Abstract
Caenorhabditis elegans gelsolin-like protein-1 (GSNL-1) is an unconventional member of the gelsolin family of actin-regulatory proteins. Unlike typical gelsolin-related proteins with three or six G domains, GSNL-1 has four gelsolin-like (G) domains (G1–G4) and exhibits calcium-dependent actin filament severing and capping activities. The first G domain (G1) of GSNL-1 is necessary for its actin-regulatory activities. However, how other domains in GSNL-1 participate in regulation of its functions is not understood. Here, we report biochemical evidence that the second G domain (G2) of GSNL-1 has a regulatory role in its calcium-dependent conformation and actin-regulatory activities. Comparison of the sequences of gelsolin-related proteins from various species indicates that sequences of G2 are highly conserved. Among the conserved residues in G2, we focused on D162 of GSNL-1, since equivalent residues in gelsolin and severin are part of the calcium-binding sites and is a pathogenic mutation site in human gelsolin causing familial amyloidosis, Finnish-type. The D162N mutation does not alter the inactive and fully calcium-activated states of GSNL-1 for actin filament severing (at 20 nM GSNL-1) and capping activities (at 50 nM GSNL-1). However, under these conditions, the mutant shows reduced calcium sensitivity for activation. By contrast, the D162N mutation strongly enhances susceptibility of GSNL-1 to chymotrypsin digestion only at high calcium concentrations but not at low calcium concentrations. The mutation also reduces affinity of GSNL-1 with actin monomers. These results suggest that G2 of GSNL-1 functions as a regulatory domain for its calcium-dependent actin-regulatory activities by mediating conformational changes of the GSNL-1 molecule.
Keywords: Actin dynamics, calcium sensitivity, capping, mutagenesis, severing
INTRODUCTION
Dynamics of the actin cytoskeleton are often accelerated by disassembly of actin filaments (Ono 2007). Once polymerized, actin filaments are relatively stable and only exchange actin monomers at filament ends. Therefore, severing of actin filaments can enhance actin turnover by increasing the number of filament ends where exchange of actin subunits occur. The gelsolin family of actin regulatory proteins is one of the major classes of actin-filament severing proteins (McGough et al. 2003; Ono 2007; Silacci et al. 2004; Sun et al. 1999). Gelsolin in vertebrates is the most extensively characterized member of the gelsolin family. It is inactive in the absence of Ca2+, whereas Ca2+ activates its actin filament severing and barbed-end capping functions. Gelsolin has six repeats of homologous domains of 100–120 amino acids, which are denoted as gelsolin-like domains (G domains) or segments (G1–G6) (Kwiatkowski et al. 1989; Kwiatkowski et al. 1986; Way et al. 1989; Way and Weeds 1988). In the absence of Ca2+, these G domains interact and form a compact globular structure such that actin-binding sites are masked (Burtnick et al. 1997). Binding of Ca2+ to gelsolin induces conformational changes to open up the molecule and expose actin-binding sites (Burtnick et al. 2004; Choe et al. 2002; Pope et al. 1997; Robinson et al. 1999). Recent biophysical studies on gelsolin revealed three conformational stages: a Ca2+-free closed conformation, an intermediate semi-open conformation (0.1~10 μM Ca2+), and an extended open conformation (100~1000 μM Ca2+) (Ashish et al. 2007; Kiselar et al. 2003). Therefore, Ca2+ is required to initiate opening of the gelsolin molecule and also to stabilize some conformational states. However, dynamic aspects of the Ca2+-dependent conformational changes and their regulation are not clearly understood. Moreover, mechanisms of Ca2+-regulation of other members of the gelsolin family with variable numbers of G domains have not been extensively studied.
The smallest naturally occurring gelsolin-related protein is ABP29 from Lilium pollen, which has only two G domains (Xiang et al. 2007). Physarum fragmin (Ampe and Vandekerckhove 1987), Dictyostelium severin (Andre et al. 1988; Schleicher et al. 1988), and vertebrate CapG (Dabiri et al. 1992; Prendergast and Ziff 1991; Yu et al. 1990) have three G domains. Caenorhabditis elegans gelsolin-like protein 1 (GSNL-1) has four G domains (Klaavuniemi et al. 2008). Regardless of the number of G domains, all these proteins are commonly regulated by Ca2+. Structure-function analyses of G domains in different gelsolin-related proteins show that G1 is a conserved essential actin-monomer binding domain (Eichinger et al. 1991; Kwiatkowski et al. 1989; Liu et al. 2010; McLaughlin et al. 1993; Yu et al. 1991), and that G2 of gelsolin or severin binds to the side of F-actin and potentiates actin filament severing activity (McGough et al. 1998; Puius et al. 2000; Van Troys et al. 1997; Way et al. 1992). G2 of gelsolin or severin also has a Ca2+-binding site (Chumnarnsilpa et al. 2006; Nag et al. 2009; Schnuchel et al. 1995), but role of G2 in Ca2+-regulation of the activities of the gelsolin family is not clearly understood.
In Ca2+-free gelsolin, unoccupied Ca2+-binding sites in G2 and G6 interact to maintain a closed conformation (Nag et al. 2009). Simultaneous disruption of both Ca2+-binding sites in G2 and G6 by mutations makes the mutant gelsolin open and active even in the absence of Ca2+ (Nag et al. 2009). Asp-187 is a part of the Ca2+-binding sites in G2, and mutation at this residue (D187N or D187Y) in human gelsolin causes familial amyloidosis, Finnish-type (also known as gelsolin amyloidosis or Meretoja's syndrome) (Pihlamaa et al. 2012; Solomon et al. 2012). These mutations make a secreted form of gelsolin susceptible to a series of proteolytic cleavages that generates amyloidogenic fragments (Page et al. 2004). Ca2+ binding to G2 stabilizes a conformation such that gelsolin becomes resistant to proteolysis (Chen et al. 2001; Huff et al. 2003; Kazmirski et al. 2000; Kazmirski et al. 2002; Ratnaswamy et al. 2001). Thus, the Ca2+-binding site in G2 is clearly important for Ca2+-dependent conformational changes of gelsolin. However, role of G2 in Ca2+-dependent actin-regulatory functions of gelsolin remains unclear. Mutant plasma gelsolin from amyloidosis patients, which had already been proteolytically processed, lacks actin filament severing activity (Weeds et al. 1993), while recombinant full-length gelsolin with the D187N mutation shows no detectable alterations in Ca2+-dependent actin-severing activity (Nag et al. 2009). Moreover, regulatory role of G2 is unclear in unconventional gelsolin-related proteins with two to four G domains.
