Abstract
Preserving isolated islets at low temperature appears attractive because it can keep islet quantity comparable to freshly isolated islets. In this study, we evaluated the effect of serum as an additive to preservation solutions on islet quality after short-term hypothermic storage. Isolated mouse islets were preserved at 4°C in University of Wisconsin solution (UW) alone, UW with serum, M-Kyoto solution (MK) alone or MK with serum. We then assessed islet quantity, morphology, viability and function in vitro as well as in vivo. Islet quantity after storage in all four solutions was well maintained for up to 120 h. However, islets functioned for different duration; glucose-stimulated insulin release assay revealed that the duration was 72 h when islets were stored in UW with serum and MK with serum, but only 24 h in UW alone, and the islet function disappeared immediately in MK alone. Viability assay confirmed that more than 70% islet cells survived for up to 48 h when islets are preserved in UW with serum and MK with serum, but the viability decreased rapidly in UW alone and MK alone. In in vivo bioassays using 48-h preserved isogeneic islets, all recipient mice restored normal blood glucose concentrations by transplants preserved in UW with serum or MK with serum, whereas 33.3% recipients and no recipient restored diabetes by transplants preserved in UW alone and in MK alone respectively. Adding serum to both UW and MK improves their capability to store isolated islets by maintaining islet functional viability.
Keywords: hypothermic short-term storage, islet, M-Kyoto solution, serum, University of Wisconsin solution
Introduction
Human-to-human transplantation of islets of Langerhans is on the way to an established therapy for patients with insulin-dependent diabetes mellitus and severe hypoglycemia.1 The Edmonton protocol, which provided the basis for the current worldwide clinical islet transplantation, transplanted fresh islets immediately after isolation in order to minimize the risk of their injury during storage.2 However, short-term storage of isolated islets has been recognized necessary to advance islet transplantations that require additional quality control of islets as grafts and recipient preparation before transplantation.
Culturing islets is now common for islet storage before transplantation. The reasons include its beneficial aspects on islet quality; immunogenicity reduction,3,4 metabolic efficacy improvement,5 and increased purity by reducing exocrine contamination.6 Successful clinical cases involving islet culture for over 48 h has already been reported.7 However, culturing islets is known to have disadvantageous aspects on islet quantity as follows: frequent induction of cell damage in islet central area8 and a rapid reduction of islet mass by 20% to > 50% even during short-term culture,9,10 which could lead to cancellation of planned clinical transplantation.9 Therefore, culturing islets is not recognized as the standard method for short-term islet storage before transplantation.
Preserving islets at low temperature could be an alternative method to their short-term storage before transplantation. The quantity of islets preserved at low temperature is well sustained, although these islets lost function in a short period of time.11 No preservation solution has been reported capable of maintaining islet function comparable to that of freshly isolated islets for over 24 h.10,12 These facts prompted us to improve hypothermic storage of isolated islets. This improvement seems achievable, because in the organ transplantation field, hypothermic storage instead of culture is currently the standard for organ storage before transplantation13,14 and successful kidney and liver transplantations were reported even after over 24-h hypothermic preservations.15 Improved solution could allow us to store islets beyond 24 h and expect therapeutic outcome comparable to that when using freshly isolated islets in transplantations.
Some conceivable factors contributing to the deterioration of isolated islets during hypothermic storage might include injury of cell membrane.16 Serum albumin is an essential component to stabilize cell membrane during cell culture.17,18 Moreover, nutrients-enriched organ preservation solution has been reported to result in improved quality of liver preservation for transplantation, suggesting that nutrients play an important role even in hypothermic organ preservation.19
Accordingly, we hypothesized that serum, which contains both albumin and nutrients, can prevent islet deterioration during hypothermic storage. In this study, we evaluated the effect of serum as an additive to preservation solution for hypothermic storage of isolated mouse islets. Using University of Wisconsin solution (UW) and M-Kyoto solution (MK)20 as preservation solutions, we assessed the islets preserved in UW alone, UW with serum, MK alone or MK with serum over time for islet quantity, morphology, viability and function.
