Abstract
The transthyretin (TTR) amyloidoses are human diseases in which the misfolded TTR protein aggregates in tissues with subsequent visceral, peripheral, and autonomic nerve dysfunction. Recent reports have stressed the importance of oligomeric intermediates as major cytotoxic species in various forms of amyloidogenesis. We have examined the cytotoxic effects of several quaternary structural states of wild-type and variant TTR proteins on cells of neural lineage. TTR amyloid fibrils and soluble aggregates >100 kDa were not toxic. Incubation of TTR under the conditions of the cell assay and analysis by size-exclusion chromatography and SDS/PAGE reveal that monomeric TTR or relatively small, rapidly formed aggregates of a maximum size of six subunits were the major cytotoxic species. Small molecules that stabilize the native tetrameric state were shown to prevent toxicity. The studies are consistent with a model in which the misfolded TTR monomer rapidly aggregates to form transient low molecular mass assemblies (<100 kDa) that are highly cytotoxic in tissue culture.
The amyloidoses are human diseases, in which organ dysfunction is a result of the deposition of proteins that are normally soluble under physiologic conditions; they include Alzheimer's disease (AD), Ig light chain amyloid, and amyloid A, among others (1, 2). Transthyretin (TTR), a transport protein synthesized primarily in liver, choroid plexus, and the retina, is one of the 23 human proteins known to be associated with local or systemic amyloidosis (3–5). TTR is a homotetramer that carries retinol-binding protein loaded with retinol and thyroxine in the plasma and cerebrospinal fluid. Senile systemic amyloidosis is a sporadic disorder resulting from the deposition of wild-type TTR (WT TTR) fibrils in cardiac and other tissues. Familial amyloidotic polyneuropathy and cardiomyopathy are hereditary autosomal-dominant diseases, in which the deposited TTR fibrils are derived from one of 80 known amyloidogenic mutations, and primarily affect the peripheral and autonomic nervous systems, and heart, respectively. The TTR deposits are extracellular, with some variants exhibiting tissue-selective deposition (6). The mechanisms responsible for the tissue selectivity, damage, and the pathway of deposition in vivo are unknown. Usually, deposition occurs at a distance from the site of synthesis, although deposition in the choroid plexus has been reported (7).
In vitro, the loss of TTR quaternary structure, i.e., disassembly of the native tetramer to its component monomers, is required for aggregation and subsequent fibril formation (4, 8). Small molecules that fit in the thyroxine-binding pocket of TTR stabilize the native tetramer and prevent fibrillogenesis (9). A therapeutic strategy based on the development and administration of such compounds is a promising alternative to current treatment, i.e., replacement of the mutant TTR gene with wild type by liver transplantation, and offers the potential of prophylaxis for asymptomatic gene carriers.
In this article, we explore the use of a cell-based assay system to evaluate the TTR quaternary structural requirements for cytotoxicity: TTR monomers and/or nonnative oligomers smaller than 100 kDa induce cytotoxicity in the human neuroblastoma cell line IMR-32 through an apoptotic mechanism. We also show that two compounds that inhibit TTR fibril formation in vitro, by stabilizing TTR tetramers, inhibit the TTR-induced cytotoxicity. Thus, stabilization of the tetramer prevents the dissociation required to form the monomer and nonnative oligomers associated with maximal cytotoxicity.
Materials and Methods
Preparation of TTR. Four TTR variants were used for the studies presented: WT TTR, the amyloidogenic mutant TTR with Val30Met mutation (V30M TTR), and two engineered variants that do not tetramerize, referred to as monomeric WT (F87M/L110M TTR, named WT M-TTR), and monomeric V30M (V30M/F87M/L110M TTR, designated V30M M-TTR) TTR. The proteins were prepared and purified in an Escherichia coli expression system as described elsewhere (10–12). The four protein variants were purified by gel filtration on a Superdex 75 column (Amersham Biosciences) in 10 mM phosphate buffer (sodium) pH 7.6/100 mM KCl/1 mM EDTA before each experiment to ensure that no aggregates were present in the starting material. Liquid chromatography-electrospray ionization MS was used to confirm the molecular weight of the proteins.
V30M TTR Amyloid Fibril Preparation. Amyloid fibrils were obtained incubating V30M TTR under mildly acidic conditions as described elsewhere (13), with minor modifications. Briefly, V30M TTR, 0.5 mg/ml in 10 mM phosphate buffer, pH 7.6/100 mM KCl/1 mM EDTA was diluted with an equal volume of 200 mM acetate buffer, pH 4.8/100 mM KCl/1 mM EDTA, was mixed, and was filtered through a 0.22-μm membrane. Subsequent manipulations were performed under sterile conditions. The TTR solution was incubated for 7 days at 37°C and the fibrils were recovered by centrifugation at 14,000 × g for 30 min at 4°C (90% of starting material). The aggregates were judged to be fibrils by their Congo red-binding activity (14). The fibrils were diluted in Opti-MEM (cell medium) to the desired concentrations and tested under the cell assay conditions detailed below.
