Background: Soluble epoxide hydrolase (sEH) is a cytosolic enzyme whose pharmacological inhibition or targeted deletion in mice has beneficial effects, including improved insulin signaling in liver and adipose tissue.
Results: sEH inhibition or deficiency attenuates high fat diet- and chemical-induced endoplasmic reticulum (ER) stress in mice and cells, respectively.
Conclusion: sEH modulates ER stress in a cell-autonomous manner.
Significance: sEH may be a therapeutic target for mitigating complications associated with the metabolic syndrome.
Keywords: Endoplasmic Reticulum Stress, Hydrolases, Inflammation, Insulin Resistance, Metabolic Regulation, Soluble Epoxide Hydrolase, Epoxyeicosatrienoic Acids (EETs)
Abstract
Soluble epoxide hydrolase (sEH) is a cytosolic enzyme whose inhibition has beneficial effects in cardiovascular, inflammatory, and metabolic diseases in murine models. Mice with targeted deletion or pharmacological inhibition of sEH exhibit improved insulin signaling in liver and adipose tissue. Herein, we assessed the role of sEH in regulating endoplasmic reticulum (ER) stress in liver and adipose tissue. We report that sEH expression was increased in the livers and adipose tissue of mice fed a high fat diet, the adipose tissue of overweight humans, and palmitate-treated cells. Importantly, sEH deficiency or inhibition in mice attenuated chronic high fat diet-induced ER stress in liver and adipose tissue. Similarly, pharmacological inhibition of sEH in HepG2 cells and 3T3-L1 adipocytes mitigated chemical-induced ER stress and activation of JNK, p38, and cell death. In addition, insulin signaling was enhanced in HepG2 cells treated with sEH substrates and attenuated in cells treated with sEH products. In summary, these findings demonstrate that sEH is a physiological modulator of ER stress and a potential target for mitigating complications associated with obesity.
Introduction
Obesity and metabolic syndrome result from excess calorie intake and genetic predisposition. Obese individuals exhibit a higher risk of chronic diseases such as type 2 diabetes and cardiovascular disease (1–3). The mechanisms by which excess nutrients and adiposity trigger changes that lead to these chronic diseases are still being elucidated. A growing body of evidence indicates that endoplasmic reticulum (ER)3 dysfunction is a contributor to metabolic disease (4, 5). The ER is highly responsive to nutrients and energy status of the cell and plays an important role in folding and maturation of newly synthesized proteins. When the folding capacity of the ER is exceeded, misfolded proteins accumulate and lead to ER stress (6). Of note, high fat feeding and genetic obesity in mice lead to increased ER stress in liver and adipose tissue (4). In addition, obese individuals exhibit increased expression of multiple markers of ER stress (7). Thus, understanding the role of ER stress in adiposity and insulin resistance may be of therapeutic potential for the treatment of metabolic diseases.
Cells use adaptive mechanisms to mitigate ER stress and to restore homeostasis known as the unfolded protein response. Unfolded protein response signaling consists of three branches initiated by the ER transmembrane proteins PKR-like ER-regulated kinase (PERK), inositol-requiring enzyme 1α (IRE1α), and activating transcription factor 6 (ATF6) (5, 8, 9). These sensor proteins respond to changes in protein folding status in the ER and convey information to activate distinct and sometimes overlapping pathways. PERK phosphorylates the α-subunit of eukaryotic translation initiation factor 2 (eIF2α) at Ser51, leading to rapid and transient attenuation of protein synthesis (10–12). IRE1α activation leads to the unconventional splicing of X-box-binding protein 1 (XBP1) mRNA, leading to the synthesis of a nuclear XBP1 form that induces the transcription of genes encoding ER chaperones and ER-associated protein degradation (13–15). The third canonical branch includes ATF6α, which, upon ER stress, traffics to the Golgi apparatus, where it is cleaved to liberate a fragment that translocates to the nucleus to induce genes encoding ER chaperones and ER-associated protein degradation functions (16, 17). The distant unfolded protein response arms synergize to attenuate stress by increasing the folding capacity of the ER, translational attenuation, ER biogenesis, and ER-associated protein degradation (6). However, if the compensatory mechanisms fail to facilitate the adaptation of cells to ER stress, induction of the unfolded protein response can lead to apoptosis (18, 19).
Soluble epoxide hydrolase (sEH) is a cytosolic enzyme with C-terminal epoxide hydrolase and N-terminal lipid phosphatase activities (20, 21). sEH inhibition has beneficial effects in cardiovascular, renal, and inflammatory diseases in murine models (22, 23). Endogenous substrates for sEH include epoxy fatty acids such as epoxyeicosatrienoic acids (EETs). EETs are arachidonic acid metabolites produced by cytochrome P-450 epoxygenases. sEH plays a key role in regulating the levels of EETs by effectively degrading them into more polar and usually less potent metabolites, dihydroxyeicosatrienoic acids (DHETs) (24, 25). sEH-selective inhibitors (sEHI) stabilize EETs and other epoxy fatty acids by preventing their conversion to DHETs or the corresponding diols (26). The stabilized EETs are anti-hypertensive, anti-inflammatory, and anti-hyperalgesic in both inflammatory and neuropathic pain models (27–29). In addition to their salutary effects in the vasculature, EETs may have beneficial effects on lipid metabolism and insulin sensitivity. sEH activity is increased in epididymal fat pads of mice fed a high fat diet (HFD) (30). Moreover, sEH expression is increased in obese Zucker rats, a commonly used animal model of obesity and insulin resistance (31). Furthermore, in a type 1 diabetes model, genetic and pharmacological inhibition of sEH results in increased insulin secretion and attenuation of hyperglycemia (31). Finally, sEH deficiency or pharmacological inhibition in mice improves systemic glucose tolerance and enhances insulin signaling in liver and adipose tissue (32). In this study, we determined the effects of sEH deficiency and pharmacological inhibition on ER stress signaling in liver and adipose tissue and investigated the underlying molecular mechanism.
EXPERIMENTAL PROCEDURES
Reagents
DMEM, G418, penicillin/streptomycin, puromycin, newborn calf serum, FBS, and trypsin were purchased from Invitrogen. Antibodies for sEH were generated by in the Hammock laboratory (28). Antibodies for tubulin were purchased from Upstate Biotechnology. Antibodies for phospho-PERK (Thr980), PERK, phospho-eIF2α (Ser51), eIF2α, spliced XBP1 (sXBP1), ATF6, IRE1α, BiP, GADD34, phospho-c-Jun (Ser73), c-Jun, phospho-insulin receptor (IR) (Tyr1162/Tyr1163), and IR were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies for phospho-p38 (Thr180/Tyr182), p38, phospho-JNK (Thr183/Tyr185), JNK, phospho-AKT (Ser473), AKT, and cleaved caspase-3 were from Cell Signaling (Beverly, MA). Antibodies for phospho-IRE1α (Ser724) were purchased from Abcam (Cambridge, MA). HRP-conjugated secondary antibodies were purchased from BioResources International (Carlsbad, CA). EET, epoxyoctadecenoic acid (EpOME), and their corresponding diol products (DHET and dihydroxyoctadecenoic acid (DiHOME), respectively) were prepared in the Hammock laboratory as described previously (33). Unless indicated otherwise, all other chemicals were purchased from Sigma.
