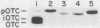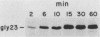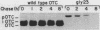Abstract
We have investigated mitochondrial import and processing of the precursor for human ornithine transcarbamylase (OTC; carbamoylphosphate:L-ornithine carbamoyltransferase, EC 2.1.3.3) in HeLa cells stably transformed with cDNA sequences encoding OTC precursors carrying mutations in their leader peptides. The mutant precursors studied included two with amino acid substitutions in the 32-amino-acid leader peptide (glycine for arginine at position 23, designated gly23; glycines for arginines at positions 15, 23, and 26, designated gly15,23,26) and two with deletions (deletion of residues 8 to 22, designated d8-22; deletion of residues 17 to 32, designated N16). Specific immunoprecipitation with anti-OTC antiserum of extracts of L-[35S]methionine-labeled cells expressing these mutations yielded only precursor species; neither mature nor intermediate-size OTC subunits were observed. Fractionation of radiolabeled cells, however, revealed important differences among the various mutants: the gly23 precursor was associated with mitochondria and was not detected in the cytosol; the d8-22 and N16 precursors were found with both the mitochondrial fraction and the cytosol; only the gly15,23,26 precursor was detected exclusively in the cytosol. A large fraction of each of the mitochondrially associated OTC species was in a trypsin-protected compartment. In particular, the gly23 precursor behaved in trypsin protection and mitochondrial fractionation studies in a manner consistent with its translocation into the mitochondrial matrix. On the other hand, the lack of binding of the gly23 protein to a delta-N-phosphonoacetyl-L-ornithine affinity column, which specifically recognizes active OTC enzyme, indicated that, despite its intramitochondrial location, the mutant protein did not assemble into the normal, active trimer. Further, the gly23 mutant precursor was unstable within the mitochondria and was degraded with a t1/2 of less further than 4 h. Thus, we have shown that, in intact HeLa cells, cleavage of the OTC leader peptide is not required for translocation into mitochondria, but is required for assembly into active enzyme.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen W. J., Douglas M. G. Phosphodiester bond cleavage outside mitochondria is required for the completion of protein import into the mitochondrial matrix. Cell. 1987 Jun 5;49(5):651–658. doi: 10.1016/0092-8674(87)90541-1. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Clarke S. The polypeptides of rat liver mitochondria: identification of a 36,000 dalton polypeptide as the subunit of ornithine transcarbamylase. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1118–1124. doi: 10.1016/0006-291x(76)90769-5. [DOI] [PubMed] [Google Scholar]
- Deshaies R. J., Koch B. D., Werner-Washburne M., Craig E. A., Schekman R. A subfamily of stress proteins facilitates translocation of secretory and mitochondrial precursor polypeptides. Nature. 1988 Apr 28;332(6167):800–805. doi: 10.1038/332800a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Oppliger W., Schatz G. Both ATP and an energized inner membrane are required to import a purified precursor protein into mitochondria. EMBO J. 1987 Apr;6(4):1073–1077. doi: 10.1002/j.1460-2075.1987.tb04860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers M., Schatz G. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature. 1986 Jul 17;322(6076):228–232. doi: 10.1038/322228a0. [DOI] [PubMed] [Google Scholar]
- Fenton W. A., Hack A. M., Helfgott D., Rosenberg L. E. Biogenesis of the mitochondrial enzyme methylmalonyl-CoA mutase. Synthesis and processing of a precursor in a cell-free system and in cultured cells. J Biol Chem. 1984 May 25;259(10):6616–6621. [PubMed] [Google Scholar]
- Hampsey D. M., Lewin A. S., Kohlhaw G. B. Submitochondrial localization, cell-free synthesis, and mitochondrial import of 2-isopropylmalate synthase of yeast. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1270–1274. doi: 10.1073/pnas.80.5.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoogenraad N. J., Sutherland T. M., Howlett G. J. Purification of ornithine transcarbamylase from rat liver by affinity chromatography with immobilized transition-state analog. Anal Biochem. 1980 Jan 1;101(1):97–102. doi: 10.1016/0003-2697(80)90045-7. [DOI] [PubMed] [Google Scholar]