Results
In vitro assessment
Islet number, volume and morphology
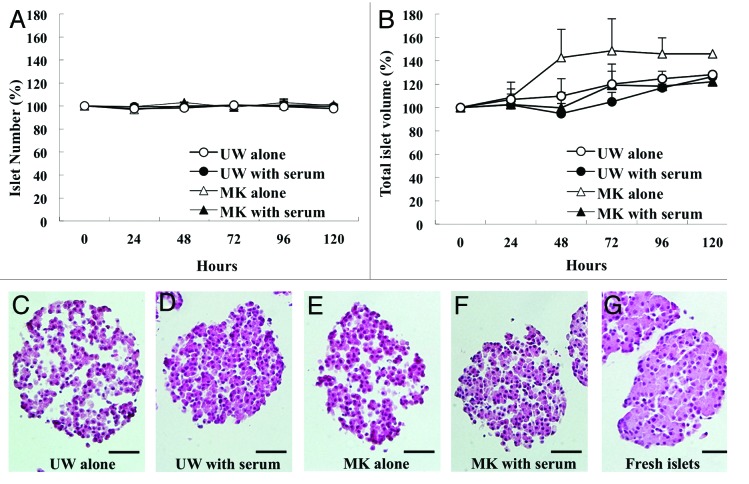
The most attractive aspect of preserving islets at low temperature is to maintain the quantity to be comparable to freshly isolated islets. To confirm that this aspect can be reproduced in our system, we counted the numbers and measured the volumes of islets preserved at 4°C up to over 120 h after isolation in four solutions: UW alone, UW with serum, MK alone and MK with serum. The numbers of islets in all four solutions were well sustained (Fig. 1A). However, the volumes of preserved islets in both preservation solutions without serum had a tendency to increase beyond 48 h (Fig. 1B). Histopathological analysis revealed gaps between the cells within islets, which contributed to those increases in volume (Fig. 1C and E). In contrast, when islets were preserved in both UW with serum and MK with serum, cell connections within the islets were well maintained and were comparable to freshly isolated islets (Fig. 1D, F and G).
Figure 1. Islet quality, total volume and morphology during hypothermic storage in preservation solutions with or without serum. Isolated mouse islets were stored at 4°C in UW alone (open circle), UW with serum (closed circle), MK alone (open triangle) or MK with serum (closed triangle). (A) Number of islets for 120 h in preservation solutions. (B) Total volume of islets for 120 h in preservation solutions. (C–F) Representative histopathological images of the islets stored for 48 h in each group. (G) Representative histopathological image of the islets freshly isolated. Scale bar = 50μm
Glucose-stimulated insulin release assay
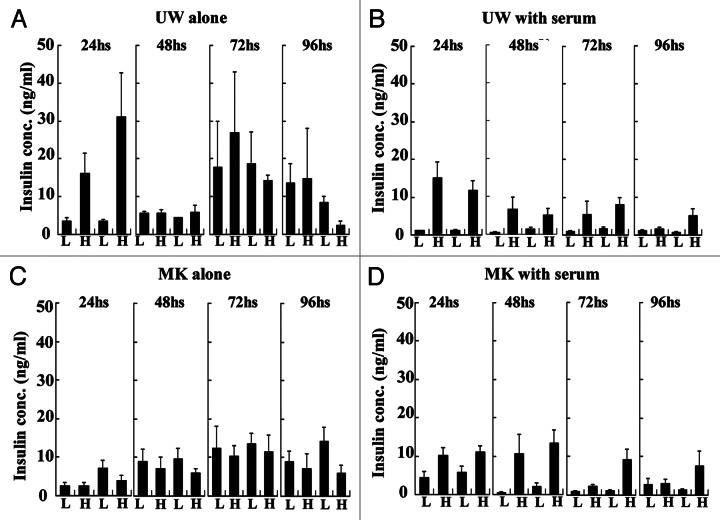
For in vitro assessment of the potency to keep islets function, we measured released insulin up to 96 h as the response to glucose stimulation. The islets that released higher amount of insulin at high glucose than low glucose were recognized as intact in function. We observed that the islets preserved in UW alone lost their function within 48 h (Fig. 2A) and the islets in MK alone lost their function within 24 h (Fig. 2C). Whereas, the islets preserved in both UW with serum and MK with serum sustained their function up to 72 h (Fig. 2B and D).
Figure 2. Glucose-stimulated insulin release of preserved islets. Preserved islets of each experimental group were subjected to static glucose challenge. One experiment contains two sequential sets of challenge in which 3.3 mmol/L of low glucose concentration (L) and 20 mmol/L of high glucose concentration (H) were used in succession.
Viability and TUNEL staining
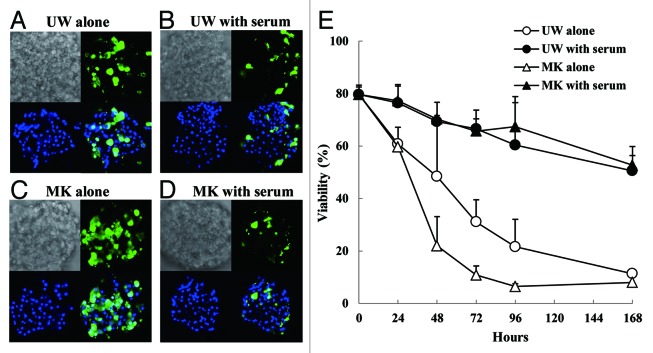
To determine whether the impaired function of the islets during hypothermic storage is attributed to islet cell death, we observed the islets using confocal microscopy after staining them with two nucleic acid dyes: Hoechst, which is membrane-permeable and stains the nuclei of all cells, and YOYO-1, which is not permeable through intact cell membrane and specifically stains the nuclei of the dead cells (Fig. 3A–D). We then calculated the viability of islets by taking the complement of the ratio of YOYO-1 positive cells to the total Hoechst positive cells. We found that the viabilities of the islets preserved in both solutions without serum decreased to < 50% after 48 h. Meanwhile, the viabilities of the islets in preservation solutions with serum were kept comparable to those of freshly isolated islets after 24 h, and they were > 70% even after 48 h (Fig. 3E).
