Abstract
Vigorous transport of cytoplasmic components along axons over substantial distances is crucial for the maintenance of neuron structure and function. The transport of mitochondria, which serves to distribute mitochondrial functions in a dynamic and non-uniform fashion, has attracted special interest in recent years following the discovery of functional connections among microtubules, motor proteins and mitochondria, and their influences on neurodegenerative diseases. Although the motor proteins that drive mitochondrial movement are now well characterized, the mechanisms by which anterograde and retrograde movement are coordinated with one another and with stationary axonal mitochondria are not yet understood. In this Commentary, we review why mitochondria move and how they move, focusing particularly on recent studies of transport regulation, which implicate control of motor activity by specific cell-signaling pathways, regulation of motor access to transport tracks and static microtubule–mitochondrion linkers. A detailed mechanism for modulating anterograde mitochondrial transport has been identified that involves Miro, a mitochondrial Ca2+-binding GTPase, which with associated proteins, can bind and control kinesin-1. Elements of the Miro complex also have important roles in mitochondrial fission–fusion dynamics, highlighting questions about the interdependence of biogenesis, transport, dynamics, maintenance and degradation.
Key words: Axonal transport, Microtubule, Mitochondria, Motor protein, Neuron, Regulation
Introduction
The dynamic ordering of cytoplasmic components, which is important in every cell, is crucial in neurons because of their length and asymmetry. The somato-dendritic and synaptic terminal regions of a human peripheral neuron have different sets of molecular machinery and distinct signaling functions, and are separated by a thin axon that can be a meter long (Fig. 1). The soma (cell body) contains a nucleus, protein synthesis machinery in the form of ribosomes, rough endoplasmic reticulum and Golgi complex, and degradation machinery, in the form of lysosomes and proteasomes. The concentration of these protein synthesis and degradation elements in axons is thought to be low; hence, it has long been accepted that after biogenesis in the cell body, new protein complexes and organelles are carried into the axon and toward the synaptic terminal by anterograde transport (Bartlett and Banker, 1984; Grafstein and Forman, 1980). During a period of service in the axon, those new materials age, accumulating oxidative damage that impairs their functions. To help maintain optimal neuron function, aged materials are returned by retrograde transport to the cell body for degradation. Despite the straightforward logic of this hypothesis, there are persistent questions about the degree of its accuracy. A complete discussion of the idea of cell-body-focused biogenesis is beyond the scope of this review. However, there is no question that organelles, protein complexes and mRNAs move vigorously along axons, often with persistent directionality over long distances (Hollenbeck and Saxton, 2005). There is also no question that such transport is important, because defects in the microtubule-based machinery that drives it can cause or contribute to a number of human neurodegenerative conditions, including spastic paraplegia, Charcot–Marie–Tooth, amyotrophic lateral sclerosis and Alzheimer's, Huntington's and Parkinson's disease (Blackstone et al., 2011; Chen and Chan, 2009; Chevalier-Larsen and Holzbaur, 2006; Crimella et al., 2011; De Vos et al., 2008; Morfini et al., 2009a; Perlson et al., 2010; Züchner and Vance, 2006).
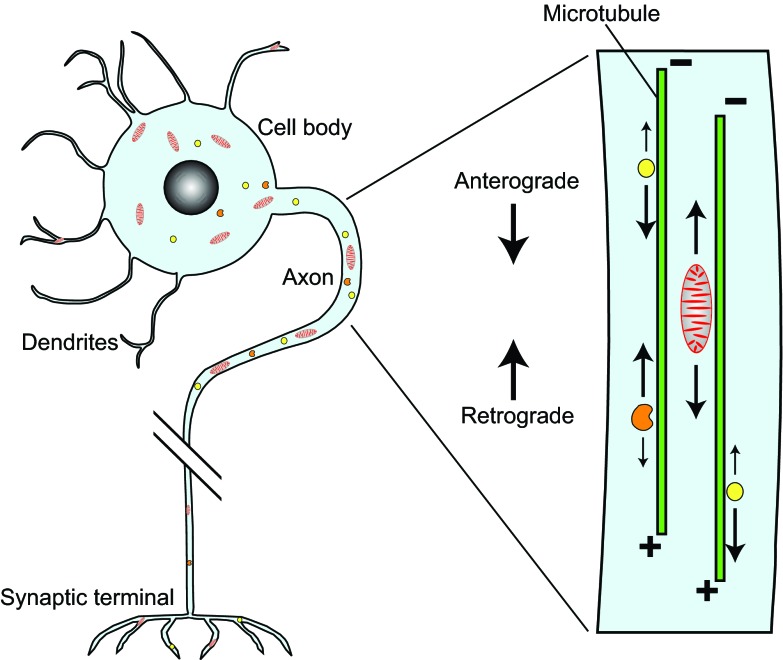
Fig. 1.
Neuron structure and axonal transport. This simplified representation of a peripheral neuron shows a somato-dendritic region (cell body) that gathers signals from presynaptic neurons, and an axon that carries waves of self-propagating membrane potential changes (action potentials) from the cell body to the synaptic terminal, which responds by releasing chemical signals to a postsynaptic neuron or muscle cell. Proper neuron development and function relies on vigorous long-distance transport of cytoplasmic components including vesicles (yellow), mitochondria (red) and endocytic organelles (orange) along the axon. It is thought that a major purpose of such transport is to deliver organelles and other materials newly synthesized in the cell body into the axon and toward the terminal (anterograde) for carrying out their various functions, and to return aged materials back to the cell body (retrograde) for degradation and recycling. Both forms of long-distance transport use motor proteins that attach to a ‘cargo’ and pull it along microtubules (green) toward either their plus ends (oriented away from the cell body) or their minus ends (oriented toward the cell body). To help grasp the scale of the transport logistics faced by a long neuron (e.g. a human upper or lower motor neuron) with a 30 μm diameter cell body and a 1 meter long by 1 μm wide axon, imagine standing in a lecture hall that is 30 meters in diameter with a 1 meter wide pipeline starting from one wall and extending off across the landscape. The pipeline terminal would be 1000 km distant. Finally, consider that the pipe, with a volume 120-fold greater than that of the lecture hall, is filled with complex machinery. Much of that machinery is fabricated in the hall, so it must be delivered to destinations that need it, then after obsolescence, much of it must be returned for recycling.
The movement and steady-state distribution of each axonal component is determined by where and how frequently it engages with and disengages from the microtubule-based transport machinery. Because axonal microtubules are organized uniformly with their plus ends toward the terminal (Fig. 1), anterograde transport involves translocation toward microtubule plus ends, using kinesin motors, whereas retrograde transport requires translocation toward minus ends, using dynein motors. The balance between anterograde and retrograde transport can be strongly biased in favor of one direction over the other; for example, movement of vesicles filled with neuropeptides is strongly biased toward sites of secretion at axon terminals (Barkus et al., 2008; Rao et al., 2001), whereas organelles that carry endocytic and signaling cargoes move mainly back toward the cell body (Hollenbeck, 1993; Lalli and Schiavo, 2002; Parton et al., 1992). The transport of mitochondria (Fig. 1) is particularly important because mitochondrial functions are needed throughout the axon and their transport can be biased strongly in either direction, depending on whether axons grow or retract, and on specific signaling stimuli (Chada and Hollenbeck, 2004; Chang et al., 2006; Morris and Hollenbeck, 1993; Pilling et al., 2006; Russo et al., 2009).
In axons, some mitochondria move persistently over long distances (tens to hundreds of micrometers), whereas others appear anchored or are otherwise stationary (Miller and Sheetz, 2004; Morris and Hollenbeck, 1993; Morris and Hollenbeck, 1995; Ohno et al., 2011; Pilling et al., 2006). During long-distance transport of mitochondria, continuous runs are interspersed with frequent brief pauses and direction changes; nevertheless, individual mitochondria are biased strongly toward runs in a primary direction (Misgeld et al., 2007; Morris and Hollenbeck, 1993; Pilling et al., 2006). For example, only 2–10% of the movement of mitochondria in axons in the intact Drosophila nervous system is in the ‘reverse’ direction for either anterograde or retrograde transport, and complete reversals in primary direction are rare (Barkus et al., 2008; Pilling et al., 2006; Russo et al., 2009; Shidara and Hollenbeck, 2010).
In this Commentary, we discuss recent research progress and ideas derived from it with regard to why axonal mitochondria move, how they move and how their behavior is regulated. Additional overviews and insights from different perspectives can be found in other reviews (Frederick and Shaw, 2007; Goldstein et al., 2008; Hirokawa et al., 2009; MacAskill and Kittler, 2010; Morfini et al., 2009a; Perlson et al., 2010; Verhey and Hammond, 2009; Zinsmaier et al., 2009).
Why do mitochondria move?
Inheritance of mitochondria by daughter cells
Mitochondria arose through engulfment of the prokaryotic mitochondrial ancestor by an ancestral eukaryotic cell. Subsequent natural selection preserved heritable changes in both organisms, which enhanced their mutual reproductive success (de Duve, 2007). Beyond surviving destruction by the host, success of the proto-mitochondrion required its growth, fission and sufficient movement to ensure distribution into host daughter cells during division. The physical linkage between mitochondria and force-generating cytoskeletal machinery is now specialized, but the initial linkage might simply have resulted from enclosure of the prokaryote by host endocytic membranes (de Duve, 2007) that already had the ability to move toward microtubule minus ends and thus could segregate with centrosomes during host cell division. From the perspective of the eukaryote, as soon as the physiological contributions of proto-mitochondria began to increase its reproductive success, selection would favor progressive modifications of its cytoplasmic transport machinery, which would then enhance the polarized delivery of healthy mitochondria to all daughter cells (Peraza-Reyes et al., 2010).
