Abstract
Schizophrenia is associated with extensive neurocognitive and behavioral impairments. Studies indicate that N-acetylaspartate (NAA), a marker of neuronal integrity, and choline, a marker of cell membrane turnover and white matter integrity, may be altered in schizophrenia. Davunetide is a neurotrophic peptide that can enhance cognitive function in animal models of neurodegeneration. Davunetide has recently demonstrated modest functional improvement in a study of people with schizophrenia. In a subset of these subjects, proton magnetic resonance spectroscopy (1H-MRS) was conducted to explore the effects of davunetide on change in NAA/creatine (NAA/Cr) and choline/creatine (choline/Cr) over 12 weeks of treatment. Of 63 outpatients with schizophrenia who received randomized davunetide (5 and 30 mg/day) or placebo in the parent clinical trial, 18 successfully completed 1H-MRS in dorsolateral prefrontal cortex (DLPFC) at baseline and at 12 weeks. Cognition was assessed using the MATRICS Consensus Cognitive Battery (MCCB). NAA/Cr was unchanged for combined high- and low-dose davunetide groups (N=11). NAA/Cr in the high-dose davunetide group (N=8) suggested a trend increase of 8.0% (P=0.072) over placebo (N=7). Choline/Cr for combined high- and low-dose davunetide groups suggested a 6.4% increase (P=0.069), while the high-dose group showed a 7.9% increase (P=0.040) over placebo. Baseline NAA/Cr correlated with the composite MCCB score (R=0.52, P=0.033), as did individual cognitive domains of attention/vigilance, verbal learning, and social cognition; however, neither metabolite correlated with functional capacity. In this exploratory study, 12 weeks of adjunctive davunetide appeared to produce modest increases in NAA/Cr and choline/Cr in DLPFC in people with schizophrenia. This is consistent with a potential neuroprotective mechanism for davunetide. The data also support use of MRS as a useful biomarker of baseline cognitive function in schizophrenia. Future clinical and preclinical studies are needed to fully define the mechanism of action and cognitive effects of davunetide in schizophrenia.
Keywords: proton magnetic resonance spectroscopy, MRS, cognition, neuroprotective, neurotrophic
INTRODUCTION
Cognitive impairment represents a core deficit in patients with schizophrenia. Neuroanatomical findings have emerged that may contribute to this deficit. For example, structural magnetic resonance imaging (MRI) studies have demonstrated reduced gray matter volume across several cortical regions subserving cognitive functions that are impaired in schizophrenia (Wright et al, 2000). Furthermore, longitudinal structural MRI studies indicate that cortical gray matter loss may be progressive, especially in the early stages of psychosis (Gur et al, 1998; Thompson et al, 2001; Van Haren et al, 2008). Consistent with MRI gray matter volume loss, postmortem studies have found reduced cortical neuropil (Rajkowska et al, 1998; Selemon et al, 1995, 1998), reduced dendritic length (Glantz and Lewis, 1997) and reduced dendritic spine density (Black et al, 2004; Garey et al, 1998; Glantz and Lewis, 2000). Increasingly, white matter neuropathology has also been implicated in schizophrenia including reduced glial cell density and reduced myelin-related mRNA expression in several cortical regions (Walterfang et al, 2011), as well as diffusion tensor imaging studies showing altered fractional anisotropy, including in fronto-temporal white matter tracts (Shenton et al, 2010). Taken together, these data provide a rationale for testing the potential cognitive benefits of compounds with neurotrophic properties.
Davunetide is an eight-amino acid peptide representing the biologically active derivative of activity-dependent neuroprotective protein (ADNP). Davunetide demonstrates neuroprotective and neurotrophic activity in several animal models of neurodegeneration (Gozes, 2011). Davunetide has demonstrated cognitive enhancement in a Phase II study in people with mild cognitive impairment and is currently being studied for progressive supranuclear palsy (Gold et al, 2012). To examine its pro-cognitive potential in schizophrenia, a randomized, placebo-controlled study of davunetide was conducted in 63 people with schizophrenia taking stable doses of antipsychotics. Although the MATRICS Consensus Cognitive Battery (MCCB) composite score did not change with treatment, the UCSD Performance-based Skills Assessment (UPSA) composite score—the functional co-primary outcome measure—demonstrated a significant improvement for davunetide over placebo (Javitt et al, 2012).
To provide in vivo insight into the mechanism of action of davunetide in schizophrenia, proton magnetic resonance spectroscopy (1H-MRS) was performed on a subset of subjects in this study. N-acetylaspartate (NAA), a neuron-specific brain metabolite synthesized from aspartic acid and acetyl-coenzyme A, is the most abundant signal in brain 1H-MRS spectra. Although the biological function of NAA remains uncertain, NAA normalized to creatine (Cr) is considered a useful measure of neuronal integrity in various neuropathological conditions (Moffett et al, 2007). For example, NAA/creatine (NAA/Cr) is consistently reduced in classic neurodegenerative disorders such as Alzheimer's disease (Soher et al, 2005) and multiple sclerosis (Sajja et al, 2009). Reduced NAA/Cr can also occur in settings of neuronal atrophy without neuronal loss, such as in a simian model of HIV dementia, in which NAA/Cr was reduced in parallel with synaptophysin, a well-established synaptic marker protein (Lentz et al, 2005). MRS has also been applied to schizophrenia, and many studies have demonstrated 5–10% reductions in NAA/Cr in frontal cortex and hippocampus, confirmed by meta-analysis (Brugger et al, 2011; Steen et al, 2005). Cross-sectional studies have demonstrated a correlation between cognitive deficits and reduced NAA/Cr in schizophrenia (Bertolino et al, 2003; Callicott et al, 2000), suggesting that NAA/Cr may represent a useful biomarker to assess longitudinal change in studies of cognitive enhancing treatments in this patient group.