The nematode Caenorhabditis elegans has three gelsolin-related proteins: FLI-1 (a homolog of Flightless-1) (Deng et al. 2007; Goshima et al. 1999), villin-like protein 1 (VILN-1), and gelsolin-like protein-1 (GSNL-1) (Klaavuniemi et al. 2008). FLI-1 is involved in a number of developmental processes (Deng et al. 2007), while functions of VILN-1 and GSNL-1 are currently unknown as mutations in genes encoding VILN-1 or GSNL-1 cause no detectable phenotypes (our unpublished data). GSNL-1 is most closely related to mammalian gelsolin, but it has only four G domains (G1–G4) (Klaavuniemi et al. 2008). GSNL-1 shares similar activities to gelsolin in Ca2+-dependent actin-filament severing and capping activities, but, unlike gelsolin, it binds to the side of actin filaments and does not nucleate actin polymerization. Analysis of functional domains of GSNL-1 shows that G1 and the linker between G1 and G2 are sufficient for actin filament severing in a similar manner to the equivalent part of gelsolin (Liu et al. 2010). However, the G1G2G3 fragment of GSNL-1 severs actin filaments in a Ca2+-dependent manner (Liu et al. 2010), whereas the equivalent fragments of gelsolin shows Ca2+-independent actin severing activity (Bryan and Hwo 1986; Chaponnier et al. 1986). We have identified two conserved aspartic acids in G1 of GSNL-1 that are important to maintain a closed conformation, suggesting the significance of a G1–G3 latch for Ca2+ regulation of the gelsolin family (Liu et al. 2011). In this study, we report identification of D162 in G2 as an important residue for Ca2+-dependent regulation of its conformation and activities. We found that the D162N mutation in GSNL-1 reduces the Ca2+ sensitivity but not the level of actin severing and capping activities at a fully activated state. These results suggest an important role of G2 of GSNL-1 in Ca2+-dependent activation and conformational changes.
MATERIALS AND METHODS
Proteins and Materials
Actin was purified from rabbit skeletal muscle acetone powder (Pel-Freeze Biologicals) as described (Pardee and Spudich 1982). Pyrene-labeled actin was prepared as described (Kouyama and Mihashi 1981). 7-chloro-4-nitrobenzeno-2-oxa-1,3-diazole (NBD)-labeled actin was prepared as described (Weeds et al. 1986). Bacterially expressed full-length wild-type GSNL-1 was purified as described previously (Klaavuniemi et al. 2008). Bovine pancreas chymotrypsin, which had been treated with 1-chloro-3-tosylamido-7-amino-2-heptanone, was purchased from Worthington Biochemical Corporation, suspended in 1 mM HCl at 1 mg/ml, and stored at −80 °C.
Site-directed Mutagenesis and Production of a GSNL-1 Mutant
Site-directed mutagenesis to convert Asp-162 of GSNL-1 to Asn was performed on the expression vector for full-length GSNL-1 (pGEX-GSNL-1) (Klaavuniemi et al. 2008) by a QuickChange mutagenesis kit (Stratagene) using a forward primer 5'-GAATTCTCTTAATTTGGGAAATGTATTCATTTTGGATCTGGG-3' and a reverse primer 5'-CCCAGATCCAAAATGAATACATTTCCCAAATTAAGAGAATTC-3'.The sequence of the protein coding region was verified by DNA sequencing. The proteins were expressed as fusion proteins with glutathione S-transferase in Escherichia coli BL21(DE3). Wild-type GSNL-1 was expressed by induction with 0.1 mM isopropyl-1-thio-β-D-galactopyranoside for 2 h at room temperature, while GST-GSNL-1(D162N) mutant was expressed over night at 15 °C. They were purified as described previously (Klaavuniemi et al. 2008). Briefly, E. coli lysates were applied to a glutathione Uniflow (Clontech) column to adsorb the GST-fusion proteins, and then the GSNL-1 variants were cleaved by thrombin (Roche Applied Science) on beads and eluted from the column. They were dialyzed against F-buffer (0.1 M KCl, 2 mM MgCl2, and 20 mM HEPES-NaOH, pH 7.5) containing 50 % glycerol and stored at −20 °C. Protein concentrations were determined by densitometry of Coomassie Blue-stained gels after SDS-PAGE using actin as a standard.
Direct Observation of Actin Filaments by Fluorescence Microscopy
Microscopic observation of actin filament severing was performed essentially as described previously (Liu et al. 2010). Unlabeled G-actin was co-polymerized with DyLight 549-labeled G-actin at 2 μM total actin (20 % labeled) for 2 hr in F-buffer. Labeled actin was diluted to 0.4 μM in F-buffer with or without GSNL-1 variants in the presence of various concentrations of CaCl2 and EGTA and incubated for 5 min at room temperature. Free Ca2+ concentrations were calculated by CHELATER (Schoenmakers et al. 1992). Then, the reactions were put on a nitrocellulose-coated coverslip, and immobilized actin filaments on the coverslip were observed by epifluorescence using a Nikon TE2000 microscope with a 60× Plan Apo objective (oil, numerical aperture of 1.4). Images were captured by a SPOT RT Monochrome CCD camera (Diagnostic Instruments) and processed by IPLab (BD Biosciences) and Adobe Photoshop CS2. Measurements of filament length were performed with IPLab.
Determination of the Apparent Critical Concentration of Actin
Varying concentrations of pyrene-labeled G-actin (20 % labeled) were polymerized overnight at room temperature in the presence of constant concentrations (50 nM) of GSNL-1 variants in F-buffer containing various concentrations of free Ca2+ as calculated by CHELATER (Schoenmakers et al. 1992). Fluorescence intensity of pyrene (excitation at 366 nm and emission at 384 nm) at the steady state was measured with a Perkin-Elmer LS50B fluorescence spectrophotometer. Apparent critical concentrations were determined by the inflection points of actin concentrations above which the fluorescence intensity was linearly increased. The apparent critical concentration values are plotted as a function of pCa and fitted to a four-parameter logistic equation by SigmaPlot 10 (Systat Software, Inc.).
Chymotryptic Digestion
Wild-type or mutant GSNL-1 (0.1 mg/ml) was incubated with 0.002 mg/ml chymotrypsin (Worthington Biochemical Corporation) in F-buffer in the presence of various free Ca2+ concentrations or 0.2 mM EGTA at room temperature. Reactions were stopped by mixing with equal volumes of SDS-sample buffer (2 % SDS, 80 mM Tris-HCl, 5 % β-mercaptoethanol, 15 % glycerol, 0.05 % bromophenol blue, pH 6.8) and heating at 98 °C for 3 min. Digestion patterns were analyzed by SDS-PAGE. Amounts of intact proteins at time 0 was set as 100 %, and remaining intact proteins at each time point were quantified by densitometry. Data were fitted to exponential decay curves by SigmaPlot 10 for calculation of rates of digestion (k).
