Abstract
In plants, the nitrate transporters, NRT1.1 and NRT2.1, are mainly responsible for nitrate uptake. Intriguingly, both nitrate transporters are located in a complementary manner in different cells layers of the mature root suggesting that their coordination should occur during nitrate uptake and plant growth. This hypothesis was examined on 5-d-old rape seedlings grown on agar medium supplemented with 1 or 5mM nitrate. Seedlings were treated with increasing potassium glutamate concentrations in order to uncouple the two nitrate transporters by inhibiting BnNRT2.1 expression and activity specifically. In both nitrate treatments, increasing the glutamate concentrations from 0.5 to 10mM induced a reduction in 15NO3- uptake and an inhibition of N assimilation. The decrease in 15NO3- uptake was caused by downregulation of BnNRT2.1 expression but surprisingly it was not compensated by the upregulation of BnNRT1.1. This created an unprecedented physiological situation where the effects of the nitrate signal on shoot growth were solely modulated by nitrate absorption. In these conditions, the osmotic water flow for volumetric shoot growth was mainly dependent on active nitrate transport and nitrate signaling. This behavior was confirmed by the allometric relationships found between changes in the root length with 15N and water accumulation in the shoot. These findings demonstrate that the BnNRT2.1 transporter is essential for nitrate uptake and growth, and renew the question of the respective roles of the NRT2.1 and NRT1.1 transporters in nitrate uptake and sensing at the whole plant level.
Keywords: Brassica napus, nitrate uptake, water uptake, cell expansion, organ growth, nitrate signaling, NRT2.1 and NRT1.1 nitrate transporters
Introduction
Central questions regarding the coordination between nitrate transporters and water fluxes for growth in intact plants deserve more attention in order to improve relations between water use efficiency (WUE) and nitrogen use efficiency (NUE).1,2 One puzzle concerns the relationship between the coordination and the functional overlap in expression and activities of nitrate transporters and the developmental changes in the root and shoot during water flow for growth and transpiration. In the 1970s a model based on a flux-force relationship with two flow components and two compartments was proposed to account for water flows for growth and transpiration in relation to solute fluxes through the roots.3,4 This model has been intensively tested and validated in excised roots of herbaceous and woody species using invasive methods such as pressure chambers.5-7 Thus, it has been demonstrated that water transport in plants results from two mechanisms: the hydraulic water flow in rapidly transpiring plants and the osmotic water flow in slowly transpiring plants driven by solute accumulation in the xylem.5,7,8 However, it is possible to create non-invasive experimental conditions on intact plants in order to dissociate hydraulic flow from osmotic flow so that the role of nitrate transporters in osmotic water flow during growth can be studied. Indeed, at the whole plant level, the water balance requires conservation of mass and may be characterized by the following equation9: A-t = G + H
Where A and T are the fluxes for absorption and transpiration, G is the storage flux for growth and H is the storage flux for re-hydration (or dehydration) corresponding to tissue capacitance. This relationship may be simplified depending on experimental conditions. For example, transpiration modifies water potential of plant tissues and modulates cell enlargement by turgor.10 Accordingly, when transpiration is high, growth is reduced to zero and in such cases: A = T
During the night, when transpiration is strongly reduced, water status of the plant is constant and capacitance is low and constant because of the absence of large variations in hydrostatic pressure; accordingly: A-t = G
This experimental condition, in which osmotic water flow is predominant, has already been tested in relation to mineral nutrients by supplying plants only during the night.11-13 Unfortunately these studies failed to examine the changes in activity and transcription of nutrient transporters such as nitrate transporters.
Furthermore, in very low or non-transpiring conditions, the relationship can be further simplified; in this case: A = G
In this condition, the rate-limiting resistance to water flow was the site of cell enlargement of the growing tissue because hydraulic resistance of the roots was smaller.9 In a previous study we have created this physiological situation by using an agar Petri dish system and by supplying oil seed rape plantlets with a large range of external nitrate concentrations (Le Ny et al., 2012). As expected, both the NRT1.1 and NRT2.1 nitrate transporters, which are involved predominantly in nitrate uptake by plant roots, responded differently to changes in root and shoot growth in response to nitrate availability.14-18 Expression levels of BnNrt2.1 were linearly correlated with variations in root length, suggesting that NRT2.1 expression adapts the nitrate uptake to plantlet growth.19 In contrast, expression levels of BnNrt1.1 in the root and shoot were strongly increased during growth changes of the root and shoot that were induced by increasing external nitrate concentrations. Thus, at a nitrate supply of between 1 and 5mM, the shoot surface area was increased by two, whereas the root length was reduced 2-fold. At the same time, osmotic water flow for shoot growth was increased twice. These results were consistent with recent evidence that the NRT1.1 transporter is not a nitrate transporter alone, but is also involved in nitrate sensing20 and root growth via auxin transport.21 Taken together, these findings raise questions of whether the NRT2.1 transporter is implicated in the regulation of osmotic water flow for growth during low transpiration conditions through regulation of nitrate uptake. Indeed, localization studies with GFP and GUS activities have revealed in rice and Arabidopsis that NRT1.1 and NRT2.1 are complementary and localized in different cell layers of the mature root. NRT1.1 is mainly expressed in the vascular cylinder in the endodermal and pericycle cell layers,14,22-24 whereas NRT2.1 is specifically expressed in epidermal and cortical cell layers.25-27 Because NRT2.1 has a high nitrate affinity and is inducible by nitrate, the lack of large changes in NRT2.1 transcription compared with NRT1.1 in low transpiring conditions when water for volumetric expansion and nitrate inflows reached their maximum level is very intriguing and deserves more attention.19
In order to analyze whether the NRT2.1 transporter is involved in the regulation of osmotic water flow for volumetric growth during low transpiration conditions through regulation of nitrate uptake and signaling, we created a new experimental situation in which the activity and transcription of NRT2.1 were uncoupled from those of NRT1.1. This physiological condition was obtained by co-treating plantlets over five days with increasing potassium glutamate concentrations in the presence of 1 and 5mM nitrate. Indeed, it is well demonstrated that amino acids (AA) such as glutamate (Glu) specifically inhibited NRT2.1 at the transcription and activity levels28-30 without modifying NRT1.1 activity and transcription.31,32 Because Glu is the central compound of nitrogen (N) metabolism in plants, such a pharmacological treatment is questionable with regard to possible side effects on N metabolism. However, we can assume that compared with NRT2.1 mutant analysis, such treatment may avoid compensation mechanisms in nitrate uptake by inhibiting the other redundant genes of the NRT2 family.15,16,33 Both 1 and 5mM nitrate external concentrations were chosen because significant changes in root morphology, hydraulic conductivity and BnNRT1.1 transcription levels were previously observed at these concentrations.19 We also used a differential 15N labeling of nitrate and Glu in order to measure the uptake of nitrate and Glu, to quantify the final N status obtained by the nitrate or nitrate-Glu combined treatments and to analyze the compensation mechanisms for the 15N uptake and accumulation from both N forms. Nitrate accumulation in both treatments was also measured by ion chromatography analyzes (Dionex). Nitrogen assimilation was evaluated by quantification of individual amino acids using ultra performance liquid chromatography (UPLC)-based amino acid profiling.
Results
Chronic glutamate treatment strongly inhibits root growth and to a lesser extent the shoot growth of B. napus seedlings
Under agar plate growth conditions, Brassica napus seedlings supplied with a large range of external KNO3 concentrations from 1 to 11 mM over five days exhibited a switch in the shoot growth relative to root growth between 1 and 5mM (Fig. 1A; Fig. S1 at PSB online). Beyond 5mM nitrate treatment, the shoot:root ratio remained stable (Fig. 1B). These results were consistent with previous data obtained in the same growing conditions.19 This led us to choose 1 and 5mM nitrate concentrations as threshold values of nitrate treatment to characterize the effects of Glu on nitrate and osmotic water fluxes for growth. Therefore the seedlings were co-treated over 5 d with 1 or 5mM nitrate and increasing potassium Glu concentrations. The co-treated seedlings with varying Glu concentrations in the presence of 1mM nitrate showed a reduction in the shoot:root ratio (Fig. 1A), whereas the shoot:root ratio of seedlings co-treated with 5mM nitrate was maintained (Fig. 1B). In both nitrate treatments, Glu induced growth inhibition of the shoots and roots (Fig. 2A and B) and caused a chlorotic phenotype compared with control seedlings (data not shown). However, whatever the nitrate treatment (1 or 5mM), Glu-induced inhibition was more significant in the roots than in the shoots (Fig. 2A-C). The reduction in root elongation was detectable for both nitrate treatments with the 1 mM Glu supply (Fig. 2C). However, the root growth inhibition was more pronounced in the nitrate–Glu co-treatment with 5mM nitrate (Fig. 2C).
