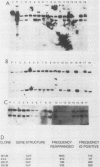
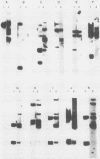
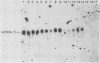
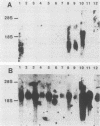
Abstract
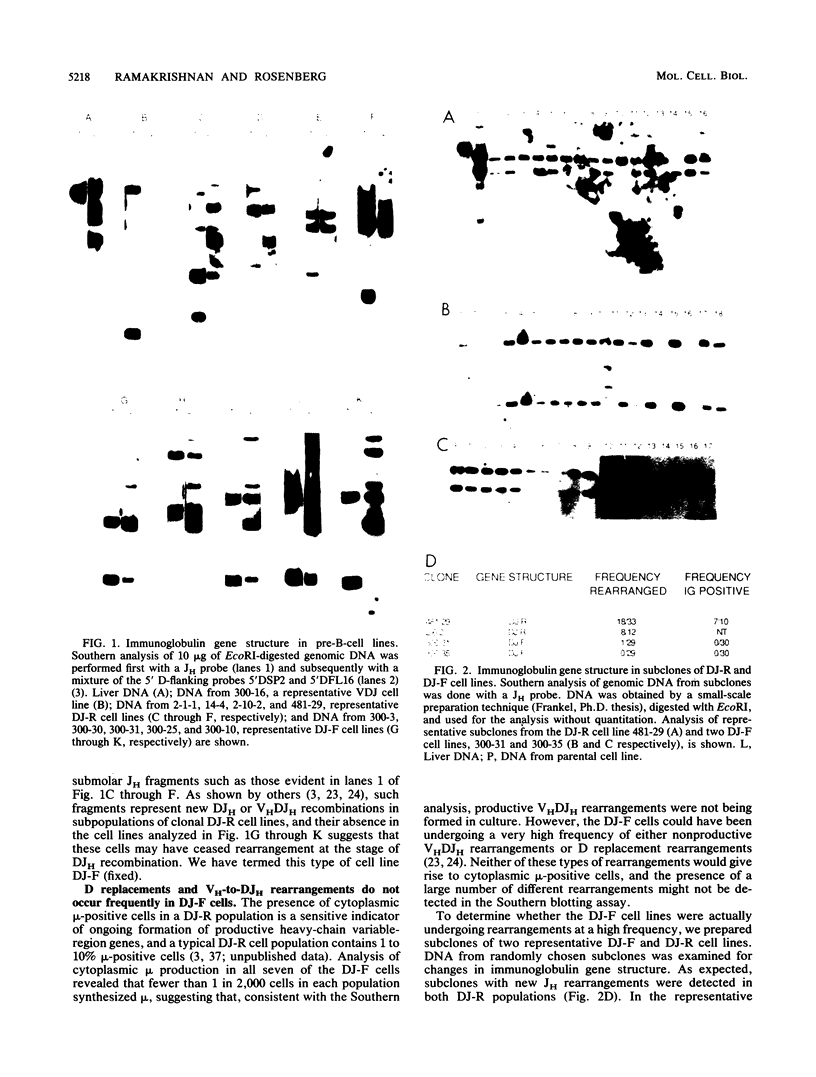
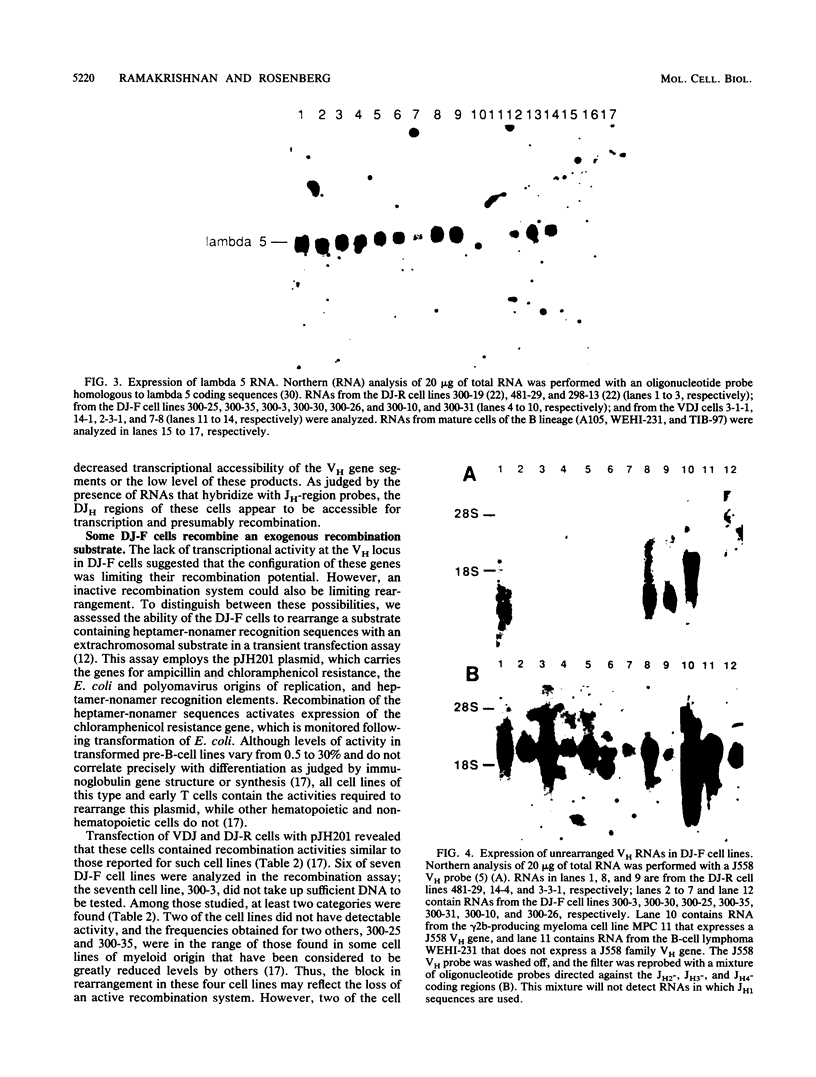
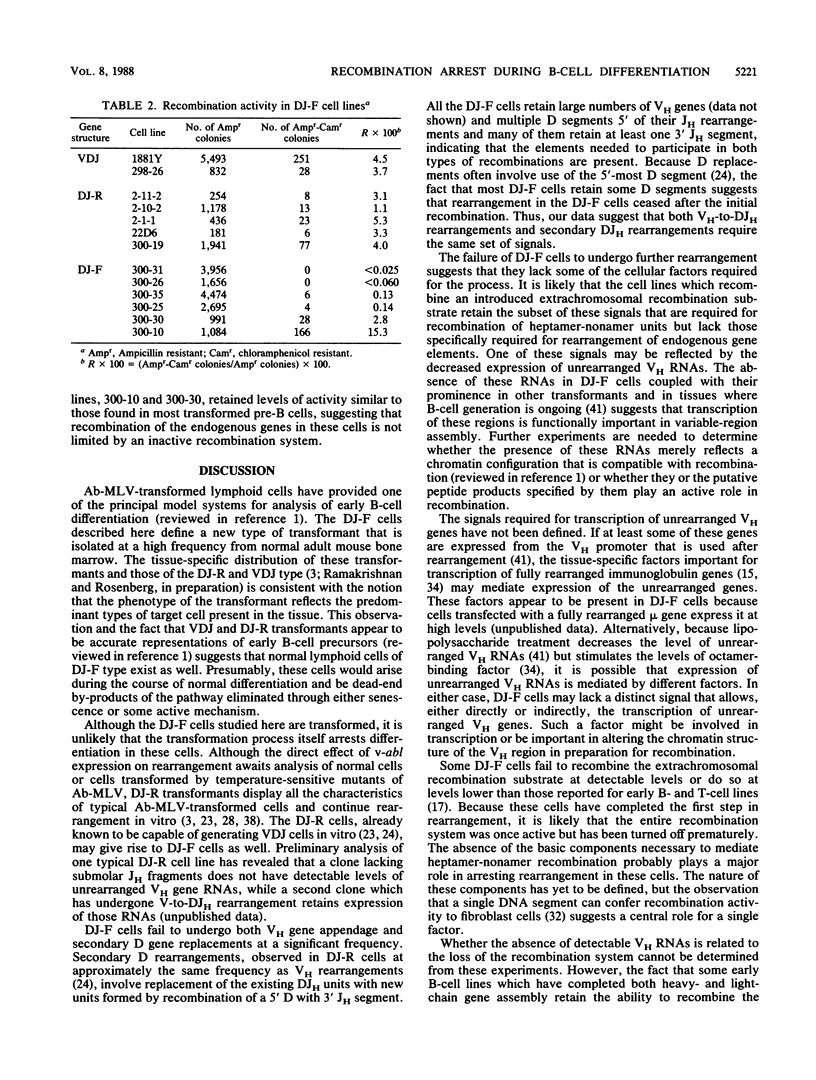
Abelson murine leukemia virus-transformed cells have provided the principal model for study of the early events in immunoglobulin gene rearrangements. In this communication, we describe a new type of Abelson virus-transformed pre-B-cell line that is arrested at the DJH stage of the recombination process. These cells differ from other pre-B transformants with respect to two properties associated with the immunoglobulin rearrangement process. First, in contrast to cell lines undergoing VH-to-DJH joining in vitro, none of these cell lines contained detectable levels of RNAs transcribed from their unrearranged VH genes. Second, only some of the cell lines recombined exogenous heptamer-nonamer sequences, indicating that many of them have lost at least a portion of the enzymatic machinery that mediates recombination. The correlation between the absence of unrearranged VH RNAs and the inability to rearrange endogenous immunoglobulin gene segments suggests that VH gene transcription is required both to maintain an active recombination system and for the final step in variable-region formation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Blackwell T. K., DePinho R. A., Reth M. G., Yancopoulos G. D. Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev. 1986 Feb;89:5–30. doi: 10.1111/j.1600-065x.1986.tb01470.x. [DOI] [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Brodeur P. H., Riblet R. The immunoglobulin heavy chain variable region (Igh-V) locus in the mouse. I. One hundred Igh-V genes comprise seven families of homologous genes. Eur J Immunol. 