI] [PubMed] [Google Scholar]
- 83.Miller JW, Urbinati CR, Teng-Umnuay P, et al. Recruitment of human muscleblind proteins to (CUG)(n) expansions associated with myotonic dystrophy. EMBO J. 2000;19(17):4439–4448. doi: 10.1093/emboj/19.17.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liquori CL, Ricker K, Moseley ML, et al. Myotonic dystrophy type 2 caused by a CCTG expansion in intron 1 of ZNF9. Science. 2001;293(5531):864–867. doi: 10.1126/science.1062125. [DOI] [PubMed] [Google Scholar]
- 85.Mankodi A, Urbinati CR, Yuan QP, et al. Muscleblind localizes to nuclear foci of aberrant RNA in myotonic dystrophy types 1 and 2. Hum Mol Genet. 2001;10(19):2165–2170. doi: 10.1093/hmg/10.19.2165. [DOI] [PubMed] [Google Scholar]
- 86.Filippova GN. Genetics and epigenetics of the multifunctional protein CTCF. Curr Top Dev Biol. 2008;80:337–360. doi: 10.1016/S0070-2153(07)80009-3. [DOI] [PubMed] [Google Scholar]
- 87.Lopez Castel A, Nakamori M, Tome S, et al. Expanded CTG repeat demarcates a boundary for abnormal CpG methylation in myotonic dystrophy patient tissues. Hum Mol Genet. 2011;20(1):1–15. doi: 10.1093/hmg/ddq427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li LB, Yu Z, Teng X, Bonini NM. RNA toxicity is a component of ataxin-3 degeneration in Drosophila. Nature. 2008;453(7198):1107–1111. doi: 10.1038/nature06909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seriola A, Spits C, Simard JP, et al. Huntington’s and myotonic dystrophy hESCs: down-regulated trinucleotide repeat instability and mismatch repair machinery expression upon differentiation. Hum Mol Genet. 2011;20(1):176–185. doi: 10.1093/hmg/ddq456. [DOI] [PubMed] [Google Scholar]
- 90.Marteyn A, Maury Y, Gauthier MM, et al. Mutant human embryonic stem cells reveal neurite and synapse formation defects in type 1 myotonic dystrophy. Cell Stem Cell. 2011;8(4):434–444. doi: 10.1016/j.stem.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 91.Nedelsky NB, Pennuto M, Smith RB, et al. Native functions of the androgen receptor are essential to pathogenesis in a Drosophila model of spinobulbar muscular atrophy. Neuron. 2010;67(6):936–952. doi: 10.1016/j.neuron.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Duvick L, Barnes J, Ebner B, et al. SCA1-like disease in mice expressing wild-type ataxin-1 with a serine to aspartic acid replacement at residue 776. Neuron. 2010;67(6):929–935. doi: 10.1016/j.neuron.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Servadio A, Koshy B, Armstrong D, et al. Expression analysis of the ataxin-1 protein in tissues from normal and spinocerebellar ataxia type 1 individuals. Nat Genet. 1995;10(1):94–98. doi: 10.1038/ng0595-94. [DOI] [PubMed] [Google Scholar]
- 94.Lin X, Antalffy B, Kang D, Orr HT, Zoghbi HY. Polyglutamine expansion down-regulates specific neuronal genes before pathologic changes in SCA1. Nat Neurosci. 2000;3(2):157–163. doi: 10.1038/72101. [DOI] [PubMed] [Google Scholar]
- 95.Serra HG, Byam CE, Lande JD, et al. Gene profiling links SCA1 pathophysiology to glutamate signaling in Purkinje cells of transgenic mice. Hum Mol Genet. 2004;13(20):2535–2543. doi: 10.1093/hmg/ddh268. [DOI] [PubMed] [Google Scholar]
- 96.Lam YC, Bowman AB, Jafar-Nejad P, et al. ATAXIN-1 interacts with the repressor Capicua in its native complex to cause SCA1 neuropathology. Cell. 2006;127(7):1335–1347. doi: 10.1016/j.cell.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 97.Serra HG, Duvick L, Zu T, et al. RORalpha-mediated Purkinje cell development determines disease severity in adult SCA1 mice. Cell. 2006;127(4):697–708. doi: 10.1016/j.cell.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 98.Brady ME, Ozanne DM, Gaughan L, et al. Tip60 is a nuclear hormone receptor coactivator. J Biol Chem. 1999;274(25):17599–17604. doi: 10.1074/jbc.274.25.17599. [DOI] [PubMed] [Google Scholar]