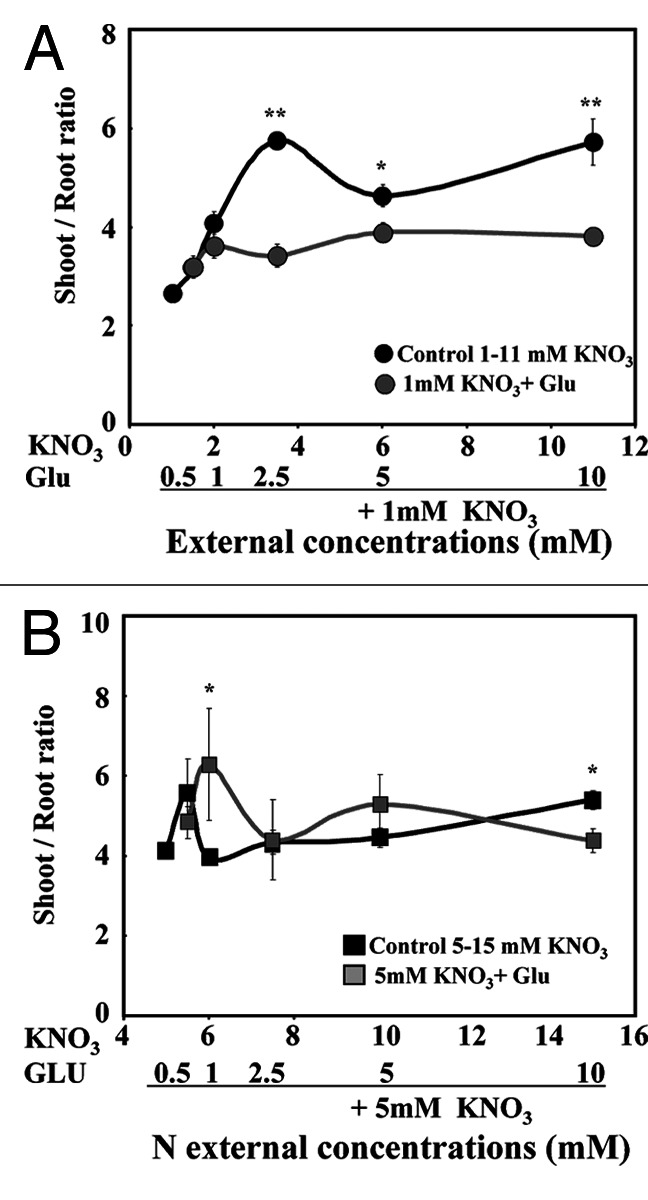
Figure 1. Comparison of the fresh weight shoot:root ratio in Brassica napus seedlings treated with nitrate and nitrate-Glu for 5 d on agar plates. (A) Effect of increasing Glu concentration on the shoot:root ratio in 1mM nitrate treated seedlings and control seedlings supplied with increasing NO3- concentration. (B) Effect of increasing Glu concentration on the shoot:root ratio in 5mM nitrate treated seedlings and control seedlings supplied with increasing NO3- concentration. Values are the average (± SE) of 3–4 replicates of four seedlings each. Significant differences between control seedlings treated with 1 or 5mM nitrate and nitrate-Glu treated seedlings are given for *p < 0.05; **p < 0.01 (t test).
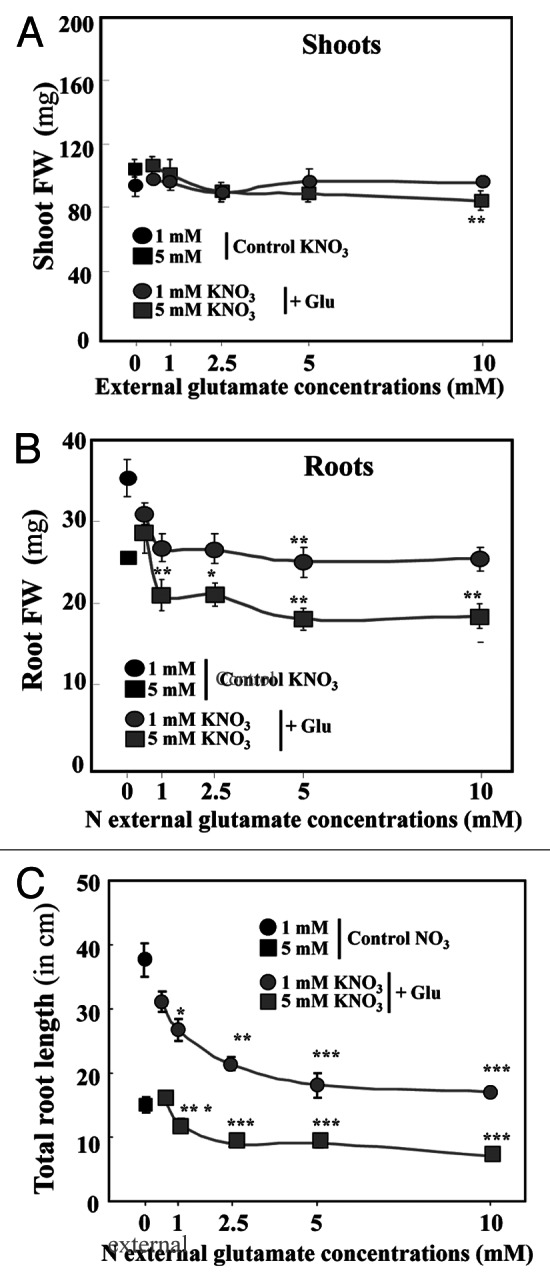
Figure 2. Changes in root and shoot fresh weights in Brassica napus seedlings treated with nitrate-Glu for 5 d on agar plates. (A) Effect of increasing Glu concentration in 1 and 5mM nitrate treated seedlings on shoot fresh weight. (B) Effect of increasing Glu concentration in 1 and 5mM nitrate treated seedlings on the root fresh weight. (C) Effect of increasing Glu concentration in 1 and 5mM nitrate treated seedlings on root elongation. Values are the average (± SE) of four replicates of four seedlings each. Significant differences between control seedlings treated with 1 or 5mM nitrate and nitrate-Glu treated seedlings are given for *p < 0.05; **p < 0.01 (t test); ***p < 0.001 (t test).
Differential 15N labeling of nitrate and glutamate demonstrates that glutamate-induced growth inhibition is not caused by a major change in N status of the seedlings
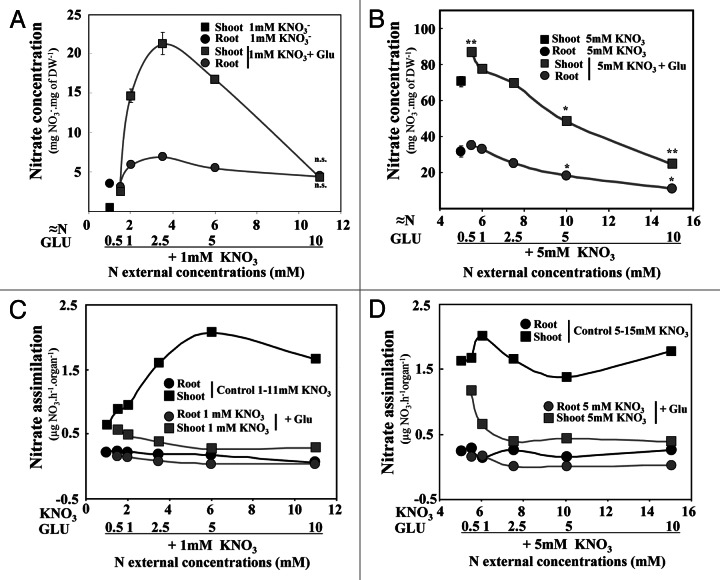
In order to analyze the effects of the Glu and nitrate nitrogen sources on N uptake and N status of the seedlings, differential 15N labeling of nitrate and Glu was performed. Calculation of the relative 15N accumulation rate via K15NO3 uptake (expressed as μg 15N.h−1.root length cm−1) compared with control seedlings was examined for 1 and 5mM nitrate treated seedlings (Fig. 3). The 15N accumulation rate was low and constant under increasing Glu concentrations in seedlings treated with 1mM nitrate (Fig. 3A). Because of Glu inhibition of the nitrate influx29,30 and the root elongation,34,36 these results did not exclude regulation of 15NO3- uptake at both the functional and structural levels. In seedlings treated with 5mM nitrate, the nitrate uptake rate was significantly reduced between the Glu concentrations of 2.5 to 10mM (Fig. 3B). However, a significant reduction in the amount of 15N accumulated per plantlet under 2.5 to 10mM Glu was observed for both nitrate treatments in spite of the reduction in the root length (Fig. S2 at PSB online). This low level of the 15NO3- uptake rate was compensated by a significant increase in the 15NGlu uptake rate (Fig. 3A and B). This compensation mechanism for the 15N accumulation rate between nitrate and Glu ensured the maintenance of a high N status in glutamate-treated seedlings compared with seedlings treated with increasing concentrations of nitrate. Hence, the results indicated that the inhibition of the shoot and root growth induced by Glu (Fig. 2A and B) was not caused by a quantitative change in the N status expressed as total 15N accumulation, but more probably by a qualitative change in the N status. This raises the question of how nitrate and Glu might exert a control on the root and shoot growth.