Special patterns of mitochondria distribution in large cells
The adaptation of specific mechanisms for the long-distance transport and positioning of mitochondria must have been crucial for the development of large cells with high, localized metabolic requirements (Hollenbeck and Saxton, 2005). Such transport in animal cells is usually achieved through motor-mediated movement along microtubules, which are sufficiently stiff to individually generate long non-branched transport paths. Thinner, more compliant actin filaments are often arranged in branched networks that are better suited to local, short-range motor movements (Kuznetsov et al., 1992; Pathak et al., 2010; Rogers and Gelfand, 1998). Proto-mitochondria, perhaps with endosome-like outer membranes, probably started with the capacity to move toward microtubule minus ends and the cell center. To move to peripheral destinations in large asymmetrical cells such as neurons, proto-mitochondria and the host needed to evolve new outer membrane links to the plus-end-directed force-generating machinery and new regulatory control mechanisms for the existing transport machinery. Stopping at points of high local energy consumption (e.g. clusters of ion pumps or cell protrusion zones) could be dictated by microtubule tracks that terminate nearby, by disengagement of mitochondria from microtubules before their ends or by specific signal-stimulated static docking. As elaborated in the section on regulation below, it is clear that complex mechanisms have indeed evolved to control embarkation, transport direction and disembarkation of axonal mitochondria at specific destinations.
Localized biogenesis of mitochondria
The hypothesis that new mitochondria are generated in the cell body, are transported to distal regions where they ‘age’ and are then eventually returned to the cell body for degradation predicts that anterograde mitochondria have a more robust morphology and functional capacity than retrograde mitochondria. Early studies in diverse systems addressed this question by using physical ligation or local cooling of axons to block transport, followed by electron microscopy to compare mitochondria on the proximal side of a block, which arrived there by anterograde transport, with those on the distal side. The results of those studies range from a clear demonstration of abnormal-looking mitochondria on the distal side of a block in squid axons (Fahim et al., 1985), through modestly different distal morphology (Hirokawa et al., 1991) or no apparent difference (Tsukita and Ishikawa, 1980), to abnormal mitochondrial morphology at the proximal side (Logroscino et al., 1980). This lack of agreement left the question of whether or not anterograde and retrograde mitochondria are morphologically distinct unanswered.
Are anterograde and retrograde populations functionally distinct? To detect differences in physiology that might underlie or control transport direction, mitochondrial inner-membrane potential, which drives ATP production and other mitochondrial processes (Box 1), has been tested for correlation with the direction of transport using potential-sensing fluorescent dyes in cultured chick neurons (Miller and Sheetz, 2004; Verburg and Hollenbeck, 2008). In one study, fluorescence intensity measurements of mitochondria stained with the aggregating dye JC-1 suggested that 90% of mitochondria with high membrane potential move anterogradely, whereas 80% of those with low potential move retrogradely (Miller and Sheetz, 2004). In another study with the less-toxic vital dye TMRM, although changes in the inner-membrane potential could be generated locally or globally by altering specific signaling pathways, no correlation was detected between membrane potential and direction of axonal transport (Verburg and Hollenbeck, 2008). To look for a causal relationship, the effects on transport of conditions that alter mitochondrial physiology were tested. A reduction of inner-membrane potential with the electron transport inhibitor antimycin A temporarily increased retrograde transport (Baqri et al., 2009). However, an inner-membrane uncoupler, CCCP, reduced transport in both directions (Baqri et al., 2009; Hollenbeck et al., 1985). Furthermore, mutation of a mitochondrial DNA polymerase subunit, which generates cumulative damage to mitochondrial DNA and hence probably causes physiological decline, increased the number of mitochondria that move in both directions rather than just retrogradely (Baqri et al., 2009). Thus, the relationship between mitochondrial ‘age’ or physiology and transport behavior remains unresolved.
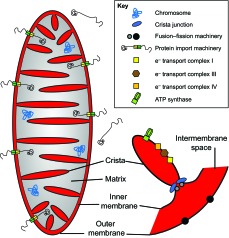
Box 1. Mitochondria overview
Mitochondria are descended from α-proteobacteria, which after engulfment by ancestral eukaryotic cells, developed an endosymbiotic relationship with them, supplying metabolites, energy in the form of ATP and complex signaling functions (de Duve, 2007). Most of the genes from the original bacterial chromosome have been transferred to the host genome in the nucleus. The proteins those genes encode are synthesized by cytoplasmic ribosomes and are then imported through the mitochondrial outer and inner membranes. However, each mitochondrion has multiple copies of a remnant chromosome that encodes a small number of essential RNAs and proteins that must be synthesized by mitochondrial ribosomes in the matrix. Energy from transfer of electrons down the electron transport chain is used to pump protons from the matrix to the intermembrane space – a process that creates an electrochemical gradient across the inner mitochondrial membrane. The potential energy of that gradient is then used to drive phosphorylation of ADP by ATP synthase. It also powers the import of many hundreds of mitochondrial protein species (black squiggly lines in the figure) synthesized in the cytosol and the import–export of metabolites. Mitochondrial shapes and sizes are determined in part by the balance between membrane fission and fusion, which is controlled by proteins such as Opa1 in the inner membrane (concentrated at the crista junction) and mitofusin and Drp1 in the outer membrane. Defects in the electron transport chain or in Opa1 can cause optic neuron degeneration and blindness. Defects in mitofusins are a common cause of inherited motor neuron disease (Cho et al., 2010; Züchner et al., 2004). Interestingly, recent research suggests that mitofusin 2 also has an important functional relationship with motor proteins that drive the axonal transport of mitochondria (Misko et al., 2010).
The central question remains: can mitochondrial biogenesis, degradation and thus the entire life cycle of the organelle occur in the axon? It has been shown that some mitochondrial DNA replication and fission–fusion can occur in axons (Amiri and Hollenbeck, 2008). However, almost all the genes that encode mitochondrial proteins reside in the nuclear genome within the cell body, so full biogenesis in the axon would require anterograde axonal transport of hundreds of different mitochondrial proteins, or their mRNAs. Axoplasm has a modest translation capacity (Lee and Hollenbeck, 2003) and some mitochondrial protein synthesis can occur there (Gioio et al., 2001; Martin et al., 1998; Sotelo-Silveira et al., 2006). Axonal mRNA microarray analysis and quantitative PCR have shown that axons can contain many mitochondrial mRNAs, some at concentrations as high as 10% of their levels measured in the cell body (Taylor et al., 2009). Furthermore, inhibitors of cytosolic translation or of mitochondrial protein import can cause marked reductions in mitochondrial function, arguing that synthesis and import of nuclear-encoded mitochondrial proteins in axons is important (Hillefors et al., 2007; Martin et al., 1998). On the degradation side, autophagic sequestration can occur in axons (Hollenbeck, 1993), but axonal mitophagy has yet to be demonstrated. Thus it remains unclear whether or not axonal translation can produce mitochondrial proteins at rates sufficient for complete biogenesis, and whether or not mitochondrial turnover in axons could match such biogenesis rates to complete the lifecycle. A compelling argument against this hypothesis is provided by the nature of axonal mitochondria transport itself. If robust biogenesis occurs locally, why do mitochondria move with such persistent directionality over such long distances?
How do mitochondria move?
For directed transport, mitochondria interact with microtubules or actin filaments, typically through force-generating motor proteins (Hollenbeck and Saxton, 2005). On the basis of structural similarities, motor proteins are classified into three families, myosins, kinesins and dyneins (Berg et al., 2001; Wickstead and Gull, 2006; Wickstead and Gull, 2007). Organelle movement by myosins can be directed toward the actin filament plus ends (e.g. myosin V) or minus ends (e.g. myosin VI), whereas kinesins move organelles toward microtubule plus ends, and dyneins move them toward minus ends (Hirokawa et al., 2009; Lister et al., 2004; Trybus, 2008; Vale, 2003). The heavy chain of each motor type has a family-specific conserved head domain that generates force and motion through cycles of ATP hydrolysis, filament binding and conformation change (Rice et al., 1999; Rayment et al., 1993; Carter et al., 2011). Non-conserved stalk sequences typically facilitate coiled-coil homodimerization of heavy chains that allows the motor to ‘walk’, alternating cycles of heavy chain to filament binding, such that one head is always attached to the filament (Vale and Milligan, 2000). Stalk and tail sequences are also thought to provide binding sites for proteins that mediate motor linkage to cargoes and regulate transport (reviewed by Akhmanova and Hammer, 2010).
Long-distance movement of mitochondria in axons is driven by kinesin and dynein motors along microtubules that are arranged with their plus ends toward synaptic terminals (Figs 1, 2). Function inhibition and biochemical studies indicate that kinesin-1 is the primary anterograde mitochondrial motor (Barkus et al., 2008; Pilling et al., 2006; Vale et al., 1985), but there is evidence from studies of tissue culture cells that the kinesin-3 motors Kif1b and Klp6 also can transport mitochondria and thus might do so in axons (Nangaku et al., 1994; Tanaka et al., 2011). Similar approaches indicate that cytoplasmic dynein is the primary retrograde mitochondrial motor (Pilling et al., 2006; Schnapp and Reese, 1989). Interestingly, genetic analyses of kinesin-1 and dynein in Drosophila revealed that the opposing motors can be interdependent (Martin et al., 1999). Although dynein mutations inhibit only retrograde mitochondrial movement, kinesin-1 mutations inhibit both anterograde and retrograde movement (Pilling et al., 2006). Likewise, in the axonal transport of neuropeptide vesicles, kinesin-3 mutations inhibit both anterograde and retrograde transport (Barkus et al., 2008). However, for neurofilament transport in axons and in a variety of other transport processes, anterograde movement driven by kinesin-1 is dependent on dynein (Ally et al., 2009; Kural et al., 2005; Ling et al., 2004; Uchida et al., 2009). These and other observations indicate that opposing axonal transport motors often have important biochemical and/or biophysical interactions with one another (Barkus et al., 2008; Brady et al., 1990; Gross, 2004; Hendricks et al., 2010; Ligon et al., 2004; Martin et al., 1999). Do multiple motor types form cooperative, biochemically linked complexes or do single motor types somehow require opposing biophysical tension? Defining the direct and indirect relationships between different types of microtubule motors on mitochondria will be crucial for the understanding of mitochondrial transport mechanisms.