Choline represents another abundant signal in 1H-MRS spectra. Choline is a primary constituent of cell membranes, especially myelin, and is often considered a marker of cell membrane turnover and white matter integrity (Sajja et al, 2009). Brain choline levels by 1H-MRS have been reported in patients with schizophrenia and although several studies reported elevated choline levels in caudate nucleus compared with controls (Bustillo et al, 2002; Fujimoto et al, 1996), a meta-analysis found no differences in choline levels in frontal cortex between patient and control subjects (Steen et al, 2005).
The current study used 1H-MRS to measure the effects of davunetide compared with placebo on change in NAA/Cr and choline/creatine (choline/Cr) over 12 weeks in dorsolateral prefrontal cortex (DLPFC) in people with schizophrenia. It was hypothesized that davunetide would increase NAA/Cr and choline/Cr.
MATERIALS AND METHODS
Setting and Subjects
The parent study was conducted at seven academic medical centers as part of the National Institute of Mental Health-funded Treatment Units for Research on Neurocognition in Schizophrenia (TURNS) consortium. The parent study was a 12-week, double-blind, parallel group, randomized clinical trial to evaluate the cognitive effects of intranasal davunetide at two doses (5 and 30 mg) or placebo in people with schizophrenia (Javitt et al, 2012). Only subjects who were enrolled in the parent protocol were eligible to participate in the MRS protocol. Subjects were enrolled in the MRS protocol at four TURNS sites (Columbia University, Washington University, Duke University, Harvard University). The study was approved by the local Institutional Review Board at each of the participating sites. Of the 63 subjects in the parent clinical trial, 31 provided informed consent to participate in the MRS protocol. 19 completed both baseline and end-of-study scans.
Inclusion criteria: people aged 18–60 years with a diagnosis of schizophrenia (DSM-IV criteria) who demonstrated clinical stability and were receiving stable doses of one or more second generation antipsychotics and/or a long-acting injectable first generation antipsychotic; Brief Psychiatric Rating Scale (BPRS) hallucinatory and unusual thought content scores ⩽5 and conceptual disorganization score ⩽4, Simpson Angus Scale score ⩽6, and Calgary Depression Rating Scale score ⩽10; Wechsler Test of Adult Reading score ⩾6; capacity to provide written informed consent. Exclusion criteria: treatment with clozapine, diagnosis of alcohol or substance abuse within the last month or alcohol or substance dependence within the last 6 months; history of significant head injury/trauma or significant medical or neurological disease. For the MRS protocol, participants were also excluded for claustrophobia, left handedness and metallic implants or paramagnetic objects in their bodies.
Study Design and Assessments
Eligible subjects entered a 2-week stabilization phase during which baseline neuropsychological ratings (including MCCB, UPSA), ratings of psychopathology, and safety measures were obtained, see Javitt et al. (2012) for details. Subjects who remained stable during the stabilization phase were randomized to low-dose (5 mg) or high-dose (30 mg) intranasal davunetide or placebo (saline solution). For the low-dose group, one intranasal puff was administered daily; for the high-dose group, three puffs were administered twice daily. For the placebo group, half of the subjects were assigned to the low-dose group and half were assigned to the high-dose group, with the number of placebo puffs matching the low and high-dose davunetide groups, respectively.
Subjects received study drug for 12 weeks. MCCB and UPSA were performed at weeks 6 and 12. Ratings of psychopathology and safety measures were obtained biweekly.
MRS Protocol
1H-MRS was performed on 3 T scanners at baseline and at the end of 12 weeks of randomized treatment. An 8 cc MRS voxel was placed in the DLPFC using a protocol developed by Dr Dikoma Shungu (Weill Cornell Medical College, NY, NY) by first acquiring a 3-plane localizer MRI series. Next, 3 oblique MRI localizer series were acquired: an axial/oblique MRI series parallel to the Sylvian fissure, a coronal/oblique localizer MRI series perpendicular to the previous axial/oblique planes, and a sagittal/oblique MRI series. To ensure correct slice prescription, the anterior commissure was located on a coronal/oblique image and the sagittal/oblique slices were oriented parallel to the brain surface at the middle frontal gyrus, forming a ∼45° angle with the inter-hemispheric fissure.
Following acquisition of the three series localizer images, an 8-cc voxel was deposited on the sagittal/oblique series, with the longest direction along the antero-posterior axis (40 mm) and the other two directions measuring 10 × 20 mm2. A screenshot of the voxel placement was captured for each subject for the quality assurance (QA) process.