1984 Oct;14(10):922–930. doi: 10.1002/eji.1830141012. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Cook W. D., Balaton A. M. T-cell receptor and immunoglobulin genes are rearranged together in Abelson virus-transformed pre-B and pre-T cells. Mol Cell Biol. 1987 Jan;7(1):266–272. doi: 10.1128/mcb.7.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper M. D., Mulvaney D., Coutinho A., Cazenave P. A. A novel cell surface molecule on early B-lineage cells. Nature. 1986 Jun 5;321(6070):616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hagiya M., Davis D. D., Takahashi T., Okuda K., Raschke W. C., Sakano H. Two types of immunoglobulin-negative Abelson murine leukemia virus-transformed cells: implications for B-lymphocyte differentiation. Proc Natl Acad Sci U S A. 1986 Jan;83(1):145–149. doi: 10.1073/pnas.83.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., von Boehmer H., Haas W., Sakano H., Trauneker A., Tonegawa S. Identification of D segments of immunoglobulin heavy-chain genes and their rearrangement in T lymphocytes. Nature. 1981 Apr 16;290(5807):565–570. doi: 10.1038/290565a0. [DOI] [PubMed] [Google Scholar]
- Landolfi N. F., Capra J. D., Tucker P. W. Interaction of cell-type-specific nuclear proteins with immunoglobulin VH promoter region sequences. Nature. 1986 Oct 9;323(6088):548–551. doi: 10.1038/323548a0. [DOI] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Lutzker S., Rothman P., Pollock R., Coffman R., Alt F. W. Mitogen- and IL-4-regulated expression of germ-line Ig gamma 2b transcripts: evidence for directed heavy chain class switching. Cell. 1988 Apr 22;53(2):177–184. doi: 10.1016/0092-8674(88)90379-0. