Abstract
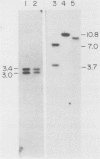
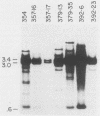
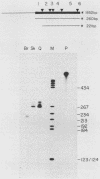
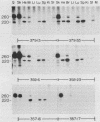
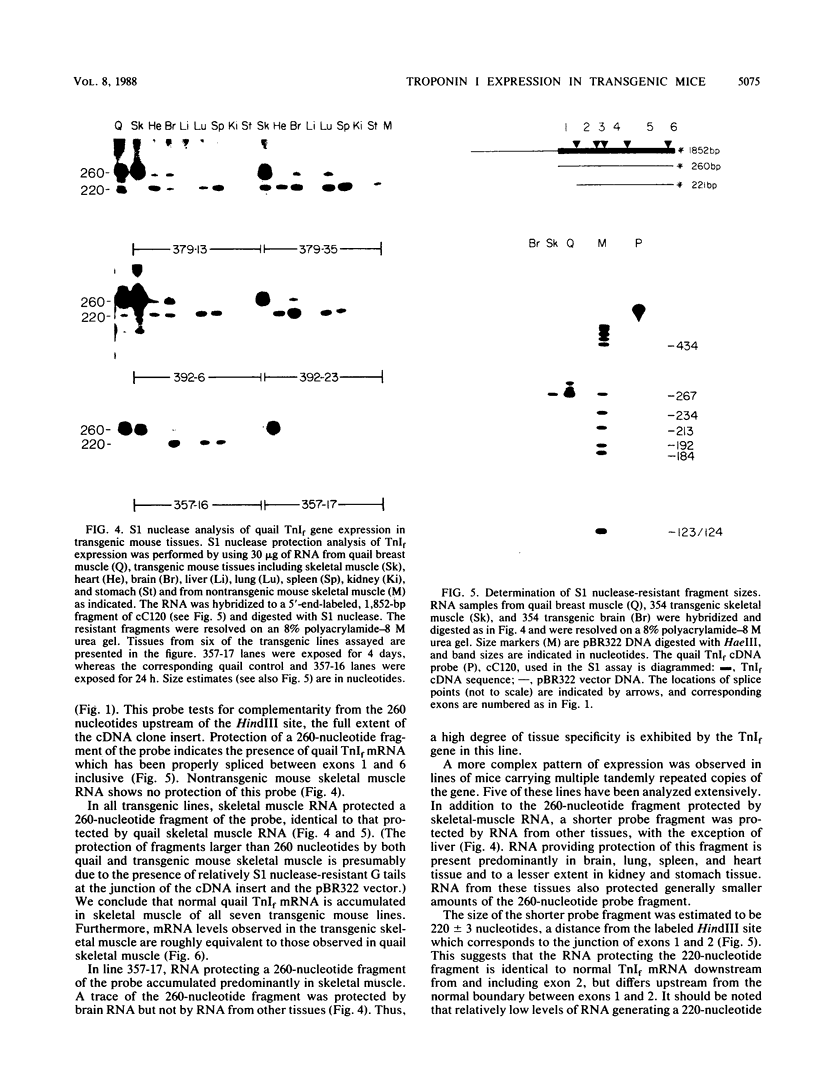
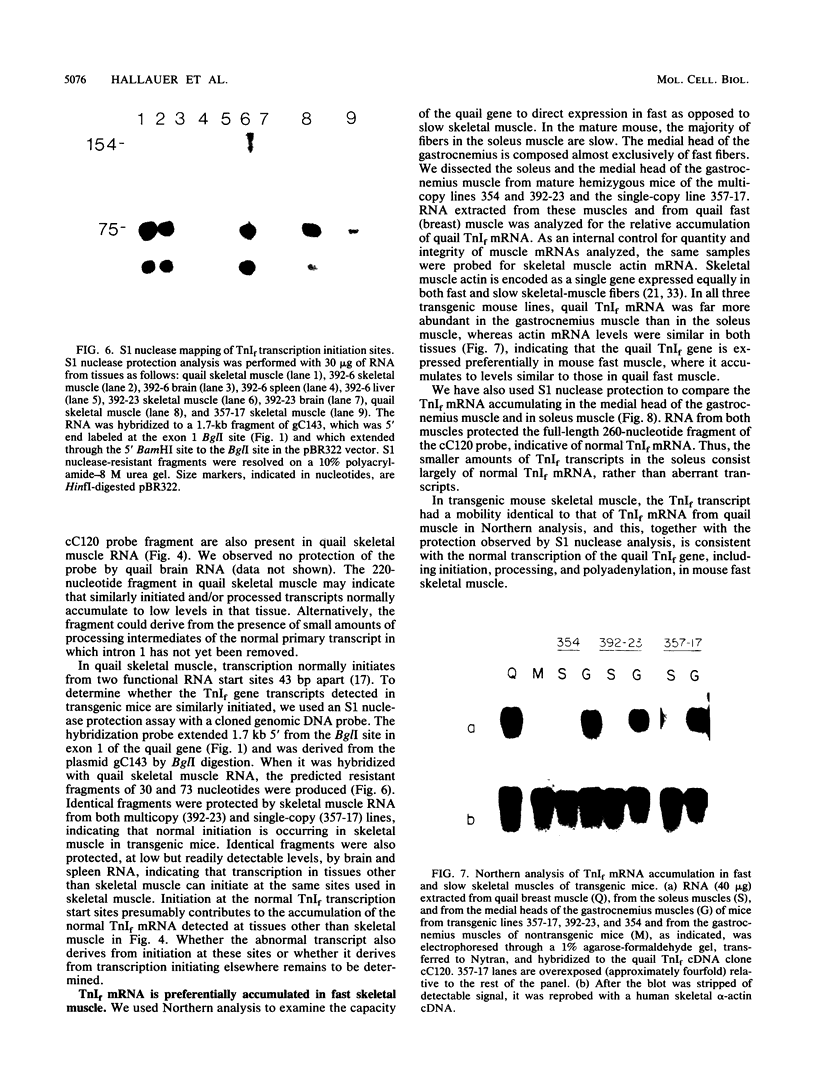
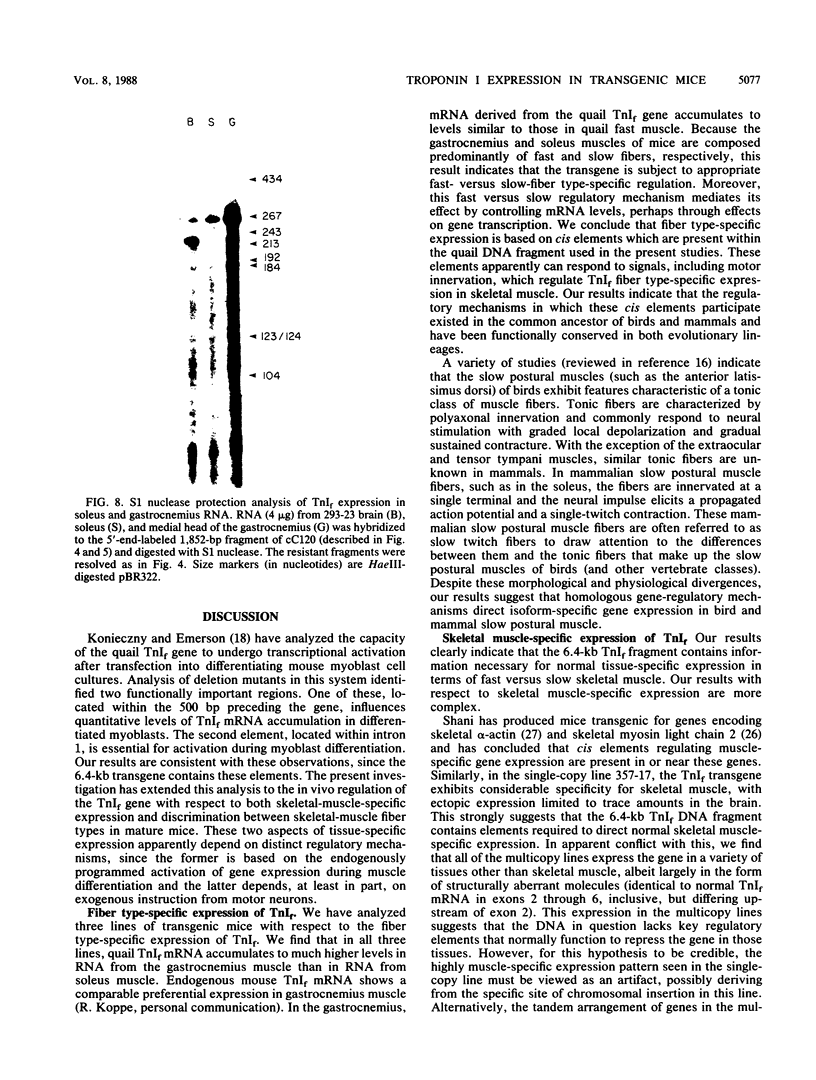
We have produced seven lines of transgenic mice carrying the quail gene encoding the fast skeletal muscle-specific isoform of troponin I (TnIf). The quail DNA included the entire TnIf gene, 530 base pairs of 5'-flanking DNA, and 1.5 kilobase pairs of 3'-flanking DNA. In all seven transgenic lines, normally initiated and processed quail TnIf mRNA was expressed in skeletal muscle, where it accumulated to levels comparable to that in quail muscle. Moreover, in the three lines tested, quail TnIf mRNA levels were manyfold higher in a fast skeletal muscle (gastrocnemius) than in a slow skeletal muscle (soleus). We conclude that the cellular mechanisms directing muscle fiber type-specific TnIf gene expression are mediated by cis-regulatory elements present on the introduced quail DNA fragment and that they control TnIf expression by affecting the accumulation of TnIf mRNA. These elements have been functionally conserved since the evolutionary divergence of birds and mammals, despite the major physiological and morphological differences existing between avian (tonic) and mammalian (twitch) slow muscles. In lines of transgenic mice carrying multiple tandemly repeated copies of the transgene, an aberrant quail TnIf transcript (differing from normal TnIf mRNA upstream of exon 2) also accumulated in certain tissues, particularly lung, brain, spleen, and heart tissues. However, this aberrant transcript was not detected in a transgenic line which carries only a single copy of the quail gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Amphlett G. W., Perry S. V., Syska H., Brown M. D., Vrbova G. Cross innervation and the regulatory protein system of rabbit soleus muscle. Nature. 1975 Oct 16;257(5527):602–604. doi: 10.1038/257602a0. [DOI] [PubMed] [Google Scholar]
