Background: The Amt family of ammonium channels does not conduct K+.
Results: E. coli AmtB variants carrying certain mutations in the conserved twin-histidine element transport K+ against a concentration gradient.
Conclusion: The twin-histidine element functions as a filter that prevents K+ conduction.
Significance: These findings provide insight into the transport mechanism of Amt channels and the ammonium species (NH3 or NH4+) that serves as their substrate.
Keywords: Escherichia coli, Ion Channels, Membrane Transport, Microbiology, Potassium Transport, Ammonium Transport, Amt Proteins
Abstract
Members of the Amt family of channels mediate the transport of ammonium. The form of ammonium, NH3 or NH4+, carried by these proteins remains controversial, and the mechanism by which they select against K+ ions is unclear. We describe here a set of Escherichia coli AmtB proteins carrying mutations at the conserved twin-histidine site within the conduction pore that have altered substrate specificity and now transport K+. Subsequent work established that AmtB-mediated K+ uptake occurred against a concentration gradient and was membrane potential-dependent. These findings indicate that the twin-histidine element serves as a filter to prevent K+ conduction and strongly support the notion that Amt proteins transport cations (NH4+ or, in mutant proteins, K+) rather than NH3 gas molecules through their conduction pores.
Introduction
Ammonium (used to designate NH3 and NH4+) is the preferred nitrogen source of many organisms, making the manner by which it traverses biological membranes of great physiological importance. The uncharged form, NH3, diffuses across lipid bilayers more than 35 times faster than water (1). When the rate of NH3 diffusion becomes growth limiting protein-mediated transport of ammonium by the Amt family of channels is required (2–5). Still subject to debate is the transport mechanism of these proteins and the species of ammonium that serves as their substrate. Based on structural studies and numerous molecular dynamic simulations (6–11), it has been argued that Amt family members are passive channels that facilitate downhill movement of NH3. We hold the opposite view, supported by the research efforts of other groups (3, 12–20), that these proteins function as active channels that mediate electrogenic ammonium uptake against a concentration gradient. Mechanisms of electrogenic transport that have been suggested include conduction of the membrane-impermeant protonated form of ammonium, NH4+, and the cotransport of NH3 and H+ (2, 3, 5, 12, 13, 16–21).
Members of the Amt family function as homotrimers (22). Structural studies indicate that these proteins and their only known homologs, the Rhesus (Rh) proteins, have a conduction pore on each monomeric subunit (7, 11, 21, 23–25) (Fig. 1). Positioned near the center of each of these otherwise hydrophobic pores is a pair of conserved histidines. It has been proposed that this twin-histidine element plays a critical role in mediating substrate transport (7, 11, 28). However, recent work by one of us demonstrated that the E. coli AmtB protein can accommodate acidic residues at one or both positions of its His168/His318 twin-histidine pairing while retaining good to excellent ammonium transport activity (14). This finding led us to question why the twin-histidine element is conserved within the Amt family. Here, we report that certain AmtB twin-histidine variants are gain-of-function mutants that conduct K+, a cation having an ionic radius similar to that of NH4+ but normally not transported by Amt proteins (17, 18, 29, 30). Our analysis shows that the twin-histidine element serves as a substrate selectivity filter and leads us to suggest that Amt channels utilize an electrogenic transport mechanism in which ammonium crosses the phenyl ring constriction as NH4+, deprotonates and traverses the conduction pore in close association with its H+, and finally reprotonates prior to entering the cytoplasm.
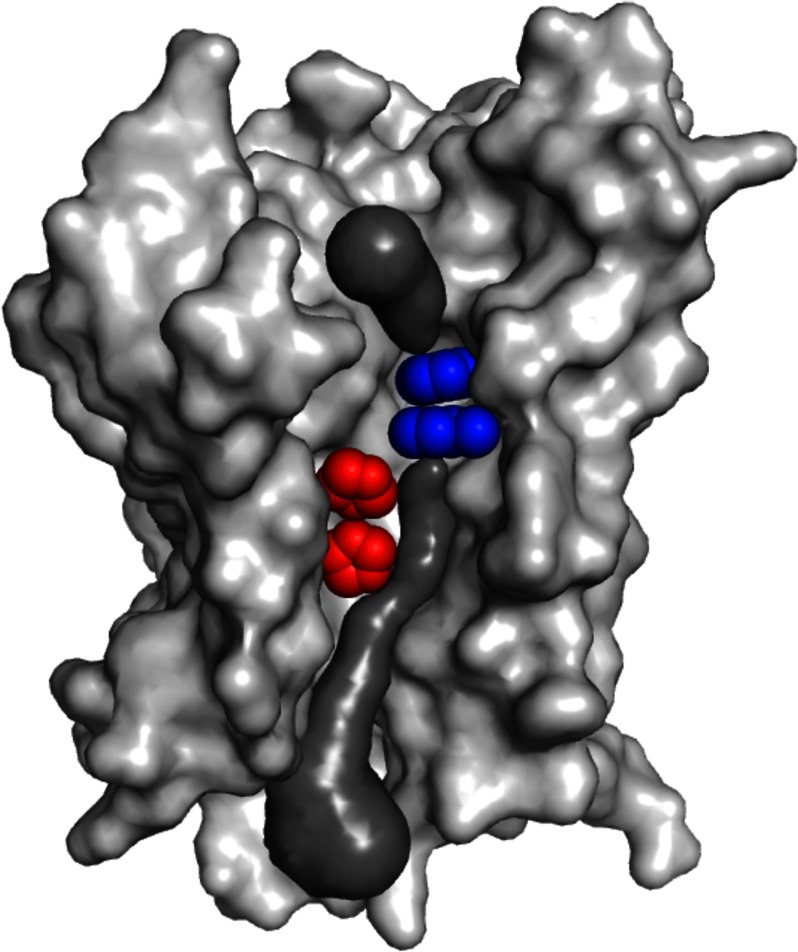
FIGURE 1.
Substrate conduction path of E. coli AmtB. The AmtB monomer as viewed from the membrane, with the periplasmic surface at the top and cytoplasmic surface at the bottom. Forward facing transmembrane helices have been removed to reveal the substrate transport path (modeled in dark gray). Components of the conserved twin-histidine element (His168 on the top and His318 on the bottom) are highlighted in red. A constriction composed of two conserved and presumably mobile phenylalanine residues (Phe107 on the top and Phe215 on the bottom) that separates the periplasmic vestibule of the transport pathway from the conduction pore is shown in blue. Whether ammonium crosses the phenyl ring constriction and traverses the conduction pore as NH3 or NH4+ remains a point of debate. The AmtB model was created from Protein Data Bank entry 2NS1 (26) using PyMOL, and the substrate conduction pathway was visualized using the program CAVER (27).