Mouse Studies
Mice with a targeted disruption in exon 1 of the Ephx2 gene (34) were back-crossed onto a C57BL/6 background (The Jackson Laboratory) 10 generations prior to use in this study (32). Mice were maintained on a 12-h light/dark cycle in a temperature-controlled facility with free access to water and food. For experiments assessing nutritional regulation of sEH expression (see Fig. 1), mice were fed a HFD (60% kcal from fat; D12492, Research Diets, New Brunswick, NJ) for the indicated times. For all other studies, Ephx2 knock-out (KO) and WT mice were fed a regular chow diet (13.5% kcal from fat; Purina LabDiet 5001) at weaning or a HFD (42% kcal from fat; Harlan Teklad TD.88137) at 6 weeks of age. In addition, mice were treated with a selective sEHI, 1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea (TUPS). Mice were provided TUPS (10 μg/ml in 1% polyethylene glycol 400) in drinking water starting from 6 weeks of age throughout the study, and control mice were provided drinking water containing 1% polyethylene glycol 400 as described previously (32). All mouse studies were approved by the Institutional Animal Care and Use Committee of the University of California, Davis.
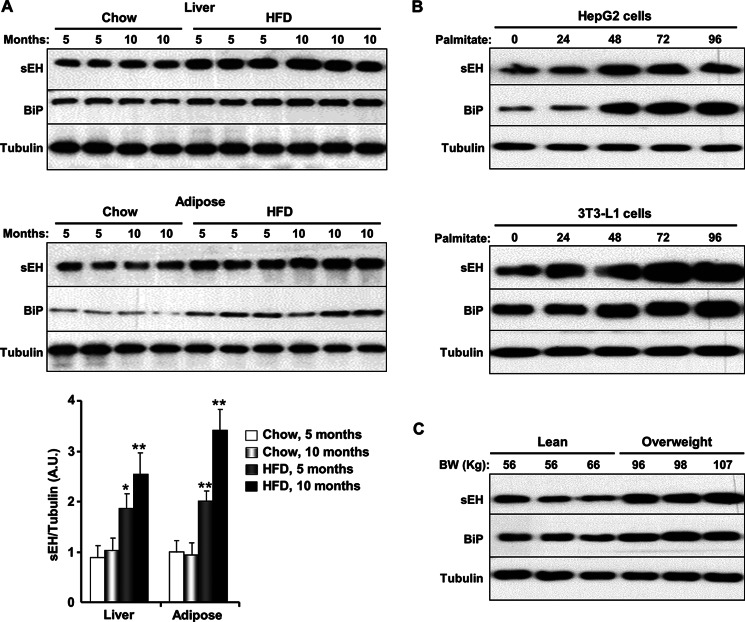
FIGURE 1.
HFD- and chemical-induced ER stress increases sEH protein expression. A, liver (upper panel) and subcutaneous adipose tissue (lower panel) were isolated from male mice fed a regular chow diet or a HFD (60% kcal from fat) for 5 and 10 months, and lysates were immunoblotted for sEH, BiP, and tubulin. Each lane represents a sample from a separate animal. The bar graph represents normalized data expressed as arbitrary units (A.U.) for sEH/tubulin from six mice per group and presented as means ± S.E. *, p ≤ 0.05, significant difference between mice fed a HFD and those fed a regular chow diet for the corresponding duration; **, p ≤ 0.01. B, HepG2 cells (upper panel) and differentiated 3T3-L1 adipocytes (lower panel) were treated with palmitate for the indicated times, and lysates were immunoblotted for sEH, BiP, and tubulin. C, adipose tissue lysates from lean and overweight human male subjects were immunoblotted for sEH, BiP, and tubulin. BW, body weight.
Cell Culture
HepG2 cells were maintained in 5% CO2 at 37 °C in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. 3T3-L1 cells were maintained in high glucose (25 mm) DMEM supplemented with 10% newborn calf serum and penicillin/streptomycin. Confluent 3T3-L1 cells were cultured in differentiation medium (1.7 μm insulin and high glucose DMEM supplemented with 10% FBS). After 2 days, cells were cultured in induction medium (1.7 μm insulin and high glucose DMEM supplemented with 10% FBS, 1 μm dexamethasone, and 0.5 mm 3-isobutyl-l-methylxanthine) for 48 h. After induction, cells were cultured in differentiation medium until they exhibited a differentiated phenotype. ER stress was induced in cells by treatment with thapsigargin (TG; 2 μm) for 2 h or palmitate (0.5 mm) for the indicated times. Palmitate was freshly prepared in HEPES/DMEM buffer containing 10.5% fatty acid-free BSA. When indicated, cells were pretreated with TUPS (1 μm) alone or in combination with EET, EpOME, DHET, or DiHOME (1 μm each) (35, 36) for 1 h, and then ER stress was induced by treating cells with palmitate (0.5 mm) for 24 h. For insulin signaling experiments, HepG2 cells were starved overnight in low glucose (5.5 mm) medium containing TUPS (1 μm) alone or in combination with 4-phenylbutyric acid at 20 mm or with EET, EpOME, DHET, or DiHOME at 1 μm and then stimulated with 100 nm insulin for 10 min.
Biochemical Analyses
Mouse tissues were dissected and immediately frozen in liquid nitrogen. Tissues were ground in the presence of liquid nitrogen and lysed using radioimmunoprecipitation assay buffer (10 mm Tris-HCl (pH 7.4), 150 mm NaCl, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate, 5 mm EDTA, 1 mm NaF, 1 mm sodium orthovanadate, and protease inhibitors). Lysates were clarified by centrifugation at 13,000 rpm for 10 min, and protein concentrations were determined using a bicinchoninic acid protein assay kit (Pierce). HepG2 cells, 3T3-L1 adipocytes, and human abdominal subcutaneous adipose tissue (from the National Human Tissue Resource Center) were lysed using radioimmunoprecipitation assay buffer. Proteins were resolved by SDS-PAGE and transferred to PVDF membranes. Immunoblotting was performed with the relevant antibodies, and proteins were visualized using LuminataTM Forte (Millipore, Billerica, MA). For quantitation purposes, pixel intensities of immunoreactive bands from blots that were in the linear range of loading and exposure were quantified using FluorChem 9900 (Alpha Innotech).
RNA was extracted from liver and subcutaneous white adipose tissue using TRIzol reagent (Invitrogen). cDNA was generated using a high-capacity cDNA archive kit (SuperScriptTM III reverse transcriptase, Invitrogen). mRNAs from BiP, sXBP1, and CHOP were assessed by RT-PCR (iCycler, Bio-Rad) and normalized to TATA box-binding protein. For RT-PCR, ABsolute Blue qPCR premix (Thermo Scientific) was mixed with each primer. The primers used were as follows: CHOP, 5′-CCCTGCCTTTCACCTTGG-3′ (forward) and 5′-CCGCTCGTTCTCCTGCTC-3′ (reverse); BiP, 5′-ACTTGGGGACCACCTATTCCT-3′ (forward) and 5′-ATCGCCAATCAGACGCTCC-3′ (reverse); sXBP1, 5′-GGTCTGCTGAGTCCGCAGCAGG-3′ (forward) and 5′-AGGCTTGGTGTATACATGG-3′ (reverse); and TATA box-binding protein, 5′-TTGGCTAGGTTTCTGCGGTC-3′ (forward) and 5′-TGCTGTAGCCGTATTCATTG-3′ (reverse).
Statistical Analyses
Data are expressed as means ± S.E. All statistical analyses were performed using JMP Statistical Discovery (SAS Institute). Comparisons among multiple groups were made using one-way analysis of variance and the Bonferroni-Holmes method for post hoc analysis. For experiments on cells, statistical analyses were performed using Student's t test.