Cell Culture. The human neuroblastoma cell line IMR-32 (CCL-127; American Type Culture Collection), was grown in 75-cm2 flasks in Opti-MEM (Life Technologies), supplemented with 5% FBS, 1 mM Hepes buffer/2 mM l-glutamine/100 units/ml penicillin/100 μg/ml streptomycin (complete cell media), and incubated at 37°C in a 5% CO2 atmosphere. The cells are adherent and were replated every 2–3 days after treatment with cell dissociation buffer (Life Technologies) and dilution at a 1:4 to 1:6 ratio with complete cell media.
Cell Assay. IMR-32 cells (2- to 3-day-old culture, up to 80% confluent) were plated into one-half-area 96-well plates in complete cell media at a density of 6,000–10,000 cells per well, depending on the assay, and were incubated overnight at 37°C in a 5% CO2 atmosphere. TTR preparations were buffer-exchanged in Opti-MEM by using a Centriprep device (10-kDa pore size; Millipore). The TTR solutions were filter-sterilized, and appropriate dilutions were prepared in Opti-MEM with antibiotics, l-glutamine, and Hepes buffer (cell assay media). The media from the cells was removed, and 100 μl of the TTR solutions was immediately added to each well. At least six wells containing cells and six wells without cells received 100 μl of media without TTR to serve as controls and blanks, respectively. After addition of TTR solutions (or media), the plates were incubated at 37°C in a 5% CO2 atmosphere until the cytotoxicity assays were performed.
Cell Toxicity Assay. Twenty-five microliters per well of 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) (5 mg/ml in PBS) were added to the wells (samples, blanks, and controls), and the plates were incubated for 3–4 h at 37°C. Then, 25 μl per well of lysis buffer (20% SDS in 1:1 N,N′-dimethylformamide:water/2% acetic acid/2.5% HCl 1 M) was added, and the plates were incubated overnight at 37°C to solubilize the formazan produced, which was quantified by OD at 570 nm in a 96-well plate reader (Spectramax 384 plus, Molecular Devices). All of the assays have been done at least twice in triplicate. We show representative results for each of the assays. In all of the assays, we used soluble V30M TTR (homotetramer) as a positive control for induced cytotoxicity.
MTT reduction measures metabolic activity of cells. For simplicity, we expressed the results as “percentage of living cells” (rather than the more precise term “proportion of metabolically active cells”) relative to that seen in control cells (cells with media only), this expression is 100 × (ODsample - ODblank)/(ODcontrol - ODblank). The ODblank was established from the average of the wells containing only Opti-MEM with no cells. Comparison of wells containing nontreated lysed cells (0.1% Triton X-100) with wells without cells gave the same background optical density. Average values and SD were calculated from triplicate determinations.
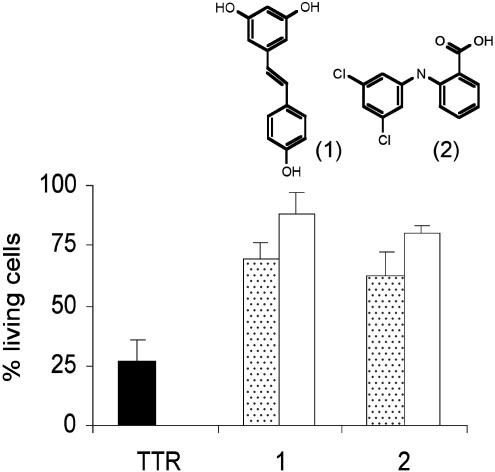
Cytotoxicity Inhibition Assay. IMR-32 cells were plated into one-half-area 96-well plates, as described above, on the day before the inhibition assay. Solutions of V30M TTR and the compounds resveratrol (1; Sigma) and biarylamine 2 (ref. 15 and Fig. 2) were prepared at 4 μM in cell assay media. Each of the compounds was mixed with equal volumes of V30M TTR, resulting in equimolar solutions. V30M TTR and compounds were also diluted with equal volumes of cell assay media to serve as controls for intrinsic TTR and drug cytotoxicity, respectively. The mixtures were incubated at 4°C for 24 h to facilitate the interaction of the compounds with the TTR. After this incubation period, all of the mixtures were diluted 1:1 with fresh cell assay media. The media from the 96-well plates was removed and 100 μl per well of test solutions (TTR, TTR with compounds, compounds alone, or cell assay media alone) were added (final concentration of V30M TTR and compounds was 1 μM). After 3 more days of incubation at 37°C, the MTT assay was performed to determine cell metabolic activity.
Fig. 2.
V30M TTR-induced cytotoxicity can be inhibited by compounds that stabilize TTR tetramer. Cells treated with V30M TTR (black bar), an equimolar mixture of V30M TTR and compounds (dotted bars), and compounds alone (white bars). Bars represent the SD of triplicate determinations.