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Media
Unless noted otherwise, all strains used in this work are derivatives of the prototrophic E. coli K-12 strain NCM3722 (3, 31). Strains NCM4236 (tesB::kan), NCM4590 (amtB::kan), NCM4833 (amtBH318D tesB::kan), NCM4889 (amtBH168D tesB::kan), and NCM4892 (amtBH168D,H318E tesB::kan) have been described (3, 14, 32). Strain NCM5020 (ΔkdpA ΔtrkA kup::cat) was constructed using successive rounds of P1 phage transduction and FLP recombinase-mediated resistance gene excision of kdpA::kan and then trkA::kan insertions (derived from Keio collection strains JW0686-5 and JW3251-2, respectively) (33), followed by a third transduction with P1 phage grown on strain NCM5019 (MG1655 kup::cat). Selected amtB alleles were introduced into strain NCM5020 by P1 phage transduction using amtB::kan or by linkage to tesB::kan. Strains NCM5030 (NCM5020 ΔglnK amtBH318D tesB::kan) and NCM5031 (NCM5020 ΔglnK amtBH168D,H318E tesB::kan) were constructed by first introducing mutant amtB tesB::kan fragments into strain NCM4589 (NCM3722 ΔglnK) using previously described methods (3, 14, 32), and then moving the ΔglnK amtBH318D and ΔglnK amtBH168D,H318E lesions into the NCM5020 strain background by P1 phage transduction using the close linkage of glnK and amtB to tesB::kan. Genetic manipulations were confirmed by PCR and sequencing. All amtB alleles were studied in single copy on the E. coli chromosome.
Strains in the NCM3722 background were maintained on LB plates, and strains in the NCM5020 background were maintained on LB plates containing 50 mm KCl. K+-based formulations of N−C− minimal medium, Neidhardt's MOPS medium, and Neidhardt's MES medium contain ∼200, 28, and 20 mm K+, respectively (3, 34, 35). Na+-based formulations of these defined media were prepared by replacing all K+-containing components with their Na+-containing counterparts.
Growth Assays
For strain growth illustrated in Fig. 2, cells grown in LB medium were diluted 100-fold into either K+-based or Na+-based N−C− minimal medium containing 0.4% glucose and 5 mm NH4Cl. After overnight incubation, cells were diluted to an A600 = 0.01 (>100-fold dilution) into either K+-based or Na+-based N−C− medium containing 0.04% glucose and 3 mm glutamine. Glutamine supports good growth as a sole source of nitrogen when cells are cultivated in this medium but its use as a sole nitrogen source elicits the nitrogen limitation response, resulting in the expression of all genes, including amtB, under control of nitrogen regulatory protein C (NtrC) (36–38). Growth was monitored by changes in absorbance at 600 nm. Strain growth was carried out with aeration at 37 °C. All Na+-based N−C− medium was supplemented with 1 mm KCl to avoid K+-limiting growth conditions.
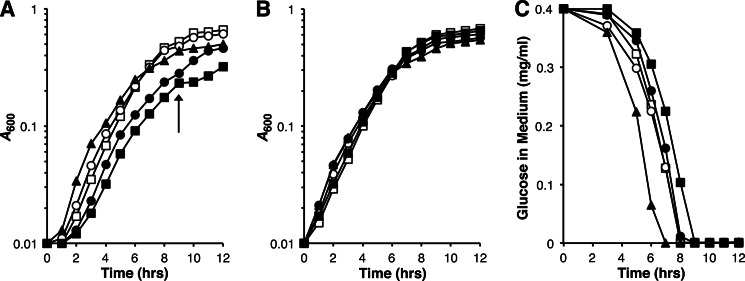
FIGURE 2.
Effect of K+ on AmtB twin-histidine variant strain growth. A and B, strains were cultivated in either K+-based N−C− minimal medium (contains 200 mm K+) (A) or Na+-based N−C− minimal medium supplemented with 1 mm KCl (B). All media contained 0.04% glucose and 3 mm glutamine. AmtB is expressed under these growth conditions (see “Experimental Procedures” for details). Strain genotypes were wild-type (○), amtB-null (□), H168D (▴), H318D (●), and H168D,H318E (■). The arrow in A denotes the point at which the glucose supply is exhausted by cells expressing AmtBH168D,H318E (see C), and the tricarboxylic acid cycle intermediates derived from glutamine begin to be used as the source of carbon. The data, from a single experiment, are representative of findings made in three independent trials. C, glucose consumption by strains illustrated in A. Culture medium glucose concentrations were determined at various times during growth. The times at which strains exhaust their glucose supply are closely approximated by their diauxic shift points in A: 7 h for H168D, 8 h for amtB-null, 8 h for wild type, 8 h for H318D, 9 h for H168D,H318E. Cell yield per glucose consumed values (shown below in units of A600/mg of glucose per ml) at the diauxic shift point for each strain were 1.2 ± 0.12 for amtB-null, 1.1 ± 0.063 for wild-type, 0.73 ± 0.052 for H168D, 0.62 ± 0.029 for H318D, and 0.55 ± 0.025 for H168D,H318E. Cell yield per glucose consumed values are reported as means ± S.D. for three independent experiments. Symbols used for strain genotypes in C are identical to those in A and B.
For strain growth illustrated in Fig. 4, cells grown in LB medium containing 100 mm KCl were diluted 100-fold into Na+-based N−C− medium supplemented with 0.4% glucose, 5 mm NH4Cl, and 100 mm KCl. After these cultures reached saturation they were again diluted 100-fold into the same Na+-based N−C− medium, except with 3 mm glutamine replacing NH4Cl as the nitrogen source. Following overnight incubation, cultures were finally diluted 200-fold into Na+-based N−C− medium supplemented with 0.4% glucose, 3 mm glutamine, and a mixture of KCl and NaCl (combined concentration of 100 mm). Growth was monitored by changes in absorbance at 600 nm. Strain growth was carried out with aeration at 37 °C.