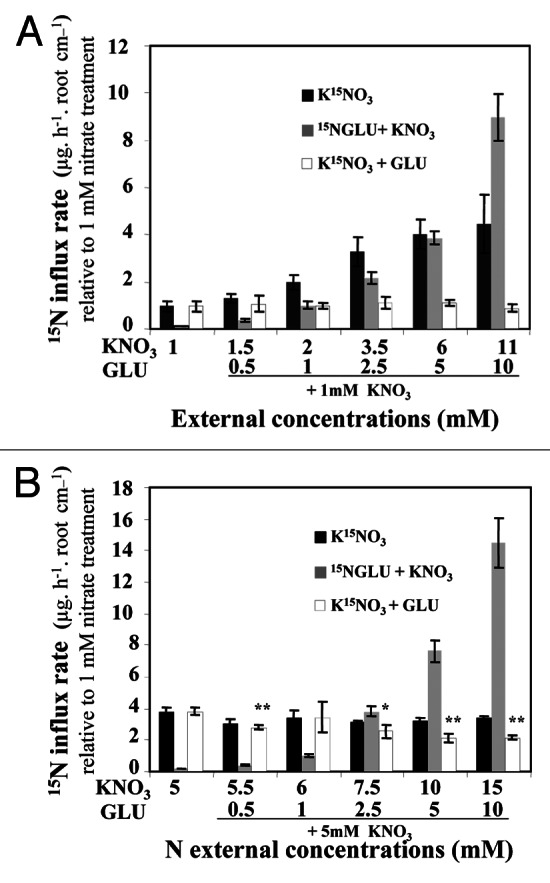
Figure 3.15N accumulation in Brassica napus seedlings after differential labeling with K15NO3 and 15NGlu in nitrate and nitrate-Glu treated plants grown for 5 d on agar plates. (A) Comparison of 15N accumulation in seedlings treated with increasing nitrate concentrations and with increasing Glu concentrations in the presence of 1mM nitrate. (B) Comparison of 15N accumulation in seedlings treated with increasing nitrate concentration and with increasing Glu concentration in the presence of 5mM nitrate. Values are the average (± SE) of four batches of four seedlings each. Data were analyzed by the nonparametric test of Kruskall Wallis, then Mood’s median test was used to compare means or medians. Bars sharing different letters are significantly different at p = 0.05. Significant differences between control seedlings treated with 1 or 5mM nitrate and nitrate-Glu treated seedlings are given for *p < 0.05; **p < 0.01; (t test).
In nitrate-glutamate treated seedlings, inhibition of nitrate uptake and assimilation corresponds to distinct but concomitant mechanisms
To further understand the effects of Glu on the root and shoot growth inhibition of the seedlings, we analyzed the nitrate accumulation and assimilation in the root and shoot tissues. Nitrate concentrations in the shoots and roots were strongly reduced in nitrate-Glu co-treated seedlings at high external Glu concentrations compared with control plants (Fig. 4A and B). In 1 and 5 mM nitrate-Glu co-treated seedlings, a significant decrease in nitrate concentrations took place with a Glu supply of 2.5 mM and higher (Fig. 4A and B). In addition, the nitrate assimilation rate in the roots and shoots was also estimated from the nitrate concentrations and the 15N accumulation derived from 15NO3 uptake (Fig. 4C and D). The data clearly indicated that in control seedlings the nitrate assimilation mainly occurred in the shoots (Fig. 4C and D). The rate of assimilation strongly increased from 0.5 to 5mM nitrate and then remained stable beyond 5mM (Fig. 4C and D). However, in nitrate-Glu co-treated plants, assimilation dropped with a Glu supply of between 0.5 and 2.5mM (Fig. 4C and D). Comparison of the nitrate-Glu co-treatments at 1 and 5mM nitrate showed that this collapse was stronger in the shoots of seedlings growing on 5mM nitrate than in those grown on 1mM nitrate (Fig. 4C and D). This was probably because endogenous nitrate concentrations were five times higher in 5mM nitrate treated seedlings than in those grown at 1mM nitrate (Fig. 4B and D). Taken together, all these results demonstrated that the inhibition by Glu of nitrate uptake and assimilation corresponds to distinct but concomitant mechanisms.
Figure 4. Nitrate accumulation and estimated nitrate assimilation rate in the shoots and roots of Brassica napus seedlings supplied with nitrate and nitrate-Glu for 5 d on agar plates. Effects of increasing nitrate concentration and increasing Glu concentration in plants supplied with 1 mM (A) and 5mM nitrate (B) on the shoot and root nitrate concentrations. Effect of increasing nitrate concentration and increasing Glue concentration in plants supplied with 1 mM (C) and 5mM nitrate (D) on the shoot and root N assimilation rate. Values are the average (± SE) of 4 replicates of four seedlings each. Significant differences between control treatments with 1 or 5mM nitrate and nitrate-Glu treatments are given for*p < 0.05; **p < 0.01; (t test). ns, not significant.
The growth inhibition induced by glutamate treatment was associated with an increase in the amounts of amino acids, NH4+ and other forms of N and a decrease in NO3-
In order to characterize the qualitative and quantitative effects of Glu treatment on the seedling N status, partitioning of the N forms between the different nitrogen compounds was analyzed from UPLC and 15N IRMS analyzes between treatments (Table 1). In 1 mM nitrate-Glu co-treated seedlings, the amounts of 15N in the different N forms revealed that compared with control, nitrate-Glu co-treatments induced a significant accumulation of free amino acids, NH4+ and other N compounds. However, NO3- increased up to the 2.5mM Glu treatment then decreased at higher external concentrations (Table 1). The nitrate-Glu co-treated seedlings at 5mM nitrate showed an increase in amino acids and NH4+ but a progressive decline in NO3- during the increase in external Glu concentrations (Table 1). The discrepancy in endogenous nitrate concentrations between the 1 and 5mM nitrate treatments is explained by the differential decrease induced by Glu between nitrate assimilation and nitrate uptake (Figs. 4A and C, 3A). Indeed, inhibition of the nitrate assimilation rate in the roots and aerial parts when increasing the concentration of exogenous Glu was less pronounced at 1mM than at 5mM nitrate (Fig. 4A and C).
Table 1. Comparison of 15N shoot partitioning in the different N nitrogen forms in Brassica napus seedlings treated with nitrate and nitrate-Glu for 5 d on agar plates. Seedlings were treated either by increasing the K15NO3- external nitrate concentration or increasing the Glu concentration in the presence of 1mM and 5mM K15NO3-. Values are the average (± SE) of four replicates of four seedlings each, with the exception of other N forms obtained from subtraction of the sum of AA, NO3- and NH4+ from the total amount of accumulated 15N .