Crosslinking
G-actin (5 μM) and wild-type or mutant GSNL-1 (5 μM) were incubated in modified G-buffer (0.2 mM ATP, 0.2 mM dithiothreitol, 2 mM HEPES-NaOH, pH 7.5) containing various free Ca2+ concentrations in the presence or absence of 0.7 mM 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) (Thermo Scientific) and 0.7 mM N-hydroxysulfosuccinimide (Sulfo-NHS) (Thermo Scientific) for 1 h at room temperature. Reactions were stopped by adding equal volumes of SDS-sample buffer and heating at 98 °C for 3 min and analyzed by SDS-PAGE.
Actin Monomer Binding Assays Using NBD-actin
The change in the fluorescence of NBD-labeled G-actin was used to detect binding of GSNL-1 to G-actin (Bryan and Kurth 1984; Weeds et al. 1986). NBD-actin (0.8 μM, 15 % labeled) was incubated with various concentrations of GSNL-1(WT) or GSNL-1(D162N) in modified G-buffer at pCa 4.0 or with 0.2 mM EGTA for 30 min at room temperature. Then, the fluorescence of NBD (F) (excitation at 480 nm and emission at 530 nm) was measured using an F-4500 fluorescence spectrophotometer (Hitachi High-Technologies), and relative fluorescence (E) was calculated as follows:
| (Equation 1) |
where F0 = fluorescence of 0.8 μM actin alone, and Fmax = the maximal fluorescence when GSNL-1 saturated binding to 0.8 μM actin.
A dissociation constant (Kd) for binding of GSNL-1 to G-actin was determined as described by Carlier et al. (Carlier et al. 1997) with modifications. Briefly, the data were fitted to equation 2:
| (Equation 2) |
where
| (Equation 3) |
and
| (Equation 4) |
Curve fitting was performed with SigmaPlot 10.
RESULTS
Conservation of Sequences of the Second Gelsolin-like Domains among Gelsolin-related Proteins
To investigate a role of G2 of C. elegans GSNL-1, we first compared the sequences of G2 of representative gelsolin-related proteins from various species (Fig. 1A). G2 sequences were extracted from human gelsolin with six G domains, human CapG with three G domains, Drosophila gelsolin with six G domains, Dictyostelium severin with three G domains, and Lilium ABP29 with two G domains, and aligned with that of C. elegans GSNL-1 using the Clustal W method (Thompson et al. 1994) (Fig. 1A). The alignment showed a number of conserved clusters of amino acids (Fig. 1A, see amino acids with black boxes). Gelsolin G2 (Burtnick et al. 1997; Burtnick et al. 2004; Kazmirski et al. 2002) and severin G2 (Puius et al. 2000; Schnuchel et al. 1995) have structures similar to other G domains with a central five-stranded β-sheet and an α-helix on each side (Fig. 1B). Many of the conserved clusters matched with positions of α helices (α1 and α2) and β strands (β1 – β5) (Fig. 1A) suggesting that these are important structural determinants.
Figure 1. Comparison of sequences of G2 from various gelsolin-related proteins.

(A) Alignment of sequences of G2 from C. elegans GSNL-1 (residues 133 – 248; Genbank accession number NM_073047), human gelsolin (residues 158 – 271; Genbank accession number NM_198252), human CapG (residues 135 – 247; Genbank accession number NP_001243068), Drosophila melanogaster gelsolin (residues 132 – 250; Genbank accession number NP_730788), Dictyostelium discoideum severin (residues 159 – 266; Genbank accession number AAA33250), and Lilium longiflorum ABP29 (residues 137 – 252; Genbank accession number ABK35296). Sequence alignment was performed by the Clustal W method using the MegAlign module within DNASTAR Lasergene 10. When four or more aligned residues are identical or similar, they are indicated by white characters and black boxes. Positions of secondary structures based on crystal structure of human gelsolin G2 are indicated below the sequences. Aspartic acid residues that participate in Ca2+-binding in human gelsolin (D187) and Dictyostelium severin (D188) are pointed by an arrow on top. (B) Crystal structure of cadmium-bound gelsolin G2 (Protein Data Bank accession number 1KCQ) is shown. Side chains of amino acids in the Cd2+/Ca2+-binding site (D187, E209, and D259) are shown by sticks. Molecular graphics were generated by PyMol (Schrödinger).
Gelsolin G2 and severin G2 also have metal-binding sites capable of binding to Ca2+ at homologous positions, which involve D187 of gelsolin (Kazmirski et al. 2002; Nag et al. 2009) and D188 of severin (Puius et al. 2000; Schnuchel et al. 1995), respectively (Fig. 1A, indicated by an arrow, and Fig. 1B). These aspartic acid residues are highly conserved among gelsolin-related proteins, including CapG that has no actin severing activity (Fig. 1A). In human gelsolin, the D187N mutation alters conformation of isolated G2 (Page et al. 2004), while the same mutation in full-length gelsolin molecule does not cause a change in its activity (Nag et al. 2009). Furthermore, role of this residue in unconventional gelsolin-related proteins with fewer numbers of G domains is unknown. Therefore, we selected an equivalent residue, D162, in GSNL-1 (Fig. 1A) for further investigation to determine whether D162 is involved in the Ca2+-regulation of the GSNL-1 molecule. We introduced a D162N mutation to full-length GSNL-1 to remove an acidic group at this residue and examined its effects on Ca2+-dependent conformational changes and activation of GSNL-1.
D162N Mutation Reduces Ca2+-sensitivity of GSNL-1 for Actin-filament Severing and Capping
Ca2+-dependent actin-filament severing activity of wild-type GSNL-1 [GSNL-1(WT)] and D162N-mutant GSNL-1 [GSNL-1(D162N)] was determined by their effects on the lengths of actin filaments (Fig. 2). In the absence of Ca2+ or at pCa 6.0, both GSNL-1(WT) and GSNL-1(D162N) had insignificant effects on the lengths of actin filaments (Fig. 2A–C; see panels J and K for quantitative data). In the presence of increasing concentrations of Ca2+, GSNL-1(WT) shortened actin filaments at or below pCa 5.5 (Fig. 2E and H; see panels L–O for quantitative data) indicating that GSNL-1(WT) severed actin filaments. GSNL-1(D162N) also shortened actin filaments in a Ca2+-dependent manner but only at or below pCa 5.0 (Fig. 2I; see panels M–O for quantitative data). At pCa 5.0, the severing activity of GSNL-1(D162N) was weaker than that of GSNL-1(WT) (Fig. 2M), suggesting that GSNL-1(D162N) was only partially activated under these conditions. GSNL-1(D162N) showed indistinguishable activity to GSNL-1(WT) at pCa 4.5 (Fig. 2H, I, and N) or pCa 4.0 (Fig. 2O). At higher concentrations, both GSNL-1(WT) and GSNL-1(D162N) showed stronger severing (our unpublished observation) indicating that the severing activity was not saturated at the examined concentration of GSNL-1 (20 nM) and suggesting that GSNL-(D162N) is not simply a mutant with reduced activity. These results suggest that the D162 in G2 is not required for Ca2+-dependent actin-filament severing activity of GSNL-1, but that disruption of this site reduces Ca2+ sensitivity for actin-filament severing.