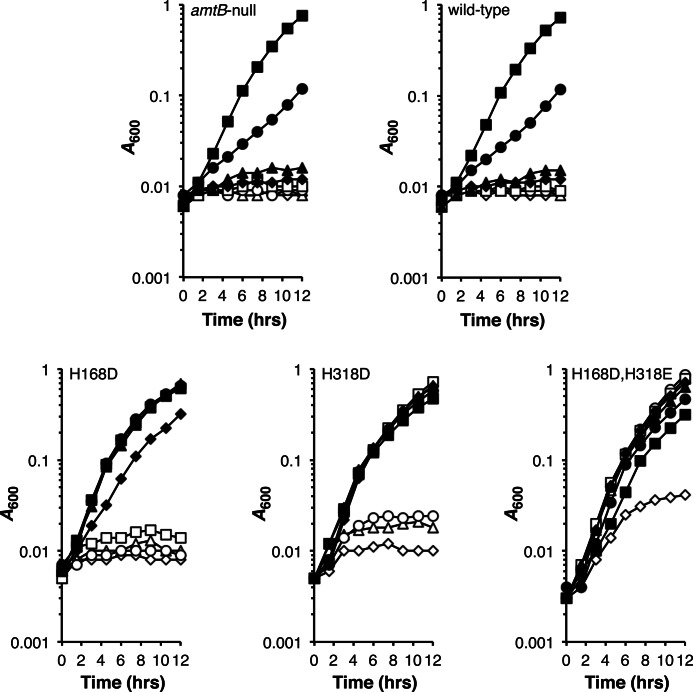
FIGURE 4.
Dependence of strain growth on K+ concentration. Strains were grown under AmtB-expressing conditions (Na+-based N−C− minimal medium containing 0.4% glucose, and 3 mm glutamine) in medium supplemented with varying concentrations of KCl (0.5 mm (♢), 1 mm (▵), 2 mm (○), 5 mm (□), 10 mm (♦), 20 mm (▴), 50 mm (●), and 100 mm (■)). Growth medium isotonicity was maintained by the addition of NaCl such that the total KCl/NaCl supplement equaled 100 mm. All amtB alleles were carried in the kdp trk kup strain background. The data, from a single experiment, are representative of findings made in three independent trials.
Growth at low NH3 concentrations (Fig. 3) was performed as previously described (14) with the following differences: cells were first adapted to minimal medium on Na+-based Neidhardt's MOPS medium (pH 7.4) and subsequently acclimated to growth at low pH by inoculation into Na+-based Neidhardt's MES medium (pH 5.5); all adaptive media contained 1 mm KCl; and cells were finally diluted into either K+-based Neidhardt's MES medium (pH 5.5) supplemented with 1 mm KCl, or Na+-based Neidhardt's medium (pH 5.5) supplemented with 0.1 mm KCl and 0.9 mm NaCl.
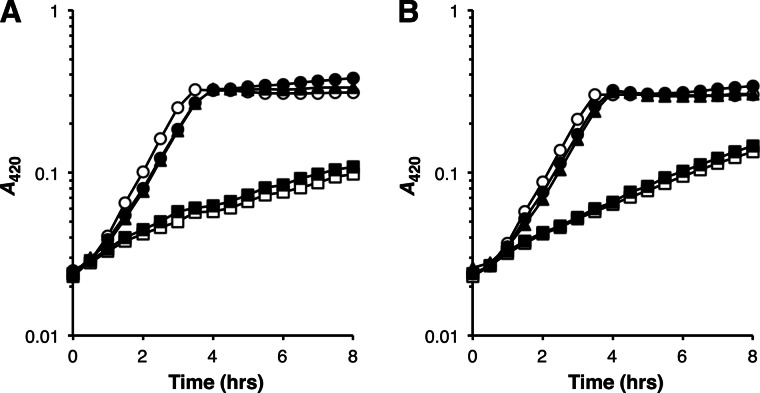
FIGURE 3.
Growth of AmtB twin-histidine variant strains at low NH3. Strain growth in low NH3 medium (0.5 mm NH4Cl, 0.1% glucose (pH 5.5)) containing 21 mm K+ (A) or 0.1 mm K+ (B) is shown. The absence of AmtB-mediated conductance of ammonium under these culture conditions results in a pronounced growth defect (3, 37). Strain genotypes were wild-type (○), amtB-null (□), H168D (▴), H318D (●), and H168D,H318E (■). Strain doubling times (in minutes) derived from these growth curves are as follows: 48 and 47 for wild-type at K+ concentrations of 21 mm and 0.1 mm, respectively; 320 and 210 for amtB-null at K+ concentrations of 21 mm and 0.1 mm, respectively; 55 and 53 for H168D at K+ concentrations of 21 mm and 0.1 mm, respectively; 55 and 55 for H318D at K+ concentrations of 21 mm and 0.1 mm, respectively; 280 and 200 for H168D,H318E at K+ concentrations of 21 mm and 0.1 mm, respectively. The data, each from single experiments, are representative of findings made in at least three independent trials.
Expression and accumulation levels of mutant proteins relative to wild-type AmtB have been reported (14). The three AmtB variants studied here were produced in amounts similar to that of the wild-type strain (60–100%) when glutamine served as the sole nitrogen source (Fig. 2 and Figs. 4–7). However, whereas AmtBH168D and AmtBH318D accumulated to 30–40% wild-type levels under this growth condition, AmtBH168D,H318E was present in amounts ≤5% of its wild-type counterpart. Similar expression and accumulation patterns were found for these three AmtB variants when low NH3 medium was used for growth studies (Fig. 3).
FIGURE 5.
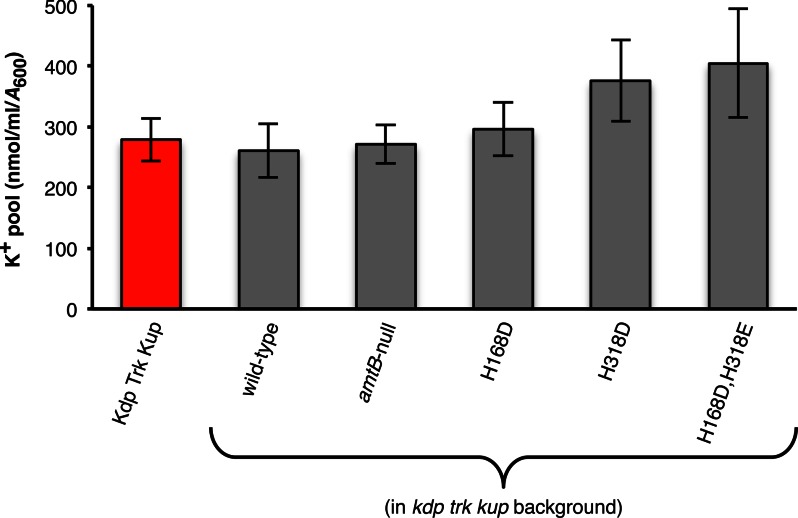
Cytoplasmic K+ pools. Intracellular K+ levels in strains grown under AmtB-expressing conditions (Na+-based N−C− minimal medium supplemented with 0.4% glucose, 3 mm glutamine, and 100 mm KCl). All amtB alleles were carried in the kdp trk kup strain background. For comparison purposes, the cytoplasmic K+ pool of the prototrophic E. coli K-12 parental strain NCM3722 (Kdp Trk Kup) (31) is shown (red bar). Values reported are means ± S.D. (error bars) for at least three independent experiments.
