Abstract
The constitutive active/androstane receptor (CAR) regulates hepatic drug metabolism by activating genes, such as cytochrome P450, and certain transferases. p38 Mitogen–activated protein kinase (MAPK) is highly activated in human primary hepatocytes but barely in human hepatoma cell lines including HepG2 cells. Liganded-CAR induced CYP2B6 mRNA in human primary hepatocytes far more effectively than in HepG2 cells ectopically expressing CAR. In the present study, we found that activation of p38 MAPK by anisomycin potentiated induction of CYP2B6 mRNA by CAR ligand in HepG2 cells to levels observed in ligand-treated human primary hepatocytes. siRNA knockdown of p38 MAPK abrogated the ability of anisomycin to synergistically induce CYP2B6 mRNA. In addition to CYP2B6, anisomycin cotreatment potentiated an increase in CYP2A7 and CYP2C9 mRNAs but not CYP3A4 or UDP-glucuronosyltransferase 1A1 mRNAs. Thus, activated p38 MAPK is required for liganded-CAR to selectively activate a set of genes that encode drug-metabolizing enzymes. Our present results suggest that CAR-mediated induction of these enzymes cannot be understood by ligand binding alone because the specificity and magnitude of induction are codetermined by a given cell signaling, such as p38 MAPK; both physiologic and pathophysiological states of cell signaling may have a strong impact in hepatic drug-metabolizing capability during treatments.
Introduction
Nuclear receptor constitutive active/androstane receptor (CAR), a xenobiotic-activated transcription factor, regulates numerous genes that encode drug-metabolizing enzymes, such as cytochrome P450 CYP2B6 and UDP-glucuronosyltransferase (UGT) 1A1 (Honkakoski et al., 1998; Sueyoshi et al., 1999; Sugatani et al., 2001). CAR activators include various therapeutic drugs, such as phenobarbital and various statins, resulting in inductions of drug-metabolizing enzymes (Sueyoshi et al., 1999; Kobayashi et al., 2005). Activation of CAR is initiated by nuclear translocation of CAR (Kawamoto et al., 1999). Subsequently, nuclear CAR forms a heterodimer with retinoid X receptor and activates its target genes by binding to response elements in promoters (Honkakoski et al., 1998). The mechanism of nuclear translocation of CAR uses various cell-signaling molecules, such as protein phosphatase 2A and extracellular signal-regulated kinase 1/2 (Yoshinari et al., 2003; Koike et al., 2007). In addition to what is observed in the cytoplasm, early growth response 1 promotes CAR to transactivate the CYP2B6 gene in HepG2 cells as effectively as that in primary hepatocytes and livers (Swales et al., 2005; Inoue and Negishi, 2008). Thus, CAR-mediated transactivation of its target genes appears to be coregulated by CAR activators and cell-signaling molecules.
p38 Mitogen–activated protein kinase (MAPK), a member of the family of MAPKs, is activated by a number of cellular stresses caused by many stimuli, such as UV, osmotic pressure, DNA damage, cytokines, and tumor necrosis factor α, and is involved in the regulation of inflammation, apoptosis, and cellular death and proliferation (Herlaar and Brown, 1999; Obata et al., 2000). Recently, a p38 MAPK inhibitor SB203580 was reported to suppress phenobarbital induction of CYP2B1/2 mRNA in rat primary hepatocytes, although the mechanism of this suppression has not been investigated (Joannard et al., 2006). Because of our unexpected finding that p38 MAPK is present but not activated in HepG2 cells, we investigated the role of p38 MAPK in CAR-mediated transcriptional activation of the CYP2B6 gene.
In the present study, we used human primary hepatocytes, hepatoma-derived cell lines (HepG2, FLC7, and Huh 7), and HepG2 ectopically expressing mouse and human CAR (called Ym17 and Yh18 cells, respectively) to demonstrate the role of p38 MAPK in the CAR-mediated activation of CYP2B6 gene. We present experimental data to characterize p38 MAPK as a regulatory cell signal in CAR activation and propose the hypothesis that CAR requires cellular signaling, such as p38 MAPK, to fully confer its transactivation activity in response to xenobiotic exposures.
Materials and Methods
Materials.