Data Acquisition
Scanners at the MRS sites: Columbia—Signa 14 × 3.0 T (GE Healthcare, Waukesha, WI); Duke—Signa EXCITE HD 3.0 T (GE); Washington U.—Siemens Trio 3.0 T (Siemens AG, Erlangen, Germany). The Harvard site did not yield usable MRS baseline/week 12 scan-pairs. Pulse sequence for single voxel MRS with PRESS localization was used for data acquisition in all sites with the following parameters: TR/TE=1700/144 ms, spectral width=2000 Hz, number of data points=2048; number of excitation=256; voxel size=8 cc. Water signal was suppressed for metabolite scans. Outer volume suppression bands were placed around the voxel.
Quality Assurance
All sites implemented the protocols using a standard spectroscopic phantom and a human phantom that were sent to each site. The phantom data were then sent to the central site for ascertainment of the pulse sequence parameters, the placement of the voxel and the quality of the spectra (including signal-to-noise ratio (SNR), linewidth, and baseline) to determine inter-site comparability and reliability.
QA scans were conducted at each site using spectroscopic phantoms on a monthly basis throughout the data acquisition period. Two measures were used to represent test–retest reliability, one was the variation of NAA/Cr and the other was the correlation of NAA and Cr. The former was 2.16% and the latter was 0.94. Both showed high test–retest reliability.
Data Processing
All data, including the screenshots for voxel placement, were processed at the central site. Preprocessing procedures entailed a combination of multichannel data, water residue removal by a matrix-pencil-based procedure (Dong et al, 2006), and spectral filtering by a Gaussian function corresponding to a 4-Hz linewidth. As an assessment of the spectral quality, the overall SNRs (mean±SD) of all valid scans were 99.56±19.38 (NAA), 56.82±15.73 (Cr), and 50.05±18.43 (choline). Spectral fitting was performed in the spectral domain with an algorithm that fitted the spectrum with several individual lines with Voigtian lineshape. Restrictions on the linewidths and frequencies were imposed according to a priori knowledge to improve the accuracy of the fitting. The Cramer Rao lower bounds for all fittings of NAA, Cr and Ch were below 20%. Areas of NAA, choline and Cr were measured based on the fitted spectral lines. All data were processed in a blinded manner.
Statistical Analyses
In order to measure the longitudinal effect of treatment, only MRS data for subjects who successfully completed both a baseline and a week 12 scan were included in the analyses. Analyses of change in NAA/Cr and choline/Cr in davunetide compared with placebo groups were performed using exact (permutation) P-values from pairwise Wilcoxon rank sum tests. This test was used given that the neurochemical metabolite data in this small sample could not be assumed to have a normal distribution. Spearman correlations were used to perform correlation analyses between metabolites and cognitive testing scores. Spearman partial correlations were also performed between end-of-study metabolites and cognitive scores, adjusting for differences in baseline metabolites and cognitive scores. Given that this was an exploratory, hypothesis-generating sub-study, outcomes were not corrected for multiple comparisons. Descriptive statistics are presented as mean±SD. All statistical analyses were performed using SAS version 9.2.3 (SAS, Cary, NC).
RESULTS
Baseline Characteristics
Nineteen subjects completed MRS scans at baseline and week 12 (11 subjects at Washington USA, 5 subjects at Columbia, 2 subjects at Duke and 1 subject at Harvard). MRS data for one subject was not usable owing to technical problems (Harvard). Of the remaining 18 subjects, 11 (age: 41.7±9.8 years) received davunetide (8 high dose (30 mg/day), 4 female/4 male; 3 low dose (5 mg/day), 1 female/2 male) and 7 subjects (age: 38.0±9.4, 5 female/2 male) received placebo.
NAA/Cr
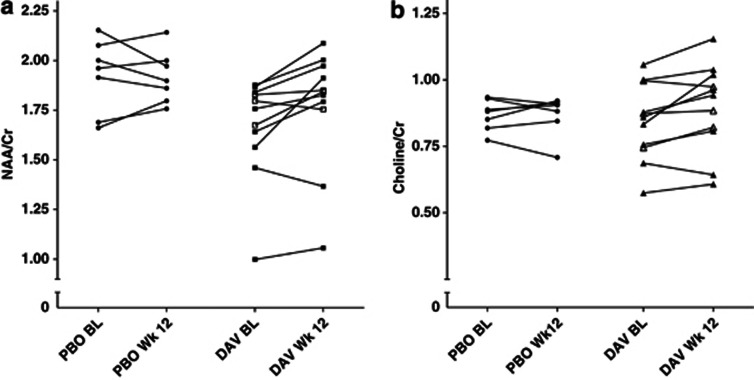
Change in NAA/Cr with davunetide (high- and low-dose groups combined) was not significantly different compared to placebo (P=0.104), see Table 1 and Figure 1a. Analyzing dosing groups separately, davunetide 30 mg/day showed a trend increase in NAA/Cr ratio (8.0% P=0.072) compared with placebo, while davunetide 5 mg/day showed no change (P=0.667).