Figure 3. Viability assay by analyzing islet membrane integrity. Preserved islets stained with fluorescent dye were evaluated using confocal laser scanning microscope. (A–D) Representative photo images of islets preserved for 48 h in UW alone (A), UW with serum (B), MK alone (C) and MK with serum (D). Each figure has four images; bright field (upper left), YOYO-1 positive cells (upper right), Hoechst 33342 positive cells (lower left), and a merged image of YOYO-1 positive with Hoechst 33342 (lower right). (E) Time course change of viability of preserved islets.
To assess whether the cell death was caused by apoptosis, we performed TUNEL staining for preserved islets. We detected no TUNEL positive cells in the islets preserved in all four solutions up to 96 h (data not shown). These findings suggest that the cells in the islets preserved in low temperature die of necrosis, in which cell membrane is impaired without passing through apoptotic pathway.
In vivo assessment
Mouse transplant bioassay using isogeneic islets
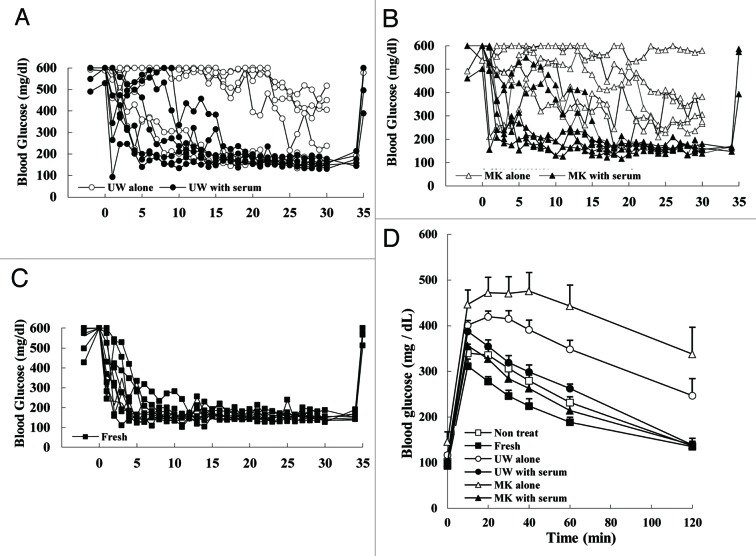
In order to confirm the contribution of serum to maintain islet function in preservation solutions, we performed transplant bioassay, which is recognized as the gold standard of function evaluation. 300 islets preserved at low-temperature were transplanted under the kidney capsule of mice rendered diabetic by streptozotocin. Based on the viability assay findings (Fig. 3E), we assumed that it is 48 h after preservation that the function becomes clearly different between the islets preserved with serum and the islets preserved without serum in both solutions. Therefore, for the bioassay we used the islets preserved for 48 h. The blood glucose concentrations of all mice that received islets preserved in either UW with serum or MK with serum decreased gradually and returned to normal within 17 d after transplantation (n = 6 in each group, Fig. 4A and B). These patterns of blood glucose after transplantation are comparable to the case of freshly isolated islets (Fig. 4C). In contrast, two out of 6 (33.3%) mice receiving islets preserved in UW alone achieved normoglycemia (Fig. 4A), and no mouse receiving islets preserved in MK alone reached normoglycemia (n = 6, Fig. 4B). Body weight of all mice increased after transplantation and no significant difference was detected between any groups (data not shown). All mice that had achieved normoglycemia reverted to hyperglycemia after removing the kidneys bearing islet grafts (Fig. 4A–C).
Figure 4. In vivo function of preserved islets. (A–C) Changes in the blood glucose concentration of mice after 300 preserved isogeneic islets were transplanted as bioassay. (A) Islets preserved for 48 h in UW alone (open circle) and in UW with serum (closed circle) were used as transplant. (B) Islets preserved for 48 h in MK alone (open triangle) and in MK with serum (closed triangle) were used as transplant. (C) Islets freshly isolated were used as transplant. (D) Intraperitoneal glucose tolerance test 32 d after transplantation.
Intraperitoneal glucose tolerance test
To further evaluate the in vivo function of the preserved islets, we performed intraperitoneal glucose tolerance test 32 d after transplantation (Fig. 4D). All the mice receiving the islets preserved in either UW with serum or MK with serum demonstrated nearly normal responses, whereas the mice receiving the islets preserved in either UW alone or MK alone indicated a nonfunctional state of the transplanted islets.