Anisomycin, 1,4-Bis-[2-(3,5-dichloropyridyloxy)]benzene, 3,3′,4,4′-Tetrachloro-1,4-bis(pyridyloxy)benzene (TCPOBOP), and dimethylsulfoxide were purchased from Sigma-Aldrich (St. Louis, MO). 6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime (CITCO) was purchased from BIOMOL (Plymouth Meeting, PA). Antibodies against phospho-p38 MAPK (Thr180/Thr182; #9211), p38 MAPK (#9212), and phospho-MAPK–activated protein kinase 2 (MAPKAPK2; Thr334; #3007) were purchased from Cell Signaling Technology (Danvers, MA). Antibodies against B-actin and horseradish peroxidase–conjugated anti-mouse or -rabbit IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ON-TARGET plus SMART pool p38 MAPK (#L-003512-00-0005) and ON-TARGET plus Non-Targeting pool (#D-001810-10) were purchased from Thermo Scientific (Lafayette, CO).
Cell Cultures, Transfections, and Chemical Exposures.
Human primary hepatocytes (from 1 male and 2 female donors) were kindly provided by Life Technologies (Carlsbad, CA). Primary hepatocytes were maintained according to the manufacturer’s instructions. Hepatocytes were placed on a 12-well plate at a density of 6 × 105 cells/well. HepG2 cells ectopically expressing mouse CAR (Ym17 cells) and human CAR (Yh18 cells) were established as described previously (Swales et al., 2005). HepG2, Ym17, and Yh18 cells were maintained as described previously (Swales et al., 2005). FLC7 cells were kindly provided by Dr. Nagamori (Kyorin University, Tokyo, Japan) (Kawada et al., 1998). FLC7 and Huh7 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% 200 mM glutamine, and antibiotics. Cells were placed on a 24-well plate at a density of 4 × 105 cells/well. For transfection, cells were transfected with siRNA (50 pmol) by LipofectAMINE RNAiMAX (Life Technologies) after cell seeding. For treatment, hepatocytes and cells were exposed with TCPOBOP (0.25 μM), CITCO (1 μM), and anisomycin (0.2 μM) for 6 hours.
Western Blotting.
Hepatocyte and cell extracts were prepared by lysis of cells with 1 × LDS buffer (Life Technologies) with 1% beta-mercaptoethanol. Protein separation and detection were performed as described previously (Inoue and Negishi, 2008).
Real-Time Quantitative Polymerase Chain Reaction.
Total hepatocyte and cell RNAs were isolated using Trizol Reagent (Life Technologies) and subjected to cDNA synthesis using High Capacity cDNA Archive Kit (Life Technologies). Real-time polymerase chain reaction (PCR) was performed using the 7900HT Fast Real-Time PCR system (Life Technologies) with the following Taq-Man probes or primers: CYP2B6 (Hs00167937_m1), CYP2C9 (Hs01682803_mH), CYP2A7 (5′-AGCCCTTGCAGCAACTTAAA-3′ and 5′-CAGTTCACGTCAACCTCAC-3′), CYP3A4 (Hs00430021_m1), and UGT1A1 (Hs02511055_m1). Human ACTB (Beta Actin) Endogenous Control (VIC/TAMRA Probe, Primer Limited: 4310881E; Life Technologies) was used as an internal control and to normalize expression levels of all other genes.
Statistics.
All numeric data are shown as mean ± S.D. The differences in data from real-time PCR were determined using one-way analysis of variance for all groups, followed by pairwise comparisons.
Results
p38 MAPK Is Not Activated in Human Hepatoma Cell Lines.