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Rosenberg N. Mapping cell surface antigens on mouse pre-B cell lines. Eur J Immunol. 1985 Mar;15(3):295–298. doi: 10.1002/eji.1830150316. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. Abelson murine leukemia virus mutants with alterations in the virus-specific P120 molecule. J Virol. 1980 Jan;33(1):340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Berger C. N., Melchers F. Isolation of a cDNA copy of an RNA species expressed in murine pre-B cells. EMBO J. 1986 Sep;5(9):2139–2147. doi: 10.1002/j.1460-2075.1986.tb04477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Baltimore D. Stable expression of immunoglobulin gene V(D)J recombinase activity by gene transfer into 3T3 fibroblasts. Cell. 1988 Apr 8;53(1):107–115. doi: 10.1016/0092-8674(88)90492-8. [DOI] [PubMed] [Google Scholar]
- Serunian L. A., Rosenberg N. Abelson virus potentiates long-term growth of mature B lymphocytes. Mol Cell Biol. 1986 Jan;6(1):183–194. doi: 10.1128/mcb.6.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavnezer-Nordgren J., Sirlin S. Specificity of immunoglobulin heavy chain switch correlates with activity of germline heavy chain genes prior to switching. EMBO J. 1986 Jan;5(1):95–102. doi: 10.1002/j.1460-2075.1986.tb04182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoye J. P., Coffin J. M. Polymorphism of murine endogenous proviruses revealed by using virus class-specific oligonucleotide probes. J Virol. 1988 Jan;62(1):168–175. doi: 10.1128/jvi.62.1.168-175.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Blackwell T. K., Suh H., Hood L., Alt F. W. Introduced T cell receptor variable region gene segments recombine in pre-B cells: evidence that B and T cells use a common recombinase. Cell. 1986 Jan 31;44(2):251–259. doi: 10.1016/0092-8674(86)90759-2. [DOI] [PubMed] [Google Scholar]