Fluorescent Microscopy. IMR-32 cells were plated into one-half-area 96-well plates, as described above, and were treated with 1 mg/ml V30M TTR (in Opti-MEM) or with Opti-MEM only (controls). After 40 h of incubation at 37°C, the cells were stained with Rhodamine 123 (Molecular Probes) and Hoechst 33342 (Alexis Biochemicals) for the visualization of mitochondrial membrane potential and nuclear condensation, respectively. Briefly, 1.8 μl per well of Rhodamine 123 solution (57 μg/ml in water) was added and incubated for 30 min at 37°C. The media was then replaced by 100 μl per well of prewarmed cell assay media and incubated at 37°C for 25 more minutes. Two microliters per well of Hoechst 33342 solution (1.5 mg/ml in water) was added, and the plates were spun at 200 × g for 5 min at room temperature. The medium was removed and replaced by fresh prewarmed cell assay media. The cells were visualized immediately with a Nikon TE300 fluorescent microscope (excitation 510 nm and emission at 534 nm for Rhodamine 123; excitation 402 nm and emission 462 nm for Hoechst 33342), and digital images captured with a Spot2 digital camera (Diagnostic Instruments).
TTR Cross-Linking. Fifty microliters of TTR samples (0.44 mg/ml) were incubated with 5 μl of glutaraldehyde (25% solution; Sigma) for 4 min at room temperature. The reaction was quenched by addition of 5 μl of a 7% solution of NaBH4 (wt/vol in 0.1 M NaOH), and the samples were analyzed by 5–15% acrylamide/bisacrylamide gradient SDS/PAGE after boiling for 5 min in SDS reducing buffer (16). The protein bands were stained with Coomassie blue.
Size-Exclusion Chromatography (SEC). Samples to be tested were filtered through a 0.22-μm filter and 50 μl were injected into an AKTA FPLC (Amersham Biosciences) with a 10/30 column Superdex 75 at 4°C. The running buffer was 10 mM phosphate (sodium) pH 7.6/100 mM KCl/1 mM EDTA, at 0.6 ml/min, and the eluted proteins were monitored at 280 nm.
Results
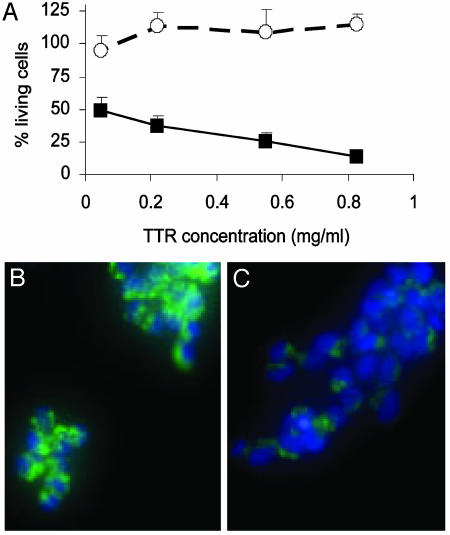
V30M TTR but Not WT TTR Is Cytotoxic to IMR-32 Cells. Native WT TTR and the amyloidogenic mutant V30M TTR were incubated with the IMR-32 cells for 3 days at 0.055, 0.22, 0.55, and 0.83 mg/ml (1, 4, 10, and 15 μM). The TTR-induced cytotoxicity was measured by MTT assay. The mutant V30M TTR was toxic to the IMR-32 cells, even at the lowest concentration tested, whereas WT TTR showed no toxicity (Fig. 1A).
Fig. 1.
(A) Cytotoxicity of WT TTR (○) and V30M TTR (▪) on IMR-32 cells after 3 days of incubation at 37°C. Bars represent the SD of triplicate determinations. (B and C) Fluorescent microscopy images of IMR-32 cells stained with Rhodamine 123 (green) and Hoechst 33342 (blue) after 40 h incubation with 1 mg/ml V30M TTR. (B) Control cells (Opti-MEM only). (C) V30M TTR-treated cells. The decrease in mitochondrial membrane potential (green) of TTR-treated cells compared with control cells, suggest that the TTR triggers apoptosis in IMR-32 cells. Some nuclei condensation (clear blue) is seen also after TTR treatment.
V30M TTR Induces Apoptosis in IMR-32 Cells. IMR-32 cells were incubated for 40 h at 37°C with 1 mg/ml solution of V30M TTR and the cells were stained with Rhodamine 163 and Hoechst 33342 as detailed in Materials and Methods. Rhodamine 163 is a cell-permeable, cationic, fluorescent dye indicative of mitochondrial membrane potential because it is internalized by active mitochondria. Hoechst 33342 stains nuclei blue. Cells that have undergone nuclear condensation saturate the image and the nuclei appear as strong clear blue spot. Fig. 1 B and C show representative images of IMR-32 cells treated with media only (B) or V30M TTR (C).