- 99.Gold DA, Baek SH, Schork NJ, et al. RORalpha coordinates reciprocal signaling in cerebellar development through sonic hedgehog and calcium-dependent pathways. Neuron. 2003;40(6):1119–1131. doi: 10.1016/s0896-6273(03)00769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gehrking KM, Andresen JM, Duvick L, et al. Partial loss of Tip60 slows mid-stage neurodegeneration in a spinocerebellar ataxia type 1 (SCA1) mouse model. Hum Mol Genet. 2011;20(11):2204–2212. doi: 10.1093/hmg/ddr108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Enevoldson TP, Sanders MD, Harding AE. Autosomal dominant cerebellar ataxia with pigmentary macular dystrophy. A clinical and genetic study of eight families. Brain. 1994;117 ( Pt 3):445–460. doi: 10.1093/brain/117.3.445. [DOI] [PubMed] [Google Scholar]
- 102.La Spada AR, Fu YH, Sopher BL, et al. Polyglutamine-expanded ataxin-7 antagonizes CRX function and induces cone-rod dystrophy in a mouse model of SCA7. Neuron. 2001;31(6):913–927. doi: 10.1016/s0896-6273(01)00422-6. [DOI] [PubMed] [Google Scholar]
- 103.Yoo SY, Pennesi ME, Weeber EJ, et al. SCA7 knockin mice model human SCA7 and reveal gradual accumulation of mutant ataxin-7 in neurons and abnormalities in short-term plasticity. Neuron. 2003;37(3):383–401. doi: 10.1016/s0896-6273(02)01190-x. [DOI] [PubMed] [Google Scholar]
- 104.Helmlinger D, Hardy S, Sasorith S, et al. Ataxin-7 is a subunit of GCN5 histone acetyltransferase-containing complexes. Hum Mol Genet. 2004;13(12):1257–1265. doi: 10.1093/hmg/ddh139. [DOI] [PubMed] [Google Scholar]
- 105.Grant PA, Duggan L, Cote J, et al. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11(13):1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 106.Helmlinger D, Hardy S, Abou-Sleymane G, et al. Glutamine-expanded ataxin-7 alters TFTC/STAGA recruitment and chromatin structure leading to photoreceptor dysfunction. PLoS Biol. 2006;4(3):e67. doi: 10.1371/journal.pbio.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McMahon SJ, Pray-Grant MG, Schieltz D, Yates JR, 3rd, Grant PA. Polyglutamine-expanded spinocerebellar ataxia-7 protein disrupts normal SAGA and SLIK histone acetyltransferase activity. Proc Natl Acad Sci U S A. 2005;102(24):8478–8482. doi: 10.1073/pnas.0503493102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palhan VB, Chen S, Peng GH, et al. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc Natl Acad Sci U S A. 2005;102(24):8472–8477. doi: 10.1073/pnas.0503505102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuccato C, Valenza M, Cattaneo E. Molecular mechanisms and potential therapeutical targets in Huntington’s disease. Physiol Rev. 2010;90(3):905–981. doi: 10.1152/physrev.00041.2009. [DOI] [PubMed] [Google Scholar]
- 110.Cook T, Gebelein B, Urrutia R. Sp1 and its likes: biochemical and functional predictions for a growing family of zinc finger transcription factors. Ann N Y Acad Sci. 1999;880:94–102. doi: 10.1111/j.1749-6632.1999.tb09513.x. [DOI] [PubMed] [Google Scholar]
- 111.Dunah AW, Jeong H, Griffin A, et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science. 2002;296(5576):2238–2243. doi: 10.1126/science.1072613. [DOI] [PubMed] [Google Scholar]
