Background: The role of protein flexibility in the C-H activation step, catalyzed by homologous thermophilic and psychrophilic alcohol dehydrogenases, is addressed.
Results: Mutation at the substrate-binding site, or at a dimer interface, alters kinetic properties and protein oligomeric structure.
Conclusion: Active site flexibility is correlated with subunit interactions 20 Å away.
Significance: A long-range network of catalytically relevant, dynamical communication is identified.
Keywords: Alcohol Dehydrogenase, Enzyme Catalysis, Isotope Effects, Mutagenesis, Protein Dynamics, Conformational Sampling, Convex Arrhenius Curves, Corresponding State Hypothesis, Extremophiles, Hydrogen Tunneling
Abstract
A tetrameric thermophilic alcohol dehydrogenase from Bacillus stearothermophilus (ht-ADH) has been mutated at an aromatic side chain in the active site (Trp-87). The ht-W87A mutation results in a loss of the Arrhenius break seen at 30 °C for the wild-type enzyme and an increase in cold lability that is attributed to destabilization of the active tetrameric form. Kinetic isotope effects (KIEs) are nearly temperature-independent over the experimental temperature range, and similar in magnitude to those measured above 30 °C for the wild-type enzyme. This suggests that the rigidification in the wild-type enzyme below 30 °C does not occur for ht-W87A. A mutation at the dimer-dimer interface in a thermolabile psychrophilic homologue of ht-ADH, ps-A25Y, leads to a more thermostable enzyme and a change in the rate-determining step at low temperature. The reciprocal mutation in ht-ADH, ht-Y25A, results in kinetic behavior similar to that of W87A. Collectively, the results indicate that flexibility at the active site is intimately connected to a subunit interaction 20 Å away. The convex Arrhenius curves previously reported for ht-ADH (Kohen, A., Cannio, R., Bartolucci, S., and Klinman, J. P. (1999) Nature 399, 496–499) are proposed to arise, at least in part, from a change in subunit interactions that rigidifies the substrate-binding domain below 30 °C, and impedes the ability of the enzyme to sample the catalytically relevant conformational landscape. These results implicate an evolutionarily conserved, long-range network of dynamical communication that controls C-H activation in the prokaryotic alcohol dehydrogenases.
Introduction
According to the corresponding state hypothesis, proteins evolve to strike a balance between stability and conformational flexibility (1). This idea has been used to explain the frequent observation that proteins are often just stable enough to operate under physiological conditions (2). It has been suggested that proteins do not evolve to maximum stability because of the requirements of the cell to achieve efficient protein synthesis and turnover, and the need for proteins to undergo rapid conformational sampling to execute their biological function (2–4). The role of conformational sampling in protein function has become the focus of intense research in the field of enzymology, as a large body of experimental data continues to illuminate the link of protein motions to bond cleavage events at enzyme active sites (5–16).
A corollary of the corresponding state hypothesis is that the introduction of stability in excess of that required under physiological conditions, due to a change in temperature or mutation, may adversely affect protein function by impairing functional conformational flexibility. Extensive investigations of proteins as a function of temperature support the expectation that proteins become increasingly rigid with decreasing temperature, and that enzymes evolved to operate at high temperatures exhibit sluggish activity relative to their mesophilic and psychrophilic counterparts at reduced temperature (3, 17–23). Evidence continues to emerge that enzymes have evolved in such a way as to optimize the influence of protein dynamics on the chemical steps of catalysis (see for example, Refs. 24 and 25).
Most enzyme reactions can be fit to the Arrhenius equation (Equation 1),
where k is the rate constant, A is the Arrhenius pre-factor, Ea is the energy of activation, and R is the molar gas constant. Generally, a plot of ln(k) against 1/T yields a straight line. However, for some enzymes, a transition to more sluggish catalysis has been seen to occur abruptly below a threshold temperature in a manner that is distinct from either a change in the rate-determining step or a decrease in enzyme stability (5, 24, 26). All of these enzymes and most other enzymes that have been shown to exhibit Arrhenius breakpoints are multimeric (see for example, Refs. 27 and 28 and references therein), raising the possibility of alterations in subunit interactions or protein oligomeric structure as the origin of the phenomenon.
One of the best-characterized examples of such behavior is an alcohol dehydrogenase from Bacillus stearothermophilus (ht-ADH).4 This enzyme shows a break in its Arrhenius plot at 30 °C that is accompanied by a significant increase in the enthalpy of activation and concomitant rapid decrease in rate with temperature. In addition, the temperature dependence of the kinetic isotope effect (KIE) is seen to undergo a transition from temperature-independent at elevated temperature to temperature-dependent below 30 °C (5). These changes have been attributed to distinct protein conformational substates above and below the break, with experimental support for this idea coming from hydrogen-deuterium exchange that demonstrates a local increase in flexibility above 30 °C within the substrate-binding domain of the enzyme (21). A psychrophilic ortholog from Moraxella TAE sp. 123 (ps-ADH) with very high-sequence identity (61%) has been shown, by contrast, to be highly thermolabile (29). The ps-ADH does not show an Arrhenius break, and exhibits local flexibility in the substrate-binding domain at 10 °C that is greater than that measured for ht-ADH at the same temperature (30).
It has been shown previously that both the Arrhenius behavior and KIEs for ht-ADH are sensitive to amino acid substitutions for conserved bulky hydrophobic residues in the NAD+ cofactor binding pocket (31, 32). We have now analyzed a variant at a conserved residue in the substrate-binding pocket of ht-ADH (ht-W87A) that is shown to abrogate the Arrhenius break in the experimental temperature range. The KIE for ht-W87A at all temperatures resembles that of the wild-type enzyme above 30 °C, with a kcat that is moderately reduced (about 5-fold). Due to the absence of a break in its activity, kcat for W87A approaches that of the wild-type enzyme at reduced temperature. However, the apparent enhancement of activity for ht-W87A relative to wild-type at low temperatures is accompanied by a loss of stability that results from dissociation of the tetramer into inactive dimers and monomers. A reciprocal relationship emerges from a mutant ps-ADH, in which subunit interactions are strengthened. The ps-ADH (ps-A25Y) variant introduces an intersubunit interaction present at the corresponding position in wild-type ht-ADH; we find that this leads to a marked stabilization of the psychrophilic enzyme that is accompanied by the emergence of an Arrhenius breakpoint at about 20 °C. Because the ht-ADH active site mutant disrupts the subunit interface, and the ps-ADH subunit interface mutation impairs catalysis at the active site, we further interrogated ht-ADH by mutating its tyrosine at the subunit interface, Y25A, uncovering kinetic behavior very similar to W87A. It is, thus, possible to identify an approximately 20-Å pathway of dynamical communication between the two targeted residues (Tyr-25 and Trp-87), comprised of the very same peptides that were previously demonstrated to rigidify below 30 °C in ht-ADH (21). We conclude that intersubunit contacts in the family of tetrameric prokaryotic alcohol dehydrogenases can alter the protein conformational landscape (31), thereby controlling the active site configurations that are directly linked to Arrhenius behavior and the properties of hydride tunneling between substrate and cofactor.
EXPERIMENTAL PROCEDURES
Materials
Benzyl alcohol was purchased from Fisher Scientific, and purified by vacuum distillation prior to use. The α,α-d2-benzyl alcohol (99.5% D) was purchased from CDN isotopes, found to be chemically pure within the detection limits of GC-MS, and thus, used without further purification. NAD+ and NADH were purchased from Sigma and used without further purification.
Cloning of ps-ADH Gene
The DNA sequence for alcohol dehydrogenase from Moraxella sp. TAE123 (ps-ADH) was obtained from the National Center for Biotechnology Information website. The gene was synthesized commercially, received in a pDrive cloning vector, and subsequently subcloned into a pET-24b expression vector. Correct insertion of the gene was confirmed by DNA sequencing.
Preparation of Mutants
Site-directed mutagenesis was carried out as previously described (22), using the QuikChange Stratagene kit for site-directed mutagenesis, with the following primers and their reverse complements: ht-W87F, 5′-CCGCGTTGGAATTCCTTTCTTATATTCTGCATGCG-3′; ht-W87L, 5′-CGCGTTGGAATTCCTTTGTTATATTCTGCATGC-3′; ht-W87A, 5′-CCGCGTTGGAATTCCTGCGTTATATTCTGCATGC-3′; ht-Y25A, 5′-GAAGTAGAAAAACCAACCATTTCAGCTGGAGAAGTATTAGTCCGC-3′; ps-A25Y, 5′-CCCAACGCCAGGTTATGGCGAGATAGTGG-3′. The mutated codon is underlined. Primers were designed using the program PrimerX and purchased from Operon. Plasmids were isolated and sequenced to confirm the mutations and the intactness of the remainder of the gene.