Figure 2. Direct observation of actin filament severing by GSNL-1(WT) and GSNL-1(D162N).
DyLight 549-labeled actin filaments (0.4 μM) were incubated without any additional protein (control) (A, D, and G) or with 20 nM GSNL-1(WT) (B, E, and H) or 20 nM GSNL-1(D162N) (C, F, and I) in a buffer containing 0.2 mM EGTA (A–C) or free Ca2+ at pCa 5.5 (D–F), or pCa 4.5 (G–I) and observed after 5 min by fluorescence microscopy. Bar, 10 μm. This assay was performed at various free Ca2+, and bar graphs of quantification of filament length from the micrographs (average length ± S. D. (μm), n=30) are shown in J–O. Data were examined by Student's t test. *, p<0.05. ns, not significant.
Next, we tested the effect of D162N mutation on the Ca2+-dependent barbed end-capping activity of GSNL-1. We determined the effects of GSNL-1(WT) or GSNL-1(D162N) on the apparent critical concentration (Cc) of actin at various free Ca2+ concentrations (Fig. 3). The Cc of uncapped actin is ~0.1 μM, and the amounts of polymerized actin will be linearly correlated with total actin concentrations above the Cc. However, because Cc at the barbed end (0.1 μM) is much lower than Cc at the pointed end (0.5 μM), the Cc value of barbed end-capped actin will be shifted near the Cc value at the pointed end (0.5 μM) as demonstrated previously (Klaavuniemi et al. 2008). In the absence of free Ca2+ (2 mM EGTA, Fig. 3A) and at pCa 7.0 (Fig. 3B) and pCa 6.5 (Fig. 3C), both GSNL-1(WT) and GSNL-1(D162N) had no effect on the Cc. At pCa 6.0 and pCa 5.5, GSNL-1(WT) shifted the Cc to ~0.4 μM (Fig. 3D and E, white circles), while GSNL-1(D162N) only weakly shifted the Cc (Fig. 3D and E, white squares). The data indicate that GSNL-1(WT) capped most of the barbed ends, whereas GSNL-1(D162N) was only partially activated for capping. At or below pCa 5.0, both GSNL-1(WT) and GSNL-1(D162N) similarly shifted the Cc to ~0.5 μM (Fig. 3F and G), indicating that GSNL-1(D162N) has normal capping activity when it is fully activated by Ca2+. Thus, GSNL-1(WT) was activated for capping in a narrow range of Ca2+ concentrations around pCa 6.0 (Fig. 3H, circles), suggesting that Ca2+ activation of GSNL-1(WT) is highly cooperative. On the other hand, GSNL-1(D162N) was activated for capping in a wider pCa range with 50 % activation occurring at ~pCa 5.5 (Fig. 3H, squares), suggesting that GSNL-1(D162N) is less sensitive to Ca2+ and less cooperative in Ca2+ activation than GSNL-1(WT).
Figure 3. Effects of GSNL-1(WT) and GSNL-1(D162N) on the apparent critical concentration of actin at various free Ca2+ concentrations.
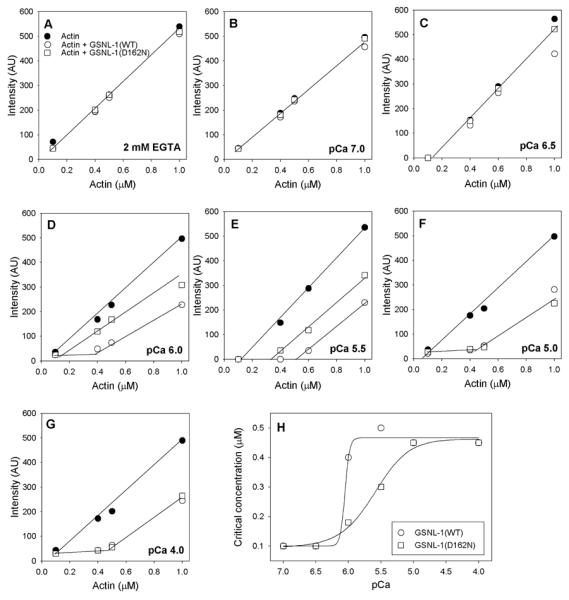
Various concentrations (0.1 – 1.0 μM) of actin (20 % pyrene-labeled) were polymerized without any additional protein (black circles) or with 50 nM GSNL-1 (WT) (white circles) or 50 nM GSNL-1(D162N) (white squares) in the presence of 0.2 mM EGTA (A) or free Ca2+ at pCa 7.0 (B), pCa 6.5 (C), pCa 6.0 (D), pCa 5.5 (E), pCa 5.0 (F), or pCa 4.0 (G). After overnight incubation, the pyrene fluorescence (arbitrary units (AU)) was measured and plotted as a function of the total actin concentrations. Apparent critical concentrations were determined by the inflection points of actin concentrations above which the fluorescence intensity was linearly increased. The apparent critical concentration values are plotted as a function of pCa in (H) (GSNL-1(WT): circles; GSNL-1(D162N): squares).
D162N Mutation Alters Ca2+-dependent Conformational Changes of GSNL-1
We then tested whether the reduced Ca2+ sensitivity of GSNL-1(D162N) for actin-filament severing and capping activities is correlated with Ca2+-dependent conformational changes. We determined susceptibility of GSNL-1(WT) and GSNL-1(D162N) to proteolysis by chymotrypsin at various free Ca2+ concentrations. We previously showed that GSNL-1(WT) is digested by chymotrypsin more rapidly in the presence of Ca2+ than in the absence of Ca2+ (Liu et al. 2011), suggesting that Ca2+ induced a conformational change of GSNL-1 from closed to open conformation. We tested the Ca2+-induced conformational change of GSNL-1(WT) and GSNL-1(D162N) at various free Ca2+ concentrations (Fig. 4). GSNL-1(WT) was only slowly digested by chymotrypsin in the absence of Ca2+ (Fig. 4A, circles) or at pCa 6.0 (Fig. 4B, circles). At pCa 5.0, GSNL-1(WT) was digested ~3-fold more rapidly digested (Fig. 4C, circles) than in the absence of Ca2+ or at pCa 6.0, suggesting that the conformation was opened. Interestingly, at pCa 4.0, it was more resistant to digestion than at pCa 5.0 (Fig. 4D, circles). This biphasic susceptibility to chymotryptic digestion suggests that the conformation of GSNL-1(WT) is opened at pCa 5.0, but that it is in another closed state or in a stabilized form at higher Ca2+ concentrations.