- Baldwin A. S., Jr, Kittler E. L., Emerson C. P., Jr Structure, evolution, and regulation of a fast skeletal muscle troponin I gene. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8080–8084. doi: 10.1073/pnas.82.23.8080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Butler J., Cosmos E., Brierley J. Differentiation of muscle fiber types in aneurogenic brachial muscles of the chick embryo. J Exp Zool. 1982 Nov 20;224(1):65–80. doi: 10.1002/jez.1402240108. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. The components of the troponin complex and development in skeletal muscle. Exp Cell Res. 1980 May;127(1):75–87. doi: 10.1016/0014-4827(80)90416-4. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V. The effect of denervation on the distribution of the polymorphic forms of troponin components in fast and slow muscles of the adult rat. Cell Tissue Res. 1982;225(1):201–215. doi: 10.1007/BF00216229. [DOI] [PubMed] [Google Scholar]
- Dhoot G. K., Perry S. V., Vrbova G. Changes in the distribution of the components of the troponin complex in muscle fibers after cross-innervation. Exp Neurol. 1981 Jun;72(3):513–530. doi: 10.1016/0014-4886(81)90001-7. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Emerson C. P., Jr cDNA clone analysis of six co-regulated mRNAs encoding skeletal muscle contractile proteins. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1553–1557. doi: 10.1073/pnas.79.5.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess A. Vertebrate slow muscle fibers. Physiol Rev. 1970 Jan;50(1):40–62. doi: 10.1152/physrev.1970.50.1.40. [DOI] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Complex regulation of the muscle-specific contractile protein (troponin I) gene. Mol Cell Biol. 1987 Sep;7(9):3065–3075. doi: 10.1128/mcb.7.9.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konieczny S. F., Emerson C. P., Jr Differentiation, not determination, regulates muscle gene activation: transfection of troponin I genes into multipotential and muscle lineages of 10T1/2 cells. Mol Cell Biol. 1985 Sep;5(9):2423–2432. doi: 10.1128/mcb.5.9.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg I. R. Skeletal myoblasts in culture. Methods Enzymol. 1979;58:511–527. doi: 10.1016/s0076-6879(79)58166-x. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Transgenic mice. Cell. 1985 Jun;41(2):343–345. doi: 10.1016/s0092-8674(85)80004-0. [DOI] [PubMed] [Google Scholar]