Expression and Purification of ht-ADH
Purification and expression of ht-ADH was carried out as previously described (21) with some modifications. For ht-W87A, an AMP-Sepharose affinity column (Amersham Biosciences) replaced the Cibicron blue column because it had a higher affinity for ht-ADH. The improved resolution of this column renders subsequent additional purification by size exclusion chromatography unnecessary. The heat treatment step (15 min at 60 °C) was also eliminated for ht-W87A because this mutant was found to lose significant activity under these conditions (see “Results” and “Discussion”). A number of minor changes were introduced into the subsequent preparation of ht-Y25A. These included inoculation of the stock into an autoinducible medium (Magic Medium, Invitrogen) with 50 μg/ml of kanamycin and 0.25 mm ZnSO4. The cells were aerobically grown for about 16 h at 37 °C, and then collected by microcentrifuge. The cell pellet was lysed by a mixture of gentle non-ionic detergents (Bugbuster®, Novagen), and the cell extract was heated at 60 °C for 15 min. Then the insoluble components were separated from the liquid phase by centrifugation at 4 °C, and the supernatant was mounted onto a fast-flow DEAE anion exchange column (GE Healthcare). The column was washed by 60 mm NaCl, and the protein was eluted by 200 mm NaCl. After buffer exchange by centrifugal filters (Amicon®, Millipore), the harvested protein was passed through an NAD+-affinity column (Blue Sepharose®, GE Healthcare) and eluted by 5 mm NAD+ from the column. The purified sample underwent buffer exchange and concentration by centrifugal filters.
Purification and Expression of ps-ADH
Because ps-ADH inactivates rapidly above 30 °C, Escherichia coli were grown to an A600 of ∼0.6 at 37 °C, and then cooled to 17 °C prior to induction with 0.5 mm isopropyl thiogalactoside. Expression of ps-ADH was monitored by gel electrophoresis, and found to reach a maximum within 48 h. Purification of ps-ADH was carried out using the protocol established for ht-ADH with the following modifications. 100 mm NaCl was maintained throughout the purification to prevent aggregation, and the 60 °C treatment was eliminated. An empty vector expression control yielded negligible endogenous ADH activity, so that the activity detected under conditions used can be assumed to arise solely from ps-ADH.
Kinetic Assays
Kinetic data were collected for the oxidation of benzyl alcohol (or its α,α-d2 isotopologue) to benzaldehyde with concomitant reduction of NAD+ to NADH, as previously described (5). With the exception of ht-W87A, enzyme stocks were maintained at 4 °C with no detectable loss of activity over the course of several hours. W87A stock solutions were preincubated at 30 °C and an enzyme concentration of 100 μm, in the presence of 1 mm NADH, in 50 mm potassium phosphate buffer, pH 7.0, for 30 min prior to use in kinetic assays (see “Results” and “Discussion” below regarding temperature-dependent inactivation and reconstitution). These solutions were then further diluted as needed for kinetic assays, and maintained at room temperature in 1 mm NADH to prevent activity loss. The final dilution of enzyme into the activity assays reduced the concentration of NADH to ≤10 μm.
Inactivation Kinetics of ht-W87A
The kinetics of activity loss upon incubation of ht-W87A was measured at multiple concentrations of enzyme and in the presence and absence of cofactor. Aliquots were removed as a function of time and their activity determined under standard assay conditions, which were 2.5 mm NAD+ and 5 mm benzyl alcohol, 50 mm potassium phosphate buffer, pH 7.0, at 20 °C. For incubation at low enzyme concentration and 0 °C (Fig. 2, panel A, lowest curve), ht-W87A was incubated under the conditions of the kinetic assay. In this case, the reaction was initiated by the addition of cofactor instead of the addition of enzyme, and the activity measured at 4 °C. Conditions were otherwise identical.
FIGURE 2.
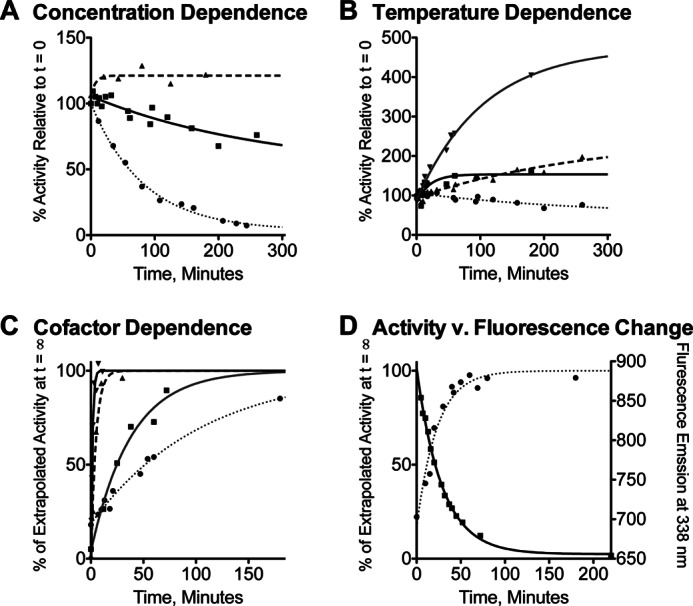
Inactivation and reconstitution of ht-W87A. A, percentage of activity remaining after incubation at 0 °C for 20 μm wild-type ht-ADH (triangles, dashed line), 20 μm ht-W87A (squares, solid line), or 0.5 μm ht-W87A (circles, dotted line). B, percentage of activity remaining for ht-W87A after incubation at 0 (circles, dotted line), 10 (squares, solid line), 20 (up triangles, dashed line), or 30 °C (down triangles, solid gray line). C, time-dependent reconstitution of activity at 30 °C as a percentage of the activity extrapolated at t = ∞, with no addition (circles, dotted line) or in the presence of 1 mm NAD+ (squares, solid gray line), 1 mm NADH (up triangles, dashed line), or 1 mm NADH + 1 mm N-butylformamide (down triangles, solid line). D, time-dependent reconstitution of activity at 10 °C in the presence of 1 mm NADH + 1 mm N-butylformamide (circles, dotted line), superimposed over time-dependent changes in fluorescence emission intensity at 338 nm under the same conditions (squares, solid line).
Fluorescence Kinetics
Fluorescence spectra were monitored at 10 and 30 °C as a function of time following the addition of 10 μm enzyme to a solution containing 100 mm N-butylformamide, and 5 eq of NADH (50 μm). Samples were excited at 295 nm, and both the excitation and detection slits were set to 1 nm. Fluorescence emission spectra were collected at 1–2-min intervals, and the intensity recorded at 338 nm, the maximum for protein fluorescence, and at 410 nm for enzyme-bound NADH, reflecting fluorescence energy transfer (FRET) from tryptophan to cofactor. Little or no fluorescence signal at 410 nm is expected from free NADH in solution for two reasons. The excitation wavelength (295 nm) gives negligible direct excitation of NADH (λmax(excitation) = 340 nm) and resonance energy transfer from protein tryptophan side chains should be very weak for free NADH due to the 1/r6 distance dependence of FRET. Additionally, the fluorescence signal from free NADH is ∼6-fold weaker than that for protein-bound NADH, due to quenching in solution.
FPLC Size Exclusion Chromatography
Protein oligomerization was estimated using a Superdex 200 FPLC column, calibrated with commercially available molecular mass standards (from 6,000 to 690,000 Da). The elution profile was monitored by UV absorption at 280 nm and activity assays were carried out for individual fractions. Chromatography and fraction collection were conducted at 4 °C. These conditions (50 mm phosphate, pH 7, with 150 mm NaCl, and 500 μg of protein) differ from previously reported preparative size exclusion chromatography, which was carried out at higher protein, lower salt, and 1 bar pressure. In all instances, activity was detected solely in the fractions containing tetrameric protein.
Circular Dichroism (CD)
Spectra were collected using an Aviv 410 spectropolarimeter in a quartz cuvette with a 1-mm path length and a protein concentration of 0.5 mg/ml in 50 mm phosphate, pH 7.0. The temperature was controlled by a Peltier temperature controller. Data points were taken every 1 nm. The signal is reported in units of mean residue elipticity.
RESULTS
Kinetic and Structural Properties of W87A
Steady-state kinetics, carried out at 30 °C for variants of ht-ADH with alternate hydrophobic side chains (at position 87), indicate relatively small changes in kcat (less than about 10-fold) with no obvious trends across the series (Table 1). The measured KIEs are all similar to the value obtained for the wild-type enzyme. A detailed analysis of both rates and KIEs as a function of temperature was carried out for ht-W87A because it represented the most drastic structural perturbation in the series, and was the only variant to exhibit cold lability (see below). An Arrhenius plot of kcat shows a complete loss of the breakpoint observed at 30 °C for the wild-type enzyme (Fig. 1). The activation parameters for ht-W87A are similar to those measured above 30 °C for the wild-type enzyme (Table 2). In addition, the KIE for ht-W87A is seen to be nearly temperature-independent throughout the experimental temperature range, in contrast to the wild-type enzyme, which exhibits a change in the temperature dependence of the KIE below 30 °C (Fig. 1, inset). (Full tabulation of the kinetic data for ht-W87A and the other mutants can be found in Table 3.)
TABLE 1.