Table 1. NAA/Cr and Choline/Cr at Baseline and Week 12.
| Arm |
Baseline |
Week 12 |
Week 12—BL |
P | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | mean | SD | N | Mean | SD | Mean | SD | ||
| NAA/Cr | |||||||||
| DAVa | 11 | 1.66 | 0.26 | 11 | 1.77 | 0.30 | 0.11 | 0.12 | 0.104 |
| PBO | 7 | 1.92 | 0.19 | 7 | 1.92 | 0.13 | −0.0 | 0.11 | |
| DAV 30 mg | 8 | 1.63 | 0.29 | 8 | 1.75 | 0.36 | 0.13 | 0.13 | 0.072 |
| DAV 5 mg | 3 | 1.77 | 0.08 | 3 | 1.82 | 0.05 | 0.05 | 0.11 | 0.667 |
| Choline/Cr | |||||||||
| DAVa | 11 | 0.842 | 0.145 | 11 | 0.896 | 0.165 | 0.054 | 0.071 | 0.069 |
| PBO | 7 | 0.868 | 0.058 | 7 | 0.869 | 0.075 | 0.0 | 0.080 | |
| DAV 30 mg | 8 | 0.831 | 0.158 | 8 | 0.897 | 0.194 | 0.066 | 0.066 | 0.040 |
| DAV 5 mg | 3 | 0.872 | 0.126 | 3 | 0.893 | 0.077 | 0.021 | 0.050 | 0.667 |
Abbreviations: BL, baseline; Cr, creatine; DAV, davunetide; NAA, N-acetylaspartate; PBO, placebo.
P=Exact Wilcoxon test P value for differences in neurochemical metabolites for davunetide compared with placebo.
DAV 5 and 30 mg doses combined.
Figure 1.
Before-and-after plot of N-acetylaspartate/creatine (NAA/Cr) and choline/creatine (choline/Cr) across all subjects. (a) Baseline (BL) and week 12 NAA/Cr values for each subject who received placebo (PBO) and davunetide (DAV). Closed squares represent high-dose DAV and open squares represent low-dose DAV. (b) BL and week 12 choline/Cr values for each subject who received PBO and DAV. Closed triangles represent high-dose DAV and open triangles represent low-dose DAV.
Choline/Cr
Davunetide treatment (high- and low-dose groups combined) showed a trend increase in choline/Cr compared with placebo (6.4% P=0.069), see Table 1 and Figure 1b. Analyzed separately, davunetide 30 mg/day was associated with a 7.9% increase in choline/Cr compared with placebo (P=0.040), while davunetide 5 mg/day showed no change (P=0.667).
Correlation between NAA/Cr, choline/Cr and Cognitive Performance
The overall MCCB composite T-score correlated with NAA/Cr at baseline (R=0.52, P=0.033), as did individual domains of attention/vigilance (R=0.69, P=0.002), verbal learning (R=0.64, P=0.004) and social cognition (R=0.48, P=0.042), see Table 2. In contrast, baseline MCCB measures did not correlate with choline/Cr ratios. The baseline UPSA summary score did not correlate with NAA/Cr (R=0.38, P=0.118) or choline/Cr (R=0.15, P=0.556).
Table 2. Spearman Correlations between NAA/Cr and Cognitive/Functional Domains at Baseline.
| N | R | P | |
|---|---|---|---|
| MCCB | |||
| Attention/vigilance | 17 | 0.69 | 0.002 |
| Processing speed | 18 | 0.30 | 0.223 |
| Trails A | 18 | 0.03 | 0.919 |
| BACS symbol coding | 18 | 0.45 | 0.062 |
| Fluency | 18 | 0.26 | 0.302 |
| Reasoning/problem solving | 18 | 0.35 | 0.156 |
| Social cognition | 18 | 0.48 | 0.042 |
| Verbal learning | 18 | 0.64 | 0.004 |
| Visual learning | 18 | 0.24 | 0.335 |
| Working memory | 18 | 0.24 | 0.348 |
| WMS III spatial span | 18 | 0.14 | 0.580 |
| Letter number sequencing | 18 | 0.37 | 0.131 |
| MCCB composite T-score | 17 | 0.52 | 0.033 |
| UPSA summary score | 18 | 0.38 | 0.118 |
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; Cr, creatine; MCCB, MATRICS consensus cognitive battery; NAA, N-acetylaspartate; UPSA, UCSD Performance-based Skills Assessment; WMS III, Wechsler Memory Scale, 3rd edition.
Correlations of change between NAA/Cr and MCCB T-scores over 12 weeks were analyzed in davunetide-treated participants. Neither change in MCCB composite T-score nor individual MCCB domain scores correlated with change in NAA/Cr. Partial correlations between end-of-study NAA/Cr and MCCB T-scores, adjusted for baseline NAA/Cr and MCCB T-scores, were also determined for davunetide-treated patients. A significant negative partial correlation emerged between NAA/Cr and the MCCB working memory battery and the WMS III Spatial Span test, see Table 3. Similar analyses of correlation of change between choline/Cr and MCCB T-scores and partial correlation analyses showed no relationship in davunetide-treated patients (data not shown).