One of the characteristics of apoptosis is the loss of mitochondrial membrane potential; thus, the lower green fluorescence seen in TTR-treated cells compared with control cells, suggests that TTR triggers apoptosis in IMR-32 cells. Moreover, some degree of nuclear condensation (clear blue), a late marker of apoptosis, is also seen after TTR treatment.
V30M TTR-Induced Cytotoxicity Can Be Inhibited by Compounds That Prevent TTR Fibril Formation in Vitro. The compounds resveratrol (1) and the chlorinated biaryl amine (2) have been shown to inhibit TTR amyloid formation in vitro by stabilizing the native tetrameric structure of TTR (9, 15, 17). These two compounds were mixed with V30M TTR and tested in the cell assay as described in Materials and Methods. Fig. 2 shows that V30M TTR-induced cytotoxicity (25% cell viability, black bar) is inhibited by preincubating the V30M homotetramer with compounds 1 or 2 (70% and 60% of cell viability respectively; dotted bars). Incubation of the cells with the compounds in the absence of V30M TTR (white bars) demonstrated that the small molecules themselves were not cytotoxic at this concentration.
The fact that V30M TTR-induced cytotoxicity can be inhibited by drugs that stabilize the TTR tetramer suggests that the native V30M TTR is not cytotoxic but is the source of some other toxic species, presumably generated during the process of fibril formation.
Only Monomers or Small Soluble TTR Aggregates Produce Cytotoxicicity. Several TTR intermediates have been identified in the course of TTR fibrillogenesis in vitro including native tetramer, free native monomer, misfolded monomer, soluble and insoluble aggregates, and possibly traces of TTR dimer and/or TTR trimer (4, 18). To further investigate the nature of the cytotoxic species, cell-based assays were performed by using TTRs of defined quaternary structure, representing potential intermediates in amyloid formation (Table 1).
Table 1. TTR variants used and their quaternary structures.
| Name | TTR variant | Quaternary structure |
|---|---|---|
| WT TTR | WT TTR | Tetramer |
| V30M TTR | V30M TTR | Tetramer |
| V30M fibrils | V30M TTR | Amyloid fibrils |
| WT M-TTR | F87M/L110M TTR | None (monomer) |
| V30M M-TTR | V30M/F87M/L110M TTR | None (monomer) |
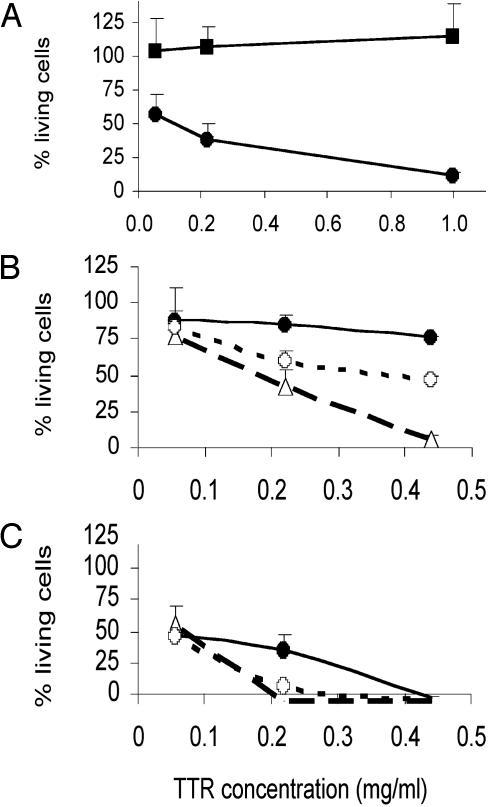
V30M TTR amyloid fibrils (see Materials and Methods) were incubated with IMR-32 cells at several concentrations (0.05–1 mg/ml) for up to 3 days. Cells exposed to soluble V30M TTR served as the positive control for cytotoxicity. Fig. 3A shows that V30M TTR-derived fibrils were not cytotoxic even at 1 mg/ml.
Fig. 3.
Cytotoxicity of quaternary structure variants of TTR in IMR-32. (A) V30M TTR fibrils (▪) versus soluble V30M TTR (•), incubated for 3 days. (B and C) V30M TTR (•) versus WT M-TTR (○) and V30M M-TTR (○), incubated for 36 h (B) or 3 days (C) at 37°C. Bars represent the SD of triplicate determinations.
Two engineered variants of TTR that are maintained in a monomeric state by strategic mutations in each of the two dimer–dimer interfaces were used (see Materials and Methods). These variants include monomeric WT M-TTR and V30M M-TTR (see Table 1). Such engineered variants do not form native tetramers; however, under conditions of mild denaturation they will aggregate to form congophilic fibrils (11). As seen in Fig. 3B (1 day of incubation with cells) and Fig. 3C (3 days of incubation with cells), the monomeric mutants were more toxic than soluble V30M TTR; the effect is observed after an incubation period as short as 1 day.