Kinetic parameters at 30 °C for ADH variants
| kcat | Km, benzyl alcohol | Km, NAD+ | KIE for kcat | |
|---|---|---|---|---|
| s−1 | mm | |||
| ht-ADHa | 24.9 (±2.5) | 6.9 (±0.5) | 1.1 (±0.1) | 3.2 (±0.2) |
| ht-W87F | 6.1 (±0.5) | 5.2 (±0.8) | 2.1 (±0.3) | 3.4 (±0.6) |
| ht-W87L | 2.2 (±0.1) | 18 (±2.2) | 4.0 (±0.7) | 3.0 (±0.3) |
| ht-W87A | 5.5 (±0.4) | 1.5 (±0.3) | 0.2 (±0.05) | 3.2 (±0.6) |
| ht-Y25A | 14.0 (±1.0) | 7.2 (±1.7) | 1.0 (±0.5) | 2.9 (±0.2) |
| ps-ADHb | 6.9 | 29.4 | 3.2 | |
| ps-A25Y | 3.8 (±0.2) | 16 (±1.5) | 0.5 (±0.06) | 3.7 (0.6) |
FIGURE 1.
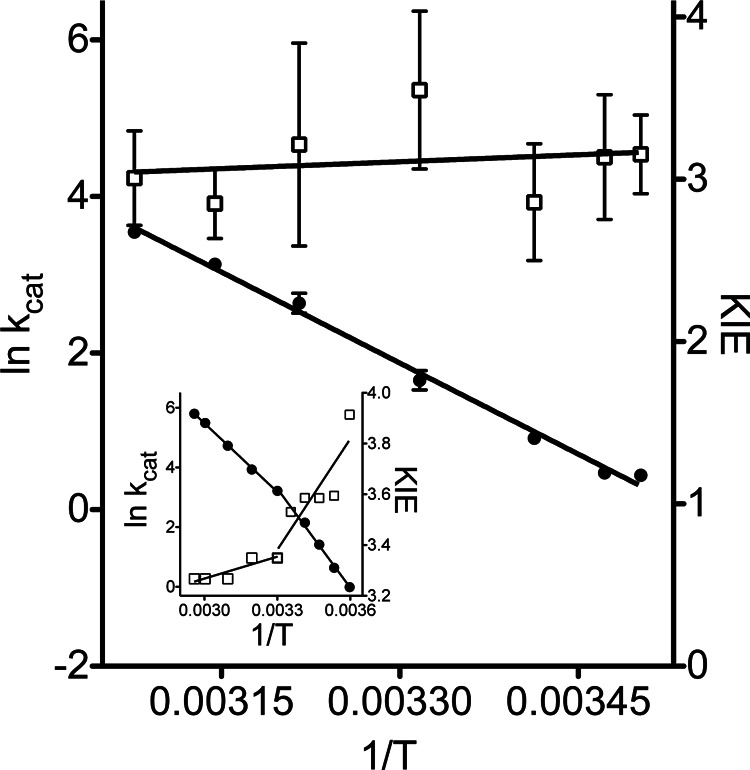
Arrhenius plot for ht-W87A between 10 and 52 °C. The temperature dependence of kcat (filled circles, left axis), KIE (open squares, right axis), and the corresponding plot for wild-type ht-ADH (inset) are presented.
TABLE 2.
Activation parameters in kcal/mol for wild-type ht-ADH and mutants for the oxidation of benzyl alcohol
| ht-ADH, | ht-ADH | ht-W87A | ps-ADH | ps-A25Y | ht-Y25A | |
|---|---|---|---|---|---|---|
| 5–30 °Ca | 30–65 °Ca | 12–52 °Cb | 5–35 °Cc | 20–52 °Cb | 10–50 °Cb,d | |
| ΔH‡ | 21.2 (±1.0) | 14.5 (±0.4) | 14.8 (±0.4) | 9.2 (±0.6) | 7.2 (±0.3) | 12.1 (±0.5) |
| TΔS‡ | 5.2 (±2.4) | −1.4 (±0.8) | −1.8 (±0.4) | −7.3 (±1.5) | −9.6 (±0.3) | −4.0 (±0.5) |
| Ea(D) − Ea(H) | 0.9 (±0.05) | 0.1 (±0.01) | 0.2 (±0.01) | 4.5 (±1.5) | NDe | 0.5 (±0.1) |
TABLE 3.
Kinetic parameters for ht-W87A, ps-A25Y, and ht-Y25A
| T | kcat | Error | Dkcata | Error |
|---|---|---|---|---|
| °C | s−1 | |||
| ht-W87A | ||||
| 12.5 | 1.5 | 0.1 | 3.2 | 0.2 |
| 15 | 1.6 | 0.1 | 3.1 | 0.4 |
| 20 | 2.5 | 0.2 | 2.9 | 0.4 |
| 28.5 | 5.2 | 0.7 | 3.6 | 0.5 |
| 38 | 14.0 | 1.8 | 3.2 | 0.6 |
| 45 | 23.0 | 1.2 | 2.9 | 0.2 |
| 52 | 34.7 | 2.5 | 3.0 | 0.3 |
| ps-A25Y | ||||
| 5 | 0.5 | 0.01 | 2.4b | 0.1 |
| 11.5 | 1.0 | 0.04 | ||
| 16 | 1.5 | 0.04 | 2.8 | 0.1 |
| 20 | 2.7 | 0.1 | ||
| 25 | 3.2 | 0.2 | ||
| 29 | 3.8 | 0.3 | 3.7 | 0.6 |
| 39 | 7.0 | 1.0 | ||
| 46 | 7.9 | 0.9 | 3.5 | 0.6 |
| 52 | 13.0 | 1.9 | 3.6 | 0.6 |
| ht-Y25A | ||||
| 10 | 2.9 | 0.4 | 2.8 | 0.2 |
| 20 | 7.4 | 0.1 | 3.4 | 0.6 |
| 30 | 13.0 | 1.0 | 2.7 | 0.1 |
| 40 | 25.2 | 2.5 | 2.4 | 0.2 |
| 50 | 51.1 | 5.7 | 2.9 | 0.3 |
a Dkcat refers to the primary KIE for oxidation of benzyl alcohol relative to its α,α-d2-isotopolog.
b KIEs were only measured at the temperatures indicated.
In the course of purification, ht-W87A was seen to exhibit cold lability that was not observed in any other variants. To establish conditions for steady-state kinetics, the temperature and concentration dependence of this phenomenon was investigated (Fig. 2). Wild-type ht-ADH incubated at 0 °C and an enzyme concentration of 20 μm showed no activity loss even after 24 h. By contrast, a freshly thawed sample of ht-W87A diluted to 20 μm lost 50% of its activity within about 5 h, with more rapid loss at lower enzyme concentration (Fig. 2, panel A). When the same sample was incubated at moderately elevated temperatures, an increase in activity was observed, with the most rapid reconstitution seen at 30 °C (Fig. 2, panel B). Slower reconstitution was seen at 40 °C, and the enzyme was inactivated upon prolonged incubation at 50 °C (data not shown). It was also found that the presence of cofactor increases the rate of reconstitution, with the most rapid reconstitution seen for a ternary complex with the reduced form of the cofactor (NADH) and the aldehyde analog inhibitor N-butylformamide (Fig. 2, panel C). Finally, ht-W87A activity is reconstituted more slowly in the presence of the inhibitor complex at 10 °C than observed at 30 °C (Fig. 2, panel D). Despite the slightly slower reconstitution than in the presence of N-butylformamide, NADH alone was found to give the largest absolute increase in specific activity (not shown), possibly due to carryover of inhibitor into the assay. Incubation of ht-W87A at 200 μm enzyme and 1 mm NADH at 30 °C for 30 min was, therefore, used to generate stable and reproducible enzyme activity for use in kinetic assays. The presence of NADH was sufficient to prevent activity loss over several hours in 2–20 μm stock solutions of ht-W87A maintained at room temperature. Kinetic assays were linear for at least 1 min and linearly dependent on enzyme concentrations between 10 and 55 °C, ruling out the possibility of rapid inactivation upon dilution, or during the course of data collection.
Additional characterization of ht-W87A was aimed at determining the mechanism of inactivation and reconstitution. Fluorescence monitored as a function of time under reconstituting conditions at 10 °C revealed a time-dependent decrease in fluorescence intensity (Fig. 2, panel D). The kinetics of fluorescence quenching were correlated with the kinetics of reconstitution at 10 (Fig. 2, panel D) and 30 °C, where both phenomena occur more rapidly. The fluorescence emission spectra are presented in Fig. 3A. A CD spectrum for ht-W87A collected for a sample cold-adapted overnight at 4 °C, and which showed no detectable activity, was nearly superimposable with a highly active sample that had been incubated under reconstituting conditions (Fig. 3B). Size exclusion chromatography indicated that the dominant oligomeric state for wild-type ht-ADH is a tetramer, whereas ht-W87A eluted primarily as a monomer (Fig. 4A). A similar experiment conducted in the presence of 1 mm NADH and 1 mm N-butylformamide, which were found to protect the enzyme from cold inactivation (data not shown) resulted in ht-W87A eluting almost exclusively as the tetramer (Fig. 4B). In all cases, activity assays performed on fractions eluting from the size exclusion column indicated that only the tetramer was active.
FIGURE 3.