FIGURE 6.
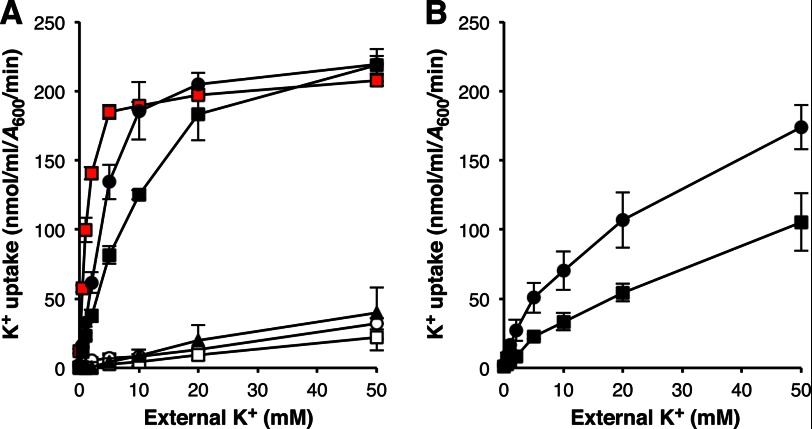
Kinetics of AmtB-mediated K+ transport. A, initial (1 min) rates of AmtB-mediated K+ uptake were measured in K+-depleted kdp trk kup cells. Strain genotypes were wild-type (○), amtB-null (□), H168D (▴), H318D (●), and H168D,H318E (■). The K+ uptake activity of the prototrophic E. coli K-12 parental strain NCM3722 (31) is shown (solid red squares). B, initial (1 min) rates of K+ uptake were measured in K+-depleted kdp trk kup glnK cells expressing AmtBH318D (●) and AmtBH168D,H318E (■). Values reported in A and B are means ± S.D. (error bars) for at least three independent experiments. Kinetic constants for K+ transport determined from the data shown in A are reported under “Results” as means ± S.D. Half-saturation constant values and maximal transport rates determined from the data shown in B were 26 ± 9.1 mm and 260 ± 8.5 nmol/ml per A600 per min for AmtBH318D and 60 ± 21 mm and 230 ± 85 nmol/ml per A600 per min for AmtBH168D,H318E.
FIGURE 7.
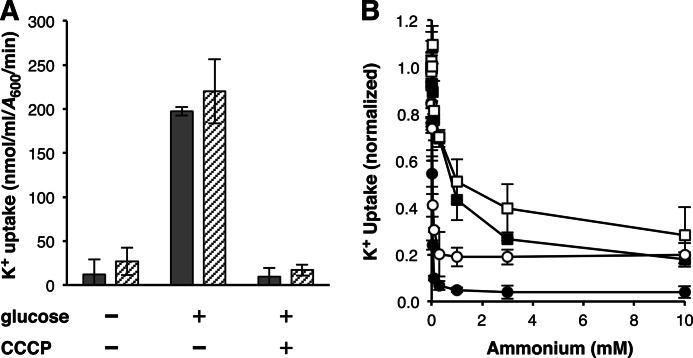
Inhibition of AmtB-mediated K+ transport. A, initial (1 min) rates of K+ uptake by AmtBH318D (shaded bars) and AmtBH168D,H318E (hatched bars) measured in K+-depleted kdp trk kup cells in the presence and absence of an energy source (0.2% glucose) and protonophore (10 μm CCCP). Values shown are means ± S.D. (error bars) of three independent experiments. B, initial (1 min) uptake rates of K+ by AmtBH318D (● and ○) and AmtBH168D,H318E (■ and □) determined in K+-depleted kdp trk kup cells (shaded symbols) or K+-depleted kdp trk kup glnK cells (open symbols) in the presence of varying concentrations of ammonium. Data from three separate trials were normalized to transport values measured in the absence of ammonium and are shown as means ± S.D. (error bars). Inhibition constants (Ki) for ammonium determined from each of these independent experiments are reported under “Results” as means ± S.D. Assay K+ concentrations for A and B and transport values measured in the absence of ammonium for B are reported under “Experimental Procedures”.
Transport Assays
Strains were grown in LB medium containing 100 mm KCl and, after overnight incubation, diluted 100-fold into Na+-based N−C− medium supplemented with 0.4% glucose, 5 mm NH4Cl, and 100 mm KCl. After these cultures reached saturation they were again diluted 100-fold into the same Na+-based N−C− medium, except with 3 mm glutamine replacing NH4Cl as the nitrogen source. Cells cultivated under these conditions were grown to exponential phase (A600 = 0.3–0.6), harvested by centrifugation, and resuspended in Na+-based N−C− medium containing 100 mm NaCl. For determination of cytoplasmic K+ pools, cells were collected and processed for inductively coupled plasma atomic emission spectroscopy (ICP-AES)2 (see below). To analyze K+ uptake, resuspended cells were washed twice and finally suspended at an A600 = 1.0 in Na+-based N−C− medium containing 100 mm NaCl, and then treated with 5 mm 2,4-dinitrophenol for 30 min. Treatment with this protonophore results in the rapid loss of cellular K+ (39, 40). K+-depleted cells were harvested, washed twice with Na+-based N−C− medium supplemented with 100 mm NaCl, resuspended at an A600 = 1.0 in the same medium supplemented with 0.2% glucose, and held on ice until use. To initiate tests of K+ transport, cell suspensions were preincubated for 20 min at 37 °C prior to the addition of an equal volume of Na+-based N−C− medium supplemented with 0.2% glucose and suitable mixtures of KCl, NH4Cl, and NaCl (combined concentration of 100 mm). After a 1 min incubation aliquots were collected on Millipore filters (0.45-μm pore size; type HAWP), rinsed twice with 5 ml of Na+-based N−C− medium supplemented with 100 mm NaCl, and dried. Washed cells (on the filter) were then incubated at room temperature (∼25 °C) for 1 h in 5 ml of 1 m HNO3 containing 50 μm NaCl to extract K+. Samples were cleared of cell debris by passage through Millipore filters (0.45-μm pore size; type HAWP) and analyzed for K+ by ICP-AES. To determine whether AmtB-mediated K+ transport required an energy source (Fig. 7A), glucose was eliminated from all assay buffer preparations. For assays with carbonyl cyanide m-chlorophenylhydrazone (CCCP), this compound or an equal volume of ethanol vehicle was added 5 min prior to the initiation of K+ uptake tests. Strain growth, K+ depletion, and K+ uptake assays were carried out with aeration at 37 °C.