Figure 4. Susceptibility of GSNL-1(WT) and GSNL-1(D162N) to chymotryptic digestion at various free Ca2+ concentrations.
GSNL-1(WT) or GSNL-1(D162N) at 0.1 mg/ml was incubated with 0.002 mg/ml chymotrypsin in the presence of 0.2 mM EGTA (A) or free Ca2+ at pCa 6.0 (B), pCa 5.0 (C), or pCa 4.0 (D), and samples from indicated time points were analyzed by SDS-PAGE on the left. Graphs on the right show relative amounts (100 % at time 0) of intact proteins that were quantified by densitometry and plotted as a function of time (GSNL-1(WT): circles; GSNL-1(D162N): squares). Data are average ± S. D. of three independent experiments. Rates of exponential decay (k) were estimated by curve fitting and shown on the graphs.
By contrast, GSNL-1(D162N) exhibited different patterns of Ca2+-dependent conformational changes from GSNL-1(WT). GSNL-1(D162N) was resistant to chymotryptic digestion in the absence of Ca2+ (Fig. 4A, squares) or at pCa 6.0 (Fig. 4B, squares) in a similar manner to GSNL-1(WT), suggesting that the mutation does not affect the closed conformation of GSNL-1. At pCa 5.0, GSNL-1(D162N) was more rapidly digested than in the absence of Ca2+ or at pCa 6.0, but in a similar manner to GSNL-1(WT) (Fig. 4C, squares). Therefore, GSNL-1(D162N) can undergo Ca2+-dependent opening of conformation as GSNL-1(WT). Remarkably, susceptibility of GSNL-1(D162N) to chymotrypsin was further enhanced at pCa 4.0 (Fig. 4D, squares), suggesting that the mutant cannot form a conformation induced at high Ca2+ concentrations. These results show that the D162 is required for conformational stability of GSNL-1 at high Ca2+ concentrations but not for Ca2+-free closed conformation or opening by low Ca2+ concentrations.
D162N Mutation Weakens G-actin Binding of GSNL-1
We examined Ca2+-dependent G-actin binding by GSNL-1(WT) or GSNL-1(D162N) by two methods. First, G-actin binding was determined by crosslinking using EDC as a zero-length crosslinker (Fig. 5). G-actin (Fig. 5, lanes 1 and 2), GSNL-1(D162N) (Fig. 5, lanes 5 and 6), or GSNL-1(WT) (Fig. 5, lanes 9 and 10) by themselves was not crosslinked by EDC in the absence or presence of Ca2+. In the mixtures of G-actin and GSNL-1(D162N) (Fig. 5, lanes 3 and 4) or GSNL-1(WT) (Fig. 5, lanes 7 and 8), one major crosslinked product of ~100 kDa (Fig. 5B, lanes 4 and 8, arrow) and one minor product of ~150 kDa (Fig. 5B, lanes 4 and 8, arrowhead) were generated by EDC in the presence of Ca2+ (Fig. 5B) but not in the absence of Ca2+ (Fig. 5A). Within the range of pCa from 4.0 to 6.0, the patterns of crosslinking were similar between GSNL-1(WT) and GSNL-1(D162N) (our unpublished observation). Size of the major crosslinked product (~100 kDa) is consistent with formation of a 1:1 complex of G-actin (42 kDa) and GSNL-1 (55 kDa), which supports our previous estimate from non-denaturing polyacrylamide gel electrophoresis (Klaavuniemi et al. 2008). Size of the minor crosslinked product (~150 kDa) suggests formation of a 2:1 or 1:2 complex of G-actin and GSNL-1, but the nature of this complex is currently unknown. Overall, these results suggest that D162N mutation does not affect stoichiometry of Ca2+-dependent G-actin binding by GSNL-1.
Figure 5. Examination of binding of GSNL-1(WT) or GSNL-1(D162N) to G-actin by chemical crosslinking.

5 μM G-actin and 5 μM GSNL-1(WT) or 5 μM GSNL-1(D162N) (shown as D) were incubated in combinations indicated on top in the presence or absence of a zero-length crosslinker (EDC) for 1 h and analyzed by SDS-PAGE. The mixtures were reacted in the presence of 0.2 mM EGTA (A) or free Ca2+ at pCa 4.0 (B). On the right, G and A indicate GSNL-1 and actin, respectively, and arrows indicate a putative 1:1 complex of actin and GSNL-1. Arrowheads indicate an additional complex (possibly 2:1 or 1:2). Molecular weight markers in kDa are shown on the left.
Next, affinity of Ca2+-activated GSNL-1 with G-actin was determined by measuring changes in the fluorescence of G-actin labeled with 7-chloro-4-nitrobenzeno-2-oxa-1,3-diazole (NBD-actin). Binding of gelsolin to NBD-actin enhances the fluorescence of NBD by 2.2 – 2.6-fold (Bryan and Kurth 1984; Weeds et al. 1986). We found that binding of GSNL-1(WT) to NBD-actin enhances the fluorescence by ~1.8-fold. When GSNL-1(WT) was fully activated at pCa 4.0, the NBD fluorescence reached a maximum at a 1:1 molar ratio of GSNL-1(WT) (0.8 μM) to NBD-actin (0.8 μM) (Fig. 6, black circles), confirming our results in Fig. 5 that they formed a 1:1 complex. Their binding under these conditions appeared to be too tight for us to estimate a dissociation constant for their interaction. However, GSNL-1(D162N) showed much weaker interaction with NBD-actin than GSNL-1(WT) with an estimated dissociation constant of 0.4 μM (Fig. 6, black triangles). In the absence of free Ca2+ (0.2 mM EGTA), either GSNL-1(WT) or GSNL-1(D162N) did not significantly enhance the NBD fluorescence (Fig. 6, white circle and triangle, respecyively). These results suggest that the D162N mutation weakens G-actin binding of GSNL-1 by affecting the conformation of Ca2+-activated GSNL-1.
Figure 6. Examination of binding of GSNL-1(WT) or GSNL-1(D162N) to G-actin by fluorescence enhancement of NBD-actin.

NBD-actin (0.8 μM, 15 % labeled) was incubated with various concentrations of GSNL-1(WT) (circles) or GSNL-1(D162N) (triangles) in modified G-buffer at pCa 4.0 (black symbols) or with 0.2 mM EGTA (white symbols) for 30 min. Then, the fluorescence of NBD was measured, and relative fluorescence was plotted as a function of total concentrations of GSNL-1(WT) or GSNL-1(D162N). Data are average ± S. D. of three independent experiments.