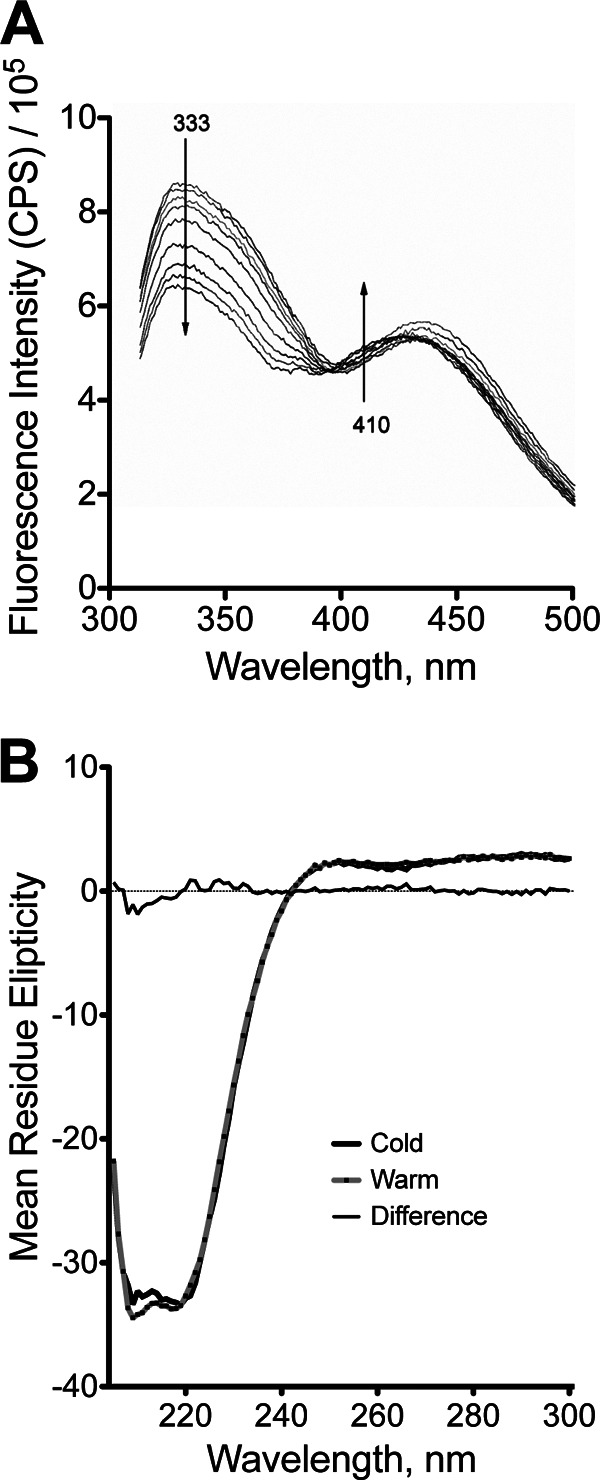
Spectroscopic characterization of ht-W87A. A, time-dependent fluorescence emission spectrum for ht-W87A. Arrows represent the direction of change observed as a function of time (min). The fluorescence data in Fig. 2D were generated from the spectra shown herein. Small, but significant changes were detected in two distinct regions of the fluorescence spectrum. Protein fluorescence (λmax ∼ 333 nm) was seen to decrease as a function of time. Fluorescence due to NADH simultaneously became blue shifted, as seen by an increase in the intensity at 410 nm. B, CD spectra at two temperatures for ht-W87A. Samples were incubated overnight at 20 μm enzyme at 0 °C (heavy black line) or for 1 h at 30 °C and 200 μm enzyme, with a spectrum collected immediately following dilution to 20 μm at 30 °C (gray line with black dots). Difference spectrum, fine black line.
FIGURE 4.
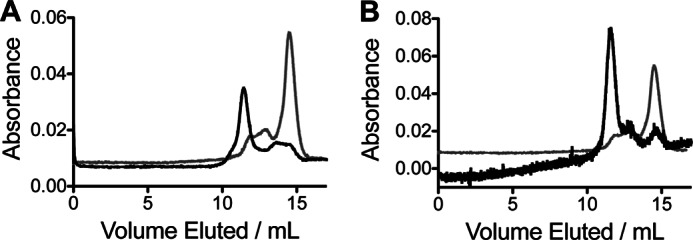
Analysis of ht-W87A by FPLC size exclusion chromatography. A, elution profile of wild-type ht-ADH (black) and ht-W87A (gray) at 4 °C showing peaks associated with the tetramer (about 11 ml), dimer (about 13 ml), and monomer (about 15 ml). B, elution profile of ht-W87A at 4 °C in the presence (black) or absence of 1 mm NADH and 100 mm N-butylformamide. High background absorption (about 0.85 absorbance units) due to NADH was subtracted from the data collected in the presence of the cofactor to superimpose the two traces for comparison.
Characterization of a ps-A25Y Variant
Turning to the ps-ADH variants, it was found that these enzymes are also only active as a tetramer. The wild-type enzyme dissociated into an inactive monomeric form when subjected to FPLC size exclusion chromatography at 4 °C, and the extreme thermolability reported previously was confirmed by incubation at 50 °C (Fig. 5). The availability of x-ray structures for both ht-ADH and ps-ADH, together with the high sequence identity between these proteins (61%), afforded the opportunity to search for structural differences at their subunit interfaces. One striking feature is the substitution of a tyrosine at position 25 in ht-ADH by alanine in ps-ADH. We therefore prepared ps-A25Y to contrast its behavior to wild-type ps-ADH. The ps-A25Y mutation significantly stabilized the tetrameric form of the enzyme at 4 °C, and led to a dramatic increase in its thermostability at 50 °C (Fig. 5). As a result of the increased thermostability, it was possible to measure steady-state kinetics up to 52 °C. An Arrhenius plot for ps-A25Y indicates a breakpoint near about 20 °C. Below this temperature, activity falls more rapidly with temperature, and the KIE on kcat becomes progressively smaller (Fig. 6A), in contrast to the wild-type ps-ADH behavior. The activation parameters for ps-A25Y above the breakpoint (between 20 and 52 °C) are similar to those measured for wild-type ps-ADH (Table 2). No attempt was made to estimate the same parameters below the breakpoint for ps-A25Y because of the apparent kinetic complexity suggested by the decrease in KIE with temperature.
FIGURE 5.
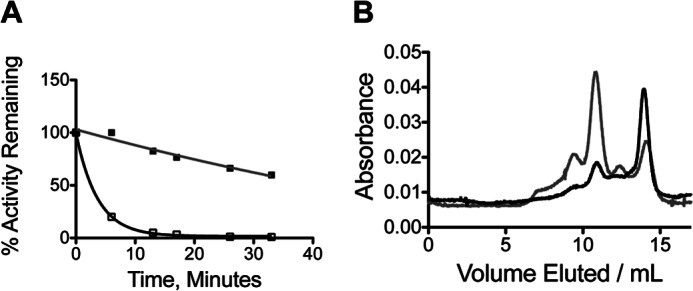
Stability and FPLC size exclusion chromatography of ps-ADH variants. A, inactivation of 20 μm ps-ADH (open squares, black line) or ps-A25Y (closed squares, gray line) at 50 °C. B, elution profile at 4 °C for wild-type ps-ADH (black) and ps-A25Y (gray) showing peaks associated with the tetramer (about 11 ml), dimer (about 12.5 ml), and monomer (about 14 ml). Peaks between the column dead volume (about 7 ml) and the tetramer represent higher order aggregates.
FIGURE 6.
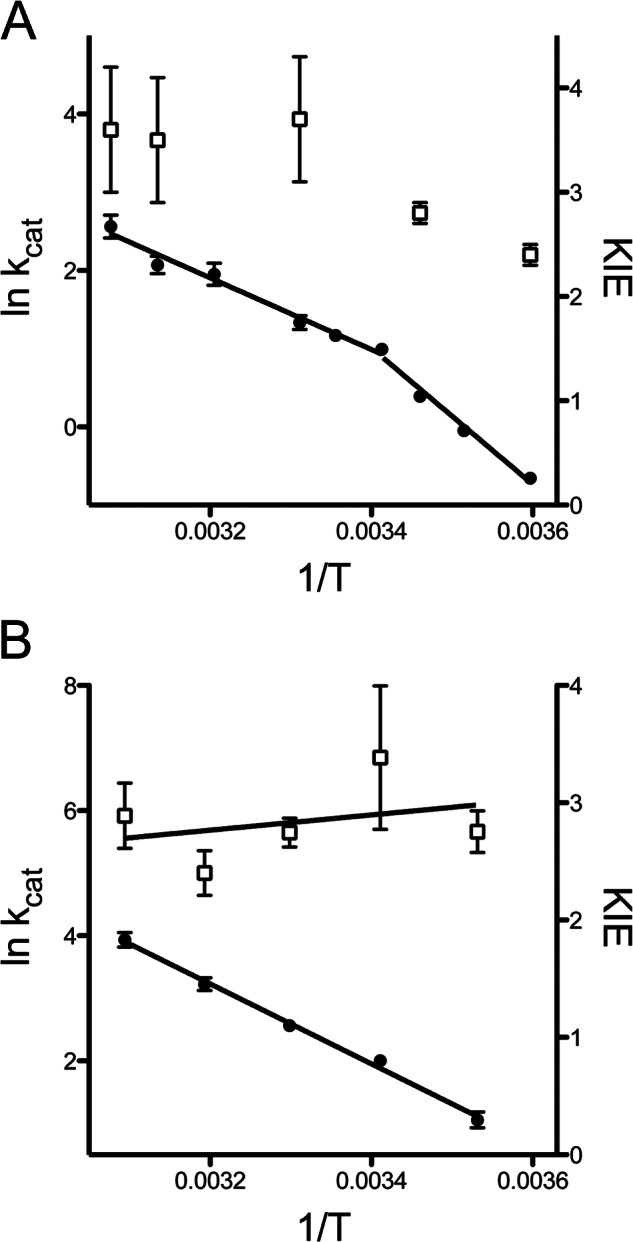
Arrhenius plots for ADH variants with amino acid substitutions at the subunit interface. A, Arrhenius plots for ps-A25Y between 5 and 52 °C. Temperature dependence of kcat (filled circles, solid line, left axis) and KIE (open squares, right axis) are shown. B, Arrhenius plot for ht-Y25A between 10 and 50 °C. The temperature dependence of kcat (filled circles, solid line, left axis) and the KIE (open squares, right axis) are shown.