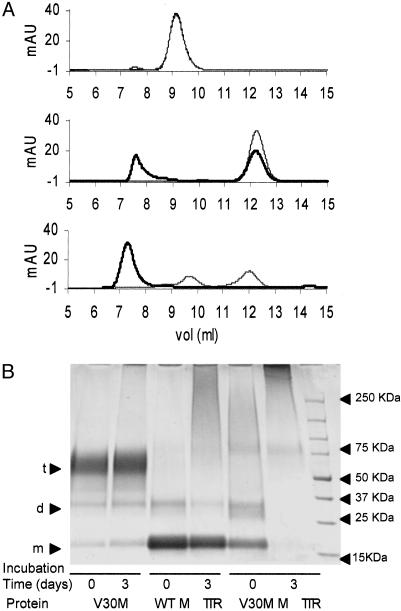
V30M TTR Is Mainly Tetramer, Whereas WT M-TTR and V30M M-TTR Aggregate Rapidly in the Conditions of the Cell Assay. We analyzed the species in solution before and after incubation of V30M TTR, WT M-TTR, and V30M M-TTR under the conditions of the cell assay. The proteins were incubated at a concentration of 0.44 mg/ml in Opti-MEM (pH 7.4) for 3 days at 37°C. Size-exclusion chromatograms of the starting material (time 0) and of the incubated samples are shown in Fig. 4A. V30M TTR (Fig. 4A Top) behaved as a tetramer (elution volume 9.2 ml) during the entire time of incubation, and WT M-TTR (Fig. 4A Middle) and V30M M-TTR (Fig. 4A Bottom) aggregated over the 3 days of the experiment (monomeric TTR elutes at 12.2 ml). V30M M-TTR was particularly sensitive to aggregation because larger soluble molecular species were already present at time 0 (immediately after buffer exchange). Mass balance was ensured chromatographically, demonstrating that no material was lost as insoluble aggregates during the incubation. By using defined molecular weight standards to calibrate the gel filtration column, we found that the soluble aggregates derived from WT M-TTR and V30M M-TTR were 100 kDa or larger. The same results were found when the solutions were analyzed with a Superdex 200 column, which is more appropriate for the characterization of larger molecular species (data not shown).
Fig. 4.
(A) SEC analysis (10/30 Superdex 75 column) of V30M TTR (Top), WT M-TTR (Middle), and V30M M-TTR (Bottom) at 0.44 mg/ml incubated at 37°C in Opti-MEM. Gray line, time 0; black line, analysis of protein solutions after 3 days of incubation. At Top (V30M TTR analysis), the two curves (time 0 and time 3 days) are superimposed. (B) SDS/PAGE of the same protein solutions than in A after cross-linking with glutaraldehyde. From left to right, V30M TTR (0 and 3 days), WT M-TTR (0 and 3 days), and V30M M-TTR (0 and 3 days) are shown. Arrowheads at right indicate the molecular weight standards. Arrowheads at left indicate sizes of monomer (m), dimer (d), and tetramer (t).
In parallel, aliquots from the same samples analyzed by gel filtration were cross-linked with glutaraldehyde to fix the aggregation state of the proteins, allowing their analysis under denaturing conditions by SDS/PAGE (Fig. 4B). Similar results were obtained with the two methods (SEC and SDS/PAGE) for WT M-TTR and V30M M-TTR. In contrast, V30M TTR appeared mainly as tetramer by gel filtration, but distinct monomer and dimer bands were observed after SDS/PAGE of the cross-linked samples. Analytical ultracentrifugation analyses (by sedimentation velocity and sedimentation equilibrium experiments) were performed on samples of V30M TTR incubated for 3 days in Opti-MEM (see Fig. 7 and Supporting Materials and Methods, which are published as supporting information on the PNAS web site). These analyses also indicated that species smaller than tetramer were in equilibrium with the native tetramer.
We also analyzed the supernatant medium of V30M TTR, WT M-TTR, and V30M M-TTR incubated for 3 days with IMR-32 cells by SEC, and compared the species in solution with the same TTR samples incubated without cells from parallel wells. No significant differences were found in the distribution of quaternary structures or in the absolute amount of material between samples incubated with and without cells (see Fig. 8, which is published as supporting information on the PNAS web site).
The results of these experiments indicate that under the conditions of the cell assay, the engineered monomers aggregate with time, whereas V30M TTR seems to be relatively stable. Furthermore, exposure of the proteins to cells does not affect the kinetics or distribution of aggregates. Finding these soluble aggregates in solution after 3 days of incubation at 37°C in the conditions of the cell assay prompted us to isolate them and test them in the IMR-32 cells to determine whether they were the cytotoxic species.