DISCUSSION
In this study, we show that D162 in G2 of GSNL-1 is important for regulation of Ca2+-dependent conformation and actin-regulatory activities. GSNL-1(D162N) was less sensitive to Ca2+ than GSNL-1(WT) in activation of actin severing and capping activities. Nonetheless, under the conditions examined, GSNL-1(D162N) had indistinguishable activities from GSNL-1(WT) in the inactive state at low Ca2+ and the fully activated state at high Ca2+. At a high Ca2+ concentration (pCa 4.0), GSNL-1(D162N) was much more susceptible to chymotrypsin digestion than GSNL-1(WT), while, at lower Ca2+ concentrations, GSNL-1(D162N) and GSNL-1(WT) showed similar patterns of chymotrypsin digestion. As a result, the D162N mutation significantly weakened binding of GSNL-1 to G-actin at a high Ca2+ concentration. The results suggest that D162 in G2 of GSNL-1 is required for maintaining a Ca2+-dependent conformation and proper Ca2+ sensitivity for severing and capping activities but not for the closed inactive conformation of GSNL-1.
Previous studies on the Ca2+-binding site in G2 of mammalian gelsolin have been mostly focused on the mechanism of familial amyloidosis, as D187N or D187Y mutation at this site causes this hereditary human disease (Pihlamaa et al. 2012; Solomon et al. 2012). The disease is caused by proteolysis of mutant plasma gelsolin that generates amyloidogenic peptides, and the effect of amyloidosis mutations on actin-regulatory activities of gelsolin was not clearly understood. Plasma gelsolin from amyloidosis patients is already proteolytically processed and lacks actin severing activity (Weeds et al. 1993). A single D187N mutation does not apparently alter Ca2+-dependent actin severing activity of recombinant human gelsolin (Nag et al. 2009). In contrast, an equivalent mutation (D162N) in GSNL-1 caused changes in Ca2+ sensitivity for actin regulation and Ca2+-dependent conformations. This discrepancy could be due to the difference in their domain organization: gelsolin has six G domains, while GSNL-1 has four G domains. G1–G3 of gelsolin and GSNL-1 share functional similarities (Liu et al. 2010). However, G4 of GSNL-1 is most similar to G6 of gelsolin. In gelsolin, G4–G6 needs to move away from G1–G3 during Ca2+ activation (Burtnick et al. 1997), but, presumably in GSNL-1, only G4 needs to move. Therefore, the activation process and conformational changes of GSNL-1 should be less complex requiring fewer elements than those of gelsolin. Indeed, G6 of gelsolin has another Ca2+-binding site, and disruption of both Ca2+-binding sites in G2 and G6 alters Ca2+ sensitivity for actin regulation and Ca2+-stablilized conformation (Nag et al. 2009). At present, three-dimensional structure of GSNL-1 has not been solved, and whether D162 is involved in Ca2+-binding is unknown. Therefore, further structural and biochemical studies are required to understand how G2 and G4 of GSNL-1 contribute to its Ca2+ activation and conformational changes.
Biochemical studies on isolated gelsolin G2 fragments show that Ca2+ stabilizes the structure of wild-type G2 but not G2 with D187N or D187Y mutation (Chen et al. 2001; Huff et al. 2003). In contrast, in the full-length GSNL-1 protein, GSNL-1(WT) was altered in a biphasic manner in response to Ca2+, while GSNL-1(D162N) was increasingly destabilized depending on Ca2+ concentrations. These results suggest that D162 is important to maintain a Ca2+-dependent conformation of GSNL-1 but not required for opening of the GSNL-1 molecule at low Ca2+. The flexible C-terminal region of gelsolin G2 is extended to the linker between G2 and G3 and contains a part of the Ca2+-binding site (Fig. 1B). Ca2+ makes a bridge between this C-terminal region and the core of G2, thereby stabilizing the flexible C-terminal region (Fig. 1B) (Kazmirski et al. 2002). Therefore, if a similar mechanism applies to GSNL-1, once GSNL-1 is opened, Ca2+ may bind to G2 and hold it in a proximity to the G2–G3 linker, which may contribute to stabilization of the open molecule. However, at pCa 4.0, GSNL-1(D162N) showed indistinguishable activities from GSNL-1(WT) in actin filament severing and capping, while GSNL-1(D162N) bound to G-actin with lower affinity than GSNL-1(WT). Thus, although the conformation of GSNL-1(D162N) is unstable at high Ca2+ concentrations affecting its G-actin binding, other actin-binding sites may be fully exposed and may not affect its actin severing and capping functions.
In conclusion, we provide evidence that D162 in G2 of GSNL-1 plays important roles in regulation of Ca2+-dependent conformation and actin-regulatory activities. Although GSNL-1 has only four G domains, the mechanism of its Ca2+-dependent activation appears to be complex. G1 of GSNL-1 is a G-actin-binding site that is essential for actin severing activity (Liu et al. 2010). However, the D162N mutation in G2 significantly affects G-actin binding of GSNL-1, suggesting that G2 is important to maintain a stable conformation for G-actin binding. GSNL-1(D162N) is less sensitive and cooperative to Ca2+ than GSNL-1(WT), suggesting that the D162 mutation in G2 influences Ca2+-dependent conformational changes of the entire molecule most likely by altering interactions of G2 with other G domains. Perhaps, it will be important to determine whether D162 is indeed a part of a Ca2+-binding site and how Ca2+ binding and the D162N mutation affect the structure of GSNL-1. Functional significance of G2 as a regulatory domain in vivo is another important problem to be addressed in the future. GSNL-1 is expressed in striated muscle in C. elegans (Fox et al. 2007). However, in vivo function of GSNL-1 remains unclear, since gsnl-1 knockout worms have superficially normal striated muscle (our unpublished observation). In mammals, expression of gelsolin in skeletal and cardiac muscles has been reported for many years (Carron et al. 1986; Dissmann and Hinssen 1994; Rouayrenc et al. 1984; Yin et al. 1981), but its function in striated muscle is unknown (Ono 2010). Thus, C. elegans is potentially a powerful system to determine functions of gelsolin-related proteins by combining different approaches in biochemistry, cell biology, and genetics.
ACKNOWLEDGMENT
This work was supported by a grant from the National Institute of Health (R01 AR48615) to S. O.