Contrasting the Behavior of ht-Y25A to ht-W87A
The reciprocal responses of ht-ADH to mutation at its active site and ps-ADH to mutation at its subunit interface suggested that ht-Y25A would produce an enzyme form very similar to ht-W87A. This prediction is confirmed by the kinetic behavior of ht-Y25A, where the breaks at 30 °C in rate and temperature dependence of the KIE for wild-type (Fig. 1, inset) are lost in ht-Y25A (Fig. 6B). The extreme susceptibility of ht-W87A to dissociation at low temperature is, however, not observed for ht-Y25A, with the fraction of tetramer being highly dependent on the concentration of enzyme analyzed by FPLC. The proportion of the enzyme found in the tetrameric form varied from about 81% at 0.5 μg to 44% at 0.3 μg, to a level below the limit of detection at 0.02 μg of protein analyzed in a 0.5 ml loading volume. In the case of ht-W87A, FPLC data (Fig. 4A, gray trace) were carried out at extremely high protein concentrations (500 μg) in an effort to suppress protein dissociation; nonetheless, the dominant enzyme form remained a monomer. This indicates the much larger effect of an active site mutant on oligomeric protein stability than the subunit interface mutant Y25A.
DISCUSSION
Activation Parameters and KIEs Indicate That a Transition Observed for ht-ADH at 30 °C Is Absent in ht-W87A and ht-Y25A
Two kinetic signatures define the Arrhenius break for the wild-type ht-ADH. First, an increase in the enthalpy of activation ΔH‡ by about 7 kcal/mol is accompanied by an equivalent increase in TΔS‡ below 30 °C. Fitting the kinetic data below this temperature to the Arrhenius equation (Eq. 1) results in an unusually large Arrhenius prefactor AOBS ∼ 1017 s−1; for the majority of chemical reactions, AOBS falls near 1013 s−1 (34). Recent characterization of ht-ADH variants mutated in the cofactor-binding pocket revealed an enhanced Arrhenius break, with values for AOBS as large as 1025 s−1 (31). A detailed characterization of these enzymes has led to the conclusion that the unusual Arrhenius curves observed for ht-ADH variants reflect a reversible distribution of the protein conformational landscape into inactive, low energy states at low temperature. The present data indicate that the Arrhenius break is completely absent for ht-W87A, as well as ht-Y25A, with values for ΔH‡ and TΔS‡ throughout the temperature range approximating the values measured above 30 °C for wild-type. We conclude that ht-Y25A and ht-W87A do not sample the low energy inactive states in the experimental temperature range.
A second signature of the Arrhenius break in the wild-type enzyme is a transition in the temperature dependence of the KIE. Above the 30 °C breakpoint, the wild-type enzyme exhibits a nearly temperature-independent KIE: the difference between the energy of activation for protium and deuterium, Ea(D) − Ea(H), approaches zero (Table 2). By contrast, below the breakpoint temperature, the KIE becomes temperature-dependent, with increasing values for Ea(D) − Ea(H). The ht-ADH enzyme is thus one of a growing number of enzymes catalyzing hydrogen transfer with KIEs that are nearly temperature-independent under native conditions, but become temperature-dependent upon the introduction of a generic perturbation, such as mutation, protein surface modification, or as in this case, a change in temperature (23). This phenomenon has increasingly been attributed to a failure of perturbed enzymes to sample conformational substates that can support ideal quantum tunneling conditions for hydrogen transfer at the active site. In the cases of both ht-W87A and ht-Y25A, the absence of a detectable break toward more temperature-dependent KIEs at low temperature indicates that conformational sampling remains nearly optimal for hydrogen tunneling throughout the experimental temperature range.
The Catalytic Properties of the ht-W87A Variant More Closely Resemble Those of ps-ADH Than ht-ADH, But the Enzyme Is Not Suited for Low Temperature Catalysis Because of Cold Lability
Extensive comparative studies of the kinetic properties of homologous enzymes from organisms adapted to different temperature niches have revealed several trends that extend to ps-ADH and ht-ADH. Thermophilic enzymes are typically more stable, and exhibit larger values for ΔH‡ and more negative values for ΔS‡ relative to their mesophilic and psychrophilic counterparts (22, 35–39). In keeping with these trends, ps-ADH shows a smaller ΔH‡ and more negative ΔS‡ than ht-ADH (22). Also, whereas the ps-ADH is extremely thermolabile, the ht-ADH is stable to the extended incubation at 65 °C.
These trends in kinetic properties are likely a consequence of co-evolutionary constraints on protein flexibility and stability. Although some studies indicate exceptions are possible (40), enzymes that have evolved to function at elevated temperature tend to be more rigid and less active at low temperature than their psychrophilic counterparts (3, 25, 30, 41, 42). As discussed in the Introduction, ht-ADH and ps-ADH can be included in this trend (30), and importantly, a decrease in local flexibility below 30 °C correlates with the Arrhenius break in ht-ADH (21).
The ht-W87A mutation converts ht-ADH to an enzyme with properties intermediate between ht-ADH (>30 °C) and ps-ADH. The mutant retains at all temperatures the lower ΔH‡ and more negative ΔS‡ associated with the wild-type enzyme at high temperature. Thus, ht-W87A activity falls off less steeply with temperature, and ht-W87A and ps-ADH yield similar rate constants at low temperature. However, whereas it remains more resistant to heat inactivation than ps-ADH, ht-W87A sacrifices thermostability relative to ht-ADH. Collectively, the data suggest that ht-W87A is overall a more flexible catalyst than the wild-type enzyme, failing to undergo rigidification in the experimental temperature range, but paying for increased flexibility via a decrease in stability.
Both ht-ADH and ps-ADH Are Active Exclusively in the Tetrameric Form, and Both the Cold Lability of ht-W87A and the Thermolability of ps-ADH Result from Dissociation of the Active Form of the Enzyme
The cold lability observed in ht-W87A is concentration-dependent, with dilute enzyme becoming inactivated more rapidly (Fig. 2A). These inactivation kinetics are consistent with dissociation of the tetrameric form of the enzyme into inactive, lower order oligomers. Size exclusion chromatography confirmed that the ht-W87A tetramer dissociates to a much larger extent than the wild-type enzyme (Fig. 4A), and activity assays indicated that the dimer and monomer are both inactive (not shown). Further evidence that the inactivation of ht-W87A at low temperature arises from reversible dissociation of the tetramer comes from the observation that enzymatic activity can be reconstituted from fractions eluting as inactive monomeric enzyme upon incubation at 30 °C in the presence of cofactor under the same conditions used to reconstitute cold-inactivated enzyme in Fig. 2 (not shown). Moreover, when size exclusion chromatography was performed at 4 °C in the presence of NADH and N-butylformamide, which prevent cold inactivation, ht-W87A eluted almost exclusively as a tetramer, and no activity was associated with minor fractions eluting as monomer or dimer (Fig. 4B). Similarly, when ps-ADH was subjected to size exclusion chromatography (also at 4 °C), activity was only associated with the tetrameric form of the enzyme. Compared with ht-ADH, a greater proportion of the psychrophilic enzyme elutes as dimer and monomer.
Fluorescence and CD Spectra Indicate No Major Changes in Secondary Structure Upon Dissociation of the ht-ADH Tetramer into Monomers
CD spectra collected for warm-adapted (active) ht-W87A as well as cold-inactivated enzyme were almost superimposable (Fig. 3B), indicating that the loss of activity is not a result of significant unfolding of the enzyme upon dissociation of the tetramer. Tryptophan fluorescence spectra collected under conditions that reconstitute activity indicated a time-dependent increase in fluorescence quenching (Fig. 2D) that correlates with the restoration of enzymatic activity. Examination of the x-ray structure for ht-ADH indicates that Trp-49 forms a π-stacking interaction with Phe-272 of an adjacent subunit (Fig. 7B). The formation of this interaction upon oligomerization could lead to a portion of the observed protein fluorescence quenching (43). Fluorescence energy transfer to the bound NADH cofactor was detected simultaneously at λemission = 410 nm, and a gradual blue-shift of this signal with a similar time dependence as the quenching of tryptophan fluorescence (Fig. 3A) suggests that the formation of subunit interactions directly alters the active site microenvironment. Taken together, the spectroscopic data support the conclusion that ht-W87A retains the overall subunit structure of the wild-type enzyme, and that minor structural changes are responsible for converting the inactive dimer to the active tetramer form.