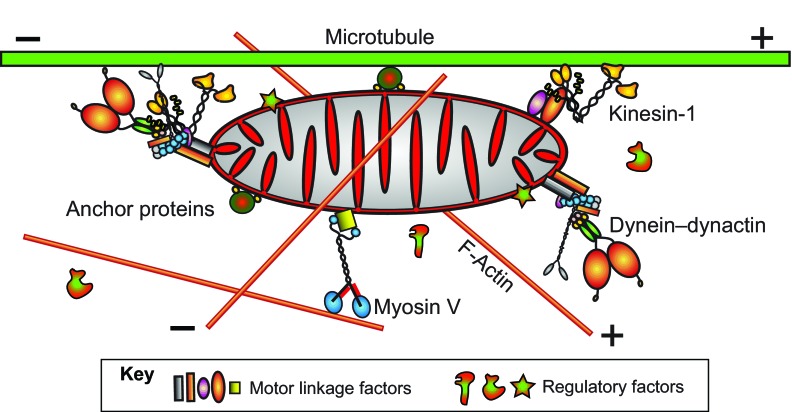
Fig. 2.
Axonal transport machinery for mitochondria. Plus- and minus-end-directed motion of mitochondria along axonal microtubules is driven by kinesin-1 and dynein, respectively. Forces from cytoplasmic myosins (e.g. myosin V) might modulate the processivity of microtubule-based long-range transport and might drive short-range local transport. There are many important questions about mitochondria transport mechanisms that need to be addressed. How are motors linked to mitochondria and how are those linkages controlled? How are motor–filament interactions controlled? What are the functional and physical relationships between motors; e.g. are different types of motors joined in multi-motor complexes (left end) or are they physically separate (right end)? Static anchorage complexes that help hold mitochondria in a stationary state can suppress motor-driven movements (center). What is the nature of those anchorage complexes and how are they controlled? Substantial progress has been made and answers are beginning to emerge. Identified linkers for kinesin-1 so far include Miro, Milton and syntabulin, and they probably have additional interacting proteins that make important contributions. One identified anchor complex includes syntaphilin and LC8, but others are expected to exist. Regulatory factors that influence linkage and motor function include Ca2+ and a variety of cytoplasmic and mitochondrial proteins, including kinases, tau and Miro.
Axons contain short actin filaments that are of mixed polarity (Bearer and Reese, 1999; Fath and Lasek, 1988; Morris and Hollenbeck, 1995), raising questions about how myosins contribute to mitochondrial transport (Bridgman, 2004). One hypothesis suggests that myosins serve an auxiliary function, helping to return mitochondria to a microtubule track when the kinesins or dyneins become disengaged, or when there are short regions of the axon that lack microtubules (Bearer and Reese, 1999; Hollenbeck and Saxton, 2005; Langford, 2002; Ligon and Steward, 2000; Morris and Hollenbeck, 1995). Another possibility, suggested by the complex two-dimensional movement of pigment organelles in melanosomes, is that myosin engagement with actin filaments actually disrupts protracted microtubule-based mitochondrial movement, thereby helping mitochondria to halt at appropriate destinations (Gross et al., 2002). This view is supported by studies in cultured Drosophila neurons, in which reduction of myosin V levels actually increased transport efficiency in both directions, with a net benefit to long anterograde runs. Reduction of myosin VI levels had similar effects, but favored retrograde runs (Pathak et al., 2010).
Regulation of mitochondria–motor–microtubule linkage and movement
The motility of mitochondria differs from that of other axonal organelles, in that it shows unique run parameters, frequent pausing and halting at stationary residence sites throughout the axon. In addition, cell signaling from specific sites can regulate mitochondrial movement and pausing behavior (Chada and Hollenbeck, 2003; Chada and Hollenbeck, 2004; Miller and Sheetz, 2004; Morris and Hollenbeck, 1993), and can control their entry into and exit from axonal branches (Ruthel and Hollenbeck, 2003). Altogether, this body of work indicates that there are indeed mitochondria-specific transport regulation mechanisms in the axon.
Which machinery controls the persistent anterograde, retrograde or stationary states of mitochondria and which mechanisms trigger switches between those states? Perhaps mitochondria can switch their binding affinities for the different motors and the resulting transport behavior is dictated simply by the identity and numbers of motors bound. Alternatively, all motor types could be concurrently bound, but differentially silenced or activated. The observation that mitochondria can rapidly reverse their direction of transport and the influences of kinesin-1 and myosin V on dynein-driven retrograde transport, as described in the preceding section, suggest that different motor proteins are simultaneously bound to the outer mitochondrial membrane and regulated there in situ (Miller and Sheetz, 2004; Morris and Hollenbeck, 1995; Pathak et al., 2010; Pilling et al., 2006; Russo et al., 2009). However, the ability of mitochondria to stop and remain stationary could entail a complete release of motor proteins. These two types of regulation are not mutually exclusive and indeed it is reasonable to expect that they co-exist.
Many proteins have been identified that, based on genetic, biochemical or functional analyses, have been proposed to bind motor proteins and link them to various cargoes, regulate their motor activity, or both (reviewed by Hirokawa et al., 2009; Karcher et al., 2002; Santama et al., 2004; Verhey and Hammond, 2009). However, a consensus on the exact mechanisms or even common themes of regulation has been slow to emerge. Defining the mechanisms that link filaments, motors and cargoes, as well as mechanisms that regulate those linkages, remains one of the major challenges in cell biology. Below, we review progress toward this goal for axonal mitochondria.
Regulation of axonal mitochondria transport
Before we consider the regulation of mitochondria-specific movement, it is worthwhile to discuss regulatory mechanisms by which axonal organelle transport in general is controlled. A number of different kinase activities are known to affect axonal organelle traffic indirectly. These include microtubule-affinity regulating kinases (MARKs), also known as Par-1 kinases (Mandelkow et al., 2004), cyclin-dependent kinases (Holzbaur, 2010; Morfini et al., 2004; Ratner et al., 1998), receptor tyrosine kinases (Chada and Hollenbeck, 2003; Chada and Hollenbeck, 2004) and phosphoinositide 3-kinases (Chada and Hollenbeck, 2003; Chada and Hollenbeck, 2004; Malaiyandi et al., 2005). Studies using in vitro and non-neuronal systems have shown that tau, a microtubule-associated protein, limits the run lengths of motors and, depending on the tau isoform, can limit the access of kinesin to microtubules such that transport is biased away from microtubule plus ends (Dixit et al., 2008; Ebneth et al., 1998; Gross et al., 2002; Trinczek et al., 1999; Vershinin et al., 2007; Vershinin et al., 2008). In neuroblastoma cells and retinal ganglion cells, a high concentration of a non-phosphorylated tau isoform has been shown to alter the transport of mitochondria and other organelles, thereby limiting their presence in neurites (Mandelkow et al., 2004; Ebneth et al., 1998; Stamer et al., 2002). Another possibility for modulating the access of motor proteins to microtubule tracks in neurons is through post-translational modifications of tubulin that alter motor microtubule binding (Reed et al., 2006).
More specific mechanisms of organelle transport regulation involve direct phosphorylation of motor subunits. C-Jun N-terminal kinase (JNK), which influences vesicle distribution in neurons (Byrd et al., 2001), phosphorylates kinesin-1 heavy chain (Khc), thereby reducing its affinity for microtubules (Morfini et al., 2006; Morfini et al., 2009b). Both glycogen synthase kinase 3 (Morfini et al., 2004; Morfini et al., 2002) and casein kinase (Pigino et al., 2009) can phosphorylate kinesin-1 light chain (Klc), which results in a reduced affinity of kinesin-1 for cargo and an inhibition of axonal organelle transport. It is notable in this context that specific splicing isoforms of Klc can associate with mitochondria, and that hyperphosphorylation of Klc coincides with aberrant distribution of mitochondria in cultured cells (De Vos et al., 2000; Khodjakov et al., 1998). Functional studies of Drosophila Klc suggest that it is not important for normal mitochondrial distribution in photoreceptor axons (Glater et al., 2006) and that it is dispensable in the binding of kinesin to mitochondrial adaptor proteins (MacAskill et al., 2009; Wang and Schwarz, 2009). However, recent work on caytaxin, a protein implicated in human ataxia, has shown that it binds to Klc, associates with mitochondria and influences mitochondria distribution in cultured cell neurites (Aoyama et al., 2009). Although caytaxin might be unique to vertebrates, further investigation of the possibility that in some cells Klc can influence mitochondria as well as vesicle transport seems warranted.