REFERENCES
- Ampe C, Vandekerckhove J. The F-actin capping proteins of Physarum polycephalum: cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J. 1987;6:4149–4157. doi: 10.1002/j.1460-2075.1987.tb02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Lottspeich F, Schleicher M, Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988;263:722–727. [PubMed] [Google Scholar]
- Ashish, Paine MS, Perryman PB, Yang L, Yin HL, Krueger JK. Global structure changes associated with Ca2+ activation of full-length human plasma gelsolin. J Biol Chem. 2007;282:25884–25892. doi: 10.1074/jbc.M702446200. [DOI] [PubMed] [Google Scholar]
- Bryan J, Hwo S. Definition of an N-terminal actin-binding domain and a C-terminal Ca2+ regulatory domain in human brevin. J Cell Biol. 1986;102:1439–1446. doi: 10.1083/jcb.102.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan J, Kurth MC. Actin-gelsolin interactions. Evidence for two actin-binding sites. J Biol Chem. 1984;259:7480–7487. [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin severing, capping, and nucleation. Cell. 1997;90:661–670. doi: 10.1016/s0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Burtnick LD, Urosev D, Irobi E, Narayan K, Robinson RC. Structure of the N-terminal half of gelsolin bound to actin: roles in severing, apoptosis and FAF. EMBO J. 2004;23:2713–2722. doi: 10.1038/sj.emboj.7600280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carron CP, Hwo SY, Dingus J, Benson DM, Meza I, Bryan J. A re-evaluation of cytoplasmic gelsolin localization. J Cell Biol. 1986;102:237–245. doi: 10.1083/jcb.102.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaponnier C, Janmey PA, Yin HL. The actin filament-severing domain of plasma gelsolin. J Cell Biol. 1986;103:1473–1481. doi: 10.1083/jcb.103.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CD, Huff ME, Matteson J, Page L, Phillips R, Kelly JW, Balch WE. Furin initiates gelsolin familial amyloidosis in the Golgi through a defect in Ca2+ stabilization. EMBO J. 2001;20:6277–6287. doi: 10.1093/emboj/20.22.6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe H, Burtnick LD, Mejillano M, Yin HL, Robinson RC, Choe S. The calcium activation of gelsolin: insights from the 3A structure of the G4–G6/actin complex. J Mol Biol. 2002;324:691–702. doi: 10.1016/s0022-2836(02)01131-2. [DOI] [PubMed] [Google Scholar]
- Chumnarnsilpa S, Loonchanta A, Xue B, Choe H, Urosev D, Wang H, Lindberg U, Burtnick LD, Robinson RC. Calcium ion exchange in crystalline gelsolin. J Mol Biol. 2006;357:773–782. doi: 10.1016/j.jmb.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Dabiri GA, Young CL, Rosenbloom J, Southwick FS. Molecular cloning of human macrophage capping protein cDNA. A unique member of the gelsolin/villin family expressed primarily in macrophages. J Biol Chem. 1992;267:16545–16552. [PubMed] [Google Scholar]
- Deng H, Xia D, Fang B, Zhang H. The Flightless I homolog, fli-1, regulates anterior/posterior polarity, asymmetric cell division and ovulation during Caenorhabditis elegans development. Genetics. 2007;177:847–860. doi: 10.1534/genetics.107.078964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissmann E, Hinssen H. Immunocytochemical localization of gelsolin in fibroblasts, myogenic cells, and isolated myofibrils. Eur J Cell Biol. 1994;63:336–344. [PubMed] [Google Scholar]
- Eichinger L, Noegel AA, Schleicher M. Domain structure in actin-binding proteins: expression and functional characterization of truncated severin. J Cell Biol. 1991;112:665–676. doi: 10.1083/jcb.112.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox RM, Watson JD, Von Stetina SE, McDermott J, Brodigan TM, Fukushige T, Krause M, Miller DM. The embryonic muscle transcriptome of Caenorhabditis elegans. Genome Biol. 2007;8:R188. doi: 10.1186/gb-2007-8-9-r188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshima M, Kariya K, Yamawaki-Kataoka Y, Okada T, Shibatohge M, Shima F, Fujimoto E, Kataoka T. Characterization of a novel Ras-binding protein Ce-FLI-1 comprising leucine-rich repeats and gelsolin-like domains. Biochem Biophys Res Commun. 1999;257:111–116. doi: 10.1006/bbrc.1999.0420. [DOI] [PubMed] [Google Scholar]
- Huff ME, Page LJ, Balch WE, Kelly JW. Gelsolin domain 2 Ca2+ affinity determines susceptibility to furin proteolysis and familial amyloidosis of finnish type. J Mol Biol. 2003;334:119–127. doi: 10.1016/j.jmb.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Kazmirski SL, Howard MJ, Isaacson RL, Fersht AR. Elucidating the mechanism of familial amyloidosis- Finnish type: NMR studies of human gelsolin domain 2. Proc Natl Acad Sci USA. 2000;97:10706–10711. doi: 10.1073/pnas.180310097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmirski SL, Isaacson RL, An C, Buckle A, Johnson CM, Daggett V, Fersht AR. Loss of a metal-binding site in gelsolin leads to familial amyloidosis-Finnish type. Nat Struct Biol. 2002;9:112–116. doi: 10.1038/nsb745. [DOI] [PubMed] [Google Scholar]
- Kiselar JG, Janmey PA, Almo SC, Chance MR. Visualizing the Ca2+-dependent activation of gelsolin by using synchrotron footprinting. Proc Natl Acad Sci USA. 2003;100:3942–3947. doi: 10.1073/pnas.0736004100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaavuniemi T, Yamashiro S, Ono S. Caenorhabditis elegans gelsolin-like protein 1 is a novel actin filament-severing protein with four gelsolin-like repeats. J Biol Chem. 2008;283:26071–26080. doi: 10.1074/jbc.M803618200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouyama T, Mihashi K. Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin. Local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur J Biochem. 1981;114:33–38. [PubMed] [Google Scholar]
- Kwiatkowski DJ, Janmey PA, Yin HL. Identification of critical functional and regulatory domains in gelsolin. J Cell Biol. 1989;108:1717–1726. doi: 10.1083/jcb.108.5.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986;323:455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Liu Z, Kanzawa N, Ono S. Calcium-sensitive activity and conformation of Caenorhabditis elegans gelsolin-like protein 1 are altered by mutations in the first gelsolin-like domain. J Biol Chem. 2011;286:34051–34059. doi: 10.1074/jbc.M111.237404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Klaavuniemi T, Ono S. Distinct roles of four gelsolin-like domains of Caenorhabditis elegans gelsolin-like protein-1 in actin filament severing, barbed end capping, and phosphoinositide binding. Biochemistry. 2010;49:4349–4360. doi: 10.1021/bi100215b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Chiu W, Way M. Determination of the gelsolin binding site on F-actin: implications for severing and capping. Biophys J. 1998;74:764–772. doi: 10.1016/S0006-3495(98)74001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough AM, Staiger CJ, Min JK, Simonetti KD. The gelsolin family of actin regulatory proteins: modular structures, versatile functions. FEBS Letters. 2003;552:75–81. doi: 10.1016/s0014-5793(03)00932-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Gooch JT, Mannherz HG, Weeds AG. Structure of gelsolin segment 1-actin complex and the mechanism of filament severing. Nature. 1993;364:685–692. doi: 10.1038/364685a0. [DOI] [PubMed] [Google Scholar]