FIGURE 7.
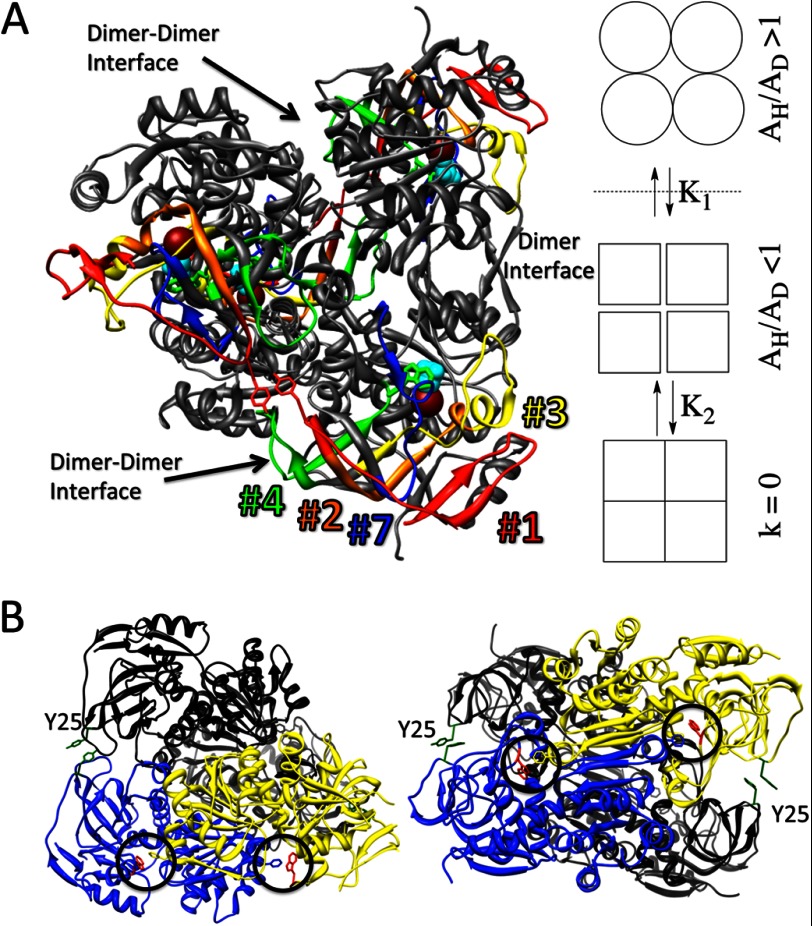
A, x-ray structure of ht-ADH and a model for the origin of the Arrhenius break. Peptides undergoing a transition in flexibility at 30 °C are illustrated in color. Trp-87 (green, near the end of peptide #4), the active site zinc (crimson sphere), and the alcohol analog trifluoroethanol (cyan) abut a contiguous β raft, which forms a contact with the same structural feature in the adjacent subunit via tyrosine 25 (red) near the C-terminal end of peptide #1. At right, three conformational landscapes of the wild-type ht-ADH tetramer are represented in schematic form. At elevated temperature (circles), a flexible tetramer exhibits weakly temperature-dependent KIEs. Below a transition temperature of 30 °C (dotted line), strong intersubunit interactions lead to a more rigid conformational landscape (squares), exhibiting temperature-dependent KIEs. These substates are in equilibrium with an additional landscape involving low energy, inactive forms of enzyme. B, x-ray structure of ht-ADH. One dimer is colored black and the monomers comprising the other, blue and yellow to emphasize interactions at the dimer-dimer interface where Tyr-25 is shown in green. The interaction between Trp-49 and Phe-272 is highlighted by a black circle. Residues from the subunit on the left are colored blue, those from the right subunit are yellow. On the right side of panel B, the image is rotated 90°. A β sheet interaction between the subunits is seen at the center of the image, flanked by the Trp-49/Phe-272 interaction.
Increased ps-A25Y Thermostability Is Due to Strengthened Intersubunit Interactions, However the Enzyme Becomes Less Suited for Catalysis at Low Temperatures Because of a Change in the Rate-limiting Step
The observed relationship between oligomeric integrity and activity in ht-W87A suggested that thermolability in ps-ADH may arise from weakened subunit interactions. Homology modeling to ht-ADH indicates a ps-ADH structure nearly superimposable with that of the thermophilic enzyme, which we regard as a dimer of dimers (44). Subunits interact strongly at a “dimer interface” in the dimeric eukaryotic alcohol dehydrogenases (45); however, the interactions that join two such dimers at what we term the “dimer-dimer interface” are relatively sparse. Notably, a π-stacking interaction at the tetramer interface between Tyr-25 and its counterpart in an adjacent subunit is lost in ps-ADH, as the psychrophilic enzyme contains alanine at position 25 (Fig. 7B). We hypothesized that restoration of this interaction in the psychrophilic enzyme would confer significant stabilization. Upon incubation at 50 °C, ps-A25Y is seen to be dramatically more stable than the wild-type enzyme, retaining nearly 75% activity after 20 min, at which time ps-ADH is completely inactivated (Fig. 5A). Analysis by size exclusion chromatography further shows that the experimental conditions promoting extensive dissociation of the wild-type ps-ADH lead to retention of the tetramer in the ps-A25Y mutant (Fig. 5B).
Kinetic characterization of ps-A25Y at 30 °C shows this enzyme is only modestly less active than ps-ADH, and similar values for the KIE indicate that the chemical step remains rate-determining (Table 1). In the elevated temperature range for ps-A25Y (between 20 and 50 °C), activation parameters are also similar to those measured for ps-ADH between 5 and 35 °C (Table 2).
Below 20 °C, ps-A25Y and ps-ADH exhibit divergent behavior. A breakpoint emerges in the ps-A25Y Arrhenius point, with an apparent increase in the enthalpy of activation, reminiscent of the ht-ADH behavior (Fig. 6A). However, examination of the KIE on kcat (Dkcat) reveals a trend toward smaller values at lower temperature, opposite what is seen for ps-ADH (22). As a result, the “break” in the ps-A25Y Arrhenius plot likely reflects increasing contributions to kcat from steps other than hydride transfer; by contrast, the hydride transfer step appears to be fully rate-limiting at all temperatures for ps-ADH. Although ps-A25Y is much more stable than ps-ADH, the mutant is significantly less active than ps-ADH at temperatures below 20 °C, consistent with the hypothesis that the need to maintain flexibility at reduced temperature places a limit on the stability of psychrophilic enzymes.
The Mutant ht-Y25A Exhibits Similar Kinetic Characteristics to ht-W87A, However, Its Quaternary Structure Is More Resistant against Cold Dissociation
The π-stacking interaction between Tyr-25 residues located on opposing subunits appears to stabilize their association in the ps-A25Y variant and the wild-type ht-ADH. Given these observations, the ht-Y25A variant was expected to exhibit stability and kinetic properties resembling those of ht-W87A. Similar to ht-W87A and the wild-type ps-ADH, the ht-Y25A variant does not yield a significant Arrhenius break at 30 °C (Fig. 6B). The activation parameters across the experimental temperature range (10–50 °C) resemble those observed for the wild-type ht-ADH above 30 °C (Table 2). This observation provides further support for the notion that Arrhenius behavior can be controlled at least in part by intersubunit interactions. However, unlike ht-W87A, which undergoes a significant loss of quaternary stability at low temperature (Fig. 4A), the ht-Y25A tetramer shows a more modest propensity to dissociate in a concentration-dependent manner (not shown). The contrasting stabilities of ht-W87A, ps-ADH, and ht-Y25A at the reduced temperatures indicate that an increase in local flexibility at the active site (expressed as a loss of the Arrhenius break) does not require, a priori, a large destabilization of intersubunit interactions.
Reciprocal Effects of Mutation to ht-ADH and ps-ADH Implicate a Network of Communication between the Active Site of the Enzyme and the Subunit Interface
Because the active site of the tetrameric bacterial alcohol dehydrogenases does not lie at a subunit interface, the exclusive activity of the tetrameric state must reflect subtle structural changes that propagate from the dimer-dimer interface to the active site of the enzyme upon formation of intersubunit interactions. The ps-A25Y mutant indicates that the effects of strengthened subunit interactions can propagate to the active site in a deleterious manner, by the introduction of a new rate-limiting step. Reciprocally, the ht-Y25A mutant with a weakened subunit interaction appears to prevent the enzyme from falling into a subset of low energy, catalytically inactive conformers that has been invoked previously to account for inflated Arrhenius prefactors in other variants of ht-ADH (31). In the case of ht-W87A, the introduction of a packing defect at the active site results in a significant weakening of subunit interactions at low temperature, indicating that changes in active site geometry likewise propagate to interactions at the subunit interface. Thus, the aggregate data indicate bi-directional communication between the active site and the dimer-dimer interface in the bacterial alcohol dehydrogenases that modulates the conformational flexibility required for efficient hydrogen tunneling. The five regions of protein (identified within peptides 1, 2–4, and 7) that have previously been shown by way of hydrogen-deuterium exchange experiments to rigidify below 30 °C in ht-ADH directly connect Tyr-25 to Trp-87 via a contiguous β sheet (Fig. 7A), presenting a pathway by which small perturbations at either position may exert structural and dynamical changes at the other position (21).