Soluble TTR Aggregates Larger Than Octamers Are Not Cytotoxic. The process of aggregation of the engineered monomers of TTR was concentration-dependent (see Fig. 9, which is published as supporting information on the PNAS web site). Both monomers, WT M-TTR and V30M M-TTR, were more cytotoxic than the V30M TTR tetramer (see above), but the kinetics of aggregation of the WT M-TTR were much slower than those of V30M M-TTR (see Fig. 4). We chose to use WT M-TTR, rather than V30M M-TTR, to isolate the putative cytotoxic species because its slower aggregation kinetics facilitated the analysis of the intermediates.
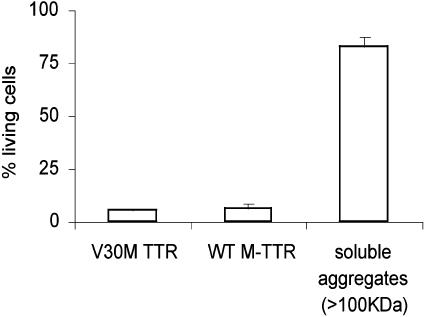
WT M-TTR was incubated at 1 mg/ml in Opti-MEM (cell medium) at 37°C for 5 days. After the incubation period, the soluble aggregates were purified by gel filtration (67% of the total peak area), were concentrated, buffer-exchanged with Opti-MEM, and added to IMR-32 cells at the same time as fresh WT M-TTR, and V30M TTR were added in parallel wells to the cells (used as cytotoxicity controls). The cells were then incubated at 37°C for 3 days and MTT assay was performed. The purified aggregates were also reanalyzed by gel filtration to confirm that they retained the same size distribution. Fig. 5 shows that the purified aggregates derived from WT M-TTR (>100kDa) were not cytotoxic to IMR-32 cells, even at 1 mg/ml concentration.
Fig. 5.
Cytotoxicity of 1-mg/ml solutions of V30M TTR, WT M-TTR, and soluble aggregates (>100 kDa) derived from WT M-TTR in IMR-32 cells after 3 days of incubation at 37°C.
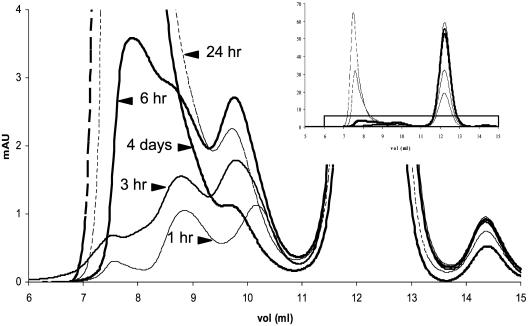
A more discriminant analysis was performed on WT M-TTR incubated at 1 mg/ml in Opti-MEM at 37°C and was analyzed by SEC after short periods of incubation up to 4 days (Fig. 6). Three intermediates leading to large soluble aggregates could be detected. A first intermediate eluting at 10.2 ml (estimated size by SEC of ≈36 kDa) was seen only after 1 h of incubation and it completely disappeared after 24 h. A second small aggregate, with an elution volume 9.75 ml (estimated size of ≈40 kDa), reached a maximum between 6 and 12 h (5% total area) and subsequently decreased. Another soluble species, with an elution volume of 8.8 ml (estimated size of ≈90 kDa), was seen after 1 h of incubation and continued to increase in concentration with time. The same intermediates were seen when the protein was incubated at 0.44 mg/ml (data not shown); however, the percentage of larger aggregates (>100 kDa, elution volume <8.2 ml) was lower at 0.44 mg/ml than when the protein was incubated at 1 mg/ml (see Fig. 9). Gel electrophoresis of the same samples cross-linked with glutaraldehyde suggest molecular weights of 28 kDa (corresponding to a dimer) for the intermediate eluting at 9.75 ml and 85 kDa (corresponding to a hexamer) for the intermediate eluting at 8.8 ml (data not shown; see representative gel in Fig. 4B). The molecular species eluting at 10.2 ml could not be determined unequivocally because its concentration is very low compared with native monomer and its presence was ephemeral. It is possible that the peak at 10.2 ml is the same size as that at 9.75 ml (probably dimer) with an alternative conformation, the differences in molecular shape resulting in the different elution volumes.
Fig. 6.
SEC analysis (10/30 Superdex 75 column) of the aggregation process of WT M-TTR at 1 mg/ml in Opti-MEM at 37°C show small aggregates forming (10.2, 9.8, and 8.8 ml). (Inset) Full chromatograms. The species seen at 10.2 ml disappears after1hof incubation. The species seen at 9.8 ml reaches a maximum between 6 and 12 h, and decreases afterward. The species at 8.8 ml increases with time.
The cytotoxicity assays, together with the SEC and SDS/PAGE analysis of the incubated samples, suggest that monomer or its very early aggregates (see Fig. 6) are the major cytotoxic species.