Table 3. Spearman Correlations and Partial Correlations between Change in NAA/Cr and MCCB T-Scores in Davunetide-treated Patients.
| MCCB domain |
Correlations between change in NAA/Cr and MCCB
T-scores |
Partial correlations between change in NAA/Cr and MCCB
T-scores, adjusted for baseline NAA/Cr and
T-scores |
||||
|---|---|---|---|---|---|---|
| N | R | P | N | R | P | |
| Processing speed | 11 | 0.01 | 0.979 | 10 | −0.43 | 0.287 |
| Trails A | 11 | 0.11 | 0.739 | 11 | 0.22 | 0.568 |
| BACS symbol coding | 11 | 0.24 | 0.473 | 11 | 0.10 | 0.801 |
| Fluency | 11 | −0.12 | 0.724 | 11 | 0.04 | 0.915 |
| Reasoning/problem solving | 11 | −0.47 | 0.145 | 11 | −0.05 | 0.905 |
| Social cognition | 11 | −0.25 | 0.465 | 11 | −0.16 | 0.687 |
| Verbal learning | 11 | −0.06 | 0.852 | 11 | −0.17 | 0.653 |
| Visual learning | 11 | −0.29 | 0.384 | 11 | 0.0 | 0.994 |
| Working memory | 11 | −0.38 | 0.244 | 11 | −0.11 | 0.784 |
| WMS III spatial span | 11 | −0.01 | 0.978 | 11 | −0.72 | 0.029 |
| Letter number sequencing | 11 | −0.42 | 0.204 | 11 | −0.69 | 0.038 |
| MCCB composite T-score | 10 | −0.30 | 0.405 | 11 | −0.36 | 0.344 |
Abbreviations: BACS, Brief Assessment of Cognition in Schizophrenia; Cr, creatine; MCCB, MATRICS consensus cognitive battery; NAA, N-acetylaspartate; WMS III, Wechsler Memory Scale, 3rd edition.
In davunetide-treated patients, change in UPSA summary score and change in NAA/Cr showed no correlation (R=−0.26, P=0.43). Similarly, change in UPSA summary score and change in choline/Cr were not correlated (R=−0.25, P=0.45).
DISCUSSION
1H-MRS was used to measure neurochemical metabolites in DLPFC before and after 12 weeks of davunetide treatment in clinically stable patients with schizophrenia. The study suggested a modest increase in both NAA/Cr and choline/Cr with high-dose davunetide compared with placebo, although the change in NAA/Cr remained a statistical trend. Although the exact biological function of NAA remains unclear, low NAA/Cr has been identified as a marker of impaired neuronal function in neuropathological disorders ranging from schizophrenia to Alzheimer's disease (Moffett et al, 2007). The current results showing davunetide-associated increase in NAA/Cr and choline/Cr provides preliminary evidence that davunetide can exert beneficial effects in a brain region previously found to have low NAA/Cr, reduced synaptic content and dendritic atrophy in people with schizophrenia (Glantz and Lewis, 1997, 2000).
Earlier studies in people with schizophrenia have demonstrated correlations between reduced NAA and deficits in cognitive performance, including verbal learning (Ohrmann et al, 2007) and working memory (Bertolino et al, 2003; Callicott et al, 2000). Baseline cognitive performance in this study was consistent with prior studies showing a correlation between NAA/Cr and cognitive performance. To our knowledge, this represents the first use of the MCCB in conjunction with MRS assessment. The MCCB is a frequently applied cognitive testing battery for people with schizophrenia, and the current data support NAA/Cr as a useful biomarker for cognitive dysfunction together with the MCCB.
Although cognitive function correlated with NAA/Cr at baseline, change in NAA/Cr or choline/Cr did not correlate with change in cognitive performance. Furthermore, although the parent trial showed modest functional improvement based on the UPSA score for davunetide 5 mg/day, the UPSA score did not correlate with NAA/Cr or choline/Cr for either the lower or higher davunetide doses. Given the limited statistical power in this exploratory study, the overall lack of correlation between change in neurochemical metabolites and change in cognitive function or change in functional capacity may not be surprising and larger cohorts are needed to test whether such relationships exist.
Furthermore, the duration of treatment that may be required for cognitive enhancement is uncertain. To activate neurotrophic mechanisms and for new or reinforced synaptic connectivity to translate into clinically measurable effects may require considerably longer exposure than 12 weeks in people with schizophrenia. Evidence from the current study of modestly higher NAA/Cr and choline/Cr may reflect activation of neurotrophic mechanisms, but that such effects may only have started to affect synaptic connectivity and broader circuit function. It is possible that davunetide and comparable neurotrophic agents may require considerably longer exposures (possibly up to 1 year or longer) to demonstrate robust functional improvements in people with schizophrenia.