- Hoogenraad N. J. Synthesis and properties of delta-N-(phosphonacetyl)-L-ornithine. A transition-state analog inhibitor of ornithine transcarbamylase. Arch Biochem Biophys. 1978 May;188(1):137–144. doi: 10.1016/0003-9861(78)90366-1. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Firgaira F. A., Fox J. E., Kolansky D., Mellman I. S., Rosenberg L. E. Expression of amplified DNA sequences for ornithine transcarbamylase in HeLa cells: arginine residues may be required for mitochondrial import of enzyme precursor. J Cell Biol. 1985 May;100(5):1515–1521. doi: 10.1083/jcb.100.5.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Fenton W. A., Williams K. R., Kalousek F., Kraus J. P., Doolittle R. F., Konigsberg W., Rosenberg L. E. Structure and expression of a complementary DNA for the nuclear coded precursor of human mitochondrial ornithine transcarbamylase. Science. 1984 Jun 8;224(4653):1068–1074. doi: 10.1126/science.6372096. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Furtak K., Pollock R. A., Rosenberg L. E. The ornithine transcarbamylase leader peptide directs mitochondrial import through both its midportion structure and net positive charge. J Cell Biol. 1987 Aug;105(2):669–677. doi: 10.1083/jcb.105.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Fenton W. A., Pollock R. A., Rosenberg L. E. Targeting of pre-ornithine transcarbamylase to mitochondria: definition of critical regions and residues in the leader peptide. Cell. 1986 Feb 14;44(3):451–459. doi: 10.1016/0092-8674(86)90466-6. [DOI] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Mellman I., Rosenberg L. E. A leader peptide is sufficient to direct mitochondrial import of a chimeric protein. EMBO J. 1985 May;4(5):1129–1135. doi: 10.1002/j.1460-2075.1985.tb03750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwich A. L., Kalousek F., Rosenberg L. E. Arginine in the leader peptide is required for both import and proteolytic cleavage of a mitochondrial precursor. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4930–4933. doi: 10.1073/pnas.82.15.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt E. C., Pesold-Hurt B., Suda K., Oppliger W., Schatz G. The first twelve amino acids (less than half of the pre-sequence) of an imported mitochondrial protein can direct mouse cytosolic dihydrofolate reductase into the yeast mitochondrial matrix. EMBO J. 1985 Aug;4(8):2061–2068. doi: 10.1002/j.1460-2075.1985.tb03892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalousek F., François B., Rosenberg L. E. Isolation and characterization of ornithine transcarbamylase from normal human liver. J Biol Chem. 1978 Jun 10;253(11):3939–3944. [PubMed] [Google Scholar]
- Kalousek F., Orsulak M. D., Rosenberg L. E. Newly processed ornithine transcarbamylase subunits are assembled to trimers in rat liver mitochondria. J Biol Chem. 1984 May 10;259(9):5392–5395. [PubMed] [Google Scholar]
- Keng T., Alani E., Guarente L. The nine amino-terminal residues of delta-aminolevulinate synthase direct beta-galactosidase into the mitochondrial matrix. Mol Cell Biol. 1986 Feb;6(2):355–364. doi: 10.1128/mcb.6.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleene R., Pfanner N., Pfaller R., Link T. A., Sebald W., Neupert W., Tropschug M. Mitochondrial porin of Neurospora crassa: cDNA cloning, in vitro expression and import into mitochondria. EMBO J. 1987 Sep;6(9):2627–2633. doi: 10.1002/j.1460-2075.1987.tb02553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolansky D. M., Conboy J. G., Fenton W. A., Rosenberg L. E. Energy-dependent translocation of the precursor of ornithine transcarbamylase by isolated rat liver mitochondria. J Biol Chem. 1982 Jul 25;257(14):8467–8471. [PubMed] [Google Scholar]
- Kraus J. P., Hodges P. E., Williamson C. L., Horwich A. L., Kalousek F., Williams K. R., Rosenberg L. E. A cDNA clone for the precursor of rat mitochondrial ornithine transcarbamylase: comparison of rat and human leader sequences and conservation of catalytic sites. Nucleic Acids Res. 1985 Feb 11;13(3):943–952. doi: 10.1093/nar/13.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingelbach K. R., Graf L. J., Dunn A. R., Hoogenraad N. J. Effect of deletions within the leader peptide of pre-ornithine transcarbamylase on mitochondrial import. Eur J Biochem. 1986 Nov 17;161(1):19–23. doi: 10.1111/j.1432-1033.1986.tb10119.x. [DOI] [PubMed] [Google Scholar]