Histological analysis
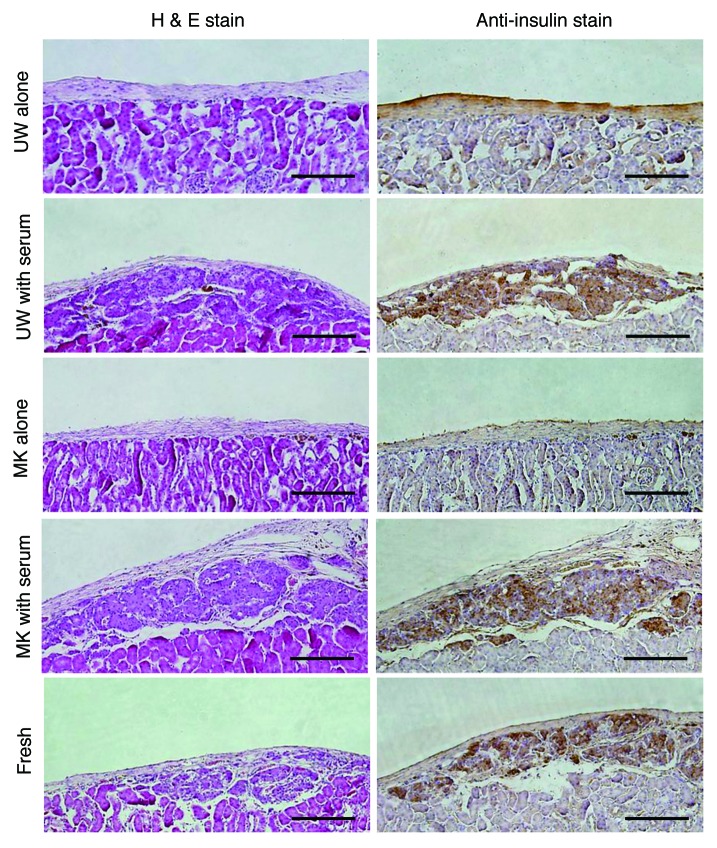
Immunohistochemical staining for insulin demonstrated that insulin-positive islet grafts with well-preserved islet structure were found when the islets had been preserved in the preservation solutions with serum, whereas almost no islet grafts were detected when the islets had been preserved without serum (Fig. 5).
Figure 5. Histopathological analysis for sections of the resected left kidney bearing transplanted islets in its subrenal capsule. Hematoxylin and eosin (H&E) stain and immunohistochemical anti-insulin stain were performed. Original magnification 100x; Scale bar = 150 μm.
Discussion
In this study, we demonstrated that serum enhances the capability of preservation solutions to maintain mouse islet function at low temperature and that it does so by decreasing the rate of islet cells suffering from necrosis. Evidence showing that serum is the factor that adds value to the maintenance of islet function includes that both glucose-stimulated insulin release assay and transplant bioassay indicated the islets preserved in UW with serum and MK with serum possessed intact function for longer period than the islets in UW alone and MK alone, respectively (Fig. 2 and Fig. 4). Evidence showing the rate of islet cells suffering from necrosis decreases is that viability assay indicated that impaired cellular membrane existed over time in less cells of the islets preserved in UW with serum and MK with serum than in UW alone and MK alone, respectively (Fig. 3).
In addition to the effect of serum on the islet function during hypothermic storage, we found that serum prevents preserved islets from showing morphological abnormality (Fig. 1C–F), namely gaps between the cells within the islets. Although we could not detect any significant differences in the content of E-cadherin or connexin expression between islets preserved with serum and without serum (data not shown), this morphological abnormality suggests that the islets suffer from interstitial edema and damage of adhesion molecules, which are frequently observed at hypothermic storage.21,22
These results clearly show that serum improves hypothermic storage of islets in preservation solutions. Some factors are considered to explain this improvement. While serum contains many kinds of proteins, minerals, lipids, growth factors, hormones and nutrients,23 albumin is the most abundant protein in serum and has anti-oxidative activity.17,18,24 Albumin could deactivate free radicals formed during cold storage25-28 and contribute to prevention of cell death due to free radicals, resulting in improvement of islet hypothermic storage. We recognize that albumin plays a key role but albumin is not the single factor in this improvement based on our additional experiment, in which adding albumin alone instead of serum to preservation solutions did not completely reproduce the outcome found in the case of adding serum in this study (data not shown). We speculate that nutrients within serum could be another factor for the improvement because 7 to 35% of cell metabolism remains even in hypothermic cell preservation condition.29 Actually, nutrients-enriched organ preservation solution named Polysol exhibited superior outcome to UW, which does not contain any nutrients, in the preservation of liver19 and small intestine.30
For in vitro evaluation of islet viability, we adopted fluorescent staining assay for cellular membrane integrity because this assay is recognized as rapid and semi-quantitative assay for islet viability, and is routinely used in clinical setting before islet transplantation.31 However, Barnett et al.32 reported that islet viability has not been evaluated properly by the routine method, in which islets are stained with fluorescein diacetate/propidium iodide (FDA/PI) and observed by epifluorescence microscopy. They attributed one of the factors that compromised proper evaluation of islet viability to inadequate visual quality caused by both FDA’s staining, intense enough to obscure PI signal to detect dead cells, and a high degree of background fluorescence. To perform proper viability assay based upon cellular membrane integrity, we modified the method reported by Marchant et al.33 and Grochowiecki et al.34 After we stained islets with both YOYO-1 and Hoechst 33342, we observed them by confocal microscopy. Because YOYO-1 becomes fluorescent when it intercalates into double stranded DNA and it is impermeant to live cells, it can limitedly detect membrane-impaired cells 35. Whereas Hoechst 33342 is permeant even to live cells and can stain the nuclei of live and dead cells. Confocal microscopy serves to minimize background fluorescence and to visualize the nuclei of virtually sliced cells and enables quantification of the ratio of dead cell by detecting the nuclei stained with YOYO-1 and the nuclei stained with Hoechst 33342. The reliability of this viability assay is confirmed to some extent by its good correlation with transplant bioassay when islets preserved for 48 h were used.