Discussion
The mechanism by which the process of TTR amyloid fibril formation occurs in humans is not fully understood. In vitro biophysical experiments indicate that the process is likely to require the dissociation of the native tetramer into its constituent monomers. A conformational change within the monomer (misfolding) enables the formation of soluble aggregates, which become insoluble as they grow (protofilaments). The insoluble aggregates eventually become amyloid fibrils by the lateral assembly of four protofilaments (4, 8).
Cytotoxic effects are seen after incubation of IMR-32 cells with the amyloidogenic mutant V30M TTR but not with WT TTR. We attribute these differences in cytotoxicity to the different stabilities of the native tetramers of the two proteins, the wild-type being substantially more stable, hence less likely to populate the misfolded monomer pool (13). The cytotoxicity is likely to be mediated by an apoptotic mechanism (Fig. 1 B and C).
We used several TTR molecules with defined quaternary structures to further characterize the molecular species that mediate the cytotoxicity (Table 1): Soluble V30M TTR (native tetramer), amyloid fibrils derived from V30M TTR, the engineered monomers WT M-TTR and V30M M-TTR, and purified soluble aggregates >100 kDa were studied in the cell assay. Only the engineered monomers and the tetrameric soluble V30M TTR produced cytotoxicity to IMR-32 cells, the monomers being more cytotoxic than soluble V30M TTR. V30M TTR-amyloid fibrils and purified soluble aggregates (100 kDa or larger) formed from WT M-TTR were not cytotoxic under the conditions of the assay (Figs. 3A and 5), even at 1 mg/ml.
Additional information was obtained from the inhibition assays depicted in Fig. 2 showing that native tetrameric V30M TTR stabilization by means of small-molecule binding (9) inhibits cytotoxicity. Thus it is almost certain that the V30M TTR tetramer must dissociate to be cytotoxic. The results are also consistent with the idea that the greater tendency of mutant tetramers to release monomer is responsible for the earlier onset of the autosomal-dominant diseases relative to the later-in-life deposition of the wild-type protein (13).
Under the conditions of the cell assay, small aggregates derived from the engineered monomer (WT M-TTR), with the apparent sizes of dimer (≈28 kDa by SDS/PAGE) and hexamer (85 kDa, by SDS/PAGE) form (Figs. 4B and 6). Because the concentrations of these intermediates are very low during the incubation, if they are the only cytotoxic species, they must be very active. Alternatively, the monomer itself may be the dominant cytotoxic molecule.
Our data are consistent with the recent observations of Kayed et al. (19) describing an antibody that binds soluble oligomers, but not native folded or amyloid fibrils, of several other amyloidogenic proteins (TTR was not included in the reported results). The authors suggest that there may also be a shared prefibrillar conformation, not detectable in mature fibrils, which is responsible for cytotoxicity in tissue culture, and perhaps in vivo. This hypothesis is supported by other reports where solutions containing early aggregates (as well as their precursors) of TTR amyloidogenic mutants (20, 21), and of amyloid-β (22), have shown cytotoxic effects on neurons and other cell lines. Our data suggest that the size of such cytotoxic intermediates can range from that of the monomer to something approaching a hexamer (<100 kDa). In earlier studies with TTR, the putative cytotoxic species were neither isolated nor characterized further.
The implications of these studies for potential treatments of the TTR amyloidoses, and other extracellular disorders of protein conformation, where tissue damage occurs at a distance from the site of synthesis, are profound. If cell damage in vivo, as in tissue culture, is produced primarily by subfibrillar aggregates then therapies that reduce fibril deposition or promote fibril clearance without reducing aggregation may accelerate the disease progression. The results also suggest that the pathologic effects of amyloidosis might best be prevented by native-state stabilization of the precursor protein: In the case of TTR, addition of small molecules that stabilize the tetramer would block the dissociation, which leads to the formation of the monomeric cytotoxic species or its nonnative early oligomers (9). In other amyloidoses like AD or amyloid A, where cleavage of a precursor protein is required (1), inhibition of the enzymes responsible for such processing would prevent not only the fibril deposition but the formation of the cytotoxic aggregates by reducing the concentration of precursor available to populate the oligomer pool.
The notion of relatively small oligomers as the primary toxic species in tissue culture seems to be well established in findings previously reported for Aβ1–42 in AD. Its validity as an in vivo pathogenetic mechanism may be easier to accept in AD, where the polymerizing fragments are synthesized and processed in proximity to the disease targets. A similar process occurring in those deposition diseases where synthesis and deposition are asynchronous in time and space may be more difficult to demonstrate in vivo (23). The present results are but a step in this direction.
Supplementary Material
Acknowledgments
We thank Kelly Fields for excellent technical assistance and Dr. R. Gottlieb and R. Sayen for assistance with the acquisition of fluorescent microscopy images. The work was supported by National Institutes of Health Grant RO1 AG15916 (to J.N.B.).