Mitochondria linkage to motors and microtubules
An understanding of specific mechanisms for the regulation of long-distance transport of mitochondria requires molecular definition of their structural connections with the motors that move them and with proteins that serve as stationary anchors. For retrograde movement, dynein intermediate chain (DIC) is a candidate for linking the motor to its numerous cargoes. DIC can bind both the cytoplasmic dynein heavy chain and the dynein activator complex, dynactin, which contains P150(Glued), Arp1, P50 and several other proteins (Gill et al., 1991; Karki and Holzbaur, 1995; Paschal et al., 1992; Vaughan and Vallee, 1995). Biochemical and immunoelectron microscopy analyses have demonstrated that dynein and dynactin associate with mitochondria (Habermann et al., 2001; Pilling et al., 2006; Varadi et al., 2004), and that blocking the interaction between DIC and dynactin components in Xenopus extracts reduces the association of dynein heavy chains with cytoplasmic membranes (Steffen et al., 1997). Finally, genetic inhibition of dynactin components in Drosophila causes axon degeneration and results in defective axonal transport of mitochondria (Haghnia et al., 2007; Martin et al., 1999; Pilling et al., 2006). Although these observations support the notion that an interaction between dynein and mitochondria is mediated through DIC and dynactin, direct evidence is lacking. Neither an outer membrane receptor that would complete such a linkage nor specific dynein control pathways have been identified thus far.
Syntabulin, which was first identified as a syntaxin-binding protein, has been proposed to act as a linker between kinesin and both vesicles and mitochondria. It binds kinesin-1, can associate with dense core vesicles (Su et al., 2004) and its disruption inhibits vesicle transport and synaptic function (Cai et al., 2007; Ma et al., 2009). Syntabulin can also associate with mitochondria, and disruption of syntabulin function specifically inhibits their anterograde transport (Cai et al., 2005). Because its mitochondria and vesicle binding sites are distinct, it is possible that syntabulin modulates the attachment of kinesin-1 to vesicles or mitochondria under different regulatory conditions (Cai and Sheng, 2009).
Another syntaxin-binding protein, syntaphilin (Lao et al., 2000), which was originally reported as a presynaptic membrane protein that regulates synaptic vesicle exocytosis and endocytosis, is a good candidate for a mitochondria-microtubule anchor (Das et al., 2003; Kang et al., 2008; Lao et al., 2000). Syntaphilin is transported along the axon together with mitochondria; it can bind microtubules and it associates strongly with stationary but not motile mitochondria (Kang et al., 2008). Interestingly, the dynein light chain LC8, which is known to have many binding partners (Rapali et al., 2011), facilitates interactions between syntaphilin and microtubules (Chen et al., 2009). Lowering the expression levels of LC8 or syntaphilin reduces the fraction of axonal mitochondria that are stationary, suggesting that these proteins create static links between mitochondria and microtubules (Chen et al., 2009; Kang et al., 2008).
Another multi-functional protein found to associate with neuronal mitochondria is Ran-binding protein 2 (RanBP2). It is highly expressed in retinal neurons, where it associates with proteasomes and the nuclear pore complex, and is thought to play a role in coordinating nucleocytoplasmic transport (Mavlyutov et al., 2002). RanBP2 also assembles a complex that includes neuron-specific kinesin-1 isoforms (Cai et al., 2001) and associates with mitochondria in non-neuronal cells (Cho et al., 2007). However, the significance of these functions for mitochondria traffic in neurons remains to be elucidated.
Axonal transport regulation by calcium
Substantial progress has been made in research on Ca2+ control of mitochondrial transport by kinesin-1. It has been known for some time that neuronal activity regulates mitochondrial movement and distribution (Chang et al., 2006; Hollenbeck and Saxton, 2005; Li et al., 2004; Morris and Hollenbeck, 1993; Ohno et al., 2011; Zhang et al., 2010), but a specific regulatory role for Ca2+ has been somewhat contentious. Classic observations of reactivated organelle transport in axons or in extruded axoplasm did not detect any influence of Ca2+ (Adams, 1982; Brady et al., 1985; Brady et al., 1984). However, subsequent studies in vertebrate neurons have shown that neurotransmitter-mediated elevation of Ca2+ can inhibit the motility of mitochondria without affecting that of other organelles (Mironov, 2006; Rintoul et al., 2003; Rintoul and Reynolds, 2010). For example, recent work shows that in myelinated axons, increased electrical activity halts mitochondria movement near plasma membrane ion pumps and that ion pump activity and elevated cytoplasmic Ca2+ are responsible (Ohno et al., 2011; Zhang et al., 2010). These and other studies (Rintoul et al., 2003; Yi et al., 2004) are consistent with the existence of a Ca2+ sensor that is involved in the regulation of mitochondrial motility.
A candidate sensor, identified in a genetic screen as being necessary for mitochondrial transport to synapses (Guo et al., 2005), turned out to be the Drosophila homolog of Miro (Fransson et al., 2006), a conserved outer membrane Ca2+-binding Rho-like GTPase (reviewed by Reis et al., 2009). Another Drosophila screen for synaptic insufficiency had earlier identified a mitochondrion-kinesin linker protein, Milton (Górska-Andrzejak et al., 2003; Stowers et al., 2002). Together, Miro and Milton form a complex with the heavy chain of kinesin-1 that attaches the motor to mitochondria and mediates the effects of Ca2+ on mitochondrial movement (Wang and Schwarz, 2009). In Drosophila, this complex is proposed to proceed from Miro through Milton to Khc. Milton binds to the stalk of Khc by competing with Klc for its binding site (Glater et al., 2006). When Ca2+ levels are elevated, a conformational change is induced in the Ca2+-binding EF hand domains of Miro that causes it to bind the Khc motor domains, thereby blocking Khc binding to microtubules, but leaving the Miro–Milton–Khc complex on the mitochondrial surface (Wang and Schwarz, 2009).
Studies of related proteins in vertebrate dendrites suggest a somewhat different mechanism; highly conserved Miro, in a GTPase-sensitive manner, binds Grif-1 (also known as TRAK2 or OIP106), which has ~30% amino acid identity with Drosophila Milton. The interaction recruits Grif-1 to mitochondria thereby enhancing their transport (MacAskill et al., 2009a). However, in contrast to the Drosophila model, Miro binds to Khc directly (MacAskill et al., 2009b). In addition, at high but physiological Ca2+ levels (in the micromolar range), Miro releases kinesin-1 from the mitochondrial surface, thereby halting mitochondria transport in the postsynaptic region of dendrites (MacAskill et al., 2009b). However, other studies indicate that Grif-1 does bind to Khc directly and that it can also form a complex that includes both Khc and Klc (Smith et al., 2006). These conflicting details of how Khc, Klc, Miro and Milton-related proteins interact with one another could be due to evolutionary divergence of mechanisms as evidenced by the substantial sequence differences between Milton and Grif-1. Alternatively, the contrasting results could reflect some variable and non-physiological binding interactions as a result of approaches that rely on overexpression of tagged proteins. Despite the contrasts, it is clear that an important molecular mechanism for control of kinesin-1-driven transport of mitochondria in neurons has been identified.
In retrospect, a regulatory function for Miro should not be surprising, because monomeric GTPases have long been viewed as nodes that integrate diverse signals (e.g. Jordan et al., 2000), they have a broad regulatory role in organelle membrane dynamics, and they have been implicated previously in axonal transport (Bloom et al., 1993). Consistent with a broad role, Miro is known to associate with a number of other proteins implicated in mitochondria behavior, including hypoxia upregulated mitochondrial movement regulator (HUMMR), which facilitates anterograde and represses retrograde mitochondria transport under conditions of low oxygen (Li et al., 2009), N-acetylglucosamine transferase (Iyer and Hart, 2003), which can influence the distribution of mitochondria in COS-7 cells when co-overexpressed with Milton-related TRAK1 or TRAK2 (Grif-1) plus the kinesin-1 KIF5C (Brickley et al., 2011) and PINK1, a protein implicated in Parkinson's disease, which influences mitochondrial dynamics and autophagy (Weihofen et al., 2009). It is also particularly interesting that Miro-mediated inhibition of axonal mitochondria transport by Ca2+ affects not only anterograde but also retrograde movement (Russo et al., 2009; Wang and Schwarz, 2009), suggesting that Miro influences dynein, perhaps through kinesin–dynein interdependence or perhaps more directly. These observations, combined with an influence of Miro on mitochondrial fission–fusion (see below), raise the possibility that Miro integrates both anterograde and retrograde mitochondrial transport with fusion–fission dynamics and degradation.
Mitochondrial transport and fusion–fission dynamics
Cycles of outer and inner membrane fission and fusion result in an exchange of genomes, proteins and lipids between mitochondria that is crucial for sustaining their robust structure and function (Cho et al., 2010; Lackner and Nunnari, 2009; Tatsuta and Langer, 2008). A growing number of observations suggest that there are important functional connections between mitochondrial fission–fusion dynamics and axonal transport. These include reports that overexpression of either Miro or Milton can enhance mitochondrial fusion (Fransson et al., 2006; Koutsopoulos et al., 2010; Saotome et al., 2008) and that inhibition of the mitochondrial fission protein Drp1 (Labrousse et al., 1999; Smirnova et al., 2001) greatly reduces the number of mitochondria in synaptic terminals (Verstreken et al., 2005). In addition, dynein and dynactin help recruit Drp1 to the outer membrane (Varadi et al., 2004), and inhibition of myosin V increases the lengths of axonal mitochondria (Pathak et al., 2010). It is particularly striking that mitofusin 2, a disease gene involved in Charcot–Marie–Tooth neurodegeneration that is well known for having a central role in outer membrane fusion, facilitates the axonal transport of mitochondria in both directions and it can form a complex with Miro (Baloh et al., 2007; Misko et al., 2010).