RESULTS
Chronic ER Stress Increases sEH Protein Levels in Liver and Adipose Tissue
We evaluated sEH expression in the livers and adipose tissue of mice fed a regular chow diet (Purina lab chow) versus mice fed a HFD (60% kcal from fat) for 5 and 10 months to induce chronic ER stress. Consistent with published data (4, 7, 37), high fat feeding led to elevated ER stress in liver and adipose tissue. Immunoblot analyses indicated that BiP expression was increased in lysates of liver and subcutaneous adipose tissue of mice fed a HFD compared with those fed a regular chow diet (Fig. 1A). In addition, sEH protein expression was increased in liver (1.8- and 2.6-fold) and adipose tissue (1.9- and 3.4-fold) after 5 and 10 months of high fat feeding, respectively (Fig. 1A). To investigate whether chemical-induced ER stress also leads to comparable alterations in sEH expression, HepG2 cells and 3T3-L1 adipocytes were treated with palmitate, which induces ER stress through degradation of carboxypeptidase E (38). Palmitate treatment led to increased BiP and sEH protein levels in HepG2 cells and 3T3-L1 adipocytes (Fig. 1B). Finally, to determine whether findings from rodent and cell models translate to humans, we analyzed sEH expression in human adipose tissues from lean and overweight subjects. Immunoblot analyses revealed increased sEH protein levels in lysates of adipose tissue from overweight subjects compared with lean controls (Fig. 1C). Together, these findings indicate that obesity induces an increase in sEH expression in vivo. Moreover, these results identify HepG2 and 3T3-L1 as suitable cell models for the assessment of sEH function in ER stress.
sEH Regulates ER Stress Signaling in Vivo
The increased sEH expression upon HFD- and chemical-induced ER stress raised the possibility that sEH may play a role in regulating ER stress in liver and adipose tissue. To test this, we determined the effects of sEH deficiency and pharmacological inhibition on HFD-induced ER stress in vivo. sEH KO and WT mice were fed a regular chow diet and a HFD for 10 months as described previously (32). As a complementary approach for assessing the role of sEH in ER stress, pharmacological inhibition was achieved by treating mice with a sEHI (TUPS) (32). TUPS is an effective sEHI (IC50 = 5 and 3 nm for murine and human sEH, respectively) (21, 39). The TUPS concentration in the plasma of treated mice was higher than its IC50, as we reported previously (32). Mice exhibited comparable body weights when fed a regular chow diet (WT, 32.7 ± 1.5 g; and KO, 33.5 ± 1.1 g) and a HFD (WT, 48.5 ± 1.7 g; and KO, 47.8 ± 2.2 g). In addition, TUPS treatment did not significantly alter the body weights of mice fed a regular chow diet (WT, 34.7 ± 1.5 g; KO, 34.2 ± 1.9 g) and a HFD (WT, 47.8 ± 2.5 g; and KO, 48.7 ± 2.1 g). Thus, any alterations in ER stress signaling upon sEH deficiency and inhibition are likely primary effects and not secondary to differences in body weight.
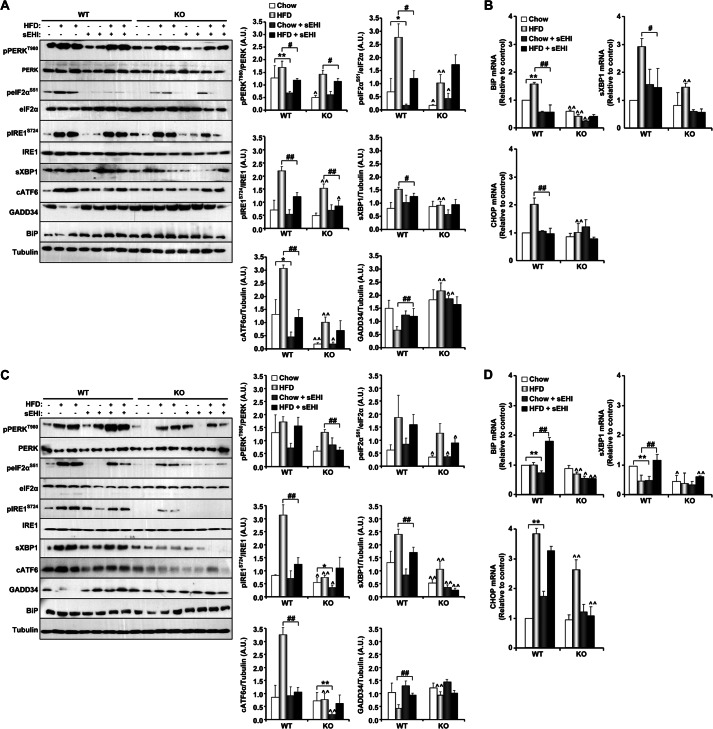
The effects of sEH deficiency and pharmacological inhibition on ER stress signaling were evaluated in the livers and subcutaneous adipose tissue of mice fed a regular chow diet and a HFD. As reported previously (37, 40, 41), prolonged high fat feeding induced ER stress, as evidenced by increased PERK (Thr980), eIF2α (Ser51), and IRE1α (Ser724) phosphorylation and increased sXBP1 and cATF6 expression in liver lysates of WT and KO mice fed a HFD compared with those fed a regular chow diet (Fig. 2A, white versus light gray bars). Expression of GADD34 (an eIF2α phosphatase) was reduced in WT mice fed a HFD compared with those fed chow, in line with published data (42), but was elevated in KO mice, in line with eIF2α phosphorylation (Fig. 2A). Importantly, sEH KO mice exhibited attenuated hepatic ER stress compared with WT mice fed a regular chow diet, and this attenuation was more apparent when mice were fed a HFD (Fig. 2A). Notably, sEH pharmacological inhibition also attenuated hepatic ER stress. sEHI-treated WT mice fed a regular chow diet and a HFD exhibited attenuated ER stress compared with non-treated WT mice (Fig. 2A). In addition, sEHI-treated KO mice exhibited comparable ER stress signaling, for most but not all markers, to non-treated KO mice (Fig. 2A). In line with these findings, hepatic mRNAs from BiP, sXBP1, and CHOP were decreased upon sEH deficiency and inhibition compared with controls, indicating attenuated ER stress (Fig. 2B). Finally, similar to what was observed in liver, sEH deficiency and pharmacological inhibition mitigated HFD-induced ER stress in adipose tissue (Fig. 2, C and D). Collectively, these findings indicate a role for sEH in regulating ER stress in liver and adipose tissue.
FIGURE 2.
sEH deficiency and pharmacological inhibition mitigate HFD-induced ER stress in vivo. sEH KO and WT mice were fed a regular chow diet or a HFD for 10 months as described under “Experimental Procedures.” In addition, KO and WT mice were treated with a sEHI (TUPS) or vehicle control (PEG 400) in drinking water for 10 months. Lysates from liver (A) and subcutaneous adipose tissue (C) were immunoblotted for phospho-PERK (Thr980), PERK, phospho-eIF2α (Ser51), eIF2α, phospho-IRE1α (Ser724), IRE1α, sXBP1, cATF6α, GADD34, BiP, and tubulin. Each lane represents a sample from a separate animal. The bar graphs represent normalized data expressed as arbitrary units (A.U.) for phospho-PERK/PERK, phospho-eIF2α/eIF2α, phospho-IRE1α/IRE1α, sXBP1/tubulin, ATF6α/tubulin, and GADD34/tubulin from three independent experiments and presented as means ± S.E. BiP, CHOP, and sXBP1 mRNAs from liver (B) and subcutaneous adipose tissue (D) were measured by quantitative real-time PCR and normalized against TATA box-binding protein. Data represent means ± S.E. of six mice. *, p ≤ 0.05, significant difference between sEHI-treated and non-treated mice fed a regular chow diet; **, p ≤ 0.01; #, significant difference between sEHI-treated and non-treated mice fed a HFD diet; ∧, significant difference between KO and WT mice fed the same diet.