When considering clinical setting for human islet transplantation, several options are available as a source of serum; autologous serum obtained from the islet recipient, human allogeneic serum and fetal calf serum.36-38 From the viewpoint of quality control and safety issue, adding only effective components instead of whole serum to preservation solution is desirable, and identification of the components is currently under investigation in our laboratory. Moreover, as a source of preservation solution for clinical use, Kyoto solution might be preferable based upon our experience, as crystal formation was often observed in UW solution when mixed with serum but not at all in Kyoto solution even with serum (data not shown). The mechanism is not certain but crystal formation does not seem rare in UW as previously reported.39-41
In conclusion, serum added to both UW and MK improves their capability to preserve islets in quantity and functional viability by maintaining the integrity of islet cell membrane. Further study to determine essential components in serum for this effect may allow hypothermic preservation of islets to be a useful storage procedure in clinical islet transplantation.
Materials and Methods
Preservation solutions
The following two commercially available preservation solutions were used: UW solution (ViaSpan, DuPont Pharmaceuticals) and ET-Kyoto solution (Kyoto solution, Otsuka Pharmaceutical Factory, Inc.). MK solution is a mixture of ET-Kyoto solution and ulinastatin (Mochida Pharmaceutical). By adding fetal bovine serum (FBS) (cat.no.S1820–500) at 10% concentration to UW and MK solution, we prepared four solutions: UW alone, UW with serum, MK alone and MK with serum.
Animals
All experiments were performed under the control of the Animal Research Committee in accordance with the Guideline on Animal Experiments at Kyoto University. 11–13 week-old C57BL/6 male mice (Japan SLC, Inc.) were maintained under specific pathogen-free environment, allowed free access to food and water, and used for experiments.
Islet isolation and preservation
The islets were isolated using a similar protocol to that reported previously.42 The final islet purity was nearly 100%, which was determined after the sampled part was stained with dithizone (2mg/mL final concentration; Sigma-Aldrich). Purified islets were stored in prepared preservation solutions at 4°C.
Islet count and volume estimation
Islet number and volume were determined based upon the photos taken using a CCD digital camera (Olympus, DP20–5) attached to stereoscopic microscope (Olympus SZX16–315). To determine islet volume, assuming that islet is a sphere,43 we measured the islet projection area A on the photo by using Java’s Image J software and estimated islet volume V as V = (4/3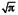 ) A1.5. Seventy islets were used in each experimental group at every experiment and experiments were run three times. These islets were evaluated at determined time points up to 120 h.
) A1.5. Seventy islets were used in each experimental group at every experiment and experiments were run three times. These islets were evaluated at determined time points up to 120 h.
Islet morphology assessment
Islets were immersion-fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Fixed islets were embedded in paraffin by conventional procedure and stained with hematoxylin/eosin.
Glucose-stimulated insulin release
Preserved islets of each experimental group were subjected to static glucose challenge in Krebs-Ringer bicarbonate buffer (KRBB) (pH 7.35) containing 10 mmol/l HEPES and 0.2% BSA (Sigma). After conditioning, three or four sets of 15 islets were incubated at 37°C in KRBB containing low (3.3 mmol/L) and high (20 mmol/L) glucose concentrations sequentially for 1 h in each solution. We repeated this challenge in succession to complete a single experiment. At the end of the incubation period, the supernatant was collected and frozen at -30°C for later analysis using a mouse insulin enzyme-linked immunosorbent assay kit (Shibayagi, Japan).
Islet viability
Islets were incubated with 100nM YOYO-1 iodide (Molecular Probes) and 10 mg/mL Hoechst 33342 (Molecular Probes) for 30 min at room temperature. Islets were then washed for three times, placed on glass-based dishes and examined using confocal laser scanning microscope. Images of stained islets were captured by LSM 510 Meta NLO imaging system of Carl Zeiss. At least 40 islets and more than 1000 islet cells were examined for each experimental group. The number of YOYO-labeled nuclei and Hoechst-labeled nuclei per islet were counted by the MetaMorph software program (Molecular Devices).