- 112.Li SH, Cheng AL, Zhou H, et al. Interaction of Huntington disease protein with transcriptional activator Sp1. Mol Cell Biol. 2002;22(5):1277–1287. doi: 10.1128/mcb.22.5.1277-1287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen-Plotkin AS, Sadri-Vakili G, Yohrling GJ, et al. Decreased association of the transcription factor Sp1 with genes downregulated in Huntington’s disease. Neurobiol Dis. 2006;22(2):233–241. doi: 10.1016/j.nbd.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 114.Zuccato C, Tartari M, Crotti A, et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nat Genet. 2003;35(1):76–83. doi: 10.1038/ng1219. [DOI] [PubMed] [Google Scholar]
- 115.Schoenherr CJ, Anderson DJ. The neuron-restrictive silencer factor (NRSF): a coordinate repressor of multiple neuron-specific genes. Science. 1995;267(5202):1360–1363. doi: 10.1126/science.7871435. [DOI] [PubMed] [Google Scholar]
- 116.Schoenherr CJ, Paquette AJ, Anderson DJ. Identification of potential target genes for the neuron-restrictive silencer factor. Proc Natl Acad Sci U S A. 1996;93(18):9881–9886. doi: 10.1073/pnas.93.18.9881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen ZF, Paquette AJ, Anderson DJ. NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet. 1998;20(2):136–142. doi: 10.1038/2431. [DOI] [PubMed] [Google Scholar]
- 118.Ooi L, Wood IC. Chromatin crosstalk in development and disease: lessons from REST. Nat Rev Genet. 2007;8(7):544–554. doi: 10.1038/nrg2100. [DOI] [PubMed] [Google Scholar]
- 119.Zuccato C, Belyaev N, Conforti P, et al. Widespread disruption of repressor element-1 silencing transcription factor/neuron-restrictive silencer factor occupancy at its target genes in Huntington’s disease. J Neurosci. 2007;27(26):6972–6983. doi: 10.1523/JNEUROSCI.4278-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tahiliani M, Mei P, Fang R, et al. The histone H3K4 demethylase SMCX links REST target genes to X-linked mental retardation. Nature. 2007;447(7144):601–605. doi: 10.1038/nature05823. [DOI] [PubMed] [Google Scholar]
- 121.Mulligan P, Westbrook TF, Ottinger M, et al. CDYL bridges REST and histone methyltransferases for gene repression and suppression of cellular transformation. Mol Cell. 2008;32(5):718–726. doi: 10.1016/j.molcel.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lepagnol-Bestel AM, Zvara A, Maussion G, et al. DYRK1A interacts with the REST/NRSF-SWI/SNF chromatin remodelling complex to deregulate gene clusters involved in the neuronal phenotypic traits of Down syndrome. Hum Mol Genet. 2009;18(8):1405–1414. doi: 10.1093/hmg/ddp047. [DOI] [PubMed] [Google Scholar]
- 123.Chrivia JC, Kwok RP, Lamb N, et al. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365(6449):855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 124.Kwok RP, Lundblad JR, Chrivia JC, et al. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370(6486):223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 125.Kazantsev A, Preisinger E, Dranovsky A, Goldgaber D, Housman D. Insoluble detergent-resistant aggregates form between pathological and nonpathological lengths of polyglutamine in mammalian cells. Proc Natl Acad Sci U S A. 1999;96(20):11404–11409. doi: 10.1073/pnas.96.20.11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nucifora FC, Jr, Sasaki M, Peters MF, et al. Interference by huntingtin and atrophin-1 with cbp-mediated transcription leading to cellular toxicity. Science. 2001;291(5512):2423–2428. doi: 10.1126/science.1056784. [DOI] [PubMed] [Google Scholar]