The balance between fusion and fission determines whether mitochondria exist as a single large reticulum or as discrete single organelles. Unlike the reticular networks seen in neuronal cell bodies (e.g. Li et al., 2004; Popov et al., 2005), mitochondria in axons are discrete bean-shaped organelles, ranging from ~100 nm to several micrometers long (Pilling et al., 2006). Although the different lengths within this range do not correlate with differing run velocities or other behavior in Drosophila neurons (Pathak et al., 2010; Pilling et al., 2006; Russo et al., 2009), extremely long axonal mitochondria that are generated by gross perturbation of fission–fusion do not move (Amiri and Hollenbeck, 2008). These observations suggest that appropriately balanced mitochondrial fission and fusion generates discrete mitochondria that are suitable for transport over long distances, providing one explanation for cross-talk between transport and fission–fusion processes. Conversely, perhaps transport supports fission–fusion dynamics in axons. Although mitochondrial fusion and associated mixing of components is crucial for robust physiology, mitochondria in axons are often widely spaced. Such spacing precludes fusion for isolated stationary mitochondria; a problem that might be solved by long-distance transport. But does fusion occur in axons? Discrete fusion events are difficult to discern in the narrow confines of axons, because the superimposition of discrete moving mitochondria is easy to confuse with bona fide fusion. However, inhibition of the fission factor Drp1 or overexpression of the fusion protein Mfn1 in vertebrate neurons both generate unusually long mitochondria in the distal axon (Amiri and Hollenbeck, 2008). Furthermore, it is notable that studies with non-neuronal cells have shown that transported mitochondria can mix their contents substantially during contacts that are as brief as 2 seconds and that moving mitochondria are more likely to fuse (Liu et al., 2009; Twig et al., 2010). If such ‘kiss-and-run’ interactions occur as mitochondria pass one another in the axon, fusion-like mixing of components might be extensive – and indeed could be one purpose of their long-distance axonal transport. Unraveling the functional relationships between mitochondrial fission–fusion and motility, and clarifying the structural interactions between the fission–fusion and motor-adaptor-regulator machineries should generate some exciting new insights.
Conclusions
Axonal transport, which is crucial for the development and proper function of neurons, offers powerful insights into the fundamental mechanisms of cytoplasmic transport, and is an important focus for deciphering the pathology of human neurodegenerative diseases. There are numerous types of axonal transport cargoes with distinct functions and different life cycles. Thus despite the relatively simple anterograde-retrograde linear geometry of axons and their uniformly ordered microtubules, molecular mechanisms of axonal transport are proving to be complex. The mitochondrion, in its evolutionary path from free bacterium to semi-autonomous symbiotic organelle, has developed diverse functions that are central to a variety of metabolic pathways and signaling processes, including those that control ATP supplies, ion homeostasis and cell death. Because of the complex roles of mitochondria and the extraordinary architecture of neurons, the pathways that control mitochondria transport have probably evolved to sense, integrate and respond to many cellular and extracellular cues so as to sustain optimal mitochondrial distribution. Challenges for the near future are to continue to elucidate the detailed mechanisms by which mitochondrial movement in axons is accomplished, and to determine which of the features of this process are common with other organelle transport mechanisms and which are unique. The studies mentioned here, and others we lacked the space to discuss, have identified a range of interesting candidates for components of the machinery that produces the characteristic movements and distribution of axonal mitochondria. However, mitochondrial movement varies among taxa, cell types and even neuron types, so it should not surprise us if the molecules and mechanisms that regulate this movement also vary. Indeed, this field has benefitted and will undoubtedly continue to benefit from the use of different experimental systems, ranging from those that lack physiological landmarks or surface signaling, such as cultured neurons or squid axoplasm (Brady et al., 1985), thus clearing the decks for molecular analysis, to ‘noisier’, more physiologically complex systems that retain local signaling and landmarks that are important for mitochondrial distribution, such as the intact Drosophila nervous system (Pilling et al., 2006; Shidara and Hollenbeck, 2010) or myelinating vertebrate cultures (Ohno et al., 2011; Zhang et al., 2010).
References
- Adams R. J. (1982). Organelle movement in axons depends on ATP. Nature 297, 327-329 [DOI] [PubMed] [Google Scholar]
- Akhmanova A., Hammer J. A., 3rd (2010). Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 22, 479-487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally S., Larson A. G., Barlan K., Rice S. E., Gelfand V. I. (2009). Opposite-polarity motors activate one another to trigger cargo transport in live cells. J. Cell Biol. 187, 1071-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri M., Hollenbeck P. J. (2008). Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev. Neurobiol. 68, 1348-1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama T., Hata S., Nakao T., Tanigawa Y., Oka C., Kawaichi M. (2009). Cayman ataxia protein caytaxin is transported by kinesin along neurites through binding to kinesin light chains. J. Cell Sci. 122, 4177-4185 [DOI] [PubMed] [Google Scholar]
- Baloh R. H., Schmidt R. E., Pestronk A., Milbrandt J. (2007). Altered axonal mitochondrial transport in the pathogenesis of Charcot-Marie-Tooth disease from mitofusin 2 mutations. J. Neurosci. 27, 422-430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baqri R. M., Turner B. A., Rheuben M. B., Hammond B. D., Kaguni L. S., Miller K. E. (2009). Disruption of mitochondrial DNA replication in Drosophila increases mitochondrial fast axonal transport in vivo. PLoS ONE 4, e7874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus R. V., Klyachko O., Horiuchi D., Dickson B. J., Saxton W. M. (2008). Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol. Biol. Cell 19, 274-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett W. P., Banker G. A. (1984). An electron microscopic study of the development of axons and dendrites by hippocampal neurons in culture. I. Cells which develop without intercellular contacts. J. Neurosci. 4, 1944-1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearer E. L., Reese T. S. (1999). Association of actin filaments with axonal microtubule tracts. J. Neurocytol. 28, 85-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg J. S., Powell B. C., Cheney R. E. (2001). A millennial myosin census. Mol. Biol. Cell 12, 780-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackstone C., O'Kane C. J., Reid E. (2011). Hereditary spastic paraplegias: membrane traffic and the motor pathway. Nature Rev. Neurosci. 12, 31-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom G. S., Richards B. W., Leopold P. L., Ritchey D. M., Brady S. T. (1993). GTP gamma S inhibits organelle transport along axonal microtubules. J. Cell Biol. 120, 467-476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D., Yin H. L., Stossel T. P. (1984). Gelsolin inhibition of fast axonal transport indicates a requirement for actin microfilaments. Nature 310, 56-58 [DOI] [PubMed] [Google Scholar]
- Brady S. T., Lasek R. J., Allen R. D. (1985). Video microscopy of fast axonal transport in extruded axoplasm: a new model for study of molecular mechanisms. Cell Motil. 5, 81-101 [DOI] [PubMed] [Google Scholar]
- Brady S. T., Pfister K. K., Bloom G. S. (1990). A monoclonal antibody against kinesin inhibits both anterograde and retrograde fast axonal transport in squid axoplasm. Proc. Natl. Acad. Sci. USA 87, 1061-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley K., Pozo K., Stephenson F. A. (2011). N-acetylglucosamine transferase is an integral component of a kinesin-directed mitochondrial trafficking complex. Biochim. Biophys. Acta 1813, 269-281 [DOI] [PubMed] [Google Scholar]
- Bridgman P. C. (2004). Myosin-dependent transport in neurons. J. Neurobiol. 58, 164-174 [DOI] [PubMed] [Google Scholar]
- Byrd D. T., Kawasaki M., Walcoff M., Hisamoto N., Matsumoto K., Jin Y. (2001). UNC-16, a JNK-signaling scaffold protein, regulates vesicle transport in C. elegans. Neuron 32, 787-800 [DOI] [PubMed] [Google Scholar]
- Cai Q., Sheng Z. H. (2009). Mitochondrial transport and docking in axons. Exp. Neurol. 218, 257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Gerwin C., Sheng Z. H. (2005). Syntabulin-mediated anterograde transport of mitochondria along neuronal processes. J. Cell Biol. 170, 959-969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Pan P. Y., Sheng Z. H. (2007). Syntabulin-kinesin-1 family member 5B-mediated axonal transport contributes to activity-dependent presynaptic assembly. J. Neurosci. 27, 7284-7296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Singh B. B., Aslanukov A., Zhao H., Ferreira P. A. (2001). The docking of kinesins, KIF5B and KIF5C, to Ran-binding protein 2 (RanBP2) is mediated via a novel RanBP2 domain. J. Biol. Chem. 276, 41594-41602 [DOI] [PubMed] [Google Scholar]
- Carter A. P., Cho C., Jin L., Vale R. D. (2011). Crystal structure of the dynein motor domain. Science 331, 1159-1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chada S. R., Hollenbeck P. J. (2003). Mitochondrial movement and positioning in axons: the role of growth factor signaling. J. Exp. Biol. 206, 1985-1992 [DOI] [PubMed] [Google Scholar]