sEH Regulates ER Stress Signaling in Vitro
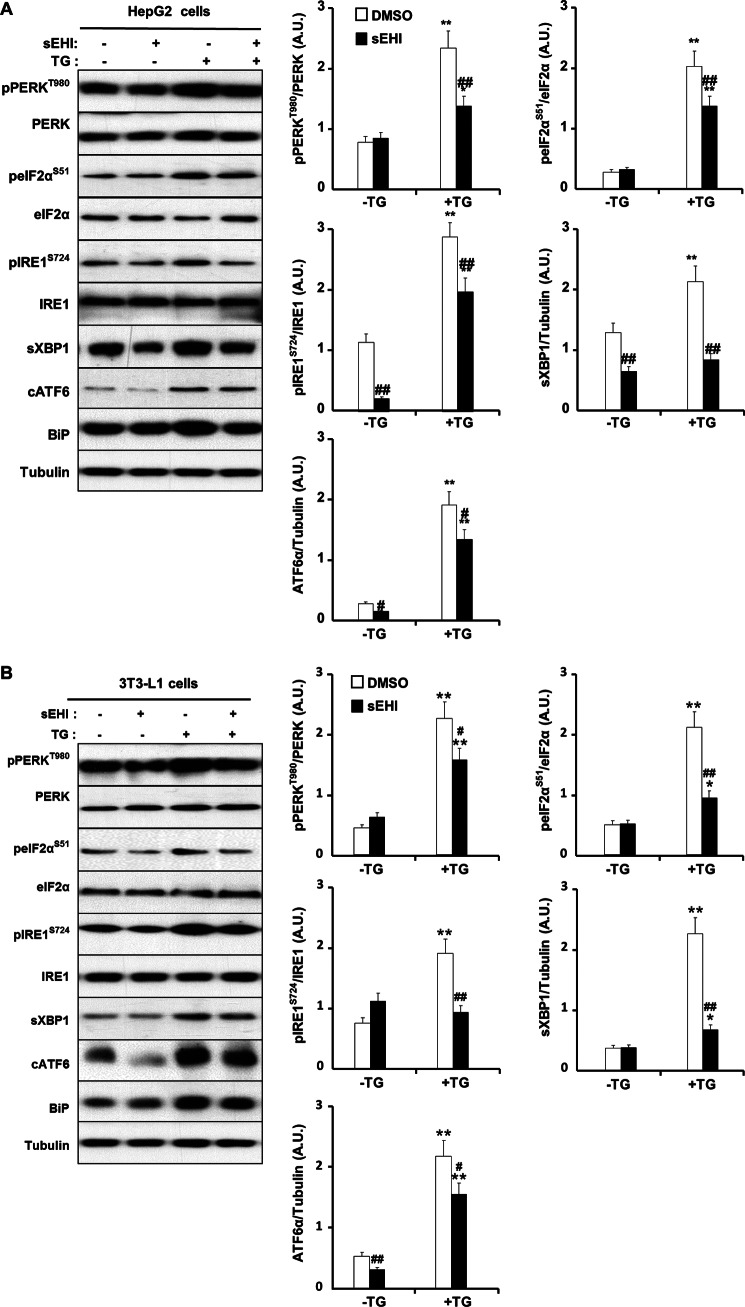
To establish whether the observed effects of sEH on ER stress in vivo were cell-autonomous, we determined the effects of sEH inhibition on ER stress in HepG2 cells and 3T3-L1 adipocytes. Cells were treated with the sEHI TUPS, and then ER stress was induced with TG as described previously (43, 44). Under basal conditions (without TG), sEH inhibition led to mild attenuation of several markers of ER stress in both cell lines (Fig. 3, A and B). As expected, TG treatment led to a significant increase in the three subarms of ER stress signaling. Importantly, sEH inhibition led to significant attenuation of TG-induced ER stress signaling in both cell lines (Fig. 3, A and B). Similarly, inhibition of sEH using another inhibitor, 3,4,4′-trichlorocarbanilide (an antimicrobial agent that is a potent inhibitor of recombinant murine and human sEH in vitro) (33, 45), also attenuated TG-induced ER stress (data not shown). This indicates that the effects of sEH inhibition on TG-induced ER stress are not unique to a specific inhibitor. Collectively, these findings demonstrate that pharmacological inhibition of sEH attenuates chemical-induced ER stress in a cell-autonomous manner.
FIGURE 3.
sEH pharmacological inhibition mitigates chemical-induced ER stress in vitro. HepG2 cells (A) and differentiated 3T3-L1 adipocytes (B) were pretreated with a sEHI (TUPS) or vehicle control (dimethyl sulfoxide (DMSO)) for 1 h. ER stress was induced by treating cells with TG (2 μm) for 2 h. Lysates were immunoblotted for phospho-PERK (Thr980), PERK, phospho-eIF2α (Ser51), eIF2α, phospho-IRE1α (Ser724), IRE1α, sXBP1, cATF6α, BiP, and tubulin. The bar graphs represent normalized data expressed as arbitrary units (A.U.) for phospho-PERK/PERK, phospho-eIF2α/eIF2α, phospho-IRE1α/IRE1α, sXBP1/tubulin, and ATF6α/tubulin from three independent experiments and presented as means ± S.E. *, p ≤ 0.05, significant difference between TG-treated and non-treated cells; **, p ≤ 0.01; #, significant difference between sEHI-treated and non-treated cells; ##, p ≤ 0.01.
sEH Inhibition Mitigates ER Stress-mediated Inflammation and Apoptosis
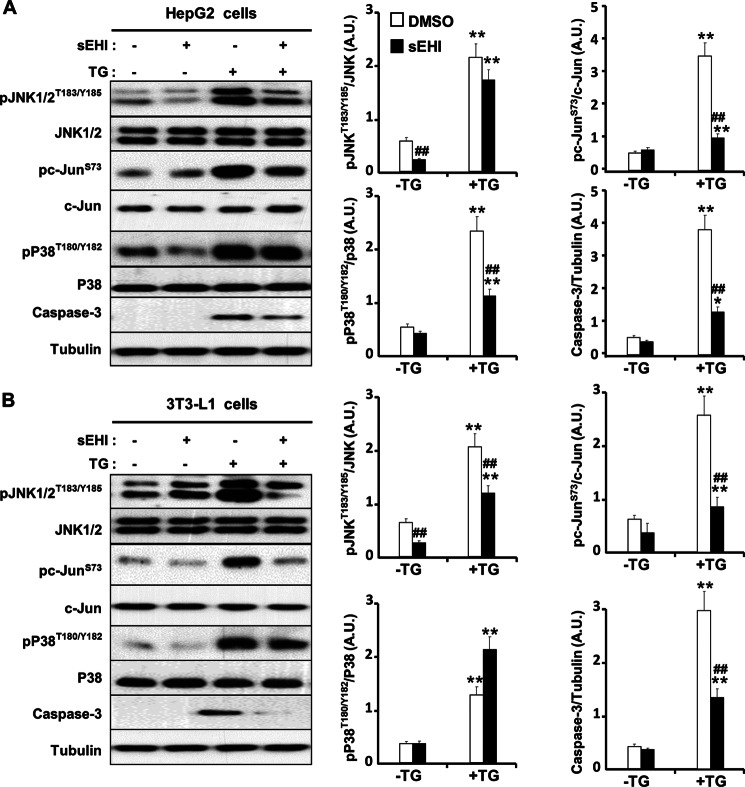
ER stress is deployed by hepatocytes and adipocytes to cope with nutrient-induced changes in protein synthesis and secretion and to balance newly synthesized protein load against the ER folding capacity. However, excessive ER stress has been linked to inflammatory responses and eventually cell death (46). We investigated the effects of sEH pharmacological inhibition on the inflammatory response of HepG2 cells and 3T3-L1 adipocytes after TG-induced ER stress. Consistent with previous reports, TG treatment induced JNK phosphorylation in HepG2 cells (47) and 3T3-L1 adipocytes (48) (Fig. 4, A and B). Importantly, sEH inhibition reduced TG-induced phosphorylation of JNK, its substrate c-Jun, and p38 in both cell lines (Fig. 4, A and B). Caspase-3 is implicated in ER stress-induced cell death (49, 50). After exposure to apoptotic stimuli, cells activate initiator caspases that proteolytically cleave and activate effector caspases (such as caspase-3) to dismantle the dying cell. Accordingly, we determined ER stress-induced expression of caspase-3 in control versus sEHI-treated cells. As expected, TG treatment led to a significant increase in caspase-3 expression (Fig. 4, A and B). However, sEH inhibition significantly decreased TG-induced caspase-3 expression. Together, these results indicate that sEH inhibition can be a protective mechanism against ER stress-induced inflammation and apoptosis.