- 127.Taylor JP, Taye AA, Campbell C, et al. Aberrant histone acetylation, altered transcription, and retinal degeneration in a Drosophila model of polyglutamine disease are rescued by CREB-binding protein. Genes Dev. 2003;17(12):1463–1468. doi: 10.1101/gad.1087503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Steffan JS, Kazantsev A, Spasic-Boskovic O, et al. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc Natl Acad Sci U S A. 2000;97(12):6763–6768. doi: 10.1073/pnas.100110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Steffan JS, Bodai L, Pallos J, et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature. 2001;413(6857):739–743. doi: 10.1038/35099568. [DOI] [PubMed] [Google Scholar]
- 130.Sadri-Vakili G, Bouzou B, Benn CL, et al. Histones associated with downregulated genes are hypo-acetylated in Huntington’s disease models. Hum Mol Genet. 2007;16(11):1293–1306. doi: 10.1093/hmg/ddm078. [DOI] [PubMed] [Google Scholar]
- 131.Durr A, Cossee M, Agid Y, et al. Clinical and genetic abnormalities in patients with Friedreich’s ataxia. N Engl J Med. 1996;335(16):1169–1175. doi: 10.1056/NEJM199610173351601. [DOI] [PubMed] [Google Scholar]
- 132.Bidichandani SI, Ashizawa T, Patel PI. The GAA triplet-repeat expansion in Friedreich ataxia interferes with transcription and may be associated with an unusual DNA structure. Am J Hum Genet. 1998;62(1):111–121. doi: 10.1086/301680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herman D, Jenssen K, Burnett R, et al. Histone deacetylase inhibitors reverse gene silencing in Friedreich’s ataxia. Nat Chem Biol. 2006;2(10):551–558. doi: 10.1038/nchembio815. [DOI] [PubMed] [Google Scholar]
- 134.Rai M, Soragni E, Jenssen K, et al. HDAC inhibitors correct frataxin deficiency in a Friedreich ataxia mouse model. PLoS One. 2008;3(4):e1958. doi: 10.1371/journal.pone.0001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rai M, Soragni E, Chou CJ, et al. Two new pimelic diphenylamide HDAC inhibitors induce sustained frataxin upregulation in cells from Friedreich’s ataxia patients and in a mouse model. PLoS One. 2010;5(1):e8825. doi: 10.1371/journal.pone.0008825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sandi C, Pinto RM, Al-Mahdawi S, et al. Prolonged treatment with pimelic o-aminobenzamide HDAC inhibitors ameliorates the disease phenotype of a Friedreich ataxia mouse model. Neurobiol Dis. 2011;42(3):496–505. doi: 10.1016/j.nbd.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hodges A, Strand AD, Aragaki AK, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15(6):965–977. doi: 10.1093/hmg/ddl013. [DOI] [PubMed] [Google Scholar]
- 138.Hockly E, Richon VM, Woodman B, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor, ameliorates motor deficits in a mouse model of Huntington’s disease. Proc Natl Acad Sci U S A. 2003;100(4):2041–2046. doi: 10.1073/pnas.0437870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pallos J, Bodai L, Lukacsovich T, et al. Inhibition of specific HDACs and sirtuins suppresses pathogenesis in a Drosophila model of Huntington’s disease. Hum Mol Genet. 2008;17(23):3767–3775. doi: 10.1093/hmg/ddn273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hathorn T, Snyder-Keller A, Messer A. Nicotinamide improves motor deficits and upregulates PGC-1alpha and BDNF gene expression in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;41(1):43–50. doi: 10.1016/j.nbd.