- Lusty C. J., Jilka R. L., Nietsch E. H. Ornithine transcarbamylase of rat liver. Kinetic, physical, and chemical properties. J Biol Chem. 1979 Oct 25;254(20):10030–10036. [PubMed] [Google Scholar]
- Mackall J., Meredith M., Lane M. D. A mild procedure for the rapid release of cytoplasmic enzymes from cultured animal cells. Anal Biochem. 1979 May;95(1):270–274. doi: 10.1016/0003-2697(79)90216-1. [DOI] [PubMed] [Google Scholar]
- Mori M., Miura S., Tatibana M., Cohen P. P. Processing of a putative precursor of rat liver ornithine transcarbamylase, a mitochondrial matrix enzyme. J Biochem. 1980 Dec;88(6):1829–1836. doi: 10.1093/oxfordjournals.jbchem.a133158. [DOI] [PubMed] [Google Scholar]
- Mori M., Morita T., Miura S., Tatibana M. Uptake and processing of the precursor for rat liver ornithine transcarbamylase by isolated mitochondria. Inhibition by uncouplers. J Biol Chem. 1981 Aug 25;256(16):8263–8266. [PubMed] [Google Scholar]
- Morita T., Mori M., Tatibana M., Cohen P. P. Site of synthesis and intracellular transport of the precursor of mitochondrial ornithine carbamoyltransferase. Biochem Biophys Res Commun. 1981 Mar 31;99(2):623–629. doi: 10.1016/0006-291x(81)91790-3. [DOI] [PubMed] [Google Scholar]
- Park S., Liu G., Topping T. B., Cover W. H., Randall L. L. Modulation of folding pathways of exported proteins by the leader sequence. Science. 1988 Feb 26;239(4843):1033–1035. doi: 10.1126/science.3278378. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Neupert W. Transport of F1-ATPase subunit beta into mitochondria depends on both a membrane potential and nucleoside triphosphates. FEBS Lett. 1986 Dec 15;209(2):152–156. doi: 10.1016/0014-5793(86)81101-2. [DOI] [PubMed] [Google Scholar]
- Pfanner N., Tropschug M., Neupert W. Mitochondrial protein import: nucleoside triphosphates are involved in conferring import-competence to precursors. Cell. 1987 Jun 19;49(6):815–823. doi: 10.1016/0092-8674(87)90619-2. [DOI] [PubMed] [Google Scholar]
- Powers-Lee S. G., Mastico R. A., Bendayan M. The interaction of rat liver carbamoyl phosphate synthetase and ornithine transcarbamoylase with inner mitochondrial membranes. J Biol Chem. 1987 Nov 15;262(32):15683–15688. [PubMed] [Google Scholar]
- Rosenberg L. E., Fenton W. A., Horwich A. L., Kalousek F., Kraus J. P. Targeting of nuclear-encoded proteins to the mitochondrial matrix: implications for human genetic defects. Ann N Y Acad Sci. 1986;488:99–108. doi: 10.1111/j.1749-6632.1986.tb46550.x. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Kornberg R. D. Cell biology. An unfolding story of protein translocation. Nature. 1986 Jul 17;322(6076):209–210. doi: 10.1038/322209a0. [DOI] [PubMed] [Google Scholar]
- Schleyer M., Neupert W. Transport of proteins into mitochondria: translocational intermediates spanning contact sites between outer and inner membranes. Cell. 1985 Nov;43(1):339–350. doi: 10.1016/0092-8674(85)90039-x. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztul E. S., Hendrick J. P., Kraus J. P., Wall D., Kalousek F., Rosenberg L. E. Import of rat ornithine transcarbamylase precursor into mitochondria: two-step processing of the leader peptide. J Cell Biol. 1987 Dec;105(6 Pt 1):2631–2639. doi: 10.1083/jcb.105.6.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Vassarotti A., Chen W. J., Smagula C., Douglas M. G. Sequences distal to the mitochondrial targeting sequences are necessary for the maturation of the F1-ATPase beta-subunit precursor in mitochondria. J Biol Chem. 1987 Jan 5;262(1):411–418. [PubMed] [Google Scholar]
- Verner K., Schatz G. Import of an incompletely folded precursor protein into isolated mitochondria requires an energized inner membrane, but no added ATP. EMBO J. 1987 Aug;6(8):2449–2456. doi: 10.1002/j.1460-2075.1987.tb02524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe M. P., Ohta S., Schatz G. A yeast mutant temperature-sensitive for mitochondrial assembly is deficient in a mitochondrial protease activity that cleaves imported precursor polypeptides. EMBO J. 1985 Aug;4(8):2069–2074. doi: 10.1002/j.1460-2075.1985.tb03893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwizinski C., Neupert W. Precursor proteins are transported into mitochondria in the absence of proteolytic cleavage of the additional sequences. J Biol Chem. 1983 Nov 10;258(21):13340–13346. [PubMed] [Google Scholar]