FIGURE 4.
Modulation of ER stress-induced apoptosis signaling by sEH inhibition in vitro. HepG2 cells (A) and differentiated 3T3-L1 adipocytes (B) were pretreated with a sEHI (TUPS) or vehicle control (dimethyl sulfoxide (DMSO)) for 1 h. ER stress was induced by treating cells with TG (2 μm). Lysates were immunoblotted for phospho-JNK (Thr183/Tyr185), JNK, caspase-3, phospho-c-Jun (Ser73), c-Jun, phospho-p38 (Thr180/Tyr182), p38, and tubulin. The bar graphs represent normalized data expressed as arbitrary units (A.U.) for phospho-JNK/JNK, phospho-c-Jun/Jun, phospho-p38/p38, and caspase-3/tubulin from three independent experiments. *, p ≤ 0.05, significant difference between TG-treated and non-treated cells; **, p ≤ 0.01; ##, significant difference between sEHI-treated and non-treated cells.
EET and Epoxy Fatty Acid Treatment Enhances, whereas Diol Treatment Attenuates Insulin Signaling in Vitro
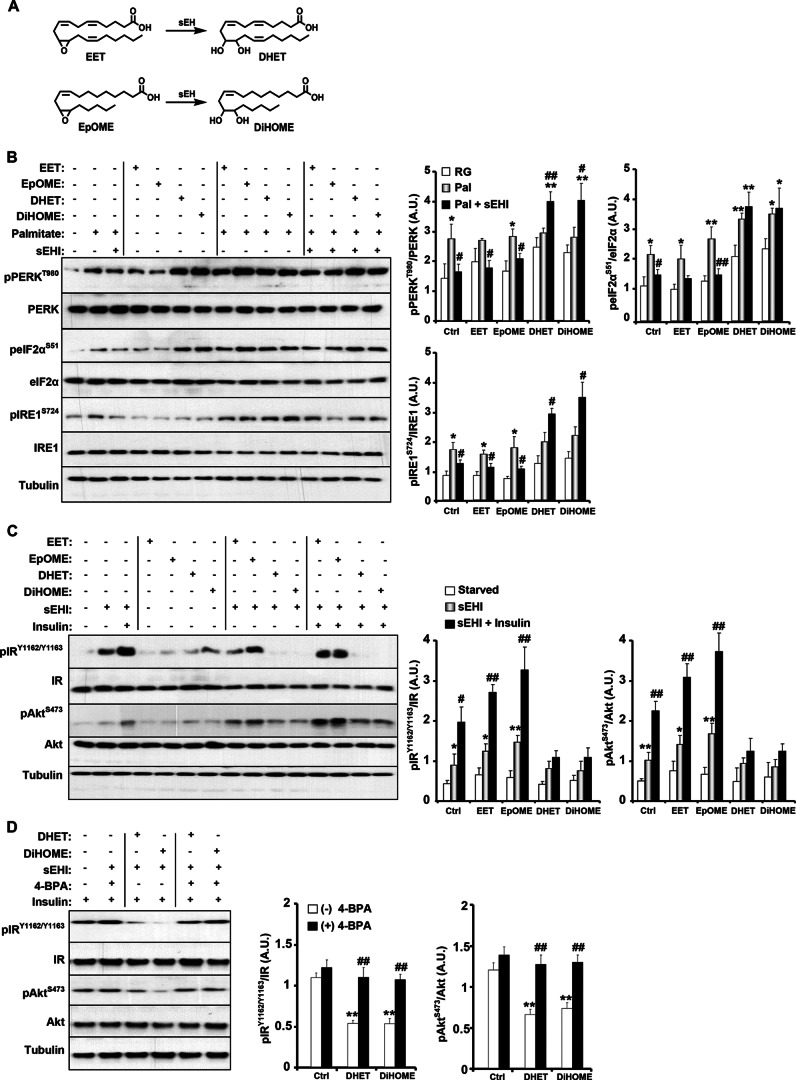
sEH plays a key role in regulating the levels of EETs by degrading them into less potent metabolites, DHETs (24, 25). sEHIs stabilize EETs and other epoxy fatty acids, such as EpOMEs from linoleic acid, by preventing their conversion to DHETs or the corresponding diols (Fig. 5A) (26). To gain insight into the molecular mechanisms underlying sEH action, we determined the effects of treatment with EET and EpOME and their corresponding diols (DHET and DiHOME, respectively) on chemical-induced ER stress in HepG2 cells. As expected, sEH inhibition attenuated palmitate-induced ER stress, as evidenced by decreased PERK, eIF2α, and IRE1α phosphorylation to levels comparable with basal levels (Fig. 5B, control (Ctrl)). EET and EpOME treatment did not further attenuate palmitate-induced ER stress and exhibited comparable effects on ER stress signaling to control cells. On the other hand, DHET and DiHOME treatment increased ER stress and counteracted the effects of sEH inhibition (Fig. 5B).
FIGURE 5.
Epoxy fatty acid treatment enhances and diol treatment attenuates insulin signaling in vitro. A, chemical structure of sEH substrates (EET and EpOME) and their conversion to the corresponding diols (DHET and DiHOME) by sEH. B, HepG2 cells were pretreated with a sEHI (TUPS) alone or in combination with EET, EpOME, DHET, or DiHOME (1 μm each) for 1 h, and then ER stress was induced by treating cells with palmitate (0.5 mm) for 24 h. Lysates were immunoblotted for phospho-PERK (Thr980), PERK, phospho-eIF2α (Ser51), eIF2α, phospho-IRE1α (Ser724), IRE1α, and tubulin. The bar graphs represent normalized data expressed as arbitrary units (A.U.) for phospho-PERK/PERK, phospho-eIF2α/eIF2α, and phospho-IRE1α/IRE1α from six independent experiments and presented as means ± S.E. *, p ≤ 0.05, significant difference between palmitate-treated and non-treated randomly growing (RG) cells; **, p ≤ 0.01; #, significant difference between palmitate (Pal)-treated and palmitate/sEHI (Pal + sEHI)-treated cells. Ctrl, control. C, HepG2 cells were starved overnight in medium containing sEHI in combination with EET, EpOME, DHET, or DiHOME and then stimulated with insulin for 10 min. Lysates were immunoblotted for phospho-IR (Tyr1162/Tyr1163), IR, phospho-AKT (Ser473), and AKT. The bar graphs represent normalized data expressed as arbitrary units for phospho-IR/IR and phospho-AKT/AKT from six independent experiments and presented as means ± S.E. *, p ≤ 0.05, significant difference between sEHI-treated and non-treated (starved) cells; #, significant difference between sEHI-treated and sEHI/insulin-treated cells. D, HepG2 cells were starved overnight in medium containing sEHI in combination with DHET or DiHOME and then stimulated with insulin for 10 min. Where indicated, 4-phenylbutyric acid (4-BPA; 20 mm) was added. Lysates were immunoblotted for phospho-IR (Tyr1162/Tyr1163), IR, phospho-AKT (Ser473), and AKT. The bar graphs represent normalized data expressed as arbitrary units for phospho-IR/IR, and phospho-AKT/AKT from five independent experiments and presented as means ± S.E. *, p ≤ 0.05, significant difference between control and DHET/DiHOME-treated cells without 4-phenylbutyric acid; #, significant difference between 4-phenylbutyric acid-treated and non-treated cells.