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Raheem O, Olufemi SE, Bachinski LL, et al. Mutant (CCTG)n expansion causes abnormal expression of zinc finger protein 9 (ZNF9) in myotonic dystrophy type 2. Am J Pathol. 2010;177(6):3025–3036. doi: 10.2353/ajpath.2010.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Winnepenninckx B, Debacker K, Ramsay J, et al. CGG-repeat expansion in the DIP2B gene is associated with the fragile site FRA12A on chromosome 12q13. 1. Am J Hum Genet. 2007;80(2):221–231. doi: 10.1086/510800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gecz J, Oostra BA, Hockey A, et al. FMR2 expression in families with FRAXE mental retardation. Hum Mol Genet. 1997;6(3):435–441. doi: 10.1093/hmg/6.3.435. [DOI] [PubMed] [Google Scholar]
- 144.Gu Y, Shen Y, Gibbs RA, Nelson DL. Identification of FMR2, a novel gene associated with the FRAXE CCG repeat and CpG island. Nat Genet. 1996;13(1):109–113. doi: 10.1038/ng0596-109. [DOI] [PubMed] [Google Scholar]
- 145.Al-Mahdawi S, Pinto RM, Ismail O, et al. The Friedreich ataxia GAA repeat expansion mutation induces comparable epigenetic changes in human and transgenic mouse brain and heart tissues. Hum Mol Genet. 2008;17(5):735–746. doi: 10.1093/hmg/ddm346. [DOI] [PubMed] [Google Scholar]
- 146.Campuzano V, Montermini L, Lutz Y, et al. Frataxin is reduced in Friedreich ataxia patients and is associated with mitochondrial membranes. Hum Mol Genet. 1997;6(11):1771–1780. doi: 10.1093/hmg/6.11.1771. [DOI] [PubMed] [Google Scholar]
- 147.Lin CH, Chen CM, Hou YT, et al. The CAG repeat in SCA12 functions as a cis element to up-regulate PPP2R2B expression. Hum Genet. 2010;128(2):205–212. doi: 10.1007/s00439-010-0843-2. [DOI] [PubMed] [Google Scholar]
- 148.Zhang S, Xu L, Lee J, Xu T. Drosophila atrophin homolog functions as a transcriptional corepressor in multiple developmental processes. Cell. 2002;108(1):45–56. doi: 10.1016/s0092-8674(01)00630-4. [DOI] [PubMed] [Google Scholar]
- 149.La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- 150.Hallen L, Klein H, Stoschek C, et al. The KRAB-containing zinc-finger transcriptional regulator ZBRK1 activates SCA2 gene transcription through direct interaction with its gene product, ataxin-2. Hum Mol Genet. 2011;20(1):104–114. doi: 10.1093/hmg/ddq436. [DOI] [PubMed] [Google Scholar]
- 151.Satterfield TF, Pallanck LJ. Ataxin-2 and its Drosophila homolog, ATX2, physically assemble with polyribosomes. Hum Mol Genet. 2006;15(16):2523–2532. doi: 10.1093/hmg/ddl173. [DOI] [PubMed] [Google Scholar]
- 152.Evert BO, Araujo J, Vieira-Saecker AM, et al. Ataxin-3 represses transcription via chromatin binding, interaction with histone deacetylase 3, and histone deacetylation. J Neurosci. 2006;26(44):11474–11486. doi: 10.1523/JNEUROSCI.2053-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burnett B, Li F, Pittman RN. The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet. 2003;12(23):3195–3205. doi: 10.1093/hmg/ddg344. [DOI] [PubMed] [Google Scholar]
- 154.Kordasiewicz HB, Thompson RM, Clark HB, Gomez CM. C-termini of P/Q-type Ca2+ channel alpha1A subunits translocate to nuclei and promote polyglutamine-mediated toxicity. Hum Mol Genet. 2006;15(10):1587–1599. doi: 10.1093/hmg/ddl080. [DOI] [PubMed] [Google Scholar]
- 155.Zhuchenko O, Bailey J, Bonnen P, et al. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15(1):62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]
- 156.Gill G, Tjian R. Eukaryotic coactivators associated with the TATA box binding protein. Curr Opin Genet Dev. 1992;2(2):236–242. doi: 10.1016/s0959-437x(05)80279-5. [DOI] [PubMed] [Google Scholar]