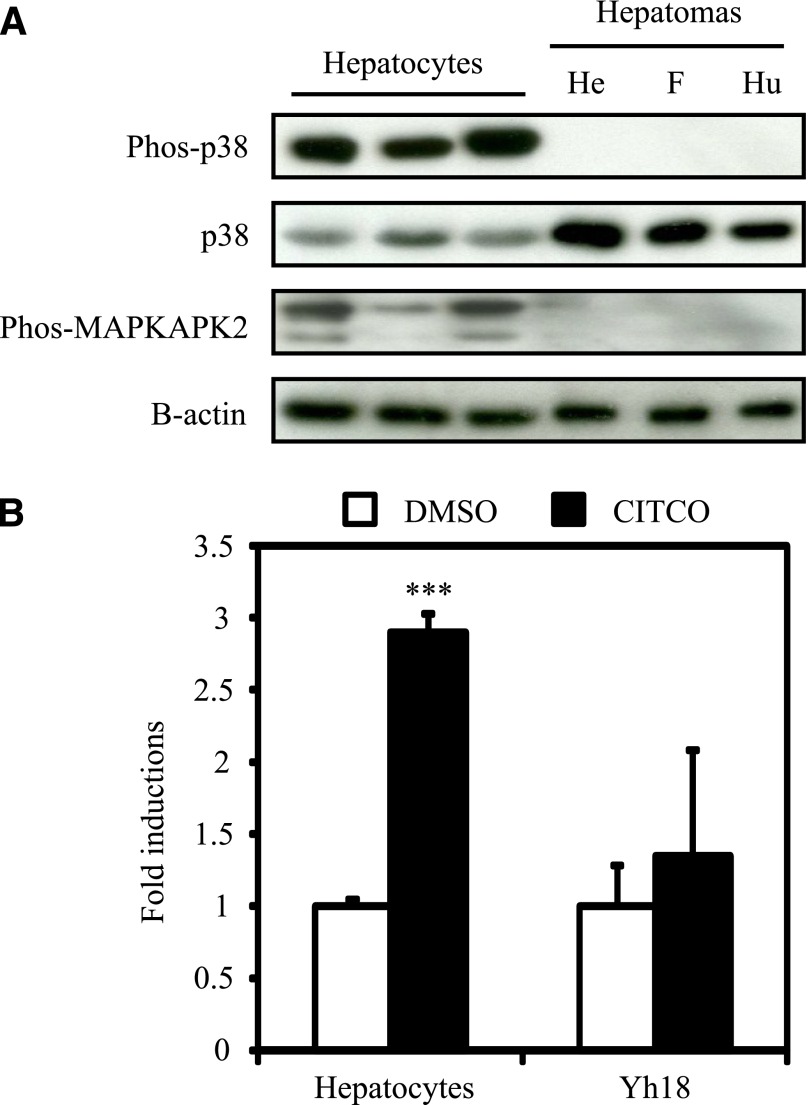
To examine whether p38 MAPK signaling is activated in human primary hepatocytes and human hepatoma cell lines, whole hepatocyte and cell extracts were prepared and subjected to Western blotting. As shown in Fig. 1A, phosphorylated p38 MAPK was detected in all human primary hepatocytes from these three different donors. In contrast, phosphorylated p38 MAPK was not detected in the human hepatoma cell lines (HepG2, FLC7, and Huh7 cells). The detected levels of the total amount of p38 MAPK protein were much weaker in all hepatocytes, compared with hepatoma cell lines. In addition, levels of phosphorylated MAPKAPK2, a downstream target of p38 MAPK, demonstrated far stronger intensity in human primary hepatocytes rather than in human hepatoma cell lines. These results indicate that p38 MAPK is constitutively activated in human primary hepatocytes but not in human hepatoma cell lines. In addition, ligand activation of human CAR by CITCO increased CYP2B6 mRNA by 2.9-fold in human primary hepatocytes but did not increase it in HepG2 cells ectopically expressing human CAR (Yh18 cells) (Fig. 1B). This result prompted us to investigate the role of p38 MAPK in CAR-mediated CYP2B6 induction.
Fig. 1.
Phosphorylation of p38 MAPK and CAR-mediated activation of CYP2B6 in human primary hepatocytes and hepatoma cell lines. (A) Hepatocyte and cell extracts were prepared as described in Materials and Methods from seeded hepatocytes and cells without any exposure. Protein levels of phosphorylated p38 MAPK (Phos-p38), p38 MAPK (p38), and phosphorylated MAPKAPK2 (Phos-MAPKAPK2). Protein levels of B-actin were determined as an internal control. Data shown are three individual donors of primary hepatocytes and three different hepatoma cell-lines (HepG2, FLC7, and Huh7 cells from left to right). He, HepG2 cells; F, FLC7 cells; Hu, Huh7 cells. (B) Total RNAs were prepared as described in Materials and Methods from one female donor of hepatocytes and Yh18 cells treated as described in Materials and Methods. Expression level of CYP2B6 mRNA was determined. Values are expressed as fold inductions relative to that of CYP2B6 mRNA level normalized to the expression levels of B-actin (ACTB) mRNA in dimethylsulfoxide-treated hepatocytes or Yh18 cells. Data are mean ± S.D. (n = 3 in each group). ***P < 0.005 for comparison between with and without CITCO exposure, Newman-Keuls multiple comparison test.
p38 MAPK Regulates CAR-Mediated Induction of CYP2B6 mRNA.