- Chada S. R., Hollenbeck P. J. (2004). Nerve growth factor signaling regulates motility and docking of axonal mitochondria. Curr. Biol. 14, 1272-1276 [DOI] [PubMed] [Google Scholar]
- Chang D. T., Honick A. S., Reynolds I. J. (2006). Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 26, 7035-7045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Chan D. C. (2009). Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Hum. Mol. Genet. 18, R169-R176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. M., Gerwin C., Sheng Z. H. (2009). Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons. J. Neurosci. 29, 9429-9438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier-Larsen E., Holzbaur E. L. (2006). Axonal transport and neurodegenerative disease. Biochim. Biophys. Acta 1762, 1094-1108 [DOI] [PubMed] [Google Scholar]
- Cho D. H., Nakamura T., Lipton S. A. (2010). Mitochondrial dynamics in cell death and neurodegeneration. Cell. Mol. Life Sci. 67, 3435-3447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. I., Cai Y., Yi H., Yeh A., Aslanukov A., Ferreira P. A. (2007). Association of the kinesin-binding domain of RanBP2 to KIF5B and KIF5C determines mitochondria localization and function. Traffic 8, 1722-1735 [DOI] [PubMed] [Google Scholar]
- Crimella C., Baschirotto C., Arnoldi A., Tonelli A., Tenderini E., Airoldi G., Martinuzzi A., Trabacca A., Losito L., Scarlato M., et al. (2011). Mutations in the motor and stalk domains of KIF5A in spastic paraplegia type 10 and in axonal Charcot-Marie-Tooth type 2. Clin. Genet. [Epub ahead of print] doi:10.1111/j.1399-0004.2011.01717.x [DOI] [PubMed] [Google Scholar]
- Das S., Boczan J., Gerwin C., Zald P. B., Sheng Z. H. (2003). Regional and developmental regulation of syntaphilin expression in the brain: a candidate molecular element of synaptic functional differentiation. Brain Res. Mol. Brain Res. 116, 38-49 [DOI] [PubMed] [Google Scholar]
- de Duve C. (2007). The origin of eukaryotes: a reappraisal. Nat. Rev. Genet. 8, 395-403 [DOI] [PubMed] [Google Scholar]
- De Vos K., Severin F., Van Herreweghe F., Vancompernolle K., Goossens V., Hyman A., Grooten J. (2000). Tumor necrosis factor induces hyperphosphorylation of kinesin light chain and inhibits kinesin-mediated transport of mitochondria. J. Cell Biol. 149, 1207-1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos K. J., Grierson A. J., Ackerley S., Miller C. C. (2008). Role of axonal transport in neurodegenerative diseases. Annu. Rev. Neurosci. 31, 151-173 [DOI] [PubMed] [Google Scholar]
- Dixit R., Ross J. L., Goldman Y. E., Holzbaur E. L. (2008). Differential regulation of dynein and kinesin motor proteins by tau. Science 319, 1086-1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebneth A., Godemann R., Stamer K., Illenberger S., Trinczek B., Mandelkow E. (1998). Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer's disease. J. Cell Biol. 143, 777-794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim M. A., Lasek R. J., Brady S. T., Hodge A. J. (1985). AVEC-DIC and electron microscopic analyses of axonally transported particles in cold-blocked squid giant axons. J. Neurocytol. 14, 689-704 [DOI] [PubMed] [Google Scholar]
- Fath K. R., Lasek R. J. (1988). Two classes of actin microfilaments are associated with the inner cytoskeleton of axons. J. Cell Biol. 107, 613-621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson S., Ruusala A., Aspenström P. (2006). The atypical Rho GTPases Miro-1 and Miro-2 have essential roles in mitochondrial trafficking. Biochem. Biophys. Res. Commun. 344, 500-510 [DOI] [PubMed] [Google Scholar]
- Frederick R. L., Shaw J. M. (2007). Moving mitochondria: establishing distribution of an essential organelle. Traffic 8, 1668-1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S. R., Schroer T. A., Szilak I., Steuer E. R., Sheetz M. P., Cleveland D. W. (1991). Dynactin, a conserved, ubiquitously expressed component of an activator of vesicle motility mediated by cytoplasmic dynein. J. Cell Biol. 115, 1639-1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gioio A. E., Eyman M., Zhang H., Lavina Z. S., Giuditta A., Kaplan B. B. (2001). Local synthesis of nuclear-encoded mitochondrial proteins in the presynaptic nerve terminal. J. Neurosci. Res. 64, 447-453 [DOI] [PubMed] [Google Scholar]
- Glater E. E., Megeath L. J., Stowers R. S., Schwarz T. L. (2006). Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell Biol. 173, 545-557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. Y., Wang X., Schwarz T. L. (2008). Axonal transport and the delivery of pre-synaptic components. Curr. Opin. Neurobiol. 18, 495-503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Górska-Andrzejak J., Stowers R. S., Borycz J., Kostyleva R., Schwarz T. L., Meinertzhagen I. A. (2003). Mitochondria are redistributed in Drosophila photoreceptors lacking milton, a kinesin-associated protein. J. Comp. Neurol. 463, 372-388 [DOI] [PubMed] [Google Scholar]
- Grafstein B., Forman D. S. (1980). Intracellular transport in neurons. Physiol. Rev. 60, 1167-1283 [DOI] [PubMed] [Google Scholar]
- Gross S. P. (2004). Hither and yon: a review of bi-directional microtubule-based transport. Phys. Biol. 1, R1-R11 [DOI] [PubMed] [Google Scholar]
- Gross S. P., Tuma M. C., Deacon S. W., Serpinskaya A. S., Reilein A. R., Gelfand V. I. (2002). Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855-865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Macleod G. T., Wellington A., Hu F., Panchumarthi S., Schoenfield M., Marin L., Charlton M. P., Atwood H. L., Zinsmaier K. E. (2005). The GTPase dMiro is required for axonal transport of mitochondria to Drosophila synapses. Neuron 47, 379-393 [DOI] [PubMed] [Google Scholar]
- Habermann A., Schroer T. A., Griffiths G., Burkhardt J. K. (2001). Immunolocalization of cytoplasmic dynein and dynactin subunits in cultured macrophages: enrichment on early endocytic organelles. J. Cell Sci. 114, 229-240 [DOI] [PubMed] [Google Scholar]
- Haghnia M., Cavalli V., Shah S. B., Schimmelpfeng K., Brusch R., Yang G., Herrera C., Pilling A., Goldstein L. S. (2007). Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment. Mol. Biol. Cell 18, 2081-2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks A. G., Perlson E., Ross J. L., Schroeder H. W., 3rd, Tokito M., Holzbaur E. L. (2010). Motor coordination via a tug-of-war mechanism drives bidirectional vesicle transport. Curr. Biol. 20, 697-702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillefors M., Gioio A. E., Mameza M. G., Kaplan B. B. (2007). Axon viability and mitochondrial function are dependent on local protein synthesis in sympathetic neurons. Cell. Mol. Neurobiol. 27, 701-716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Sato-Yoshitake R., Kobayashi N., Pfister K. K., Bloom G. S., Brady S. T. (1991). Kinesin associates with anterogradely transported membranous organelles in vivo. J. Cell Biol. 114, 295-302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa N., Noda Y., Tanaka Y., Niwa S. (2009). Kinesin superfamily motor proteins and intracellular transport. Nat. Rev. Mol. Cell Biol. 10, 682-696 [DOI] [PubMed] [Google Scholar]
- Hollenbeck P. J. (1993). Products of endocytosis and autophagy are retrieved from axons by regulated retrograde organelle transport. J. Cell Biol. 121, 305-315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck P. J., Saxton W. M. (2005). The axonal transport of mitochondria. J. Cell Sci. 118, 5411-5419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbeck P. J., Bray D., Adams R. J. (1985). Effects of the uncoupling agents FCCP and CCCP on the saltatory movements of cytoplasmic organelles. Cell Biol. Int. Rep. 9, 193-199 [DOI] [PubMed] [Google Scholar]
- Holzbaur E. (2010). Axonal transport: CDKs as traffic signals for motor-ists along the axon? Curr. Biol. 20, R641-R642 [DOI] [PubMed] [Google Scholar]
- Iyer S. P., Hart G. W. (2003). Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J. Biol. Chem. 278, 24608-24616 [DOI] [PubMed] [Google Scholar]
- Jordan J. D., Landau E. M., Iyengar R. (2000). Signaling networks: the origins of cellular multitasking. Cell 103, 193-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J. S., Tian J. H., Pan P. Y., Zald P., Li C., Deng C., Sheng Z. H. (2008). Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karcher R. L., Deacon S. W., Gelfand V. I. (2002). Motor-cargo interactions: the key to transport specificity. Trends Cell Biol. 12, 21-27 [DOI] [PubMed] [Google Scholar]
- Karki S., Holzbaur E. L. (1995). Affinity chromatography demonstrates a direct binding between cytoplasmic dynein and the dynactin complex. J. Biol. Chem. 270, 28806-28811 [DOI] [PubMed] [Google Scholar]
- Khodjakov A., Lizunova E. M., Minin A. A., Koonce M. P., Gyoeva F. K. (1998). A specific light chain of kinesin associates with mitochondria in cultured cells. Mol. Biol. Cell 9, 333-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos O. S., Laine D., Osellame L., Chudakov D. M., Parton R. G., Frazier A. E., Ryan M. T. (2010). Human Miltons associate with mitochondria and induce microtubule-dependent remodeling of mitochondrial networks. Biochim. Biophys. Acta 1803, 564-574 [DOI] [PubMed] [Google Scholar]
- Kural C., Kim H., Syed S., Goshima G., Gelfand V. I., Selvin P. R. (2005). Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science 308, 1469-1472 [DOI] [PubMed] [Google Scholar]
- Kuznetsov S. A., Langford G. M., Weiss D. G. (1992). Actin-dependent organelle movement in squid axoplasm. Nature 356, 722-725 [DOI] [PubMed] [Google Scholar]
- Labrousse A. M., Zappaterra M. D., Rube D. A., van der Bliek A. M. (1999). C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol. Cell 4, 815-826 [DOI] [PubMed] [Google Scholar]