- Pette D., Vrbová G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985 Oct;8(8):676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Schafer D. A., Miller J. B., Stockdale F. E. Cell diversification within the myogenic lineage: in vitro generation of two types of myoblasts from a single myogenic progenitor cell. Cell. 1987 Feb 27;48(4):659–670. doi: 10.1016/0092-8674(87)90244-3. [DOI] [PubMed] [Google Scholar]
- Shani M. Tissue-specific and developmentally regulated expression of a chimeric actin-globin gene in transgenic mice. Mol Cell Biol. 1986 Jul;6(7):2624–2631. doi: 10.1128/mcb.6.7.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani M. Tissue-specific expression of rat myosin light-chain 2 gene in transgenic mice. Nature. 1985 Mar 21;314(6008):283–286. doi: 10.1038/314283a0. [DOI] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M., Arriza J., Hammer R., Brinster R., Rosenfeld M. G., Evans R. M. Novel developmental specificity in the nervous system of transgenic animals expressing growth hormone fusion genes. 1985 Sep 26-Oct 2Nature. 317(6035):363–366. doi: 10.1038/317363a0. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota N., Shimada Y. Differentiation of troponin in cardiac and skeletal muscles in chicken embryos as studied by immunofluorescence microscopy. J Cell Biol. 1981 Nov;91(2 Pt 1):497–504. doi: 10.1083/jcb.91.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyota N., Shimada Y. Isoform variants of troponin in skeletal and cardiac muscle cells cultured with and without nerves. Cell. 1983 May;33(1):297–304. doi: 10.1016/0092-8674(83)90358-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson J. M., Grand R. J. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978 Jan 5;271(5640):31–35. doi: 10.1038/271031a0. [DOI] [PubMed] [Google Scholar]