The functional link between structural features at the active site and the subunit interface is reminiscent of allostery, wherein the role of ligand binding as the allosteric signal has been replaced by a change in temperature. Furthermore, the lack of major rearrangements in the secondary structure of the protein as a function of temperature (31), or upon disruption of intersubunit interactions (W87A) indicates that conformational sampling may carry the “allosteric” signal in place of a large change in the average conformation of the protein. Transmission of an allosteric signal via dynamic fluctuations, and in the absence of detectable conformational change, has been proposed for other proteins recently and has been discussed in detail (46). The present data show that prokaryotic alcohol dehydrogenases have evolved to tune the long-range interactions between the subunit interface and the active site in support of optimal catalysis within their respective temperature niches.
Convex Arrhenius Curves Observed for ht-ADH and ps-A25Y Appear to Arise from a Temperature-dependent Change in Subunit Interactions
Although the Arrhenius break for ps-A25Y arises from kinetic complexity, it contributes to a pattern in which stronger subunit interactions tend to be associated with convex Arrhenius curves. Weakened subunit interactions in ps-ADH and ht-W87A, or the direct removal of an intersubunit interaction in the case of ht-Y25A, are all associated with the absence of an Arrhenius break. A similar relationship has been observed recently for a thermophilic, dimeric dihydrofolate reductase (27). In those experiments, the wild-type enzyme showed a modest breakpoint at 25 °C below which both ΔH‡ and the temperature dependence of the KIE increased, as seen with ht-ADH. The introduction of a mutation at the subunit interface resulted in an active monomeric enzyme, and abrogated the break. However, unlike the ht-ADH and more similar to ps-A25Y, the break in the Arrhenius plot was found to arise from a change in the rate-determining step. Convex Arrhenius curves in which the hydrogen transfer step remains rate-determining at all temperatures have been proposed to arise from conformational pre-equilibria (28, 47, 48), and we have shown that impaired conformational sampling can be used to model the enormously inflated Arrhenius pre-factors measured for a series of ht-ADH mutants at reduced temperatures (31). The molecular basis for these Arrhenius breakpoints has been elusive, although a clue may lie in the observation that the transition from low to high temperature conformational equilibria in ht-ADH requires both a large positive ΔH°, and a large positive ΔS° (31), as have been measured for the dissociation of protein subunits (33).
The results presented herein, taken together with extensive previous characterization of the ht-ADH support a model for the observed kinetic properties, presented in Fig. 7A. At elevated temperatures, ht-ADH adopts a subset of relatively flexible conformational substates that support efficient tunneling. Below 30 °C, the enzyme adopts more rigid conformational substates that still support catalysis, however, with altered tunneling parameters. The ability of the enzyme to adopt these conformers depends upon intersubunit interactions at position 25. As the temperature decreases, the rigidified subunits can enter into a second, lower energy interaction with one another that forces the enzyme into a catalytically inactive conformational space via K2. As this occurs, the rate of catalysis decreases rapidly with temperature, resulting in inflated Arrhenius parameters (31). We propose that the packing defect caused by the ht-W87A mutation introduces flexibility into the protein that overwhelms the stabilizing effect of interactions at Tyr-25. This prevents the enzyme from rigidifying at reduced temperature, so that it is able to sample a conformational subspace compatible with efficient tunneling at all temperatures (K1 in the model approaches zero). In complementary fashion, the absence of a π-stacking interaction at the subunit interface in ht-Y25A likewise prevents the enzyme from rigidifying at reduced temperature (K1 in the model again approaches zero). Because ht-Y25A remains more cold-stable than the ht-W87A mutation, the latter appears to weaken additional intersubunit interactions, possibly through a process of mild deformation of the protein about the resulting cavity in the active site. This process can be prevented or reversed by the binding of NAD cofactor, NADH, or a combination of NADH and the aldehyde analog inhibitor, N-butylformamide. Cofactor binding to alcohol dehydrogenase is known to induce a conformational change. This could offset the extreme destabilizing effect of the ht-W87A by introducing intersubunit interactions that are unavailable in the conformational substates adopted in the absence of cofactor. In the case of ps-A25Y, an increase in subunit interactions analogous to the process represented by K2 is suggested to take place below 20 °C. Because the enzyme remains catalytically active, the consequence of these interactions must be to slow down a step other than hydride transfer (possibly product release). The observation of Arrhenius breaks in multimeric enzymes may, thus, be a consequence of a temperature-dependent change in the strength of subunit interactions, which imposes restrictions on the conformational landscape, and thereby distorts the active site away from its optimal geometry. The ability of subunit interactions to regulate enzyme activity in a reversible and temperature-dependent manner may also serve a previously unrecognized biological role, presenting a strategy by which organisms can respond rapidly to temperature changes at the level of enzyme activity without the need for cycles of protein degradation and replenishment.
Conclusion
An evolutionary trade-off among stability, local flexibility, and catalytic activity is evident in ps-ADH, ht-ADH, and their respective mutant variants. The data support a model that involves communication between the dimer-dimer interface and the enzyme active site in prokaryotic alcohol dehydrogenases. The two enzyme variants for which a catalytically deleterious rigidification appears to occur, ht-ADH and ps-A25Y, share the feature of relatively strong intersubunit interactions. In both cases, subunit interactions propagate to the active site, with ht-ADH exhibiting impairment of hydride transfer, and ps-ADH a change in rate-limiting step. Reciprocally, attenuated subunit interactions in ps-ADH, ht-Y25A, and ht-W87A permit hydrogen tunneling to proceed unimpaired at reduced temperature. These observations provide new insight into the molecular origins of unusual Arrhenius curves seen in ht-ADH and other oligomeric proteins. A more detailed understanding of the molecular basis for subtle, correlated changes between catalysis and protein flexibility may yield novel targets for the disruption of protein-protein interactions and the inhibition of enzymatic activity.
This work was supported, in whole or in part, by National Institutes of Health Grants GM025765 (to J. P. K.) and GM008295 (to Z. D. N.) and the National Science Foundation Grant MCB0446395 (to J. P. K.).
- ht-ADH
- high temperature-alcohol dehydrogenase
- FPLC
- fast protein liquid chromatography
- FRET
- fluorescence energy transfer
- ps
- psychrophilic
- KIEs
- kinetic isotope effects.