| Treatments |
AAtot |
NH4+ |
NO3- |
Other N forms |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNO3 1mM |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+GLU |
|||
| (mM) | Glu (mM) | µg. shoot−1 | ||||||||||
|
1 |
|
30.6 ± 1.2 - |
|
0.7 ± 0.2 - |
|
0.03 ± 0.02 - |
|
45.8 - |
|
|||
|
1.5 |
0.5 |
29.1 ± 1 |
22.6 ± 2.2 |
0.3 ± 0.001 |
0.5 ± 0.02 |
0.4 ± 0.1 |
0.1 ± 0.0 |
80.6 |
50.4 |
|||
|
2 |
1 |
25.3 ± 1.9 |
22.7 ± 1.2 |
0.5 ± 0.1 |
0.5 ± 0.03 |
2.3 ± 0.2 |
0.6 ± 0.1 |
112.3 |
59.8 |
|||
|
3.5 |
2.5 |
23.8 ± 0.6 |
19 ± 0.7 |
0.3 ± 0.01 |
0.4 ± 0.01 |
1.7 ± 0.2 |
1.0 ± 0.1 |
185.4 |
74.3 |
|||
|
6 |
5 |
19.7 ± 0.9 |
34 ± 4 |
0.4 ± 0.04 |
0.8 ± 0.1 |
1.9 ± 0.04 |
0.7 ± 0.1 |
249.6 |
86.1 |
|||
| 11 | 10 | 23.5 | 85 ± 16.5 | 0.3 | 0.7 ± 0.1 | 3.1 ± 0.5 | 0.2 ± 0.1 | 209.9 | 125.2 | |||
| Treatments |
AAtot |
NH4+ |
NO3- |
Other N forms |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KNO3 1mM |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+Glu |
KNO3 |
KNO3+GLU |
|||||
| (mM) | Glu (mM) | µg. shoot−1 | ||||||||||||
|
5 |
|
11 ± 0,4 - |
|
0.12 ± 0.003 - |
|
3.1 ± 0.4 - |
|
222.2 - |
|
|||||
|
5.5 |
0.5 |
9.6 ± 0,2 |
11.3 ± 0,3 |
0.11 ± 0.01 |
0.1 ± 0.01 |
4.1 ± 0.2 |
4.1 ± 0.1 |
236.1 |
182.9 |
|||||
|
6 |
1 |
10.6 ± 0,7 |
11.7 ± 0,1 |
0.11 ± 0.004 |
0.11 ± 0.01 |
2.0 ± 0.3 |
3.8 ± 0.5 |
233.3 |
130.5 |
|||||
|
7.5 |
2.5 |
10.7 ± 0,7 |
13.4 ± 0,6 |
0.11 ± 0.01 |
0.12 ± 0.002 |
3.7 ± 0.3 |
3.2 ± 0.1 |
233.0 |
121.6 |
|||||
|
10 |
5 |
9.8 ± 0,4 |
26.3 ± 1,7 |
0.12 ± 0.01 |
0.44 ± 0.05 |
3.3 ± 0.5 |
2.2 ± 0.1 |
195.0 |
157.1 |
|||||
| 15 | 10 | 10.9 ± 0.2 | 55.6 ± 9 | 0.11 ± 0.002 | 0.61 ± 0.1 | 4.8 ± 0.5 | 1.1 ± 0.2 | 259.4 | 210.5 | |||||
Moreover, a closer examination of the AA profiling data showed that treatment with 10 mM external Glu increased the endogenous Glu concentrations 1.8 times while it increased the level of Gln 10 times (Figs. S3 and S4 at PSB online). This strong Gln accumulation was observed with Glu supplies of 2.5 mM and higher (Fig. S5 at PSB online) and suggested that inhibition of nitrate assimilation caused by Glu probably occurred during its conversion from Gln by glutamine amino transferase enzymes (GOGAT) rather than glutamine synthase (Fig. S4 at PSB online). Indeed, these results are consistent with previous studies in Arabidopsis, barley and tobacco with Fd-glutamine amino transferase enzyme (Fd-GOGAT) mutants or antisense lines showing an accumulation of Gln and a reduction in Glu levels.37-40
The treatment with glutamate upregulated BnNRT1.1 and downregulated BnNRT2.1 expression in the roots
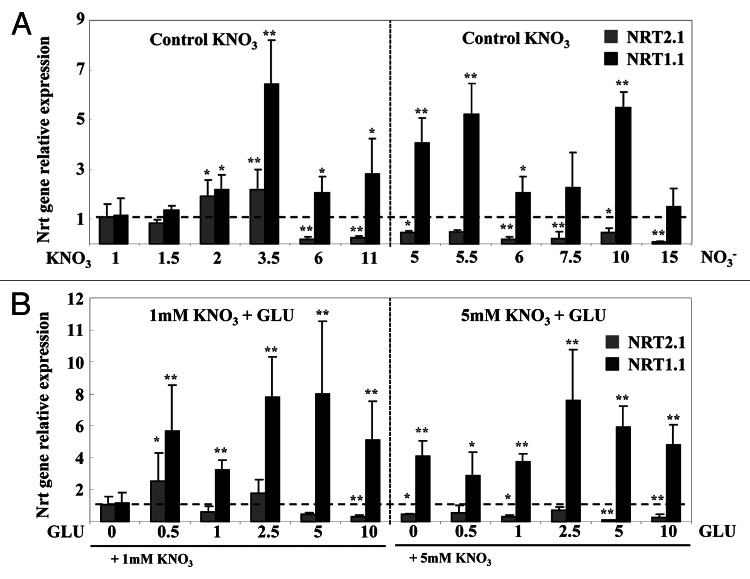
To further characterize the effect of Glu on nitrate uptake, the mRNA abundance of both the BnNRT1.1 and BnNRT2.1 nitrate transporter genes were analyzed in seedlings treated for 5 d with nitrate and nitrate-Glu (Fig. 5). In nitrate treated seedlings, BnNRT2.1 and BnNRT1.1 transcript levels showed similar expression patterns to those previously reported by Le Ny et al.19 in the same growing conditions. Both genes were upregulated between nitrate concentrations of 1 and 3.5mM (Fig. 5A). Then, between the 6 and 15 mM external nitrate concentrations, BnNRT2.1 expression was downregulated while BnNRT1.1 expression remained higher (Fig. 5A). Moreover, nitrate induction of BnNRT1.1 transcript levels was always 3 to 10-fold higher than BnNRT2.1 for all nitrate concentrations used (Fig. 5A).
Figure 5. Changes in the expression patterns of BnNRT2.1 and BnNRT1.1 transcripts in the roots of Brassica napus seedlings treated with nitrate and nitrate-Glu for 5 d on agar plates. (A) Effect of increasing nitrate concentration on BnNRT2.1 and BnNRT1.1 transcript levels. (B) Effect of increasing Glu concentration in the presence of 1 and 5 mM nitrate on BnNRT2.1 and BnNRT1.1 transcript levels. Values are the average (± SE) of 3–5 replicates of four seedlings each. Significant differences between control seedlings treated with 1 or 5mM nitrate and nitrate-Glu treated seedlings are given for *p < 0.05; **p < 0.01; (t test).
A comparison of seedlings treated either with nitrate or nitrate-Glu indicated that Glu treatment downregulated BnNRT2.1 expression but strongly upregulated BnNRT1.1 expression (Fig. 5A and B). Because the Glu supply induced a large increase in endogenous Gln concentrations (Fig. S4 at PSB online) we cannot determine which of the amino acid pools (e.g., exogenous increase of the Glu supply or endogenous increase of Gln concentrations) was responsible for downregulation of BnNRT2.1 expression. However, these results demonstrated that chronic treatment with high Gln concentrations allows uncoupling of the BnNRT2.1 and BnNRT1.1 transporters at the transcriptional level. Moreover, comparison of BnNRT2.1 nitrate transporter expression and the 15N nitrate uptake rate indicated that beyond 2.5mM, Glu treatment probably repressed both the activity and transcription of BnNRT2.1 (Figs. 3 and 5; Fig. S2 at PSB online). Indeed, inhibition of the nitrate uptake rate was observed when a large increase in Gln concentrations occurred (Figs. 3 and 5; Fig. S4 at PSB online). These results were consistent with the fact that NRT2.1 transporter activity is inhibited by endogenous or exogenous Gln concentrations.29,30 Likewise, a closer examination of seedlings treated either with nitrate or nitrate-Glu revealed that nitrate and Glu upregulated BnNRT1.1 expression. However, if the effects have the same intensity level, they are completely different in nature because the effectors are not the same (Fig. 5A and B).
The impairment of the nitrate-signaling cascade by chronic glutamate treatment reduces the osmotic water flow and 15N accumulation during shoot growth
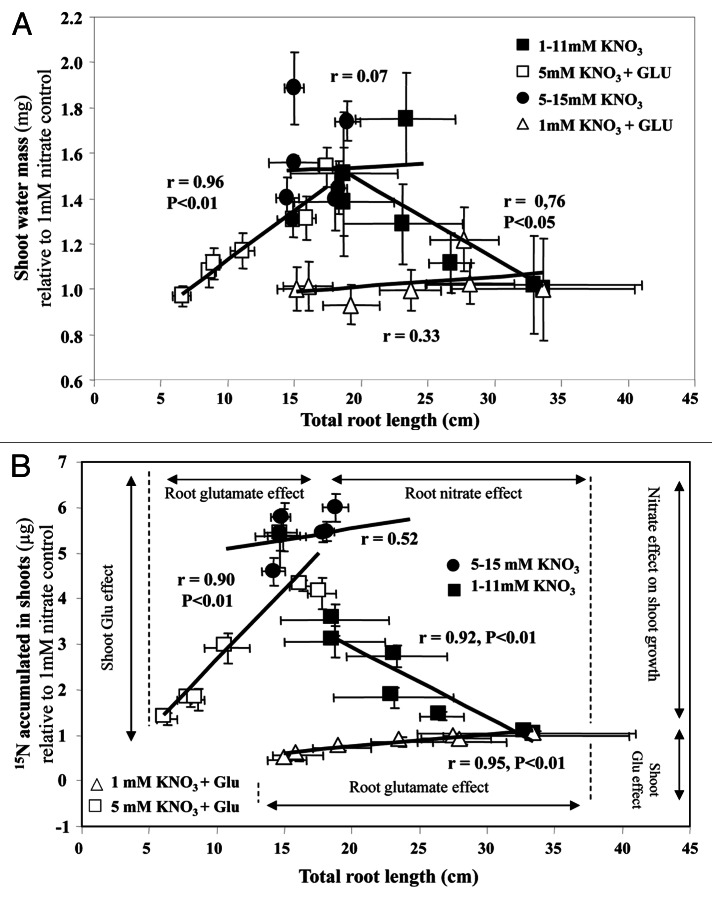
In low transpiring conditions, plantlets submitted to increasing external nitrate concentrations have revealed that the osmotic water flow for growth is solely driven by nitrate signaling via nitrate transporter activity.19 In order to determine if Glu treatment can modify this behavior by impairment of the nitrate-signaling cascade through BnNRT2.1 inhibition, we examined the effects of nitrate-Glu co-treatments on allometric relationships between shoot variables (15N and water accumulation) and the root length (Fig. 6). Comparison of these relationships between all the treatments confirmed the essential role of BnNRT2.1 nitrate transporters when large changes in nitrate accumulation occurred in the shoot (Figs. 4A and B, 6). This behavior is perfectly illustrated by the geometric relationship (isosceles triangle) obtained between water accumulation in the shoot and the root length (Fig. 6A). This was particularly striking in control seedlings treated with increasing external nitrate concentrations from 1 to 11mM. In this physiological situation, the accumulation of water and 15N were strongly correlated (Fig. 6A and B; Fig. S5 at PSB online). This behavior was also observed in the nitrate-Glu co-treatment with 5mM nitrate where reduction in nitrate concentrations in the shoot (Fig. 4A and B) was associated with the decline in 15N and water accumulation in the tissue (Fig. 6A and B). However, this plantlet behavior disappeared when the relationships between water and 15N were lost, as observed for high nitrate concentrations from 5 to 15mM (Fig. 6A and B; Fig S5 at PSB online). Finally, this geometric behavior (isosceles triangle) was caused by two Glu effects as indicated in Figure 6B: the specific inhibition by Glu of root elongation and the modulation of nitrate-signaling cascade necessary for the shoot expansion through the Glu-induced reduction of BnNRT2.1 nitrate absorption (Fig. 3) and nitrate assimilation (Fig. 4).