Apparent kinetic constants (half-saturation constant values and maximal transport rates) for AmtB-mediated K+ uptake were estimated by curve fitting initial transport rates to the Michaelis-Menten equation using the program CurveExpert Professional, version 1.6.2. The concentration range of K+ used to approximate these kinetic constants was 0.5–50 mm. Inhibition constant (Ki) values for ammonium inhibition of AmtB-mediated K+ transport were determined using linear Dixon plots (41), with the assumption of competitive inhibition between ammonium and K+. For energy requirement assays and Ki determinations (Fig. 7), K+ was present at twice half-saturation constant values for AmtBH318D and AmtBH168D,H318E in the glnK+ background, and 40 mm for AmtBH318D and AmtBH168D,H318E in the glnK background. Transport values measured in the absence of ammonium using these K+ concentrations were 180 ± 38 nmol/ml per A600 per min for AmtBH318D in the glnK+ background, 210 ± 51 nmol/ml per A600 per min for AmtBH168D,H318E in the glnK+ background, 140 ± 21 nmol/ml per A600 per min for AmtBH318D in the glnK background, and 96 ± 6.3 nmol/ml per A600 per min for AmtBH168D,H318E in the glnK background. The concentration range of ammonium used to approximate Ki values was 1 μm to 10 mm.
Glucose Determination
Samples from cultures illustrated in Fig. 2A were subjected to filtration (0.22-μm pore size; Millipore type Millex-GV) to remove cells. Glucose concentrations in cell-free medium were determined enzymatically using a glucose oxidase/peroxidase-coupled assay monitoring the oxidation of o-dianisidine (Sigma-Aldrich product GAGO-20).
RESULTS
K+-dependent Growth Defect in AmtB Twin-histidine Variant Strains
A number of E. coli AmtB mutant proteins carrying acidic residues at the His168/His318 twin-histidine site cause a pronounced growth defect when expressed under nitrogen-limiting conditions in media devoid of ammonium and of high K+ content (Fig. 2, A and C). This defect is characterized by a decreased carbon yield relative to both the wild-type and amtB-null strains. We reasoned that the twin-histidine variants exhibiting the growth defect were wasting energy in the futile active transport of K+. Several mutants were selected to test this hypothesis. Two of these, AmtBH168D and AmtBH318D, have near wild-type ammonium transport activity even in the presence of ∼20 mm K+ (Fig. 3 and Ref. 14). Like wild-type AmtB, the AmtBH168D protein also transports the ammonium analog methylammonium (used to designate CH3NH2 and CH3NH3+) in the absence of K+, whereas AmtBH318D does not (14). A third mutant, AmtBH168D,H318E, that exhibits neither ammonium nor methylammonium transport activity even at low K+ (Fig. 3 and Ref. 14) was also examined. Studies conducted on cells expressing these variants showed that the growth defect was eliminated if Na+ replaced most of the K+ in the culture medium (1 mm K+ supplied to avoid K+-limiting growth conditions) (Fig. 2B).
AmtB Twin-histidine Variants Conduct K+
Having established a link between the external K+ concentration and the growth defect observed in certain twin-histidine mutants, we next looked to see whether this defect resulted from AmtB-mediated inward K+ leakage. A strain lacking the three major K+ import systems (Kdp, Trk, and Kup) was constructed for this work. In the absence of these transport systems E. coli possesses only a residual K+ uptake activity and requires an elevated concentration of this cation for growth (39, 40). We found that the kdp trk kup triple mutant failed to grow appreciably on media containing less than ∼50 mm K+, and expression of wild-type AmtB had no effect on this growth phenotype (Fig. 4; compare amtB-null and wild-type strains). Expression of AmtBH168D, AmtBH318D, or AmtBH168D,H318E, on the other hand, reduced the K+ requirement to ∼10, 5, and 1 mm, respectively.
The ability of AmtB to conduct K+ was also assayed directly using ICP-AES. Initial experiments indicated that cytoplasmic K+ pools of kdp trk kup triple mutants expressing a twin-histidine variant protein, in particular AmtBH318D or AmtBH168D,H318E, were not only higher than their wild-type AmtB-expressing and amtB-null counterparts, but also larger than that of a wild-type AmtB-expressing strain carrying the Kdp, Trk, and Kup systems (Fig. 5). We next depleted each of these strains of their K+ to study the net uptake of this cation. As expected, the K+ transport rate of a kdp trk kup triple mutant expressing wild-type AmtB was no better than that of an otherwise isogenic amtB-null strain (Fig. 6A). Only a slight improvement (<2-fold) in K+ uptake was observed when AmtBH168D was expressed in this background. The initial K+ transport rates of wild-type AmtB and AmtBH168D were linearly proportional to the external K+ concentration over the range tested (up to 50 mm) and markedly lower than the combined activities of Trk and Kup (Kdp not expressed under the conditions (100 mm K+ in the growth medium) used for this assay; Refs. 39, 40). AmtBH318D and AmtBH168D,H318E, on the other hand, exhibited considerable K+ uptake activity. These two twin-histidine variants were found to have 5–10-fold higher half-saturation constant values (4.9 ± 0.93 mm and 11 ± 1.4 mm for AmtBH318D and AmtBH168D,H318E, respectively) and elevated maximal transport rates (250 ± 6.0 nmol/ml per A600 per min and 270 ± 22 nmol/ml per A600 per min for AmtBH318D and AmtBH168D,H318E, respectively) relative to the aggregate kinetic properties of the Trk and Kup systems (half-saturation constant of 1.1 ± 0.07 mm; maximal transport rate of 210 ± 2.1 nmol/ml per A600 per min; also see Ref. 40). Given that a 1 ml E. coli culture of A600 = 1 has a total cell volume of ∼3.6 μl (42), we calculate that AmtBH318D and AmtBH168D,H318E concentrate K+ 3–10-fold during the first min of the transport reaction when K+ is present at 0.5–10 mm in the external medium.