Islet transplantation
Three hundred islets were transplanted into the renal subcapsular space of six STZ-induced diabetic mice in each group with the method as described previously.44 Diabetes was induced by intraperitoneal administration of streptozotocin (Sigma-Aldrich Co.) (120 mg/kg). The recipients were mice with nonfasting blood glucose levels of over 400 mg/dL based on two consecutive measurements. An islet suspension was injected under the renal capsule of the left kidney. Blood glucose levels were measured every day and nephrectomies were performed 34 d after transplantation. Cure of diabetes was defined as blood glucose levels of less than 200 mg/dL in two consecutive days. Nephrectomy of the graft-bearing kidney was performed to confirm that islet graft contributes directly to the normalizing blood glucose after transplantation.
Intraperitoneal glucose tolerance test (IPGTT)
The mice were fasted for 16 h, and glucose (2.0 g/kg body weight) was intraperitoneally injected. Their blood glucose levels were then measured before and 10, 20, 30, 40, 60 and 120 min after injection. Five naive C57BL/6 male mice were tested as controls for this procedure.
Immunostaining
Removed islet-bearing kidneys were fixed in 4% paraformaldehyde and embedded with paraffin. Guinea pig anti-insulin antibody (Dako Cytomation) and HRP-Labeled anti-guinea pig antibody (Dako EnVision + System) were used to detect insulin as primary and secondary antibody, respectively.
Statistical analysis
All data are presented as mean ± standard deviation. Statistical analyses were performed by unpaired t-test. A value of p less than 0.05 was considered significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr. Masaharu Akao and Dr. Moritake Iguchi for use of confocal laser scanning microscope, Ms. Kae Furukawa for her technical assistance and Ms. Amy Hsiao for her careful review of this manuscript.
Glossary
Abbreviations:
- UW
University of Wisconsin solution
- MK
M-Kyoto solution
- STZ
streptozotocin
- TUNEL
TdT-mediated dUTP Nick-End Labeling
- YOYO-1
1,1'-(4,4,7,7-tetramethyl-4,7-diazaundecamethylene)- bis- 4-3-methyl-2,3-dihydro-(benzo-1,3-oxazole)- 2-methylidene-quinolinium tetraiodide
- FBS
fetal bovine serum
- ATP
adenosine triphosphate
- ADP
adenosine diphosphate
- FDA
fluorescein diacetate
- PI
propidium iodide
- IPGTT
intraperitoneal glucose tolerance test
- KRBB
Krebs-Ringer bicarbonate buffer
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/24025
References
- 1.Alejandro R, Barton FB, Hering BJ, Wease S, Collaborative Islet Transplant Registry Investigators 2008 Update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86:1783–8. doi: 10.1097/TP.0b013e3181913f6a. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Markmann JF, Tomaszewski J, Posselt AM, Levy MM, Woehrle M, Barker CF, et al. The effect of islet cell culture in vitro at 24 degrees C on graft survival and MHC antigen expression. Transplantation. 1990;49:272–7. doi: 10.1097/00007890-199002000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Jahr H, Hussmann B, Eckhardt T, Bretzel RG. Successful single donor islet allotransplantation in the streptozotocin diabetes rat model. Cell Transplant. 2002;11:513–8. [PubMed] [Google Scholar]
- 5.Ihm SH, Matsumoto I, Zhang HJ, Ansite JD, Hering BJ. Effect of short-term culture on functional and stress-related parameters in isolated human islets. Transpl Int. 2009;22:207–16. doi: 10.1111/j.1432-2277.2008.00769.x. [DOI] [PubMed] [Google Scholar]
- 6.Weber CJ, Hardy MA, Lerner RL, Reemtsma K. Tissue culture isolation and preservation of human cadaveric pancreatic islets. Surgery. 1977;81:270–3. [PubMed] [Google Scholar]
- 7.Hering BJ, Kandaswamy R, Harmon JV, Ansite JD, Clemmings SM, Sakai T, et al. Transplantation of cultured islets from two-layer preserved pancreases in type 1 diabetes with anti-CD3 antibody. Am J Transplant. 2004;4:390–401. doi: 10.1046/j.1600-6143.2003.00351.x. [DOI] [PubMed] [Google Scholar]
- 8.Ilieva A, Yuan S, Wang RN, Agapitos D, Hill DJ, Rosenberg L. Pancreatic islet cell survival following islet isolation: the role of cellular interactions in the pancreas. J Endocrinol. 1999;161:357–64. doi: 10.1677/joe.0.1610357. [DOI] [PubMed] [Google Scholar]
- 9.Kin T, Senior P, O’Gorman D, Richer B, Salam A, Shapiro AM. Risk factors for islet loss during culture prior to transplantation. Transpl Int. 2008;21:1029–35. doi: 10.1111/j.1432-2277.2008.00719.x. [DOI] [PubMed] [Google Scholar]