- Lackner L. L., Nunnari J. M. (2009). The molecular mechanism and cellular functions of mitochondrial division. Biochim. Biophys. Acta 1792, 1138-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli G., Schiavo G. (2002). Analysis of retrograde transport in motor neurons reveals common endocytic carriers for tetanus toxin and neurotrophin receptor p75NTR. J. Cell Biol. 156, 233-240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford G. M. (2002). Myosin-V, a versatile motor for short-range vesicle transport. Traffic 3, 859-865 [DOI] [PubMed] [Google Scholar]
- Lao G., Scheuss V., Gerwin C. M., Su Q., Mochida S., Rettig J., Sheng Z. H. (2000). Syntaphilin: a syntaxin-1 clamp that controls SNARE assembly. Neuron 25, 191-201 [DOI] [PubMed] [Google Scholar]
- Lee S. K., Hollenbeck P. J. (2003). Organization and translation of mRNA in sympathetic neurons. J. Cell Sci. 116, 4467-4478 [DOI] [PubMed] [Google Scholar]
- Li Y., Lim S., Hoffman D., Aspenstrom P., Federoff H. J., Rempe D. A. (2009). HUMMR, a hypoxia- and HIF-1alpha-inducible protein, alters mitochondrial distribution and transport. J. Cell Biol. 185, 1065-1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Okamoto K., Hayashi Y., Sheng M. (2004). The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 119, 873-887 [DOI] [PubMed] [Google Scholar]
- Ligon L. A., Steward O. (2000). Role of microtubules and actin filaments in the movement of mitochondria in the axons and dendrites of cultured hippocampal neurons. J. Comp. Neurol. 427, 351-361 [DOI] [PubMed] [Google Scholar]
- Ligon L. A., Tokito M., Finklestein J. M., Grossman F. E., Holzbaur E. L. (2004). A direct interaction between cytoplasmic dynein and kinesin I may coordinate motor activity. J. Biol. Chem. 279, 19201-19208 [DOI] [PubMed] [Google Scholar]
- Ling S. C., Fahrner P. S., Greenough W. T., Gelfand V. I. (2004). Transport of Drosophila fragile X mental retardation protein-containing ribonucleoprotein granules by kinesin-1 and cytoplasmic dynein. Proc. Natl. Acad. Sci. USA 101, 17428-17433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister I., Roberts R., Schmitz S., Walker M., Trinick J., Veigel C., Buss F., Kendrick-Jones J. (2004). Myosin VI: a multifunctional motor. Biochem. Soc. Trans. 32, 685-688 [DOI] [PubMed] [Google Scholar]
- Liu X., Weaver D., Shirihai O., Hajnóczky G. (2009). Mitochondrial ‘kiss-and-run’: interplay between mitochondrial motility and fusion-fission dynamics. EMBO J. 28, 3074-3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logroscino C. A., Sanguinetti C., Catalano F. (1980). Electron microscopic study of the early changes proximal to a constriction in myelinated nerve. Int. Orthop. 4, 121-125 [DOI] [PubMed] [Google Scholar]
- Ma H., Cai Q., Lu W., Sheng Z. H., Mochida S. (2009). KIF5B motor adaptor syntabulin maintains synaptic transmission in sympathetic neurons. J. Neurosci. 29, 13019-13029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill A. F., Kittler J. T. (2010). Control of mitochondrial transport and localization in neurons. Trends Cell Biol. 20, 102-112 [DOI] [PubMed] [Google Scholar]
- MacAskill A. F., Brickley K., Stephenson F. A., Kittler J. T. (2009a). GTPase dependent recruitment of Grif-1 by Miro1 regulates mitochondrial trafficking in hippocampal neurons. Mol. Cell. Neurosci. 40, 301-312 [DOI] [PubMed] [Google Scholar]
- MacAskill A. F., Rinholm J. E., Twelvetrees A. E., Arancibia-Carcamo I. L., Muir J., Fransson A., Aspenstrom P., Attwell D., Kittler J. T. (2009b). Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541-555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaiyandi L. M., Honick A. S., Rintoul G. L., Wang Q. J., Reynolds I. J. (2005). Zn2+ inhibits mitochondrial movement in neurons by phosphatidylinositol 3-kinase activation. J. Neurosci. 25, 9507-9514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E. M., Thies E., Trinczek B., Biernat J., Mandelkow E. (2004). MARK/PAR1 kinase is a regulator of microtubule-dependent transport in axons. J. Cell Biol. 167, 99-110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M., Iyadurai S. J., Gassman A., Gindhart J. G., Jr, Hays T. S., Saxton W. M. (1999). Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport. Mol. Biol. Cell 10, 3717-3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., Vaida B., Bleher R., Crispino M., Giuditta A. (1998). Protein synthesizing units in presynaptic and postsynaptic domains of squid neurons. J. Cell Sci. 111, 3157-3166 [DOI] [PubMed] [Google Scholar]
- Mavlyutov T. A., Cai Y., Ferreira P. A. (2002). Identification of RanBP2- and kinesin-mediated transport pathways with restricted neuronal and subcellular localization. Traffic 3, 630-640 [DOI] [PubMed] [Google Scholar]
- Miller K. E., Sheetz M. P. (2004). Axonal mitochondrial transport and potential are correlated. J. Cell Sci. 117, 2791-2804 [DOI] [PubMed] [Google Scholar]
- Mironov S. L. (2006). Spontaneous and evoked neuronal activities regulate movements of single neuronal mitochondria. Synapse 59, 403-411 [DOI] [PubMed] [Google Scholar]
- Misgeld T., Kerschensteiner M., Bareyre F. M., Burgess R. W., Lichtman J. W. (2007). Imaging axonal transport of mitochondria in vivo. Nat. Methods 4, 559-561 [DOI] [PubMed] [Google Scholar]
- Misko A., Jiang S., Wegorzewska I., Milbrandt J., Baloh R. H. (2010). Mitofusin 2 is necessary for transport of axonal mitochondria and interacts with the Miro/Milton complex. J. Neurosci. 30, 4232-4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G., Szebenyi G., Elluru R., Ratner N., Brady S. T. (2002). Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J. 21, 281-293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G., Szebenyi G., Brown H., Pant H. C., Pigino G., DeBoer S., Beffert U., Brady S. T. (2004). A novel CDK5-dependent pathway for regulating GSK3 activity and kinesin-driven motility in neurons. EMBO J. 23, 2235-2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G., Pigino G., Szebenyi G., You Y., Pollema S., Brady S. T. (2006). JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat. Neurosci. 9, 907-916 [DOI] [PubMed] [Google Scholar]
- Morfini G. A., Burns M., Binder L. I., Kanaan N. M., LaPointe N., Bosco D. A., Brown R. H., Jr, Brown H., Tiwari A., Hayward L., et al. (2009a). Axonal transport defects in neurodegenerative diseases. J. Neurosci. 29, 12776-12786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini G. A., You Y. M., Pollema S. L., Kaminska A., Liu K., Yoshioka K., Björkblom B., Coffey E. T., Bagnato C., Han D., et al. (2009b). Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat. Neurosci. 12, 864-871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R. L., Hollenbeck P. J. (1993). The regulation of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J. Cell Sci. 104, 917-927 [DOI] [PubMed] [Google Scholar]
- Morris R. L., Hollenbeck P. J. (1995). Axonal transport of mitochondria along microtubules and F-actin in living vertebrate neurons. J. Cell Biol. 131, 1315-1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangaku M., Sato-Yoshitake R., Okada Y., Noda Y., Takemura R., Yamazaki H., Hirokawa N. (1994). KIF1B, a novel microtubule plus end-directed monomeric motor protein for transport of mitochondria. Cell 79, 1209-1220 [DOI] [PubMed] [Google Scholar]
- Ohno N., Kidd G. J., Mahad D., Kiryu-Seo S., Avishai A., Komuro H., Trapp B. D. (2011). Myelination and axonal electrical activity modulate the distribution and motility of mitochondria at CNS nodes of Ranvier. J. Neurosci. 31, 7249-7258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Simons K., Dotti C. G. (1992). Axonal and dendritic endocytic pathways in cultured neurons. J. Cell Biol. 119, 123-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Mikami A., Pfister K. K., Vallee R. B. (1992). Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J. Cell Biol. 118, 1133-1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak D., Sepp K. J., Hollenbeck P. J. (2010). Evidence that myosin activity opposes microtubule-based axonal transport of mitochondria. J. Neurosci. 30, 8984-8992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraza-Reyes L., Crider D. G., Pon L. A. (2010). Mitochondrial manoeuvres: latest insights and hypotheses on mitochondrial partitioning during mitosis in Saccharomyces cerevisiae. Bioessays 32, 1040-1049 [DOI] [PubMed] [Google Scholar]
- Perlson E., Maday S., Fu M. M., Moughamian A. J., Holzbaur E. L. (2010). Retrograde axonal transport: pathways to cell death? Trends Neurosci. 33, 335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigino G., Morfini G., Atagi Y., Deshpande A., Yu C., Jungbauer L., LaDu M., Busciglio J., Brady S. (2009). Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc. Natl. Acad. Sci. USA 106, 5907-5912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilling A. D., Horiuchi D., Lively C. M., Saxton W. M. (2006). Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057-2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov V., Medvedev N. I., Davies H. A., Stewart M. G. (2005). Mitochondria form a filamentous reticular network in hippocampal dendrites but are present as discrete bodies in axons: a three-dimensional ultrastructural study. J. Comp. Neurol. 492, 50-65 [DOI] [PubMed] [Google Scholar]
- Rao S., Lang C., Levitan E. S., Deitcher D. L. (2001). Visualization of neuropeptide expression, transport, and exocytosis in Drosophila melanogaster. J. Neurobiol. 49, 159-172 [DOI] [PubMed] [Google Scholar]
- Rapali P., Radnai L., Süveges D., Harmat V., Tölgyesi F., Wahlgren W. Y., Katona G., Nyitray L., Pál G. (2011). Directed evolution reveals the binding motif preference of the LC8/DYNLL hub protein and predicts large numbers of novel binders in the human proteome. PLoS ONE 6, e18818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratner N., Bloom G. S., Brady S. T. (1998). A role for cyclin-dependent kinase(s) in the modulation of fast anterograde axonal transport: effects defined by olomoucine and the APC tumor suppressor protein. J. Neurosci. 18, 7717-7726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. (1993). Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261, 50-58 [DOI] [PubMed] [Google Scholar]