Davunetide is an eight-amino acid chain peptide contained within ADNP. ADNP is an essential protein for normal brain development (Pinhasov et al, 2003). Preclinical studies have demonstrated that davunetide can promote neurite outgrowth and synaptogenesis (Smith-Swintosky et al, 2005). Animal studies suggest a potential therapeutic role for davunetide through its ability to stabilize microtubules and rescue cognitive deficits in several models of neurodegeneration (Merenlender-Wagner et al, 2010; Vulih-Shultzman et al, 2007). Although speculative, the evidence for a modest davunetide-associated increase in NAA/Cr in the high-dose group is consistent with a neurotrophic mechanism of action. A similar argument can be made for the small but significant increase in choline/Cr in the high-dose davunetide group. Choline is often considered a marker of membrane phospholipid turnover. In Alzheimer's disease, studies consistently find elevated choline/Cr together with low NAA/Cr, which is thought to reflect active membrane phospholipid breakdown associated with neuronal cell loss (Soher et al, 2005). Similarly, reports indicate that choline/Cr is elevated and NAA/Cr is reduced during multiple sclerosis lesion exacerbation, likely reflecting active demyelination/remyelination (Sajja et al, 2009). In classic neurodegenerative disorders, elevated choline/Cr and low NAA/Cr co-occur during clinical worsening. This is in contrast to the current study where both markers increase in tandem following davunetide treatment, together with at least some evidence for functional improvement. As the pathophysiology of schizophrenia is not thought to be associated with neuronal cell loss or demyelination, nor was there any evidence of physical or psychiatric deterioration associated with davunetide treatment in the current study, a plausible interpretation of the modest parallel increase in choline/Cr and NAA/Cr is that these represent davunetide-mediated neurotrophic/neuroprotective effects. Further insight into this issue may be gained preclinically by probing a davunetide-treated animal model with high-field MRS and postmortem quantitative histochemistry.
The current study has several limitations. First, the sample size was small and statistical power was therefore limited to demonstrating changes with large effect sizes. Second, the treatment duration may have been too short to demonstrate a measurable response in cognitive and functional outcomes from a drug that may require longer brain exposure to produce structural neuronal changes. Third, the voxel placed in the DLPFC encompassed both gray and white matter and the relative contributions from each compartment could not be assessed. Assessing changes in brain metabolites in gray and white matter separately would represent an important focus for future studies. Fourth, antipsychotic treatment was not standardized for enrolled subjects. Several studies have suggested that antipsychotic treatment or withdrawal of treatment may raise or reduce cortical NAA levels, respectively (Bertolino et al, 2001; Ertugrul et al, 2009). To limit potential antipsychotic treatment confounds, only patients taking stable antipsychotic drug regimens were enrolled. Furthermore, a 6-month longitudinal MRS study in haloperidol-treated rats found no effect of antipsychotic treatment on NAA or choline in cortical brain regions (Bustillo et al, 2006).
In summary, 12 weeks of davunetide was associated with a modest increase in choline/Cr and a suggestive increase in NAA/Cr in DLPFC in clinically stable people with schizophrenia taking antipsychotic medication. These data provide preliminary evidence that davunetide can exert neurotrophic effects in people with schizophrenia in a brain region with known dendritic and synaptic deficits. The study also supports prior reports that NAA represents a useful biomarker of baseline cognitive function in this population. Future studies with larger sample sizes and longer treatment exposure are needed to better establish the potential benefits of davunetide in schizophrenia.
Acknowledgments
This study was funded by a NARSAD Distinguished Investigator Award (Lieberman), National Institute of Mental Health contract HHSN278200441003C (Marder), and Allon Therapeutics, Inc. Double-blind medications were provided by Allon Therapeutics, Inc. The sponsors had no role in study design, data collection, and data analysis, or in manuscript preparation, revision, and final approval. The authors wish to acknowledge Dr Dikoma Shungu (Weill Cornell Medical College, NY, NY) for his assistance with the protocol for voxel placement. This work was presented in part at the 13th International Congress on Schizophrenia Research, Colorado Springs, CO 04/04/2011. Clinicaltrials.gov registry no. NCT00505765. The trial was conducted under a FDA IND and oversight provided by a NIMH Drug Safety and Monitoring Board.