Figure 6. Relationships between water, 15N and nitrate accumulation in the shoots of Brassica napus seedlings treated with nitrate and nitrate-Glu for 5 d on agar plates. Effect of increasing nitrate concentration and increasing Glu concentration in plants supplied with 1 or 5 mM nitrate on the relationship between the root length and water accumulation (A), nitrate accumulation (B) or 15N accumulation (C). Values are the average (± SE) of 4 replicates of four seedlings each. The regression coefficient and significance for each relationship are shown.
Discussion
The aim of Glu treatment was to examine in low transpiring conditions the interaction of BnNRT2.1 and BnNRT1.1 nitrate transporters in the mature root in order to measure the effects of coupled nitrate and osmotic water flow in plantlet growth. In our experimental conditions the hydraulic water flow caused by the transpiration stream was experimentally dissociated from the osmotic water flow induced by nitrate availability by using an agar Petri dish system. Indeed, the high relative humidity in the Petri dishes prevents transpiration, thus allowing transpiration and nitrate uptake to be separated.19 Two homogeneous external concentrations of 1 and 5mM nitrate were used to analyze the effect of increasing external Glu concentrations on the relationship between NRT1.1 and NRT2.1 transporters in nitrate and osmotic water flow. Indeed, we have previously shown that both of the external nitrate concentrations used correspond to concentrations where significant changes in root and shoot morphology, root hydraulic conductivity and BnNRT2.1 and BnNRT1.1 transcription levels were observed.19
Chronic glutamate treatment inhibits root elongation through a specific effect that is distinct from the nitrate signaling effect
Our findings revealed that Glu inhibition of nitrate uptake was compensated by a strong 15N-Glu uptake. Although this compensation mechanism maintained a high N status in the seedlings, Glu-treated seedlings were strongly impaired in their root elongation and shoot growth. Intriguingly, the homeostasis for Glu was preserved in the roots and shoots (Fig. S3 at PSB on line) but seedlings were unable to use this N source instead of nitrate to restore seedling growth. These results were consistent with previous studies where the Glu pool in the root is relatively unaffected by exogenously applied Glu.30,41,42 In our long-term experiment, the reduction in nitrate uptake and nitrate assimilation suggested that Glu inhibits specifically the enzymatic activities of glutamate synthase (GOGAT) and nitrate transporters.29,40,41 This did not discount the possibility that Glu could also act as an exogenous signal to modulate root growth through a possible interaction between Glu and auxin signaling.43 Indeed, the sensitivity of root elongation in response to L-Glu treatment showed large differences between auxin mutants.43 Because NRT1.1 expression is regulated by auxin, and NRT1.1 acts also as an auxin carrier, all these results could explain the upregulation of NRT1.1 expression induced by Glu that was observed in our experiments.21,23 However, whatever the signaling effects of Glu involved in NRT1.1 expression, our findings clearly demonstrated that the inhibition of root elongation by Glu was a specific process distinct from the reduction in root elongation induced by nitrate (Fig. S6 at PSB online). This observation is also consistent with the stimulation by Glu of radial expansion of the root and the slender-root phenotype observed in rice GLR3.1 T-DNA mutant seedlings.44,45 Moreover, our findings contrast with a previous study reporting that nitrate signaling mediated by the NRT1.1 nitrate transporter antagonizes Glu-induced changes in the primary root elongation of Arabidopsis seedlings.36 Indeed, in our experimental conditions, Glu reduced root elongation in low (1 mM) and high (5 mM) external nitrate concentrations and in both cases the Glu effect was distinct and additive to the nitrate effect, which contrasts with previous results and conclusions (Fig. S6 at PSB online). Furthermore, NRT1.1 transcription was significantly induced instead of being reduced by nitrate-Glu treated seedlings.
Glutamate-induced reduction in nitrate uptake modulates nitrate signaling effects on shoot growth and accordingly reduces osmotic water flow
The co-treatments with nitrate and Glu created an unprecedented physiological situation where endogenous nitrate concentrations were modulated by inhibition of the nitrate uptake rate and nitrate assimilation in relation to nitrate availability. Indeed, the Glu treatments reduced not only the nitrate uptake and accumulation but also strongly inhibited nitrate assimilation. This physiological situation was complementary to the pioneer works of Mark Stitt and collaborators using nitrate reductase-transformants of tobacco plants submitted to varying external nitrate concentrations.46-48 Indeed, in our experimental conditions, the modulation of nitrate fluxes and endogenous nitrate concentrations by nitrate-Glu treatments leads to better characterization of the role of nitrate transporters and nitrate signaling in both shoot and root growth. This was demonstratively illustrated by the changes in the allometric relationships between accumulation of nitrate, 15N, and water with changes in the root length. The results revealed that BnNRT2.1 plays an essential role in the coordination of nitrate transporters that is involved in the nitrate-signaling cascade during shoot growth. According to previous results, in low transpiring conditions shoot cell and organ enlargement is the greatest rate-limiting resistance site to osmotic water flow.9,19 In our experimental conditions, shoot growth mainly depends on nitrate signaling,19,48 and this confirms that the cell and organ enlargement in shoots induced by nitrate is the rate-limiting step for the osmotic water flow. Indeed, as already shown, it is the growth processes in the cell wall that constrain turgor pressure and water status.49-52 Taken together, all these results corroborated the conclusion that nitrate acts at first as a signal to regulate shoot and root growth, probably via hormonal regulation rather than turgor pressure.19,46 This conclusion fits well with the acid-growth theory of cell elongation, where auxin causes an excretion of protons in the apoplasm causing wall loosening and cell expansion.53 H+-ATPase energizes ion transport through a variety of secondary transporters and channels such as nitrate transporters, but also potassium transporters.54,55 If nitrate is involved in primary metabolism activation, K+ accumulation leads to malate synthesis in order to increase osmoticum for cell water uptake.56 Hence, with potassium Glu treatment, reduction in endogenous nitrate concentrations was not compensated by the presence of high concentrations of K+, suggesting that the osmoticum function of K+ in cell enlargement cannot be exerted without the prior effects of nitrate signaling on shoot growth. Furthermore, this conclusion is also consistent with transcriptomic analyzes in Arabidopsis showing that the synthesis and transport of hormones such as auxin, ABA and cytokinins may be directly regulated by nitrate or may act as an enhancer of nitrate signaling/induction in developmental changes of the shoot and root cells.57-59 In addition, the recent discovery that the NRT1.1 nitrate transporter also acts as an auxin transporter in the root and facilitates auxin transport and fine-tuning of auxin transport by nitrate is also in line with this conclusion.21
In low transpiring conditions, the inhibition of BnNRT2.1 by glutamate treatment reveals that BnNRT2.1 plays a major role in nitrate uptake and seedling growth
A better understanding of coupling between BnNRT2.1 and BnNRT1.1 transporters in terms of structure-function related to plant growth is becoming more crucial because of recent localization and functional data on NRT1.1 and NRT2.1 nitrate transporters. Indeed, it has been shown by using pNRT::GUS and pNRT::GFP transgenic Arabidopsis and rice seedlings that NRT2.1 activity is mainly expressed in the epidermis and cortical layers,24,26,60 whereas NRT1.1 activity is mainly expressed in the cellular layers of the vascular cylinder in the mature root.14,22,23,27 It has also been clearly demonstrated that nitrate absorption takes place at the mature root level and not at the root tip where the vascular system is still immature.61-63 In this context, because NRT1.1 is not under the control of feedback regulation exerted by downstream metabolites such as amino acids,31,32 the aim of the Glu treatment was to inhibit specifically the BnNRT2.1 transporters in order to examine the interactions between BnNRT2.1 and BnNRT1.1 in nitrate uptake during seedling growth. Compared with the mutant approach, we can assume that the use of this pharmacological strategy prevents compensatory mechanisms for nitrate uptake caused by other transporters of the NRT2 family such as NRT2.2.17,18,33 Furthermore, in amphidiploid species such as Brassica napus where the number of NRT2 genes is probably twice that of Arabidopsis (e.g., 14 genes), in the first instance a pharmacological strategy was the most convenient. Our findings revealed the essential role of the BnNRT2.1 nitrate transporter in nitrate uptake and accordingly, osmotic water flow during shoot growth. Several lines of evidence support this assumption. First, although NRT1.1 expression was upregulated by Glu treatment, no compensatory effect for nitrate uptake was observed. This result is consistent with the localization of NRT1.1 in the cellular layers of the vascular cylinder14,22,23 and suggests that the NRT2.1 transporter will be the first involved in nitrate sensing and transport at the epidermal cell level in the mature root.24,64 The high-affinity character of the NRT2.1 transporter and the linear correlations found between induced changes in total root length and BnNRT2.1 expression reinforced this assumption.19,34,65 Second, changes in the allometric relationships found between the root length and 15N or water accumulation in the shoot clearly demonstrated that inhibition of NRT2.1 expression and activity by chronic Glu treatment modifies the water status through nitrate uptake and nitrate signaling effects on shoot growth. Third, studies with NRT1.1 transporter mutants showed a deregulation of NRT2.1 suggesting that coordination between both of these transporters is essential for seedling growth in response to nitrate availability.66,67 Fourth, our results confirmed the previous work of Orsel et al.33 who reported that disruption of the nitrate transporters, AtNRT2.1 and AtNRT2.2, restricts growth at low external nitrate concentrations.