REFERENCES
- 1. Somero G. N. (1978) Temperature adaptation of enzymes. Biological optimization through structure-function comrpomises. Annu. Rev. Ecol. Syst. 9, 1–29 [Google Scholar]
- 2. Somero G. N. (1995) Proteins and temperature. Annu. Rev. Physiol. 57, 43–68 [DOI] [PubMed] [Google Scholar]
- 3. Závodszky P., Kardos J., Svingor A., Petsko G. A. (1998) Adjustment of conformational flexibility is a key event in the thermal adaptation of proteins. Proc. Natl. Acad. Sci. U.S.A. 95, 7406–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feller G. (2010) Protein stability and enzyme activity at extreme biological temperatures. J. Phys. Condes. Matter 22, 323101–323117 [DOI] [PubMed] [Google Scholar]
- 5. Kohen A., Cannio R., Bartolucci S., Klinman J. P. (1999) Enzyme dynamics and hydrogen tunnelling in a thermophilic alcohol dehydrogenase. Nature 399, 496–499 [DOI] [PubMed] [Google Scholar]
- 6. Benkovic S. J., Hammes-Schiffer S. (2003) A perspective on enzyme catalysis. Science 301, 1196–1202 [DOI] [PubMed] [Google Scholar]
- 7. Hammes-Schiffer S., Benkovic S. J. (2006) Relating protein motion to catalysis. Annu. Rev. Biochem. 75, 519–541 [DOI] [PubMed] [Google Scholar]
- 8. Fan F., Gadda G. (2007) An internal equilibrium preorganizes the enzyme-substrate complex for hydride tunneling in choline oxidase. Biochemistry 46, 6402–6408 [DOI] [PubMed] [Google Scholar]
- 9. Henzler-Wildman K., Kern D. (2007) Dynamic personalities of proteins. Nature 450, 964–972 [DOI] [PubMed] [Google Scholar]
- 10. Benkovic S. J., Hammes G. G., Hammes-Schiffer S. (2008) Free-energy landscape of enzyme catalysis. Biochemistry 47, 3317–3321 [DOI] [PubMed] [Google Scholar]
- 11. Klinman J. P. (2009) An integrated model for enzyme catalysis emerges from studies of hydrogen tunneling. Chem. Phys. Lett. 471, 179–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pudney C. R., Hay S., Levy C., Pang J., Sutcliffe M. J., Leys D., Scrutton N. S. (2009) Evidence to support the hypothesis that promoting vibrations enhance the rate of an enzyme catalyzed H-tunneling reaction. J. Am. Chem. Soc. 131, 17072–17073 [DOI] [PubMed] [Google Scholar]
- 13. Bhabha G., Lee J., Ekiert D. C., Gam J., Wilson I. A., Dyson H. J., Benkovic S. J., Wright P. E. (2011) A dynamic knockout reveals that conformational fluctuations influence the chemical step of enzyme catalysis. Science 332, 234–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schramm V. L. (2011) Enzymatic transition states, transition-state analogs, dynamics, thermodynamics, and lifetimes. Annu. Rev. Biochem. 80, 703–732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stojković V., Perissinotti L. L., Willmer D., Benkovic S. J., Kohen A. (2012) Effects of the donor-acceptor distance and dynamics on hydride tunneling in the dihydrofolate reductase catalyzed reaction. J. Am. Chem. Soc. 134, 1738–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Glowacki D. R., Harvey J. N., Mulholland A. J. (2012) Exciton dynamics. Electrons take an unexpected turn. Nat. Chem. 4, 169–176 [DOI] [PubMed] [Google Scholar]
- 17. Tang K. E., Dill K. A. (1998) Native protein fluctuations. The conformational-motion temperature and the inverse correlation of protein flexibility with protein stability. J. Biomol. Struct. Dyn. 16, 397–411 [DOI] [PubMed] [Google Scholar]
- 18. Fields P. A., Somero G. N. (1998) Hot spots in cold adaptation. Localized increases in conformational flexibility in lactate dehydrogenase A4 orthologs of Antarctic notothenioid fishes. Proc. Natl. Acad. Sci. U.S.A. 95, 11476–11481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vihinen M. (1987) Relationship of protein flexibility to thermostability. Protein Eng. 1, 477–480 [DOI] [PubMed] [Google Scholar]
- 20. Varley P. G., Pain R. H. (1991) Relation between stability, dynamics and enzyme activity in 3-phosphoglycerate kinases from yeast and Thermus thermophilus. J. Mol. Biol. 220, 531–538 [DOI] [PubMed] [Google Scholar]
- 21. Liang Z. X., Lee T., Resing K. A., Ahn N. G., Klinman J. P. (2004) Thermal-activated protein mobility and its correlation with catalysis in thermophilic alcohol dehydrogenase. Proc. Natl. Acad. Sci. U.S.A. 101, 9556–9561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liang Z. X., Tsigos I., Bouriotis V., Klinman J. P. (2004) Impact of protein flexibility on hydride-transfer parameters in thermophilic and psychrophilic alcohol dehydrogenases. J. Am. Chem. Soc. 126, 9500–9501 [DOI] [PubMed] [Google Scholar]
- 23. Nagel Z. D., Klinman J. P. (2009) A 21st century revisionist's view at a turning point in enzymology. Nat. Chem. Biol. 5, 543–550 [DOI] [PubMed] [Google Scholar]
- 24. Heyes D. J., Levy C., Sakuma M., Robertson D. L., Scrutton N. S. (2011) A twin-track approach has optimized proton and hydride transfer by dynamically coupled tunneling during the evolution of protochlorophyllide oxidoreductase. J. Biol. Chem. 286, 11849–11854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oyeyemi O. A., Sours K. M., Lee T., Kohen A., Resing K. A., Ahn N. G., Klinman J. P. (2011) Comparative hydrogen-deuterium exchange for a mesophilic vs thermophilic dihydrofolate reductase at 25 °C. Identification of a single active site region with enhanced flexibility in the mesophilic protein. Biochemistry 50, 8251–8260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anandarajah K., Schowen K. B., Schowen R. L. (2008) Hydrogen tunneling in glucose oxidation by the Archaeon Thermoplasma acidophilum. Int. J. Res. Phys. Chem. Chem. Phys. 222, 1333–1347 [Google Scholar]
- 27. Loveridge E. J., Rodriguez R. J., Swanwick R. S., Allemann R. K. (2009) Effect of dimerization on the stability and catalytic activity of dihydrofolate reductase from the hyperthermophile Thermotoga maritima. Biochemistry 48, 5922–5933 [DOI] [PubMed] [Google Scholar]
- 28. Massey V., Curti B., Ganther H. (1966) A temperature-dependent conformational change in d-amino acid oxidase and its effect on catalysis. J. Biol. Chem. 241, 2347–2357 [PubMed] [Google Scholar]
- 29. Tsigos I., Velonia K., Smonou I., Bouriotis V. (1998) Purification and characterization of an alcohol dehydrogenase from the Antarctic psychrophile Moraxella sp. TAE123. Eur. J. Biochem. 254, 356–362 [DOI] [PubMed] [Google Scholar]
- 30. Liang Z. X., Tsigos I., Lee T., Bouriotis V., Resing K. A., Ahn N. G., Klinman J. P. (2004) Evidence for increased local flexibility in psychrophilic alcohol dehydrogenase relative to its thermophilic homologue. Biochemistry 43, 14676–14683 [DOI] [PubMed] [Google Scholar]
- 31. Nagel Z. D., Dong M., Bahnson B. J., Klinman J. P. (2011) Impaired protein conformational landscapes as revealed in anomalous Arrhenius prefactors. Proc. Natl. Acad. Sci. U.S.A. 108, 10520–10525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nagel Z. D., Meadows C. W., Dong M., Bahnson B. J., Klinman J. P. (2012) Active site hydrophobic residues impact hydrogen tunneling differently in a thermophilic alcohol dehydrogenase at optimal versus nonoptimal temperatures. Biochemistry 51, 4147–4156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Weber G. (1995) Revisited, enthalpy of association of protein subunits. J. Phys. Chem. 99, 1052–1059 [Google Scholar]
- 34. Forst W. (1973) Theory of unimolecular reactions, Academic Press, New York [Google Scholar]
- 35. Lonhienne T., Gerday C., Feller G. (2000) Psychrophilic enzymes. Revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim. Biophys. Acta 1543, 1–10 [DOI] [PubMed] [Google Scholar]
- 36. D'Amico S., Collins T., Marx J. C., Feller G., Gerday C. (2006) Psychrophilic microorganisms. Challenges for life. EMBO Rep. 7, 385–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feller G. (2007) Life at low temperatures. Is disorder the driving force? Extremophiles 11, 211–216 [DOI] [PubMed] [Google Scholar]
- 38. Nagel Z. D., Klinman J. P. (2006) Tunneling and dynamics in enzymatic hydride transfer. Chem. Rev. 106, 3095–3118 [DOI] [PubMed] [Google Scholar]
- 39. Lam S. Y., Yeung R. C. Y., Yu T. H., Sze K. H., Wong K. B. (2011) A rigidifying salt-bridge favors the activity of thermophilic enzyme at high temperatures at the expense of low-temperature activity. PLoS Biol. 9, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Merkley E. D., Parson W. W., Daggett V. (2010) Temperature dependence of the flexibility of thermophilic and mesophilic flavoenzymes of the nitroreductase fold. Protein Eng. Des. Sel. 23, 327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kohen A., Klinman J. P. (2000) Protein flexibility correlates with degree of hydrogen tunneling in thermophilic and mesophilic alcohol dehydrogenases. J. Am. Chem. Soc. 122, 10738–10739 [Google Scholar]
- 42. Wolf-Watz M., Thai V., Henzler-Wildman K., Hadjipavlou G., Eisenmesser E. Z., Kern D. (2004) Linkage between dynamics and catalysis in a thermophilic-mesophilic enzyme pair. Nat. Struct. Mol. Biol. 11, 945–949 [DOI] [PubMed] [Google Scholar]
- 43. Nanda V., Brand L. (2000) Aromatic interactions in homeodomains contribute to the low quantum yield of a conserved, buried tryptophan. Proteins 40, 112–125 [DOI] [PubMed] [Google Scholar]
- 44. Ceccarelli C., Liang Z. X., Strickler M., Prehna G., Goldstein B. M., Klinman J. P., Bahnson B. J. (2004) Crystal structure and amide H/D exchange of binary complexes of alcohol dehydrogenase from Bacillus stearothermophilus. Insight into thermostability and cofactor binding. Biochemistry 43, 5266–5277 [DOI] [PubMed] [Google Scholar]
- 45. Eklund H., Samma J. P., Wallén L., Brändén C. I., Akeson A., Jones T. A. (1981) Structure of a triclinic ternary complex of horse liver alcohol dehydrogenase at 2.9-Å resolution. J. Mol. Biol. 146, 561–587 [DOI] [PubMed] [Google Scholar]
- 46. Tzeng S. R., Kalodimos C. G. (2011) Protein dynamics and allostery. An NMR view. Curr. Opin. Struct. Biol. 21, 62–67 [DOI] [PubMed] [Google Scholar]
- 47. Truhlar D., Kohen A. (2001) Convex Arrhenius plots and their interpretation. Proc. Natl. Acad. Sci. U.S.A. 98, 848–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Limbach H. H., Lopez J. M., Kohen A. (2006) Arrhenius curves of hydrogen transfers. Tunnel effects, isotope effects and effects of pre-equilibria. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 1399–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]