During the past 3 years, the authors declare the following financial disclosures. Dr Jarskog has received research funding from GlaxoSmithKline, Novartis, Sunovion and Genentech and has served as a DSMB member for Janssen. Dr Girgis has received research funding from Eli Lilly. Dr Kegeles has received research funding from Pfizer and Amgen. Dr Barch has received research funding from National Institute of Mental Health, Allon and Novartis and has served as a consultant for Pfizer. Dr Buchanan has served on advisory boards for Abbott, Amgen, Astellas, Janssen Pharmaceuticals Inc., Merck, NuPathe, Pfizer, Roche, Solvay Pharmaceuticals, Inc. and Takeda; he has served as a consultant for Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb, Cypress Bioscience, EnVivo, GlaxoSmithKline, Pfizer, and Takeda; he serves as a DSMB member for Pfizer, Cephalon and Otsuka. Dr Csernansky has served as a DSMB member for Eli Lilly and Sanofi-Aventis. Dr Goff has received research funding from Janssen, Pfizer, GlaxoSmithKline, PamLab and Novartis; he has served as a consultant to Eli Lilly, Bristol-Myers Squibb, Roche, Endo Pharmaceuticals, Genentech, Cypress Bioscience, Dainippon Sumitomo, Solvay, Biovail, and Takeda Pharmaceuticals; he has served as a DSMB member for Otsuka; he has applied for patents regarding genetic predictors of response to glutamatergic agents and folate. Dr Javitt has received research funding from Jazz, Pfizer, Roche; he has served as a consultant for NPS, Solvay, Sepracor, AstraZeneca, Pfizer, Cypress, Merck, Sunovion, Bristol-Myers Squibb, Eli Lilly, and Takeda; he has served on advisory boards for Promentis; he has equity in Glytech and AASI. Dr Keefe has received research funding from the Department of Veterans Affairs, GlaxoSmithKline, National Institute of Mental Health, Novartis, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council; he has received honoraria, served as a consultant, or advisory board member for Abbott, Amgen, Astellas, Asubio, BiolineRx, Boehringer-Ingelheim, BrainCells, Bristol-Myers Squibb, Eli Lilly, EnVivo, Helicon, Lundbeck, Merck, Mitsubishi, Novartis, Otsuka, Pfizer, Roche, Sanofi-Aventis, Shire, Solvay, Sunovion, Takeda, Targacept and Wyeth; he receives royalties from the BACS testing battery and the MATRICS Battery (BACS Symbol Coding); he is a shareholder in NeuroCog Trials, Inc.; Duke University holds the copyright for the SCoRS, and licenses are issued by NeuroCog Trials, Inc., however, there is currently no license fee to use the SCoRS. Dr McEvoy has received research grants from Roche/Genentech, Psychogenics, Merck and has received honoraria for speaking from Eli Lilly, Merck and Sunovion. Dr McMahon has served as a consultant for Amgen. Dr Marder has received research funding from Allon, GlaxoSmithKline, Novartis, Sunovion, and Psychogenics; he has served as a consultant for Amgen, Abbott, Astellas, Otsuka, Pfizer, Roche, Genentech, Lundbeck, Shire and Targacept. Dr Lieberman does not receive direct financial compensation or salary support for participation in research, consulting, or advisory board activities. He serves or has served on advisory boards for Alkermes, Bioline, Intracellular Therapies, Pierre Fabre and PsychoGenics. He receives grant support from Allon, F. Hoffman-La Roche LTD, GlaxoSmithKline, Eli Lilly, Merck, Novartis, Pfizer, Psychogenics, Sunovion and Targacept; and he holds a patent from Repligen. Drs Peterson, Kangarlu, Dong, Colibazzi and Harms declare no conflict of interest.
References
- Bertolino A, Callicott JH, Mattay VS, Weidenhammer KM, Rakow R, Egan MF, et al. The effect of treatment with antipsychotic drugs on brain N-acetylaspartate measures in patients with schizophrenia. Biol Psychiatry. 2001;49:39–46. doi: 10.1016/s0006-3223(00)00997-5. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Sciota D, Brudaglio F, Altamura M, Blasi G, Bellomo A, et al. Working memory deficits and levels of N-acetylaspartate in patients with schizophreniform disorder. Am J Psychiatry. 2003;160:483–489. doi: 10.1176/appi.ajp.160.3.483. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, et al. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Brugger S, Davis JM, Leucht S, Stone JM. Proton magnetic resonance spectroscopy and illness stage in schizophrenia--a systematic review and meta-analysis. Biol Psychiatry. 2011;69:495–503. doi: 10.1016/j.biopsych.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Bustillo J, Barrow R, Paz R, Tang J, Seraji-Bozorgzad N, Moore GJ, et al. Long-term treatment of rats with haloperidol: lack of an effect on brain N-acetyl aspartate levels. Neuropsychopharmacology. 2006;31:751–756. doi: 10.1038/sj.npp.1300874. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B, et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry. 2002;159:130–133. doi: 10.1176/appi.ajp.159.1.130. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Bertolino A, Mattay VS, Langheim FJ, Duyn J, Coppola R, et al. Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex. 2000;10:1078–1092. doi: 10.1093/cercor/10.11.1078. [DOI] [PubMed] [Google Scholar]
- Dong Z, Dreher W, Leibfritz D. Toward quantitative short-echo-time in vivo proton MR spectroscopy without water suppression. Magn Reson Med. 2006;55:1441–1446. doi: 10.1002/mrm.20887. [DOI] [PubMed] [Google Scholar]