Inhibition of AmtB-mediated K+ Transport
We used AmtBH318D and AmtBH168D,H318E as vehicles to analyze the energy requirements of AmtB-mediated K+ conduction and the effect ammonium has on this transport reaction. The rate of K+ uptake by these two proteins was reduced 90–95% when the protonophore CCCP was present at a concentration of 10 μm (Fig. 7A), indicating that K+ uptake by AmtB was dependent on the proton motive force, presumably the membrane potential component, across the cytoplasmic membrane. In support of this conclusion is the finding that neither twin-histidine variant conducted K+ after treatment with 2,4-dinitrophenol, a process that both depletes the cytoplasmic K+ pool and discharges the proton motive force (39, 40), without subsequent addition of an energy source. Competition tests showed that K+ transport by these mutant proteins was also reduced in the presence of ammonium (Fig. 7B). The inhibitory effect was far greater for AmtBH318D (Ki = 4.9 ± 1.9 μm), which conducts ammonium, than for AmtBH168D,H318E (Ki = 0.77 ± 0.37 mm), which lacks ammonium uptake activity. Because the GlnK protein quickly inactivates AmtB in the presence of micromolar quantities of external ammonium (43), the impact this compound had on AmtB-mediated K+ transport was also examined in a kdp trk kup glnK strain background. Half-saturation constant values for AmtBH318D and AmtBH168D,H318E K+ uptake were increased ∼5-fold when GlnK was not present (Fig. 6B). Similar-sized decreases in ammonium inhibition of K+ transport by AmtBH318D (Ki = 22 ± 5.3 μm) and AmtBH168D,H318E (Ki = 2.9 ± 1.6 mm) were observed in the absence of the GlnK protein (Fig. 7B). These results suggest that the uptake of K+ is influenced by AmtB-GlnK interaction and, when taken together with the observation that AmtBH318D K+ conduction was strongly inhibited by ammonium, provides support for the idea that K+ and ammonium share the same transport pathway through AmtB.
DISCUSSION
Controversy remains over the form of ammonium carried by members of the Amt family. Due to the strongly hydrophobic nature of the conduction pore it was proposed that these proteins facilitated diffusion of NH3 (7, 11). A study of AmtB function in a reconstituted system provided evidence for such a transport mechanism (7), but the findings of that work have been criticized for being interpreted incorrectly and could not be corroborated (4, 44). Other observations question the plausibility of the facilitated NH3 diffusion model. Quantitative analyses of ammonium flux have shown that this transport mechanism would not sustain the rates of growth observed under the low external ammonium conditions at which Amt proteins operate (15, 44). Also, given that these proteins are active when the internal to external ammonium concentration ratio is >1 (15), facilitated diffusion would lead to the export rather than import of ammonium without the involvement of a linked energy source. The alternative view of ammonium transport holds that Amt channels actively conduct NH4+ or cotransport NH3 and H+. In support of these mechanisms, all plant members of the Amt family functionally characterized to date, save for those from the distantly related AMT2 subfamily, have been found to mediate electrogenic ammonium uptake (4, 17, 18, 30, 45, 46). Moreover, Amt proteins from a number of other organisms, including E. coli, concentrate methylammonium in a membrane potential-dependent manner (3, 13, 16, 20). That certain AmtB twin-histidine variants conduct K+ against its concentration gradient (Fig. 6) is compatible with these active transport mechanisms. In addition, because K+ cannot separate from its charge, our results imply that ammonium migrates through the AmtB pore as either NH4+ or as a closely associated NH3/H+ pairing (see below). This is in contrast to a net NH4+ uptake mechanism that proceeds via a symport reaction in which NH3 passes through the pore alone while a H+ follows a separate unidentified transport pathway.
In work presented here, we have described a set of AmtB twin-histidine variants that have altered substrate specificity and now conduct K+. The spectrum of compounds (methylammonium, ammonium, and K+) carried by each mutant depends on the position and number of acidic residues present in the conduction pore. Thus, AmtBH168D transports all three compounds, AmtBH318D conducts both ammonium and K+ but no longer exhibits methylammonium uptake activity, and AmtBH168D,H318E carries only K+ (Figs. 3, 4 , and 6; and Ref. 14). These findings lead us to conclude that the His168/His318 twin-histidine element serves as a substrate selectivity filter that prevents K+ transport. How might this element enable members of the Amt family to discriminate between K+ and ammonium? If, as our results predict, ammonium crosses the phenyl ring constriction and enters the conduction pore as NH4+ its charge will need to be masked to migrate across this hydrophobic environment. By accepting a H+ from NH4+ and only transferring it back just prior to substrate release into the cytoplasm, a transport mechanism shown to be plausible in a recent simulation study (47), the twin-histidine element allows the H+ to remain delocalized as it moves in parallel with NH3 through the conduction pore. The transport of K+ is prohibited because, unlike NH4+ and CH3NH3+, this cation is incapable of separating from its charge. Certain acidic amino acid substitutions of the twin-histidine element reduce the need for such charge separation by increasing pore hydrophilicity and, as a consequence, allow K+ to be carried. It is not clear why these mutations would also cause progressive decreases in CH3NH3+ and then NH4+ transport. However, the underlying trend suggests that this behavior results from the differences in the manner by which the introduced acidic residues handle H+ and K+ as well as changes to the hydrophobic character of the pore.
The strong preference of Amt family members for ammonium over K+ (3, 17, 18, 29, 30, 48) implies that this selectivity is physiologically important. Our current analysis highlights why these proteins do not carry K+. The inward leakage of this cation through AmtB, although able to substitute for the K+ uptake activities of the Trk and Kup systems, is associated with a large energy cost (Fig. 2, A and C). We suggest that this cost is incurred by the action of two processes. First, K+ movement across the cytoplasmic membrane is known to play an important role in regulating pH homeostasis (39, 49, 50). Given the elevated K+ pools found in cells expressing AmtB twin-histidine variants, substantial energy reserves would likely be used to preserve normal intracellular pH. Similarly, energy would be required to maintain the membrane potential that would otherwise be dissipated by illicit AmtB-mediated K+ conduction. These energy expenditures can prove problematic because members of the Amt family oftentimes need to operate in nutrient-poor environments having high K+ levels. For instance, the two Amt proteins of Nitrosopumilus maritimus, a member of a group of ammonium-oxidizing marine archaea that are key intermediates in the global nitrogen cycle, are likely active in the open ocean where ammonium and K+ concentrations are <1 μm and ∼10 mm, respectively (51–53). Growth and survival under such energy- and nitrogen-limited conditions would be severely compromised if Amt proteins did not discriminate against K+.