Abbreviations: TTR, transthyretin; V30M TTR, TTR with Val30Met mutation; WT M-TTR, monomeric WT TTR; V30M M-TTR, monomeric V30M TTR; AD, Alzheimer's disease; SEC, size-exclusion chromatography; MTT, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide.
References
- 1.Buxbaum, J. N. & Tagoe, C. E. (2000) Annu. Rev. Med. 51, 543-569. [DOI] [PubMed] [Google Scholar]
- 2.Merlini, G. & Bellotti, V. (2003) N. Engl. J. Med. 349, 583-596. [DOI] [PubMed] [Google Scholar]
- 3.Sacchettini, J. C. & Kelly, J. W. (2002) Nat. Rev. Drug Discov. 1, 267-275. [DOI] [PubMed] [Google Scholar]
- 4.Kelly, J. W. (1998) Curr. Opin. Struct. Biol. 8, 101-106. [DOI] [PubMed] [Google Scholar]
- 5.Damas, A. M. & Saraiva, M. J. (2000) J. Struct. Biol. 130, 290-299. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson, D. R., Pastore, R. D., Yaghoubian, R., Kane, I., Gallo, G., Buck, F. S. & Buxbaum, J. N. (1997) N. Engl. J. Med. 336, 466-473. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson, L. & Westermark, P. (1990) Acta Neuropathol. 80, 597-603. [DOI] [PubMed] [Google Scholar]
- 8.Quintas, A., Vaz, D. C., Cardoso, I., Saraiva, M. J. & Brito, R. M. (2001) J. Biol. Chem. 276, 27207-27213. [DOI] [PubMed] [Google Scholar]
- 9.Hammarstrom, P., Wiseman, R. L., Powers, E. T. & Kelly, J. W. (2003) Science 299, 713-716. [DOI] [PubMed] [Google Scholar]
- 10.White, J. T. & Kelly, J. W. (2001) Proc. Natl. Acad. Sci. USA 98, 13019-13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, X., Smith, C. S., Petrassi, H. M., Hammarstrom, P., White, J. T., Sacchettini, J. C. & Kelly, J. W. (2001) Biochemistry 40, 11442-11452. [DOI] [PubMed] [Google Scholar]
- 12.Hammarstrom, P., Sekijima, Y., White, J. T., Wiseman, R. L., Lim, A., Costello, C. E., Altland, K., Garzuly, F., Budka, H. & Kelly, J. W. (2003) Biochemistry 42, 6656-6663. [DOI] [PubMed] [Google Scholar]
- 13.Hammarström, P., Jiang, X., Hurshman, A. R., Powers, E. T. & Kelly, J. W. (2002) Proc. Natl. Acad. Sci. USA 99, 16427-16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lashuel, H. A., Wurth, C., Woo, L. & Kelly, J. W. (1999) Biochemistry 38, 13560-13573. [DOI] [PubMed] [Google Scholar]
- 15.Oza, V. B., Petrassi, H. M., Purkey, H. E. & Kelly, J. W. (1999) Bioorg. Med. Chem. Lett. 9, 1-6. [DOI] [PubMed] [Google Scholar]
- 16.Hammarstrom, P., Jiang, X., Deechongkit, S. & Kelly, J. W. (2001) Biochemistry 40, 11453-11459. [DOI] [PubMed] [Google Scholar]
- 17.Klabunde, T., Petrassi, H. M., Oza, V. B., Raman, P., Kelly, J. W. & Sacchettini, J. C. (2000) Nat. Struct. Biol. 7, 312-321. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso, I., Goldsbury, C. S., Muller, S. A., Olivieri, V., Wirtz, S., Damas, A. M., Aebi, U. & Saraiva, M. J. (2002) J. Mol. Biol. 317, 683-695. [DOI] [PubMed] [Google Scholar]
- 19.Kayed, R., Head, E., Thompson, J. L., McIntire, T. M., Milton, S. C., Cotman, C. W. & Glabe, C. G. (2003) Science 300, 486-489. [DOI] [PubMed] [Google Scholar]
- 20.Andersson, K., Olofsson, A., Nielsen, E. H., Svehag, S. E. & Lundgren, E. (2002) Biochem. Biophys. Res. Commun. 294, 309-314. [DOI] [PubMed] [Google Scholar]
- 21.Sousa, M. M., Cardoso, I., Fernandes, R., Guimaraes, A. & Saraiva, M. J. (2001) Am. J. Pathol. 159, 1993-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taylor, B. M., Sarver, R. W., Fici, G., Poorman, R. A., Lutzke, B. S., Molinari, A., Kawabe, T., Kappenman, K., Buhl, A. E. & Epps, D. E. (2003) J. Protein Chem. 22, 31-40. [DOI] [PubMed] [Google Scholar]
- 23.Buxbaum, J. N. (2003) Trends Biochem. Sci. 28, 585-592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.