It remains to be elucidated why the strong expression of the NRT1.1 gene induced by Glu treatment could not compensate for nitrate uptake when the NRT2.1 transporter failed to function. Indeed, this suggested either that the NRT1.1 transporter is inhibited at the posttranscriptional level or that Glu treatment also inhibits other members of the NRT2 family involved in compensatory mechanisms for nitrate uptake.14,33 Hence, a pharmacological study with nitrite, a specific inhibitor of NRT1.1 transcription and activity,32 should provide complementary information on the coordination or compensation mechanisms between NRT1.1 and NRT2.1 transporters for nitrate and water fluxes during growth and a better understanding of the relationships between nitrogen and water use efficiencies for the improvement of crop species under low N fertilization input.
Materials and Methods
Plant material and growth conditions
The Brassica napus L. seeds used in this study were the winter oil seed rape cultivar, Capitol. The seeds were treated for germination according to Leblanc et al.34 and transferred to Petri dishes (12x12 cm) filled with 50 mL of solidified agar (0.8% W/V, Sigma A-7002) culture medium. Basic medium used for seedling culture contained 0.4 mM KH2PO4, 0.15 mM K2HPO4, 1 mM K2S04, 0.5 mM MgSO4, 3 mM CaCl2, 0.2 mM Fe-Na EDTA, 14 μM H3B03, 5 μM MnSO4, 3μM ZnSO4, 0.7 μM CuSO4, 0.7μM (NH4)6 Mo7O24 and 0.1 μM CoCl2, pH 6.75. For control treatments, this basic medium was supplemented with KNO3 as the sole nitrogen source at the concentrations indicated for each individual experiment (from 1 to 15 mM). For Glu treatments, this basic medium was used with 1 or 5 mM KNO3 and supplemented with increasing potassium Glu concentrations (Sigma G-1501) from 0.5 to 10 mM in order to maintain a constant molarity in N supply compared with control treatments. The Petri dishes were half sealed with adhesive tape and placed vertically in a growth chamber at 22°C under a 16/8 light/dark regimen with a light intensity of 200 μmol m−2 s−1.
Exploratory root system analyzes
Effects of nitrate and Glu treatments on the elongation of the exploratory root system (primary and lateral roots) were measured after 5 d of each treatment. During harvest, the root and shoot parts of four seedlings corresponding to one replicate (one agar plate) were excised. Then, seedlings roots were washed in 1 mM CaSO4 solution for 1 min at room temperature before being placed in demineralized water and analyzed by a WinRHIZO scan system (Regent Instruments Inc., Canada). The mean of the total root length for each treatment was estimated from 4 replicates of 4 seedlings. The root and shoot parts of each replicate were then dried in an oven over 72h and ground to a fine powder in an Eppendorf tube with 4mm diameter inox beads in an oscillating grinder (MM301 Mixer Mill, Retsch, Haan, Germany) before stable isotope analysis by mass spectrometry.
15NO3- and 15NGlutamate analyzes
N accumulation in K15NO3- and 15NGlu treated seedlings was measured by 15N labeling and analyzed by stable isotope mass spectrometry (IRMS). The culture medium was supplemented with K15NO3 or 15NGlu (atom % 15N: 1%). The same ratio of 15NO3-/14NO3- or 15NGlu/14NGlu was used for the different KNO3 or Glu concentration treatments (from 0 to 10 mM). The total 15N amount was determined for the roots and shoots. The analyzes were performed using an analyzer (EA 3000, Eurovector, Milan, Italy) coupled with an isotopic mass spectrometer (Isoprime X, GV Instruments, Manchester, UK).
Nitrate analyzes
Anions such as nitrate and sulfate were extracted from freeze-dried shoot and root tissues of 5 d-old seedlings treated with the different nitrate and Glu concentrations. Fifteen mg dry weight of the freeze-dried tissues were resuspended at room temperature in 1 mL of extraction buffer (H2O/alcohol (1V/1V) then extracted at 40°C over 30 min. After centrifugation (1000 g for 20 min), the supernatant was removed and the pellet was re-extracted under the same conditions. Then the pellet was re-extracted 2 more times in 1 mL of milliQ water over 30 min at 95°C. After the centrifugation, all the supernatants were pooled and dried under vacuum before being suspended in 600 µL milliQ water. The sample concentration was optimized in 300 µL before analysis by ion chromatography using an ICS3000 analyzer (Dionex, Jouy en Josas, France) with an IonPac AS17 hydroxide-selective anion exchange column (Dionex). The EG40 Eluent Generator electrolytically produces high purity potassium hydroxide eluent from water, using a gradient from 12 to 40mM. Electrolytic and chemical suppression (ASRS300 4mm) greatly enhanced sensitivity (signal-to-noise ratio) by decreasing the background conductivity of the eluent while simultaneously increasing the analyte response, compared with non-suppressed ion chromatography.
Amino acid profiling
Amino acid profiling was performed on shoot and root material according to Beauclair et al.35 and Renault et al.68 After extraction of amino acids with methanol-chloroform-water and derivatization according to the AccQ•Tag Ultra Derivatization Kit protocol (Waters corp., Milford, USA), amino acids were analyzed with an ACQUITY UltraPerformance LC (UPLC) separation system (Waters corp., Milford, USA). The amount of individual amino acid was expressed in µmoles per g of dry weight by reference to internal standard BABA and to an external calibration curve for amino acids.
Calculation of the nitrate assimilation rate and the redistribution of different forms of N
The assimilation rate of nitrate was determined from the free nitrate concentrations and 15N accumulation for each treatment. 15N accumulated in the shoot corresponded to both free and structural N. The amount of nitrate assimilated with or without Glu treatment was calculated by subtracting the amounts of 15N accumulated in the root and shoot tissues from 15NO3 treatments and the free nitrate accumulated in these organs as follows: Assimilated 15NO3- (μg NO3-. h−1.seedling−1) = [amount of 15NO3- accumulated (μg.seedling−1) – amount of free NO3- (μg. seedling−1x 0.23)]/120 h, with the 0.23 factor corresponding to the proportion of N in the molecular mass of NO3-.