- 10.Noguchi H, Naziruddin B, Jackson A, Shimoda M, Ikemoto T, Fujita Y, et al. Low-temperature preservation of isolated islets is superior to conventional islet culture before islet transplantation. Transplantation. 2010;89:47–54. doi: 10.1097/TP.0b013e3181be3bf2. [DOI] [PubMed] [Google Scholar]
- 11.Gordon DA, Toledo-Pereyra LH, MacKenzie GH. Preservation for transplantation: a review of techniques of islet cell culture and storage. J Surg Res. 1982;32:182–93. doi: 10.1016/0022-4804(82)90089-0. [DOI] [PubMed] [Google Scholar]
- 12.Schulak JA, Stuart FP, Reckard CR. Physiologic aspects of intrasplenic autotransplantation of pancreatic fragments in the dog after 24 hours of cold storage. J Surg Res. 1978;24:125–31. doi: 10.1016/0022-4804(78)90164-6. [DOI] [PubMed] [Google Scholar]
- 13.Southard JH, Belzer FO. Organ preservation. Annu Rev Med. 1995;46:235–47. doi: 10.1146/annurev.med.46.1.235. [DOI] [PubMed] [Google Scholar]
- 14.Maathuis MH, Leuvenink HG, Ploeg RJ. Perspectives in organ preservation. Transplantation. 2007;83:1289–98. doi: 10.1097/01.tp.0000265586.66475.cc. [DOI] [PubMed] [Google Scholar]
- 15.Lee CY, Mangino MJ. Preservation methods for kidney and liver. Organogenesis. 2009;5:105–12. doi: 10.4161/org.5.3.9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemasters JJ, Bunzendahl H, Thurman RG. Reperfusion injury to donor livers stored for transplantation. Liver Transpl Surg. 1995;1:124–38. doi: 10.1002/lt.500010211. [DOI] [PubMed] [Google Scholar]
- 17.Roche M, Rondeau P, Singh NR, Tarnus E, Bourdon E. The antioxidant properties of serum albumin. FEBS Lett. 2008;582:1783–7. doi: 10.1016/j.febslet.2008.04.057. [DOI] [PubMed] [Google Scholar]
- 18.Faure P, Tamisier R, Baguet JP, Favier A, Halimi S, Lévy P, et al. Impairment of serum albumin antioxidant properties in obstructive sleep apnoea syndrome. Eur Respir J. 2008;31:1046–53. doi: 10.1183/09031936.00062707. [DOI] [PubMed] [Google Scholar]
- 19.Bessems M, Doorschodt BM, van Vliet AK, van Gulik TM. Improved rat liver preservation by hypothermic continuous machine perfusion using polysol, a new, enriched preservation solution. Liver Transpl. 2005;11:539–46. doi: 10.1002/lt.20388. [DOI] [PubMed] [Google Scholar]
- 20.Noguchi H, Ueda M, Nakai Y, Iwanaga Y, Okitsu T, Nagata H, et al. Modified two-layer preservation method (M-Kyoto/PFC) improves islet yields in islet isolation. Am J Transplant. 2006;6:496–504. doi: 10.1111/j.1600-6143.2006.01223.x. [DOI] [PubMed] [Google Scholar]
- 21.Trocha SD, Kevil CG, Mancini MC, Alexander JS. Organ preservation solutions increase endothelial permeability and promote loss of junctional proteins. Ann Surg. 1999;230:105–13. doi: 10.1097/00000658-199907000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Askenasy N, Navon G. Intracellular volumes and membrane permeability in rat hearts during prolonged hypothermic preservation with St Thomas and University of Wisconsin solutions. J Mol Cell Cardiol. 1998;30:1329–39. doi: 10.1006/jmcc.1998.0695. [DOI] [PubMed] [Google Scholar]
- 23.Serum-Free Media RIF. New Jersey: John Willey & Sons, Inc, 2010. [Google Scholar]
- 24.Strubelt O, Younes M, Li Y. Protection by albumin against ischaemia- and hypoxia-induced hepatic injury. Pharmacol Toxicol. 1994;75:280–4. doi: 10.1111/j.1600-0773.1994.tb00361.x. [DOI] [PubMed] [Google Scholar]
- 25.Salahudeen A, Nawaz M, Poovala V, Kanji V, Wang C, Morrow J, et al. Cold storage induces time-dependent F2-isoprostane formation in renal tubular cells and rat kidneys. Kidney Int. 1999;55:1759–62. doi: 10.1046/j.1523-1755.1999.00390.x. [DOI] [PubMed] [Google Scholar]
- 26.Katz SM, Sun S, Schechner RS, Tellis VA, Alt ER, Greenstein SM. Improved small intestinal preservation after lazaroid U74389G treatment and cold storage in University of Wisconsin solution. Transplantation. 1995;59:694–8. doi: 10.1097/00007890-199503150-00009. [DOI] [PubMed] [Google Scholar]
- 27.Brass CA, Roberts TG. Hepatic free radical production after cold storage: Kupffer cell-dependent and -independent mechanisms in rats. Gastroenterology. 1995;108:1167–75. doi: 10.1016/0016-5085(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 28.Salahudeen AK, Huang H, Patel P, Jenkins JK. Mechanism and prevention of cold storage-induced human renal tubular cell injury. Transplantation. 2000;70:1424–31. doi: 10.1097/00007890-200011270-00005. [DOI] [PubMed] [Google Scholar]