We reported previously that sEH deficiency and pharmacological inhibition in vivo enhance insulin signaling in liver and adipose tissue (32). To investigate the molecular mechanisms underlying regulation of insulin signaling by sEH inhibition, we determined the effects of treatment with the substrates (EET and EpOME) and products (DHET and DiHOME) on insulin signaling in HepG2 cells. In line with our previous report (32), sEH inhibition enhanced basal and, more robustly, insulin-stimulated IR and AKT phosphorylation (Fig. 5C, control (Ctrl)). Importantly, EET and EpOME treatment enhanced IR and AKT phosphorylation, whereas DHET and DiHOME treatment significantly attenuated insulin signaling (Fig. 5C). Remarkably, mitigation of ER stress using the chemical chaperone 4-phenylbutyric acid (51) enhanced the response of DHET- and DiHOME-treated cells to insulin (Fig. 5D). Taken together, these findings implicate sEH activity in the regulation of ER stress and insulin signaling.
DISCUSSION
The role of sEH in modulating ER stress and the physiological significance have heretofore remained largely unexplored. In this study, we demonstrated that HFD- and chemical-induced ER stress increased sEH expression in vivo and in vitro, respectively. Importantly, sEH deficiency or pharmacological inhibition attenuated HFD-induced ER stress in liver and adipose tissue and chemical-induced ER stress in cells. Moreover, epoxy fatty acid treatment enhanced and diol treatment attenuated insulin signaling in vitro. Together, these findings demonstrate that sEH is a physiologically relevant regulator of ER stress and a potential therapeutic target for mitigating complications associated with the metabolic syndrome.
HFD- and chemical-induced ER stress increased sEH expression. Chronic high fat feeding significantly increased hepatic and adipose sEH protein expression in mice. These findings appear to translate to humans because overweight subjects exhibited increased adipose sEH expression. However, it is not clear if the increased sEH expression in overweight/obese rodents and humans can be directly attributed to obesity or other factor(s) such as an elevated proinflammatory response to a HFD. In addition, the regulatory point(s) for ER stress-induced sEH protein expression remains to be determined and could be due to increased expression and/or pre-translational alterations. The current findings are in line with a previous study that reported increased hepatic sEH expression in mice fed a HFD (60% kcal from fat) for 16 weeks (52). On the other hand, another study indicates that high fat feeding (for 20 weeks) does not alter adipose sEH expression (but elevates adipose sEH activity) (30). The discrepancy in adipose sEH expression could be due to differences in HFDs (42% kcal from fat versus 60% kcal from fat used in this study) and/or duration of the challenge. Of note, acute exposure to a hypolipidemic drug, the peroxisome proliferator clofibrate, leads to increased hepatic sEH expression and activity in mice (53, 54). The effects of clofibrate-induced increases in sEH on ER stress remain to be determined and warrant additional investigation. Nevertheless, the increased expression of sEH after prolonged high fat feeding raises the possibility that hepatic and adipose sEH inhibition might mitigate chronic ER stress.
sEH deficiency or pharmacological inhibition attenuated HFD-induced ER stress in liver and adipose tissue and chemical-induced ER stress in cells. The three subarms of ER stress signaling (PERK, IRE1α, and ATF6) were affected by sEH inhibition. Importantly, in vivo studies demonstrated that the effects of sEH inhibition on ER stress were primary and not secondary to body weight differences because KO and sEHI-treated mice exhibited comparable body weights to control mice. In addition, in vitro studies established that the effects of sEH inhibition on ER stress were cell-autonomous. Moreover, inhibition of sEH hydrolase activity using two inhibitors (TUPS and 3,4,4′-trichlorocarbanilide) yielded comparable effects on ER stress, indicating that they were not unique to one inhibitor. Collectively, genetic and pharmacological studies clearly demonstrate that sEH modulates ER stress in a cell-autonomous manner.
These studies provided new insights into the molecular mechanism underlying regulation of ER stress by sEH. Hydrolysis of epoxygenated fatty acids is regulated by sEH, which degrades them into less potent metabolites. sEHIs stabilize EETs and other epoxy fatty acids by preventing their conversion to DHETs or the corresponding diols. Therefore, the sEHI-induced increase in EETs and other epoxy fatty acids and/or decrease in the corresponding diols will presumably attenuate ER stress. However, treatment of HepG2 cells with EET/EpOME failed to attenuate ER stress (Fig. 5). The underlying reason(s) is not known and could be due to rapid degradation of the added EET and EpOME, and additional studies examining various concentrations and treatment periods are warranted. Notably, DHET and DiHOME treatment increased ER stress and counteracted the effects of sEH inhibition (Fig. 5). Thus, the observed increase in sEH expression in liver and adipose tissue after chronic HFD feeding (Fig. 1) will likely increase the concentration of diols and contribute to elevated ER stress.
ER dysfunction is a contributor to chronic metabolic deterioration, and sEH inhibition-mediated attenuation of ER stress likely contributes to a favorable metabolic status, including, but not limited to, enhanced insulin signaling. Chemical alleviation of ER stress improves insulin sensitivity in animal models (55, 56) and, more importantly, in obese humans (57, 58). The mechanism by which ER stress impairs insulin action is complex and likely involves integration of ER stress response and inflammatory signaling cascades (4, 59, 60). We reported previously that sEH deficiency and inhibition lead to increased insulin signaling in liver and adipose tissue (32). In line with these observations, sEH inhibition enhanced basal and insulin-stimulated signaling in HepG2 cells. In addition, cells treated with epoxy fatty acids exhibited increased insulin signaling. Whether this is due to decreased ER stress and/or other mechanism(s) such as attenuation of inflammatory signaling remains to be determined. Conversely, cells treated with diols exhibited blunted insulin signaling that could be mitigated by the attenuation of ER stress using a chemical chaperone. Together, these findings implicate sEH activity in regulation of insulin signaling. It is important to note that sEH-mediated regulation of ER stress can modulate glucose and lipid metabolism independent of insulin action. For example, XBP1 suppresses hepatic gluconeogenesis through interaction with FOXO1 (61), and hepatic Xbp1 deficiency prevents steatosis (62). sEH inhibition attenuates HFD-induced hepatic steatosis due to a reduced inflammatory state (52); however, mitigation of ER stress cannot be excluded as a contributing factor and warrants additional investigation. Finally, ER stress has been proposed as a regulator of adipogenesis, although its precise function has not been fully elucidated (63, 64). Thus, it is reasonable to hypothesize that attenuation of ER stress by sEH inhibition in adipocytes affects adipogenesis. Indeed, treatment of adipocyte stem cells with sEHI suppresses differentiation (65, 66).
In summary, in this study, we have identified sEH as a regulator of ER stress and provided new insights into the underlying molecular mechanism and metabolic implications. A better understanding of sEH function may provide new therapeutic targets for mitigating complications associated with obesity and metabolic syndrome.
Acknowledgments
We thank members of the Haj and Hammock laboratories for technical help.
This work was supported, in whole or in part, by National Institutes of Health Grants R56 DK084317 and R01 DK090492 (to F. G. H.) and by National Institutes of Health Grant ES02710 and Superfund Basic Research Program Grant P42 ES04699 from NIEHS and Grant HL059699 from NHLBI (to B. D. H.). This work was also supported by Juvenile Diabetes Research Foundation Research Grant 1-2009-337 (to F. G. H.).
- ER
- endoplasmic reticulum
- PERK
- PKR-like ER-regulated kinase
- IRE1α
- inositol-requiring enzyme 1α
- cATF6α
- cleaved activating transcription factor 6α
- eIF2α
- eukaryotic translation initiation factor 2α
- XBP1
- X-box-binding protein 1
- sEH
- soluble epoxide hydrolase
- EET
- epoxyeicosatrienoic acid
- DHET
- dihydroxyeicosatrienoic acid
- sEHI
- sEH-selective inhibitor
- HFD
- high fat diet
- sXBP1
- spliced XBP1
- IR
- insulin receptor
- EpOME
- epoxyoctadecenoic acid
- DiHOME
- dihydroxyoctadecenoic acid
- KO
- knock-out
- TUPS
- 1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluoromethoxy)phenyl)urea
- TG
- thapsigargin.