Acknowledgments
We are grateful to Sydney Kustu for her involvement in all stages of this research project. We thank Terence Hwa for suggesting that AmtB-mediated K+ leakage was responsible for the growth defect illustrated in Fig. 2A; Paul Brooks at the University of California, Berkeley ICP Spectroscopy Facility for ICP-AES assistance; and Wolfgang Epstein, Hiroshi Nikaido, and Helen Zgurskaya for thoughtful criticisms of the manuscript.
This work was supported by National Institutes of Health Grant GM38361 (to Sydney Kustu).
- ICP-AES
- inductively coupled plasma atomic emission spectroscopy
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone.
REFERENCES
- 1. Walter A., Gutknecht J. (1986) Permeability of small nonelectrolytes through lipid bilayer membranes. J. Membr. Biol. 90, 207–217 [DOI] [PubMed] [Google Scholar]
- 2. Andrade S. L., Einsle O. (2007) The Amt/Mep/Rh family of ammonium transport proteins. Mol. Membr. Biol. 24, 357–365 [DOI] [PubMed] [Google Scholar]
- 3. Fong R. N., Kim K. S., Yoshihara C., Inwood W. B., Kustu S. (2007) The W148L substitution in the Escherichia coli ammonium channel AmtB increases flux and indicates that the substrate is an ion. Proc. Natl. Acad. Sci. U.S.A. 104, 18706–18711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Javelle A., Lupo D., Li X. D., Merrick M., Chami M., Ripoche P., Winkler F. K. (2007) Structural and mechanistic aspects of Amt/Rh proteins. J. Struct. Biol. 159, 243–252 [DOI] [PubMed] [Google Scholar]
- 5. Ludewig U. (2006) Ion transport versus gas conduction: function of AMT/Rh-type proteins. Transfus. Clin. Biol. 13, 111–116 [DOI] [PubMed] [Google Scholar]
- 6. Bostick D. L., Brooks C. L. (2007) Deprotonation by dehydration: the origin of ammonium sensing in the AmtB channel. PLoS Comput. Biol. 3, e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khademi S., O'Connell J., 3rd, Remis J., Robles-Colmenares Y., Miercke L. J., Stroud R. M. (2004) Mechanism of ammonia transport by Amt/MEP/Rh: structure of AmtB at 1.35 Å. Science 305, 1587–1594 [DOI] [PubMed] [Google Scholar]
- 8. Lin Y., Cao Z., Mo Y. (2009) Functional role of Asp160 and the deprotonation mechanism of ammonium in the Escherichia coli ammonia channel protein AmtB. J. Phys. Chem. B 113, 4922–4929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nygaard T. P., Rovira C., Peters G. H., Jensen M. Ø. (2006) Ammonium recruitment and ammonia transport by E. coli ammonia channel AmtB. Biophys. J. 91, 4401–4412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang H., Xu Y., Zhu W., Chen K., Jiang H. (2007) Detailed mechanism for AmtB conducting NH4+/NH3: molecular dynamic simulations. Biophys. J. 92, 877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zheng L., Kostrewa D., Bernèche S., Winkler F. K., Li X. D. (2004) The mechanism of ammonia transport based on the crystal structure of AmtB of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 101, 17090–17095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Boeckstaens M., André B., Marini A. M. (2008) Distinct transport mechanisms in yeast ammonium transport/sensor proteins of the Mep/Amt/Rh family and impact on filamentation. J. Biol. Chem. 283, 21362–21370 [DOI] [PubMed] [Google Scholar]
- 13. Boussiba S., Dilling W., Gibson J. (1984) Methylammonium transport in Anacystis nidulans R-2. J. Bacteriol. 160, 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hall J. A., Kustu S. (2011) The pivotal twin histidines and aromatic triad of the Escherichia coli ammonium channel AmtB can be replaced. Proc. Natl. Acad. Sci. U.S.A. 108, 13270–13274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kim M., Zhang Z., Okano H., Yan D., Groisman A., Hwa T. (2012) Need-based activation of ammonium uptake in Escherichia coli. Mol. Syst. Biol. 8, 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleiner D., Fitzke E. (1981) Some properties of a new electrogenic transport system: the ammonium (methylammonium) carrier from Clostridium pasteurianum. Biochim. Biophys. Acta 641, 138–147 [DOI] [PubMed] [Google Scholar]
- 17. Ludewig U., von Wirén N., Frommer W. B. (2002) Uniport of NH4+ by the root hair plasma membrane ammonium transporter LeAMT1;1. J. Biol. Chem. 277, 13548–13555 [DOI] [PubMed] [Google Scholar]
- 18. Søgaard R., Alsterfjord M., Macaulay N., Zeuthen T. (2009) Ammonium ion transport by the AMT/Rh homolog TaAMT1;1 is stimulated by acidic pH. Pflugers Arch. 458, 733–743 [DOI] [PubMed] [Google Scholar]
- 19. Ullmann R. T., Andrade S. L., Ullmann G. M. (2012) Thermodynamics of transport through the ammonium transporter Amt-1 investigated with free energy calculations. J. Phys. Chem. B 116, 9690–9703 [DOI] [PubMed] [Google Scholar]
- 20. Walter B., Küspert M., Ansorge D., Krämer R., Burkovski A. (2008) Dissection of ammonium uptake systems in Corynebacterium glutamicum: mechanism of action and energetics of AmtA and AmtB. J. Bacteriol. 190, 2611–2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrade S. L., Dickmanns A., Ficner R., Einsle O. (2005) Crystal structure of the archaeal ammonium transporter Amt-1 from Archaeoglobus fulgidus. Proc. Natl. Acad. Sci. U.S.A. 102, 14994–14999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blakey D., Leech A., Thomas G. H., Coutts G., Findlay K., Merrick M. (2002) Purification of the Escherichia coli ammonium transporter AmtB reveals a trimeric stoichiometry. Biochem. J. 364, 527–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gruswitz F., Chaudhary S., Ho J. D., Schlessinger A., Pezeshki B., Ho C. M., Sali A., Westhoff C. M., Stroud R. M. (2010) Function of human Rh based on structure of RhCG at 2.1 Å. Proc. Natl. Acad. Sci. U.S.A. 107, 9638–9643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X., Jayachandran S., Nguyen H. H., Chan M. K. (2007) Structure of the Nitrosomonas europaea Rh protein. Proc. Natl. Acad. Sci. U.S.A. 104, 19279–19284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lupo D., Li X. D., Durand A., Tomizaki T., Cherif-Zahar B., Matassi G., Merrick M., Winkler F. K. (2007) The 1.3-Å resolution structure of Nitrosomonas europaea Rh50 and mechanistic implications for NH3 transport by Rhesus family proteins. Proc. Natl. Acad. Sci. U.S.A. 104, 19303–19308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gruswitz F., O'Connell J., 3rd, Stroud R. M. (2007) Inhibitory complex of the transmembrane ammonia channel, AmtB, and the cytosolic regulatory protein, GlnK, at 1.96 Å. Proc. Natl. Acad. Sci. U.S.A. 104, 42–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Petrek M., Otyepka M., Banás P., Kosinová P., Koca J., Damborský J. (2006) CAVER: a new tool to explore routes from protein clefts, pockets and cavities. BMC Bioinformatics 7, 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Javelle A., Lupo D., Zheng L., Li X. D., Winkler F. K., Merrick M. (2006) An unusual twin-His arrangement in the pore of ammonia channels is essential for substrate conductance. J. Biol. Chem. 281, 39492–39498 [DOI] [PubMed] [Google Scholar]