15NGlu utilization in the shoot was calculated from 15NGlu uptake and 15N accumulation in the shoot as well as the Glu concentrations estimated from UPLC analysis. The quantities of the different N forms such as AA, NO3- and NH4+ in the roots and shoots were calculated as follows: Amount of N form (g N.h−1.seedling−1) = [µmoles. g DW−1 of N form x root or shoot DW (g) x molecular mass of each AA or NH4 or NO3- (g.mole−1) x proportion of N in the molecular mass]/ [120h x Number of seedlings]
RNA isolation and quantitative real-time PCR analysis
For gene expression analysis, after 5 d of treatment with the different nitrate or Glu concentrations the total RNA of each experiment was extracted purified and analyzed according to Leblanc et al.34 For RT, 1 µg of total RNA was converted to cDNA with an “iScript cDNA synthesis kit” using the manufacturer's protocol (Bio-Rad, Marne-la-Coquette, France). Expression levels of genes were normalized to the expression level of the 18S house-keeping gene (F, 5′-cggataaccgtagtaattctag and R, 5′-gtactcattccaattaccagac). The primers used to amplify the gene specific sequences for BnNRT2.1 were F, 5′-T ggtggaataggcggctcgagttg and R, 5′-gtatacgttttgggtcattgccat (AJ293028): and for BnNRT1.1 were F, 5′-atggtaaccgaagtgccttg and R, 5′-tgattccagctgttgaagc (AJ27896
Supplementary Material
Acknowledgments
This work was financially supported by the French Ministry of National Education, Research and Technology (MENRT) and the Regional Council of Basse-Normandie. The authors are grateful to Josiane Pichon and Julien Lecourt for their technical assistance during seedling harvest and Sandrine Rezé for help in Q-PCR analyzes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/psb/article/22904
References
- 1.Brouder SM, Volenec JJ. Impact of climate change on crop nutrient and water use efficiencies. Physiol Plant. 2008;133:705–24. doi: 10.1111/j.1399-3054.2008.01136.x. [DOI] [PubMed] [Google Scholar]
- 2.Brueck H. Effects of nitrogen supply on water-use efficiency of higher plants. J Plant Nutr Soil Sci. 2008;171:210–9. doi: 10.1002/jpln.200700080. [DOI] [Google Scholar]
- 3.Dainty J. Water relations of plant cells. Adv Bot Res. 1963;1:279–326. doi: 10.1016/S0065-2296(08)60183-4. [DOI] [Google Scholar]
- 4.Fiscus EL, Kramer PJ. General model for osmotic and pressure-induced flow in plant roots. Proc Natl Acad Sci U S A. 1975;72:3114–8. doi: 10.1073/pnas.72.8.3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer PJ. The absorption of water and root and stem pressures. In, Kramer PJ eds. Water Relations of Plants. Orlando Academic press, 1983: 215-234. [Google Scholar]
- 6.Steudle E, Peterson CA. How does water get through the roots? J Exp Bot. 1998;49:775–88. [Google Scholar]
- 7.Steudle E. Water uptake by plants roots: integration of views. Plant Soil. 2000;226:45–56. doi: 10.1023/A:1026439226716. [DOI] [Google Scholar]
- 8.Steudle E. Water uptake by roots: effects of water deficit. J Exp Bot. 2000;51:1531–42. doi: 10.1093/jexbot/51.350.1531. [DOI] [PubMed] [Google Scholar]
- 9.Boyer JS. Water transport. Annu Rev Plant Physiol. 1985;36:473–516. doi: 10.1146/annurev.pp.36.060185.002353. [DOI] [Google Scholar]
- 10.Cosgrove DJ. Growth of the plant cell wall. Nat Rev Mol Cell Biol. 2005;6:850–61. doi: 10.1038/nrm1746. [DOI] [PubMed] [Google Scholar]
- 11.Tanner W, Beevers H. Does transpiration have an essential function in long-distance ion transport in plants? Plant Cell Environ. 1990;13:745–50. doi: 10.1111/j.1365-3040.1990.tb01089.x. [DOI] [Google Scholar]
- 12.Tanner W, Beevers H. Transpiration, a prerequisite for long-distance transport of minerals in plants? Proc Natl Acad Sci U S A. 2001;98:9443–7. doi: 10.1073/pnas.161279898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macduff JH, Bakken AK. Diurnal variation in uptake and xylem contents of inorganic and assimilated N under continuous and interrupted N supply to Phleum pratense and Festuca pratensis. J Exp Bot. 2003;54:431–44. doi: 10.1093/jxb/erg058. [DOI] [PubMed] [Google Scholar]
- 14.Guo FQ, Wang R, Chen M, Crawford NM. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is activated and functions in nascent organ development during vegetative and reproductive growth. Plant Cell. 2001;13:1761–77. doi: 10.11054/TPC.010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orsel M, Krapp A, Daniel-Vedele F. Analysis of the NRT2 nitrate transporter family in Arabidopsis. Structure and gene expression. Plant Physiol. 2002;129:886–96. doi: 10.1104/pp.005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okamoto M, Vidmar JJ, Glass ADM. Regulation of NRT1 and NRT2 gene families of Arabidopsis thaliana: responses to nitrate provision. Plant Cell Physiol. 2003;44:304–17. doi: 10.1093/pcp/pcg036. [DOI] [PubMed] [Google Scholar]
- 17.Filleur S, Dorbe MF, Cerezo M, Orsel M, Granier F, Gojon A, et al. An arabidopsis T-DNA mutant affected in Nrt2 genes is impaired in nitrate uptake. FEBS Lett. 2001;489:220–4. doi: 10.1016/S0014-5793(01)02096-8. [DOI] [PubMed] [Google Scholar]
- 18.Li W, Wang Y, Okamoto M, Crawford NM, Siddiqi MY, Glass ADM. Dissection of the AtNRT2.1:AtNRT2.2 inducible high-affinity nitrate transporter gene cluster. Plant Physiol. 2007;143:425–33. doi: 10.1104/pp.106.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Ny F, Leblanc A, Beauclair P, Deleu C, Le Deunff E. In low transpiring conditions, nitrate and water fluxes for growth of B. napus plantlets correlate with changes in BnNrt2.1 and BnNrt1.1 nitrate transporter expression. Plant Si Be. 2012 doi: 10.4161/psb.22902. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho CH, Lin SH, Hu HC, Tsay YF. CHL1 functions as a nitrate sensor in plants. Cell. 2009;138:1184–94. doi: 10.1016/j.cell.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, et al. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Cell. 2010;18:1–11. doi: 10.1016/j.devcel.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 22.Guo FQ, Wang R, Crawford NM. The Arabidopsis dual-affinity nitrate transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots. J Exp Bot. 2002;53:835–44. doi: 10.1093/jexbot/53.370.835. [DOI] [PubMed] [Google Scholar]
- 23.Remans T, Nacry P, Pervent M, Girin T, Tillard P, Lepetit M, et al. A central role for the nitrate transporter NRT2.1 in the integrated morphological and physiological responses of the root system to nitrogen limitation in Arabidopsis. Plant Physiol. 2006;140:909–21. doi: 10.1104/pp.105.075721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Remans T, Nacry P, Pervent M, Filleur S, Diatloff E, Mounier E, et al. The Arabidopsis NRT1.1 transporter participates in the signaling pathway triggering root colonization of nitrate-rich patches. Proc Natl Acad Sci U S A. 2006;103:19206–11. doi: 10.1073/pnas.0605275103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Girin T, Lejay L, Wirth J, Widiez T, Palenchar PM, Nazoa P, et al. Identification of a 150 bp cis-acting element of the AtNRT2.1 promoter involved in the regulation of gene expression by the N and C status of the plant. Plant Cell Environ. 2007;30:1366–80. doi: 10.1111/j.1365-3040.2007.01712.x. [DOI] [PubMed] [Google Scholar]
- 26.Orsel M, Chopin F, Leleu O, Smith SJ, Krapp A, Daniel-Vedele F, et al. Nitrate signaling and the two component high affinity uptake system in Arabidopsis. Plant Signal Behav. 2007;2:260–2. doi: 10.4161/psb.2.4.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopin F, Wirth J, Dorbe MF, Lejay L, Krapp A, Gojon A, et al. The Arabidopsis nitrate transporter AtNRT2.1 is targeted to the root plasma membrane. Plant Physiol Biochem. 2007;45:630–5. doi: 10.1016/j.plaphy.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Vidmar JJ, Zhuo D, Siddiqi MY, Schjoerring JK, Touraine B, Glass ADM. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen pools in roots of barley. Plant Physiol. 2000;123:307–18. doi: 10.1104/pp.123.1.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beuve N, Rispail N, Lainé P, Cliquet JB, Ourry A, Le Deunff E. Putative role of gamma-aminobutyric acid (GABA) as a long distance signal in up-regulation of nitrate uptake in Brassica napus L. Plant Cell Environ. 2004;27:1035–46. doi: 10.1111/j.1365-3040.2004.01208.x. [DOI] [Google Scholar]
- 30.Li JZ, He GY, Cram WJ. The effect of amino acids on nitrate uptake by wheat roots. Cereal Res Commun. 2010;38:482–8. doi: 10.1556/CRC.38.2010.4.4. [DOI] [Google Scholar]
- 31.Lejay L, Tillard P, Lepetit M, Olive FD, Filleur S, Daniel-Vedele F, et al. Molecular and functional regulation of two NO3- uptake systems by N- and C-status of Arabidopsis plants. Plant J. 1999;18:509–19. doi: 10.1046/j.1365-313X.1999.00480.x. [DOI] [PubMed] [Google Scholar]
- 32.Loqué D, Tillard P, Gojon A, Lepetit M. Gene expression of the NO3- transporter NRT1.1 and the nitrate reductase NIA1 is repressed in Arabidopsis roots by NO2-, the product of NO3- reduction. Plant Physiol. 2003;132:958–67. doi: 10.1104/pp.102.018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orsel M, Eulenburg K, Krapp A, Daniel-Vedele F. Disruption of the nitrate transporter genes AtNRT2.1 and AtNRT2.2 restricts growth at low external nitrate concentration. Planta. 2004;219:714–21. doi: 10.1007/s00425-004-1266-x. [DOI] [PubMed] [Google Scholar]