REFERENCES
- 1. Friedman J. M. (2000) Obesity in the new millennium. Nature 404, 632–634 [DOI] [PubMed] [Google Scholar]
- 2. Spiegelman B. M., Flier J. S. (2001) Obesity and the regulation of energy balance. Cell 104, 531–543 [DOI] [PubMed] [Google Scholar]
- 3. Mensah G. A., Mokdad A. H., Ford E., Narayan K. M., Giles W. H., Vinicor F., Deedwania P. C. (2004) Obesity, metabolic syndrome, and type 2 diabetes: emerging epidemics and their cardiovascular implications. Cardiol. Clin. 22, 485–504 [DOI] [PubMed] [Google Scholar]
- 4. Ozcan U., Cao Q., Yilmaz E., Lee A. H., Iwakoshi N. N., Ozdelen E., Tuncman G., Görgün C., Glimcher L. H., Hotamisligil G. S. (2004) Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science 306, 457–461 [DOI] [PubMed] [Google Scholar]
- 5. Hummasti S., Hotamisligil G. S. (2010) Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ. Res. 107, 579–591 [DOI] [PubMed] [Google Scholar]
- 6. Schröder M., Kaufman R. J. (2005) The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 [DOI] [PubMed] [Google Scholar]
- 7. Sharma N. K., Das S. K., Mondal A. K., Hackney O. G., Chu W. S., Kern P. A., Rasouli N., Spencer H. J., Yao-Borengasser A., Elbein S. C. (2008) Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J. Clin. Endocrinol. Metab. 93, 4532–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ron D., Walter P. (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 8, 519–529 [DOI] [PubMed] [Google Scholar]
- 9. Hotamisligil G. S. (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shi Y., Vattem K. M., Sood R., An J., Liang J., Stramm L., Wek R. C. (1998) Identification and characterization of pancreatic eukaryotic initiation factor 2 α-subunit kinase, PEK, involved in translational control. Mol. Cell. Biol. 18, 7499–7509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harding H. P., Zhang Y., Ron D. (1999) Protein translation and folding are coupled by an endoplasmic reticulum-resident kinase. Nature 397, 271–274 [DOI] [PubMed] [Google Scholar]
- 12. Novoa I., Zhang Y., Zeng H., Jungreis R., Harding H. P., Ron D. (2003) Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 22, 1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sidrauski C., Walter P. (1997) The transmembrane kinase Ire1p is a site-specific endonuclease that initiates mRNA splicing in the unfolded protein response. Cell 90, 1031–1039 [DOI] [PubMed] [Google Scholar]
- 14. Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107, 881–891 [DOI] [PubMed] [Google Scholar]
- 15. Calfon M., Zeng H., Urano F., Till J. H., Hubbard S. R., Harding H. P., Clark S. G., Ron D. (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415, 92–96 [DOI] [PubMed] [Google Scholar]
- 16. Ye J., Rawson R. B., Komuro R., Chen X., Davé U. P., Prywes R., Brown M. S., Goldstein J. L. (2000) ER stress induces cleavage of membrane-bound ATF6 by the same proteases that process SREBPs. Mol. Cell 6, 1355–1364 [DOI] [PubMed] [Google Scholar]
- 17. Okada T., Yoshida H., Akazawa R., Negishi M., Mori K. (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem. J. 366, 585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zinszner H., Kuroda M., Wang X., Batchvarova N., Lightfoot R. T., Remotti H., Stevens J. L., Ron D. (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev. 12, 982–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishitoh H., Matsuzawa A., Tobiume K., Saegusa K., Takeda K., Inoue K., Hori S., Kakizuka A., Ichijo H. (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 16, 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gill S. S., Hammock B. D. (1980) Distribution and properties of a mammalian soluble epoxide hydrase. Biochem. Pharmacol. 29, 389–395 [DOI] [PubMed] [Google Scholar]
- 21. Enayetallah A. E., French R. A., Thibodeau M. S., Grant D. F. (2004) Distribution of soluble epoxide hydrolase and of cytochrome P450 2C8, 2C9, and 2J2 in human tissues. J. Histochem. Cytochem. 52, 447–454 [DOI] [PubMed] [Google Scholar]
- 22. Inceoglu B., Schmelzer K. R., Morisseau C., Jinks S. L., Hammock B. D. (2007) Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs). Prostaglandins Other Lipid Mediat. 82, 42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Imig J. D., Hammock B. D. (2009) Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat. Rev. Drug Discov. 8, 794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Spector A. A., Norris A. W. (2007) Action of epoxyeicosatrienoic acids on cellular function. Am. J. Physiol. Cell Physiol 292, C996–C1012 [DOI] [PubMed] [Google Scholar]
- 25. Newman J. W., Morisseau C., Hammock B. D. (2005) Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog. Lipid Res. 44, 1–51 [DOI] [PubMed] [Google Scholar]
- 26. Shen H. C., Hammock B. D. (2012) Discovery of inhibitors of soluble epoxide hydrolase: a target with multiple potential therapeutic indications. J. Med. Chem. 55, 1789–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Node K., Huo Y., Ruan X., Yang B., Spiecker M., Ley K., Zeldin D. C., Liao J. K. (1999) Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 285, 1276–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Imig J. D., Zhao X., Capdevila J. H., Morisseau C., Hammock B. D. (2002) Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension 39, 690–694 [DOI] [PubMed] [Google Scholar]
- 29. Wagner K., Inceoglu B., Gill S. S., Hammock B. D. (2011) Epoxygenated fatty acids and soluble epoxide hydrolase inhibition: novel mediators of pain reduction. J. Agric. Food Chem. 59, 2816–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Taeye B. M., Morisseau C., Coyle J., Covington J. W., Luria A., Yang J., Murphy S. B., Friedman D. B., Hammock B. B., Vaughan D. E. (2010) Expression and regulation of soluble epoxide hydrolase in adipose tissue. Obesity 18, 489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo P., Chang H. H., Zhou Y., Zhang S., Hwang S. H., Morisseau C., Wang C. Y., Inscho E. W., Hammock B. D., Wang M. H. (2010) Inhibition or deletion of soluble epoxide hydrolase prevents hyperglycemia, promotes insulin secretion, and reduces islet apoptosis. J. Pharmacol. Exp. Ther. 334, 430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luria A., Bettaieb A., Xi Y., Shieh G. J., Liu H. C., Inoue H., Tsai H. J., Imig J. D., Haj F. G., Hammock B. D. (2011) Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc. Natl. Acad. Sci. U.S.A. 108, 9038–9043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morisseau C., Goodrow M. H., Dowdy D., Zheng J., Greene J. F., Sanborn J. R., Hammock B. D. (1999) Potent urea and carbamate inhibitors of soluble epoxide hydrolases. Proc. Natl. Acad. Sci. U.S.A. 96, 8849–8854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sinal C. J., Miyata M., Tohkin M., Nagata K., Bend J. R., Gonzalez F. J. (2000) Targeted disruption of soluble epoxide hydrolase reveals a role in blood pressure regulation. J. Biol. Chem. 275, 40504–40510 [DOI] [PubMed] [Google Scholar]
- 35. Kundu S., Roome T., Bhattacharjee A., Carnevale K. A., Yakubenko V. P., Zhang R., Hwang S. H., Hammock B. D., Cathcart M. K. (2013) Metabolic products of soluble epoxide hydrolase are essential for monocyte chemotaxis to MCP-1 in vitro and in vivo. J. Lipid Res. 54, 436–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schmidt-Ott K. M., Yang J., Chen X., Wang H., Paragas N., Mori K., Li J. Y., Lu B., Costantini F., Schiffer M., Bottinger E., Barasch J. (2005) Novel regulators of kidney development from the tips of the ureteric bud. J. Am. Soc. Nephrol. 16, 1993–2002 [DOI] [PubMed] [Google Scholar]