- 29. Javelle A., Lupo D., Ripoche P., Fulford T., Merrick M., Winkler F. K. (2008) Substrate binding, deprotonation, and selectivity at the periplasmic entrance of the Escherichia coli ammonia channel AmtB. Proc. Natl. Acad. Sci. U.S.A. 105, 5040–5045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Loqué D., Mora S. I., Andrade S. L., Pantoja O., Frommer W. B. (2009) Pore mutations in ammonium transporter AMT1 with increased electrogenic ammonium transport activity. J. Biol. Chem. 284, 24988–24995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soupene E., van Heeswijk W. C., Plumbridge J., Stewart V., Bertenthal D., Lee H., Prasad G., Paliy O., Charernnoppakul P., Kustu S. (2003) Physiological studies of Escherichia coli strain MG1655: growth defects and apparent cross-regulation of gene expression. J. Bacteriol. 185, 5611–5626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Inwood W. B., Hall J. A., Kim K. S., Demirkhanyan L., Wemmer D., Zgurskaya H., Kustu S. (2009) Epistatic effects of the protease/chaperone HflB on some damaged forms of the Escherichia coli ammonium channel AmtB. Genetics 183, 1327–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. (1969) Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J. Bacteriol. 100, 215–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neidhardt F. C., Bloch P. L., Smith D. F. (1974) Culture medium for enterobacteria. J. Bacteriol. 119, 736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bender R. A., Magasanik B. (1977) Regulatory mutations in the Klebsiella aerogenes structural gene for glutamine synthetase. J. Bacteriol. 132, 100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Soupene E., He L., Yan D., Kustu S. (1998) Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein. Proc. Natl. Acad. Sci. U.S.A. 95, 7030–7034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zimmer D. P., Soupene E., Lee H. L., Wendisch V. F., Khodursky A. B., Peter B. J., Bender R. A., Kustu S. (2000) Nitrogen regulatory protein C-controlled genes of Escherichia coli: scavenging as a defense against nitrogen limitation. Proc. Natl. Acad. Sci. U.S.A. 97, 14674–14679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Epstein W. (2003) The roles and regulation of potassium in bacteria. Prog. Nucleic Acid Res. Mol. Biol. 75, 293–320 [DOI] [PubMed] [Google Scholar]
- 40. Rhoads D. B., Waters F. B., Epstein W. (1976) Cation transport in Escherichia coli. VIII. Potassium transport mutants. J. Gen. Physiol. 67, 325–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dixon M. (1953) The determination of enzyme inhibitor constants. Biochem. J. 55, 170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Volkmer B., Heinemann M. (2011) Condition-dependent cell volume and concentration of Escherichia coli to facilitate data conversion for systems biology modeling. PLoS ONE 6, e23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Javelle A., Severi E., Thornton J., Merrick M. (2004) Ammonium sensing in Escherichia coli. J. Biol. Chem. 279, 8530–8538 [DOI] [PubMed] [Google Scholar]
- 44. Boogerd F. C., Ma H., Bruggeman F. J., van Heeswijk W. C., García-Contreras R., Molenaar D., Krab K., Westerhoff H. V. (2011) AmtB-mediated NH3 transport in prokaryotes must be active and as a consequence regulation of transport by GlnK is mandatory to limit futile cycling of NH4+/NH3. FEBS Lett. 585, 23–28 [DOI] [PubMed] [Google Scholar]
- 45. Guether M., Neuhäuser B., Balestrini R., Dynowski M., Ludewig U., Bonfante P. (2009) A mycorrhizal-specific ammonium transporter from Lotus japonicas acquires nitrogen release by arbuscular mycorrhizal fungi. Plant Physiol. 150, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Neuhäuser B., Dynowski M., Ludewig U. (2009) Channel-like NH3 flux by ammonium transporter AtAMT2. FEBS Lett. 583, 2833–2838 [DOI] [PubMed] [Google Scholar]
- 47. Wang S., Orabi E. A., Baday S., Bernèche S., Lamoureux G. (2012) Ammonium transporters achieve charge transfer by fragmenting their substrate. J. Am. Chem. Soc. 134, 10419–10427 [DOI] [PubMed] [Google Scholar]
- 48. Marini A. M., Soussi-Boudekou S., Vissers S., Andre B. (1997) A family of ammonium transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 17, 4282–4293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Booth I. R. (1985) Regulation of cytoplasmic pH in bacteria. Microbiol. Rev. 49, 359–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Booth I. R. (1999) The regulation of intracellular pH in bacteria. Novartis Found. Symp. 221, 19–28; discussions 28–37 [DOI] [PubMed] [Google Scholar]
- 51. Capone D. G. (2000) in Microbial Ecology of the Oceans (Kirchman D.L., ed) pp. 455–493, John Wiley & Sons, New York [Google Scholar]
- 52. Millero F. J. (2006) Chemical Oceanography, pp. 55–62, 3rd Ed., CRC Press, Boca Raton, FL [Google Scholar]
- 53. Walker C. B., de la Torre J. R., Klotz M. G., Urakawa H., Pinel N., Arp D. J., Brochier-Armanet C., Chain P. S., Chan P. P., Gollabgir A., Hemp J., Hügler M., Karr E. A., Könneke M., Shin M., Lawton T. J., Lowe T., Martens-Habbena W., Sayavedra-Soto L. A., Lang D., Sievert S. M., Rosenzweig A. C., Manning G., Stahl D. A. (2010) Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc. Natl. Acad. Sci. U.S.A. 107, 8818–8823 [DOI] [PMC free article] [PubMed] [Google Scholar]