- 34.Leblanc E, Renault H, Lecourt J, Etienne P, Deleu C, Le Deunff E. Elongation changes of exploratory and root hair systems induced by ACC and AVG affect nitrate uptake and the BnNRT2.1 and BnNRT1.1 transporters genes expression in Brassica napus. Plant Physiol. 2008;146:1928–40. doi: 10.1104/pp.107.109363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Beauclair P, Leblanc A, Lemauviel-Lavenant S, Deleu C, Le Deunff E. Ethylene modifies architecture of root system in response to stomatal opening and water allocation changes between root and shoot. Plant Signal Behav. 2009;4:1–3. doi: 10.4161/psb.4.1.7268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walch-Liu P, Forde BG. Nitrate signalling mediated by the NRT1.1 nitrate transporter antagonises L-glutamate-induced changes in root architecture. Plant J. 2008;54:820–8. doi: 10.1111/j.1365-313X.2008.03443.x. [DOI] [PubMed] [Google Scholar]
- 37.Blackwell RD, Murray AJS, Lea PJ, Kendall AC, Hall NP, Turner JC, et al. The value of mutants unable to carry out photorespiration. Photosynth Res. 1988;16:155–76. doi: 10.1007/BF00039491. [DOI] [PubMed] [Google Scholar]
- 38.Leegood RC, Lea PJ, Adcock MD, Häusler RE. The regulation and control of photorespiration. J Exp Bot. 1995;46:1397–414. doi: 10.1093/jxb/46.special_issue.1397. [DOI] [Google Scholar]
- 39.Ferrario-Mery S, Hodges M, Hirel B, Foyer CH. Photorespiration-dependent increases in phospho enolpyruvate carboxylase, isocitrate dehydrogenase and glutamate dehydrogenase in transformed tobacco plants deficient in ferredoxin-dependent glutamine-alpha-ketoglutarate aminotransferase. Planta. 2002;214:877–86. doi: 10.1007/s00425-001-0692-2. [DOI] [PubMed] [Google Scholar]
- 40.Kissen R, Winge P, Tran DHT, Jørstad TS, Størseth TR, Christensen T, et al. Transcriptional profiling of an Fd-GOGAT1/GLU1 mutant in Arabidopsis thaliana reveals a multiple stress response and extensive reprogramming of the transcriptome. BMC Genomics. 2010;11:190. doi: 10.1186/1471-2164-11-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stitt M, Müller C, Matt P, Gibon Y, Carillo P, Morcuende R, et al. Steps towards an integrated view of nitrogen metabolism. J Exp Bot. 2002;53:959–70. doi: 10.1093/jexbot/53.370.959. [DOI] [PubMed] [Google Scholar]
- 42.Forde BG, Lea PJ. Glutamate in plants: metabolism, regulation, and signalling. J Exp Bot. 2007;58:2339–58. doi: 10.1093/jxb/erm121. [DOI] [PubMed] [Google Scholar]
- 43.Walch-Liu P, Liu LH, Remans T, Tester M, Forde BG. Evidence that L-glutamate can act as an exogenous signal to modulate root growth and branching in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:1045–57. doi: 10.1093/pcp/pcj075. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Zhu S, Song X, Shen Y, Chen H, Yu J, et al. A rice glutamate receptor-like gene is critical for the division and survival of individual cells in the root apical meristem. Plant Cell. 2006;18:340–9. doi: 10.1105/tpc.105.037713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sivaguru M, Pike S, Gassmann W, Baskin TI. Aluminum rapidly depolymerizes cortical microtubules and depolarizes the plasma membrane: evidence that these responses are mediated by a glutamate receptor. Plant Cell Physiol. 2003;44:667–75. doi: 10.1093/pcp/pcg094. [DOI] [PubMed] [Google Scholar]
- 46.Scheible W, Lauerer M, Schulze E, Caboche M, Stitt M. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. Plant J. 1997;11:671–91. doi: 10.1046/j.1365-313X.1997.11040671.x. a. [DOI] [Google Scholar]
- 47.Scheible WR, Gonzalez-Fontes A, Lauerer M, Muller-Rober B, Caboche M, Stitt M. Nitrate acts as a signal to induce organic acid metabolism and repress starch metabolism in tobacco. Plant Cell. 1997;9:783–98. doi: 10.1105/tpc.9.5.783. b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stitt M, Feil R. Lateral root frequency decreases when nitrate accumulates in tobacco transformants with low reductase activity: consequences for the regulation of biomass partitioning between shoots and root. Plant Soil. 1999;215:143–53. doi: 10.1023/A:1004676605336. [DOI] [Google Scholar]
- 49.Bressan RA, Handa AK, Handa S, Hasegawa PM. Growth and water relations of cultured tomato cells after adjustment to low external water potentials. Plant Physiol. 1982;70:1303–9. doi: 10.1104/pp.70.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu GL, Boyer JS. Enlargement in chara studied with a turgor clamp : growth rate is not determined by turgor. Plant Physiol. 1992;100:2071–80. doi: 10.1104/pp.100.4.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Proseus TE, Zhu GL, Boyer JS. Turgor, temperature and the growth of plant cells: using Chara corallina as a model system. J Exp Bot. 2000;51:1481–94. doi: 10.1093/jexbot/51.350.1481. [DOI] [PubMed] [Google Scholar]
- 52.Maggio A, Zhu J-K, Hasegawa PM, Bressan RA. Osmogenetics: Aristotle to Arabidopsis. Plant Cell. 2006;18:1542–57. doi: 10.1105/tpc.105.040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rayle DL, Cleland RE. The Acid Growth Theory of auxin-induced cell elongation is alive and well. Plant Physiol. 1992;99:1271–4. doi: 10.1104/pp.99.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palmgren MG. Plant plasma membrane H+-ATPases powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:817–45. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 55.Perrot-Rechenmann C. Cellular responses to auxin: division versus expansion. Cold Spring Harb Perspect Biol. 2012;•••:1–15. doi: 10.1101/cshperspect.a001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haschke H-P, Lüttge U. stoichiometric correlation of malate accumulation with auxin-dependent K+-H+ exchange and growth in Avena coleoptile segments. Plant Physiol. 1975;56:696–8. doi: 10.1104/pp.56.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gutiérrez RA, Lejay LV, Dean A, Chiaromonte F, Shasha DE, Coruzzi GM. Qualitative network models and genome-wide expression data define carbon/nitrogen-responsive molecular machines in Arabidopsis. Genome Biol. 2007;8:R7. doi: 10.1186/gb-2007-8-1-r7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nero D, Krouk G, Tranchina D, Coruzzi GM. A system biology approach highlights a hormonal enhancer effect on regulation of genes in a nitrate responsive “biomodule”. BMC Syst Biol. 2009;3:59. doi: 10.1186/1752-0509-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krouk G, Tranchina D, Lejay L, Cruikshank AA, Shasha D, Coruzzi GM, et al. A systems approach uncovers restrictions for signal interactions regulating genome-wide responses to nutritional cues in Arabidopsis. PLoS Comput Biol. 2009;5:e1000326. doi: 10.1371/journal.pcbi.1000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feng HM, Yan M, Fan XR, Li BZ, Shen QR, Miller AJ, et al. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J Exp Bot. 2011;62:2319–32. doi: 10.1093/jxb/erq403. [DOI] [PubMed] [Google Scholar]
- 61.Lazof DB, Rufty TW, Redinbaugh MG. Localization of nitrate absorption and translocation within morphological regions of the corn root. Plant Physiol. 1992;100:1251–8. doi: 10.1104/pp.100.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taylor AR, Bloom AJ. Ammonium, nitrate, and proton fluxes along the maize root. Plant Cell Environ. 1998;21:1255–63. doi: 10.1046/j.1365-3040.1998.00357.x. [DOI] [Google Scholar]
- 63.Colmer TD, Bloom AJ. A comparison of NH4+ and NO3- net fluxes along roots of rice and maize. Plant Cell Environ. 1998;21:240–6. doi: 10.1046/j.1365-3040.1998.00261.x. [DOI] [Google Scholar]
- 64.Little DY, Rao H, Oliva S, Daniel-Vedele F, Krapp A, Malamy JE. The putative high-affinity nitrate transporter NRT2.1 represses lateral root initiation in response to nutritional cues. Proc Natl Acad Sci U S A. 2005;102:13693–8. doi: 10.1073/pnas.0504219102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faure-Rabasse S, Le Deunff E, Lainé P, Macduff JH, Ourry A. Effects of nitrate pulses on BnNRT1 and BnNRT2 genes: mRNA levels and nitrate influx rates in relation to the duration of N deprivation in Brassica napus L. J Exp Bot. 2002;53:1711–21. doi: 10.1093/jxb/erf023. [DOI] [PubMed] [Google Scholar]
- 66.Krouk G, Tillard P, Gojon A. Regulation of the high-affinity NO3- uptake system by NRT1.1-mediated NO3- demand signaling in Arabidopsis. Plant Physiol. 2006;142:1075–86. doi: 10.1104/pp.106.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang R, Xing X, Wang Y, Tran A, Crawford NM. A genetic screen for nitrate regulatory mutants captures the nitrate transporter gene NRT1.1. Plant Physiol. 2009;151:472–8. doi: 10.1104/pp.109.140434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Renault H, Roussel V, El Amrani A, Arzel M, Renault D, Bouchereau A, et al. The Arabidopsis pop2-1 mutant reveals the involvement of GABA transaminase in salt stress tolerance. BMC Plant Biol. 2010;10:20. doi: 10.1186/1471-2229-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.