- Nag S, Ma Q, Wang H, Chumnarnsilpa S, Lee WL, Larsson M, Kannan B, Hernandez-Valladares M, Burtnick LD, Robinson RC. Ca2+ binding by domain 2 plays a critical role in the activation and stabilization of gelsolin. Proc Natl Acad Sci USA. 2009;106:13713–13718. doi: 10.1073/pnas.0812374106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Ono S. Dynamic regulation of sarcomeric actin filaments in striated muscle. Cytoskeleton. 2010;67:677–692. doi: 10.1002/cm.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LJ, Huff ME, Kelly JW, Balch WE. Ca2+ binding protects against gelsolin amyloidosis. Biochem Biophys Res Commun. 2004;322:1105–1110. doi: 10.1016/j.bbrc.2004.07.125. [DOI] [PubMed] [Google Scholar]
- Pardee JD, Spudich JA. Purification of muscle actin. Methods Enzymol. 1982;85:164–181. doi: 10.1016/0076-6879(82)85020-9. [DOI] [PubMed] [Google Scholar]
- Pihlamaa T, Suominen S, Kiuru-Enari S. Familial amyloidotic polyneuropathy type IV - gelsolin amyloidosis. Amyloid. 2012;(Suppl 1):30–33. doi: 10.3109/13506129.2012.674076. [DOI] [PubMed] [Google Scholar]
- Pope BJ, Gooch JT, Weeds AG. Probing the effects of calcium on gelsolin. Biochemistry. 1997;36:15848–15855. doi: 10.1021/bi972192p. [DOI] [PubMed] [Google Scholar]
- Prendergast GC, Ziff EB. Mbh 1: a novel gelsolin/severin-related protein which binds actin in vitro and exhibits nuclear localization in vivo. EMBO J. 1991;10:757–766. doi: 10.1002/j.1460-2075.1991.tb08007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puius YA, Fedorov EV, Eichinger L, Schleicher M, Almo SC. Mapping the functional surface of domain 2 in the gelsolin superfamily. Biochemistry. 2000;39:5322–5331. doi: 10.1021/bi992364d. [DOI] [PubMed] [Google Scholar]
- Ratnaswamy G, Huff ME, Su AI, Rion S, Kelly JW. Destabilization of Ca2+-free gelsolin may not be responsible for proteolysis in Familial Amyloidosis of Finnish Type. Proc Natl Acad Sci USA. 2001;98:2334–2339. doi: 10.1073/pnas.041452598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson RC, Mejillano M, Le VP, Burtnick LD, Yin HL, Choe S. Domain movement in gelsolin: a calcium-activated switch. Science. 1999;286:1939–1942. doi: 10.1126/science.286.5446.1939. [DOI] [PubMed] [Google Scholar]
- Rouayrenc JF, Fattoum A, Gabrion J, Audemard E, Kassab R. Muscle gelsolin: isolation from heart tissue and characterization as an integral myofibrillar protein. FEBS Lett. 1984;167:52–58. doi: 10.1016/0014-5793(84)80831-5. [DOI] [PubMed] [Google Scholar]
- Schleicher M, Andre E, Hartmann H, Noegel AA. Actin-binding proteins are conserved from slime molds to man. Dev Genet. 1988;9:521–530. doi: 10.1002/dvg.1020090428. [DOI] [PubMed] [Google Scholar]
- Schnuchel A, Wiltscheck R, Eichinger L, Schleicher M, Holak TA. Structure of severin domain 2 in solution. J Mol Biol. 1995;247:21–27. doi: 10.1006/jmbi.1994.0118. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TJ, Visser GJ, Flik G, Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–874. 876–879. [PubMed] [Google Scholar]
- Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci. 2004;61:2614–2623. doi: 10.1007/s00018-004-4225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JP, Page LJ, Balch WE, Kelly JW. Gelsolin amyloidosis: genetics, biochemistry, pathology and possible strategies for therapeutic intervention. Crit Rev Biochem Mol Biol. 2012;47:282–296. doi: 10.3109/10409238.2012.661401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HQ, Yamamoto M, Mejillano M, Yin HL. Gelsolin, a multifunctional actin regulatory protein. J Biol Chem. 1999;274:33179–33182. doi: 10.1074/jbc.274.47.33179. [DOI] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Dewitte D, Verschelde JL, Goethals M, Vandekerckhove J, Ampe C. Analogous F-actin binding by cofilin and gelsolin segment 2 substantiates their structural relationship. J Biol Chem. 1997;272:32750–32758. doi: 10.1074/jbc.272.52.32750. [DOI] [PubMed] [Google Scholar]
- Way M, Gooch J, Pope B, Weeds AG. Expression of human plasma gelsolin in Escherichia coli and dissection of actin binding sites by segmental deletion mutagenesis. J Cell Biol. 1989;109:593–605. doi: 10.1083/jcb.109.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M, Pope B, Weeds AG. Evidence for functional homology in the F-actin binding domains of gelsolin and alpha-actinin: implications for the requirements of severing and capping. J Cell Biol. 1992;119:835–842. doi: 10.1083/jcb.119.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M, Weeds A. Nucleotide sequence of pig plasma gelsolin. Comparison of protein sequence with human gelsolin and other actin-severing proteins shows strong homologies and evidence for large internal repeats. J Mol Biol. 1988;203:1127–1133. doi: 10.1016/0022-2836(88)90132-5. [DOI] [PubMed] [Google Scholar]
- Weeds AG, Gooch J, McLaughlin P, Maury CP. Variant plasma gelsolin responsible for familial amyloidosis (Finnish type) has defective actin severing activity. FEBS Lett. 1993;335:119–123. doi: 10.1016/0014-5793(93)80452-z. [DOI] [PubMed] [Google Scholar]
- Weeds AG, Harris H, Gratzer W, Gooch J. Interactions of pig plasma gelsolin with G-actin. Eur J Biochem. 1986;161:77–84. doi: 10.1111/j.1432-1033.1986.tb10126.x. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Huang X, Wang T, Zhang Y, Liu Q, Hussey PJ, Ren H. ACTIN BINDING PROTEIN 29 from Lilium pollen plays an important role in dynamic actin remodeling. Plant Cell. 2007;19:1930–1946. doi: 10.1105/tpc.106.048413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HL, Albrecht JH, Fattoum A. Identification of gelsolin, a Ca2+-dependent regulatory protein of actin gel-sol transformation, and its intracellular distribution in a variety of cells and tissues. J Cell Biol. 1981;91:901–906. doi: 10.1083/jcb.91.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Johnston PA, Sudhof TC, Yin HL. gCap39, a calcium ion- and polyphosphoinositide-regulated actin capping protein. Science. 1990;250:1413–1415. doi: 10.1126/science.2255912. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhou DM, Yin HL. Chimeric and truncated gCap39 elucidate the requirements for actin filament severing and end capping by the gelsolin family of proteins. J Biol Chem. 1991;266:19269–19275. [PubMed] [Google Scholar]