- 29.Rubinsky B. Principles of low temperature cell preservation. Heart Fail Rev. 2003;8:277–84. doi: 10.1023/A:1024734003814. [DOI] [PubMed] [Google Scholar]
- 30.Wei L, Hata K, Doorschodt BM, Büttner R, Minor T, Tolba RH. Experimental small bowel preservation using Polysol: a new alternative to University of Wisconsin solution, Celsior and histidine-tryptophan-ketoglutarate solution? World J Gastroenterol. 2007;13:3684–91. doi: 10.3748/wjg.v13.i27.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–95. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 32.Barnett MJ, McGhee-Wilson D, Shapiro AM, Lakey JR. Variation in human islet viability based on different membrane integrity stains. Cell Transplant. 2004;13:481–8. doi: 10.3727/000000004783983701. [DOI] [PubMed] [Google Scholar]
- 33.Merchant FA, Diller KR, Aggarwal SJ, Bovik AC. Viability analysis of cryopreserved rat pancreatic islets using laser scanning confocal microscopy. Cryobiology. 1996;33:236–52. doi: 10.1006/cryo.1996.0024. [DOI] [PubMed] [Google Scholar]
- 34.Grochowiecki T, Gotoh M, Dono K, Takeda Y, Nishihara M, Ohta Y, et al. Pretreatment of crude pancreatic islets with mitomycin C (MMC) prolongs islet graft survival in a xenogeneic rat-to-mouse model. Cell Transplant. 1998;7:411–2. doi: 10.1016/S0963-6897(98)00023-2. [DOI] [PubMed] [Google Scholar]
- 35.Weinhaus AJ, Bhagroo NV, Brelje TC, Sorenson RL. Dexamethasone counteracts the effect of prolactin on islet function: implications for islet regulation in late pregnancy. Endocrinology. 2000;141:1384–93. doi: 10.1210/en.141.4.1384. [DOI] [PubMed] [Google Scholar]
- 36.Granchi D, Devescovi V, Baglio SR, Magnani M, Donzelli O, Baldini N. A regenerative approach for bone repair in congenital pseudarthrosis of the tibia associated or not associated with type 1 neurofibromatosis: correlation between laboratory findings and clinical outcome. Cytotherapy. 2012;14:306–14. doi: 10.3109/14653249.2011.627916. [DOI] [PubMed] [Google Scholar]
- 37.Yoshikawa T, Mitsuno H, Nonaka I, Sen Y, Kawanishi K, Inada Y, et al. Wound therapy by marrow mesenchymal cell transplantation. Plast Reconstr Surg. 2008;121:860–77. doi: 10.1097/01.prs.0000299922.96006.24. [DOI] [PubMed] [Google Scholar]
- 38.Zakaria N, Koppen C, Van Tendeloo V, Berneman Z, Hopkinson A, Tassignon MJ. Standardized limbal epithelial stem cell graft generation and transplantation. Tissue Eng Part C Methods. 2010;16:921–7. doi: 10.1089/ten.tec.2009.0634. [DOI] [PubMed] [Google Scholar]
- 39.Walcher F, Marzi I, Schäfer W, Flecks U, Larsen R. Undissolved particles in UW solution cause microcirculatory disturbances after liver transplantation in the rat. Transpl Int. 1995;8:161–2. doi: 10.1111/j.1432-2277.1995.tb01494.x. [DOI] [PubMed] [Google Scholar]
- 40.Fischer JH, Jeschkeit S. Effectivity of freshly prepared or refreshed solutions for heart preservation versus commercial EuroCollins, Bretschneider’s HTK or University of Wisconsin solution. Transplantation. 1995;59:1259–62. [PubMed] [Google Scholar]
- 41.Tullius SG, Filatenkow A, Horch D, Mehlitz T, Reutzel-Selke A, Pratschke J, et al. Accumulation of crystal deposits in abdominal organs following perfusion with defrosted University of Wisconsin solutions. Am J Transplant. 2002;2:627–30. doi: 10.1034/j.1600-6143.2002.20707.x. [DOI] [PubMed] [Google Scholar]
- 42.Yonekawa Y, Okitsu T, Wake K, Iwanaga Y, Noguchi H, Nagata H, et al. A new mouse model for intraportal islet transplantation with limited hepatic lobe as a graft site. Transplantation. 2006;82:712–5. doi: 10.1097/01.tp.0000234906.29193.a6. [DOI] [PubMed] [Google Scholar]
- 43.Shintaku H, Okitsu T, Kawano S, Matsumoto S, Suzuki T, Kanno I, et al. Effects of fluid dynamic stress on fracturing of cell-aggregated tissue during purification for islets of Langerhans transplantation. J Phys D Appl Phys. 2008;41:115507–16. doi: 10.1088/0022-3727/41/11/115507. [DOI] [Google Scholar]
- 44.Okitsu T, Bartlett ST, Hadley GA, Drachenberg CB, Farney AC. Recurrent autoimmunity accelerates destruction of minor and major histoincompatible islet grafts in nonobese diabetic (NOD) mice. Am J Transplant. 2001;1:138–45. doi: 10.1034/j.1600-6143.2001.10207.x. [DOI] [PubMed] [Google Scholar]