- Ertugrul A, Volkan-Salanci B, Basar K, Karli Oguz K, Demir B, Ergun EL, et al. The effect of clozapine on regional cerebral blood flow and brain metabolite ratios in schizophrenia: relationship with treatment response. Psychiatry Res. 2009;174:121–129. doi: 10.1016/j.pscychresns.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Nakano T, Takano T, Takeuchi K, Yamada K, Fukuzako T, et al. Proton magnetic resonance spectroscopy of basal ganglia in chronic schizophrenia. Biol Psychiatry. 1996;40:14–18. doi: 10.1016/0006-3223(95)00316-9. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, et al. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Reduction of synaptophysin immunoreactivity in the prefrontal cortex of subjects with schizophrenia. Regional and diagnostic specificity. Arch Gen Psychiatry. 1997;54:943–952. doi: 10.1001/archpsyc.1997.01830220065010. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gold M, Lorenzl S, Stewart AJ, Morimoto BH, Williams DR, Gozes I. Critical appraisal of the role of davunetide in the treatment of progressive supranuclear palsy. Neuropsychiatr Dis Treat. 2012;8:85–93. doi: 10.2147/NDT.S12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I. Microtubules, schizophrenia and cognitive behavior: preclinical development of davunetide (NAP) as a peptide-drug candidate. Peptides. 2011;32:428–431. doi: 10.1016/j.peptides.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Gur RE, Cowell P, Turetsky BI, Gallacher F, Cannon T, Bilker W, et al. A follow-up magnetic resonance imaging study of schizophrenia. Relationship of neuroanatomical changes to clinical and neurobehavioral measures. Arch Gen Psychiatry. 1998;55:145–152. doi: 10.1001/archpsyc.55.2.145. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Buchanan RW, Keefe RS, Kern R, McMahon RP, Green MF, et al. Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res. 2012;136:25–31. doi: 10.1016/j.schres.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Lentz MR, Kim JP, Westmoreland SV, Greco JB, Fuller RA, Ratai EM, et al. Quantitative neuropathologic correlates of changes in ratio of N-acetylaspartate to creatine in macaque brain. Radiology. 2005;235:461–468. doi: 10.1148/radiol.2352040003. [DOI] [PubMed] [Google Scholar]
- Merenlender-Wagner A, Pikman R, Giladi E, Andrieux A, Gozes I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse--a microtubule-deficient model of schizophrenia. Peptides. 2010;31:1368–1373. doi: 10.1016/j.peptides.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. doi: 10.1016/j.pneurobio.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohrmann P, Siegmund A, Suslow T, Pedersen A, Spitzberg K, Kersting A, et al. Cognitive impairment and in vivo metabolites in first-episode neuroleptic-naive and chronic medicated schizophrenic patients: a proton magnetic resonance spectroscopy study. J Psychiatr Res. 2007;41:625–634. doi: 10.1016/j.jpsychires.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pinhasov A, Mandel S, Torchinsky A, Giladi E, Pittel Z, Goldsweig AM, et al. Activity-dependent neuroprotective protein: a novel gene essential for brain formation. Brain Res Dev Brain Res. 2003;144:83–90. doi: 10.1016/s0165-3806(03)00162-7. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Selemon LD, Goldman-Rakic PS. Neuronal and glial somal size in the prefrontal cortex: a postmortem morphometric study of schizophrenia and Huntington disease. Arch Gen Psychiatry. 1998;55:215–224. doi: 10.1001/archpsyc.55.3.215. [DOI] [PubMed] [Google Scholar]
- Sajja BR, Wolinsky JS, Narayana PA. Proton magnetic resonance spectroscopy in multiple sclerosis. Neuroimaging Clin N Am. 2009;19:45–58. doi: 10.1016/j.nic.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Abnormally high neuronal density in the schizophrenic cortex. A morphometric analysis of prefrontal area 9 and occipital area 17. Arch Gen Psychiatry. 1995;52:805–818. doi: 10.1001/archpsyc.1995.03950220015005. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Rajkowska G, Goldman-Rakic PS. Elevated neuronal density in prefrontal area 46 in brains from schizophrenic patients: application of a three-dimensional, stereologic counting method. J Comp Neurol. 1998;392:402–412. [PubMed] [Google Scholar]
- Shenton ME, Whitford TJ, Kubicki M. Structural neuroimaging in schizophrenia: from methods to insights to treatments. Dialogues Clin Neurosci. 2010;12:317–332. doi: 10.31887/DCNS.2010.12.3/mshenton. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Swintosky VL, Gozes I, Brenneman DE, D'Andrea MR, Plata-Salaman CR. Activity-dependent neurotrophic factor-9 and NAP promote neurite outgrowth in rat hippocampal and cortical cultures. J Mol Neurosci. 2005;25:225–238. doi: 10.1385/JMN:25:3:225. [DOI] [PubMed] [Google Scholar]
- Soher BJ, Doraiswamy PM, Charles HC.2005A review of 1H MR spectroscopy findings in Alzheimer's disease Neuroimaging Clin N Am 15847–852.xi. [DOI] [PubMed] [Google Scholar]
- Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Vidal C, Giedd JN, Gochman P, Blumenthal J, Nicolson R, et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci USA. 2001;98:11650–11655. doi: 10.1073/pnas.201243998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haren NE, Pol HE, Schnack HG, Cahn W, Brans R, Carati I, et al. Progressive brain volume loss in schizophrenia over the course of the illness: evidence of maturational abnormalities in early adulthood. Biol Psychiatry. 2008;63:106–113. doi: 10.1016/j.biopsych.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Vulih-Shultzman I, Pinhasov A, Mandel S, Grigoriadis N, Touloumi O, Pittel Z, et al. Activity-dependent neuroprotective protein snippet NAP reduces tau hyperphosphorylation and enhances learning in a novel transgenic mouse model. J Pharmacol Exp Ther. 2007;323:438–449. doi: 10.1124/jpet.107.129551. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Velakoulis D, Whitford TJ, Pantelis C. Understanding aberrant white matter development in schizophrenia: an avenue for therapy. Expert Rev Neurother. 2011;11:971–987. doi: 10.1586/ern.11.76. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]