- Reed N. A., Cai D., Blasius T. L., Jih G. T., Meyhofer E., Gaertig J., Verhey K. J. (2006). Microtubule acetylation promotes kinesin-1 binding and transport. Curr. Biol. 16, 2166-2172 [DOI] [PubMed] [Google Scholar]
- Reis K., Fransson A., Aspenström P. (2009). The Miro GTPases: at the heart of the mitochondrial transport machinery. FEBS Lett. 583, 1391-1398 [DOI] [PubMed] [Google Scholar]
- Rice S., Lin A. W., Safer D., Hart C. L., Naber N., Carragher B. O., Cain S. M., Pechatnikova E., Wilson-Kubalek E. M., Whittaker M., et al. (1999). A structural change in the kinesin motor protein that drives motility. Nature 402, 778-784 [DOI] [PubMed] [Google Scholar]
- Rintoul G. L., Reynolds I. J. (2010). Mitochondrial trafficking and morphology in neuronal injury. Biochim. Biophys. Acta 1802, 143-150 [DOI] [PubMed] [Google Scholar]
- Rintoul G. L., Filiano A. J., Brocard J. B., Kress G. J., Reynolds I. J. (2003). Glutamate decreases mitochondrial size and movement in primary forebrain neurons. J. Neurosci. 23, 7881-7888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S. L., Gelfand V. I. (1998). Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr. Biol. 8, 161-164 [DOI] [PubMed] [Google Scholar]
- Russo G. J., Louie K., Wellington A., Macleod G. T., Hu F., Panchumarthi S., Zinsmaier K. E. (2009). Drosophila Miro is required for both anterograde and retrograde axonal mitochondrial transport. J. Neurosci. 29, 5443-5455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruthel G., Hollenbeck P. J. (2003). Response of mitochondrial traffic to axon determination and differential branch growth. J. Neurosci. 23, 8618-8624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santama N., Er C. P., Ong L. L., Yu H. (2004). Distribution and functions of kinectin isoforms. J. Cell Sci. 117, 4537-4549 [DOI] [PubMed] [Google Scholar]
- Saotome M., Safiulina D., Szabadkai G., Das S., Fransson A., Aspenstrom P., Rizzuto R., Hajnóczky G. (2008). Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl. Acad. Sci. USA 105, 20728-20733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnapp B. J., Reese T. S. (1989). Dynein is the motor for retrograde axonal transport of organelles. Proc. Natl. Acad. Sci. USA 86, 1548-1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shidara Y., Hollenbeck P. J. (2010). Defects in mitochondrial axonal transport and membrane potential without increased reactive oxygen species production in a Drosophila model of Friedreich ataxia. J. Neurosci. 30, 11369-11378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E., Griparic L., Shurland D. L., van der Bliek A. M. (2001). Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell 12, 2245-2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. J., Pozo K., Brickley K., Stephenson F. A. (2006). Mapping the GRIF-1 binding domain of the kinesin, KIF5C, substantiates a role for GRIF-1 as an adaptor protein in the anterograde trafficking of cargoes. J. Biol. Chem. 281, 27216-27228 [DOI] [PubMed] [Google Scholar]
- Sotelo-Silveira J. R., Calliari A., Kun A., Koenig E., Sotelo J. R. (2006). RNA trafficking in axons. Traffic 7, 508-515 [DOI] [PubMed] [Google Scholar]
- Stamer K., Vogel R., Thies E., Mandelkow E., Mandelkow E. M. (2002). Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress. J. Cell Biol. 156, 1051-1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen W., Karki S., Vaughan K. T., Vallee R. B., Holzbaur E. L., Weiss D. G., Kuznetsov S. A. (1997). The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell 8, 2077-2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers R. S., Megeath L. J., Górska-Andrzejak J., Meinertzhagen I. A., Schwarz T. L. (2002). Axonal transport of mitochondria to synapses depends on milton, a novel Drosophila protein. Neuron 36, 1063-1077 [DOI] [PubMed] [Google Scholar]
- Su Q., Cai Q., Gerwin C., Smith C. L., Sheng Z. H. (2004). Syntabulin is a microtubule-associated protein implicated in syntaxin transport in neurons. Nat. Cell Biol. 6, 941-953 [DOI] [PubMed] [Google Scholar]
- Tanaka K., Sugiura Y., Ichishita R., Mihara K., Oka T. (2011). KLP6: a newly identified kinesin that regulates the morphology and transport of mitochondria in neuronal cells. J. Cell Sci. 124, 2457-2465 [DOI] [PubMed] [Google Scholar]
- Tatsuta T., Langer T. (2008). Quality control of mitochondria: protection against neurodegeneration and ageing. EMBO J. 27, 306-314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Berchtold N. C., Perreau V. M., Tu C. H., Li Jeon N., Cotman C. W. (2009). Axonal mRNA in uninjured and regenerating cortical mammalian axons. J. Neurosci. 29, 4697-4707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinczek B., Ebneth A., Mandelkow E. M., Mandelkow E. (1999). Tau regulates the attachment/detachment but not the speed of motors in microtubule-dependent transport of single vesicles and organelles. J. Cell Sci. 112, 2355-2367 [DOI] [PubMed] [Google Scholar]
- Trybus K. M. (2008). Myosin V from head to tail. Cell. Mol. Life Sci. 65, 1378-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S., Ishikawa H. (1980). The movement of membranous organelles in axons. Electron microscopic identification of anterogradely and retrogradely transported organelles. J. Cell Biol. 84, 513-530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G., Liu X., Liesa M., Wikstrom J. D., Molina A. J., Las G., Yaniv G., Hajnóczky G., Shirihai O. S. (2010). Biophysical properties of mitochondrial fusion events in pancreatic beta-cells and cardiac cells unravel potential control mechanisms of its selectivity. Am. J. Physiol. Cell Physiol. 299, C477-C487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida A., Alami N. H., Brown A. (2009). Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments. Mol. Biol. Cell 20, 4997-5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale R. D. (2003). The molecular motor toolbox for intracellular transport. Cell 112, 467-480 [DOI] [PubMed] [Google Scholar]
- Vale R. D., Milligan R. A. (2000). The way things move: looking under the hood of molecular motor proteins. Science 288, 88-95 [DOI] [PubMed] [Google Scholar]
- Vale R. D., Reese T. S., Sheetz M. P. (1985). Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A., Johnson-Cadwell L. I., Cirulli V., Yoon Y., Allan V. J., Rutter G. A. (2004). Cytoplasmic dynein regulates the subcellular distribution of mitochondria by controlling the recruitment of the fission factor dynamin-related protein-1. J. Cell Sci. 117, 4389-4400 [DOI] [PubMed] [Google Scholar]
- Vaughan K. T., Vallee R. B. (1995). Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J. Cell Biol. 131, 1507-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg J., Hollenbeck P. J. (2008). Mitochondrial membrane potential in axons increases with local nerve growth factor or semaphorin signaling. J. Neurosci. 28, 8306-8315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhey K. J., Hammond J. W. (2009). Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10, 765-777 [DOI] [PubMed] [Google Scholar]
- Vershinin M., Carter B. C., Razafsky D. S., King S. J., Gross S. P. (2007). Multiple-motor based transport and its regulation by Tau. Proc. Natl. Acad. Sci. USA 104, 87-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vershinin M., Xu J., Razafsky D. S., King S. J., Gross S. P. (2008). Tuning microtubule-based transport through filamentous MAPs: the problem of dynein. Traffic 9, 882-892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P., Ly C. V., Venken K. J., Koh T. W., Zhou Y., Bellen H. J. (2005). Synaptic mitochondria are critical for mobilization of reserve pool vesicles at Drosophila neuromuscular junctions. Neuron 47, 365-378 [DOI] [PubMed] [Google Scholar]
- Wang X., Schwarz T. L. (2009). The mechanism of Ca2+ -dependent regulation of kinesin-mediated mitochondrial motility. Cell 136, 163-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihofen A., Thomas K. J., Ostaszewski B. L., Cookson M. R., Selkoe D. J. (2009). Pink1 forms a multiprotein complex with Miro and Milton, linking Pink1 function to mitochondrial trafficking. Biochemistry 48, 2045-2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstead B., Gull K. (2006). A “holistic” kinesin phylogeny reveals new kinesin families and predicts protein functions. Mol. Biol. Cell 17, 1734-1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstead B., Gull K. (2007). Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8, 1708-1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M., Weaver D., Hajnóczky G. (2004). Control of mitochondrial motility and distribution by the calcium signal: a homeostatic circuit. J. Cell Biol. 167, 661-672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. L., Ho P. L., Kintner D. B., Sun D., Chiu S. Y. (2010). Activity-dependent regulation of mitochondrial motility by calcium and Na/K-ATPase at nodes of Ranvier of myelinated nerves. J. Neurosci. 30, 3555-3566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinsmaier K. E., Babic M., Russo G. J. (2009). Mitochondrial transport dynamics in axons and dendrites. Results Probl. Cell Differ. 48, 361-381 [DOI] [PubMed] [Google Scholar]
- Züchner S., Vance J. M. (2006). Mechanisms of disease: a molecular genetic update on hereditary axonal neuropathies. Nat. Clin. Pract. Neurol. 2, 45-53 [DOI] [PubMed] [Google Scholar]
- Züchner S., Mersiyanova I. V., Muglia M., Bissar-Tadmouri N., Rochelle J., Dadali E. L., Zappia M., Nelis E., Patitucci A., Senderek J., et al. (2004). Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot-Marie-Tooth neuropathy type 2A. Nat. Genet. 36, 449-451 [DOI] [PubMed] [Google Scholar]