- 37. Nakatani Y., Kaneto H., Kawamori D., Yoshiuchi K., Hatazaki M., Matsuoka T. A., Ozawa K., Ogawa S., Hori M., Yamasaki Y., Matsuhisa M. (2005) Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J. Biol. Chem. 280, 847–851 [DOI] [PubMed] [Google Scholar]
- 38. Jeffrey K. D., Alejandro E. U., Luciani D. S., Kalynyak T. B., Hu X., Li H., Lin Y., Townsend R. R., Polonsky K. S., Johnson J. D. (2008) Carboxypeptidase E mediates palmitate-induced beta-cell ER stress and apoptosis. Proc. Natl. Acad. Sci. U.S.A. 105, 8452–8457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jones P. D., Tsai H. J., Do Z. N., Morisseau C., Hammock B. D. (2006) Synthesis and SAR of conformationally restricted inhibitors of soluble epoxide hydrolase. Bioorg. Med. Chem. Lett. 16, 5212–5216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boden G., Duan X., Homko C., Molina E. J., Song W., Perez O., Cheung P., Merali S. (2008) Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes 57, 2438–2444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Nagata N., Matsuo K., Bettaieb A., Bakke J., Matsuo I., Graham J., Xi Y., Liu S., Tomilov A., Tomilova N., Gray S., Jung D. Y., Ramsey J. J., Kim J. K., Cortopassi G., Havel P. J., Haj F. G. (2012) Hepatic Src homology phosphatase 2 regulates energy balance in mice. Endocrinology 153, 3158–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ye R., Jung D. Y., Jun J. Y., Li J., Luo S., Ko H. J., Kim J. K., Lee A. S. (2010) Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes 59, 6–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xu C., Ma H., Inesi G., Al-Shawi M. K., Toyoshima C. (2004) Specific structural requirements for the inhibitory effect of thapsigargin on the Ca2+-ATPase SERCA. J. Biol. Chem. 279, 17973–17979 [DOI] [PubMed] [Google Scholar]
- 44. Nair S., Xu C., Shen G., Hebbar V., Gopalakrishnan A., Hu R., Jain M. R., Liew C., Chan J. Y., Kong A. N. (2007) Toxicogenomics of endoplasmic reticulum stress inducer tunicamycin in the small intestine and liver of Nrf2 knockout and C57BL/6J mice. Toxicol. Lett. 168, 21–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McElroy N. R., Jurs P. C., Morisseau C., Hammock B. D. (2003) QSAR and classification of murine and human soluble epoxide hydrolase inhibition by urea-like compounds. J. Med. Chem. 46, 1066–1080 [DOI] [PubMed] [Google Scholar]
- 46. Zha B. S., Zhou H. (2012) ER stress and lipid metabolism in adipocytes. Biochem. Res. Int. 2012, 312943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Latreille M., Laberge M. K., Bourret G., Yamani L., Larose L. (2011) Deletion of Nck1 attenuates hepatic ER stress signaling and improves glucose tolerance and insulin signaling in liver of obese mice. Am. J. Physiol. Endocrinol. Metab. 300, E423–E434 [DOI] [PubMed] [Google Scholar]
- 48. Zhou L., Zhang J., Fang Q., Liu M., Liu X., Jia W., Dong L. Q., Liu F. (2009) Autophagy-mediated insulin receptor down-regulation contributes to endoplasmic reticulum stress-induced insulin resistance. Mol. Pharmacol. 76, 596–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang Y., Dong L., Yang X., Shi H., Zhang L. (2011) α-Linolenic acid prevents endoplasmic reticulum stress-mediated apoptosis of stearic acid lipotoxicity on primary rat hepatocytes. Lipids Health Dis. 10, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang K., Wang S., Malhotra J., Hassler J. R., Back S. H., Wang G., Chang L., Xu W., Miao H., Leonardi R., Chen Y. E., Jackowski S., Kaufman R. J. (2011) The unfolded protein response transducer IRE1α prevents ER stress-induced hepatic steatosis. EMBO J. 30, 1357–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Koh I. U., Lim J. H., Joe M. K., Kim W. H., Jung M. H., Yoon J. B., Song J. (2010) AdipoR2 is transcriptionally regulated by ER stress-inducible ATF3 in HepG2 human hepatocyte cells. FEBS J. 277, 2304–2317 [DOI] [PubMed] [Google Scholar]
- 52. Liu Y., Dang H., Li D., Pang W., Hammock B. D., Zhu Y. (2012) Inhibition of soluble epoxide hydrolase attenuates high-fat-diet-induced hepatic steatosis by reduced systemic inflammatory status in mice. PLoS ONE 7, e39165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hammock B. D., Ota K. (1983) Differential induction of cytosolic epoxide hydrolase, microsomal epoxide hydrolase, and glutathione S-transferase activities. Toxicol. Appl. Pharmacol. 71, 254–265 [DOI] [PubMed] [Google Scholar]
- 54. Pinot F., Grant D. F., Spearow J. L., Parker A. G., Hammock B. D. (1995) Differential regulation of soluble epoxide hydrolase by clofibrate and sexual hormones in the liver and kidneys of mice. Biochem. Pharmacol. 50, 501–508 [DOI] [PubMed] [Google Scholar]
- 55. Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R. O., Görgün C. Z., Hotamisligil G. S. (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kammoun H. L., Chabanon H., Hainault I., Luquet S., Magnan C., Koike T., Ferré P., Foufelle F. (2009) GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 119, 1201–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kars M., Yang L., Gregor M. F., Mohammed B. S., Pietka T. A., Finck B. N., Patterson B. W., Horton J. D., Mittendorfer B., Hotamisligil G. S., Klein S. (2010) Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59, 1899–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xiao C., Giacca A., Lewis G. F. (2011) Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and beta-cell dysfunction in humans. Diabetes 60, 918–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nakamura T., Furuhashi M., Li P., Cao H., Tuncman G., Sonenberg N., Gorgun C. Z., Hotamisligil G. S. (2010) Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140, 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sabio G., Davis R. J. (2010) c-Jun NH2-terminal kinase 1 (JNK1): roles in metabolic regulation of insulin resistance. Trends Biochem. Sci. 35, 490–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhou Y., Lee J., Reno C. M., Sun C., Park S. W., Chung J., Lee J., Fisher S. J., White M. F., Biddinger S. B., Ozcan U. (2011) Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nat. Med. 17, 356–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jurczak M. J., Lee A. H., Jornayvaz F. R., Lee H. Y., Birkenfeld A. L., Guigni B. A., Kahn M., Samuel V. T., Glimcher L. H., Shulman G. I. (2012) Dissociation of inositol-requiring enzyme (IRE1α)-mediated c-Jun N-terminal kinase activation from hepatic insulin resistance in conditional X-box-binding protein-1 (XBP1) knock-out mice. J. Biol. Chem. 287, 2558–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sha H., He Y., Chen H., Wang C., Zenno A., Shi H., Yang X., Zhang X., Qi L. (2009) The IRE1α-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 9, 556–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lowe C. E., Dennis R. J., Obi U., O'Rahilly S., Rochford J. J. (2012) Investigating the involvement of the ATF6α pathway of the unfolded protein response in adipogenesis. Int. J. Obes. 36, 1248–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kim D. H., Vanella L., Inoue K., Burgess A., Gotlinger K., Manthati V. L., Koduru S. R., Zeldin D. C., Falck J. R., Schwartzman M. L., Abraham N. G. (2010) Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARγ. Stem Cells Dev. 19, 1863–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burgess A., Vanella L., Bellner L., Schwartzman M. L., Abraham N. G. (2012) Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins Other Lipid Mediat. 97, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]