We first investigated the effect of p38 MAPK activation in the CAR-mediated induction of CYP2B6 using HepG2 cells ectopically expressing human CAR (Yh18 cells). Because mouse CAR with its ligand TCPOBOP is a well-established model to investigate the ligand activation of CAR and the induction of CYP2B6 in HepG2 cells (Swales et al., 2005; Inoue and Negishi, 2008), we also used HepG2 cells ectopically expressing mouse CAR (Ym17 cells). The activation of p38 MAPK by anisomycin was verified in HepG2 cells and in Yh18 and Ym17 cells (Supplemental Fig. 1) Ligand activation of ectopically expressing human CAR by CITCO significantly increased CYP2B6 mRNA levels only in the presence of anisomycin (Fig. 2A). The extent of the CYP2B6 mRNA increase by anisomycin and CITCO was 2.8-fold, which is compatible to those in primary human hepatocytes (Fig. 1B). Although ligand activation of ectopically expressing mouse CAR significantly increased CYP2B6 mRNA level, anisomycin treatment further enhanced the increased level of CYP2B6 mRNA (Fig. 2B). Because ectopically expressing mouse CAR clearly demonstrated the potentiation effect rather than ectopically expressing human CAR, we knocked down p38 MAPK by siRNA in Ym17 cells to examine whether p38 MAPK mediates the potentiation of CAR-mediated CYP2B6 induction by anisomycin. As shown in Fig. 2C, knockdown of p38 MAPK significantly suppressed the anisomycin-potentiated effect to induce CYP2B6 with ligand activation of CAR. The remaining potentiation effect of anisomycin may be attributable to remaining p38 MAPK. The role of p38 MAPK on the potentiation effect of anisomycin was further confirmed by inhibition of p38 MAPK (Supplemental Fig. 2). These results indicate that p38 MAPK regulates CAR-mediated CYP2B6 induction.
Fig. 2.
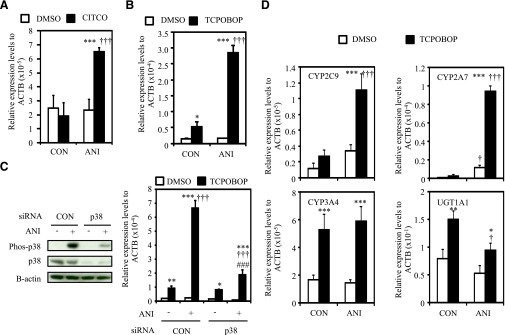
Role of p38 MAPK in CAR-mediated CYP2B6 induction. (A–D) Total RNAs were prepared as described in Materials and Methods from Yh18 (A) and Ym17 (B–D) cells treated as described in Materials and Methods. Expression levels of CYP2B6 (A–C) and CYP2C9, CYP2A7, CYP3A4, and UGT1A1 (D) mRNA were determined. Values are expressed as relative expression levels normalized to the expression levels of B-actin (ACTB) mRNA. Data are mean ± S.D. (n = 3 or 4 in each group). *P < 0.05; **P < 0.01; ***P < 0.005 for comparison between with and without CITCO or TCPOBOP exposure; †P < 0.05; †††P < 0.005 for comparison between with and without anisomycin exposure; ###P < 0.005 for comparison between control siRNA and p38 MAPK (p38) siRNA. Newman-Keuls multiple comparison test. (C) Protein levels of phosphorylated p38 MAPK (Phos-p38), p38 MAPK (p38), and B-actin were also determined to verify the effect of p38 MAPK siRNA at 2 hours of anisomycin treatment. ANI, anisomycin; CON, control.
p38 MAPK Regulates a Set of CAR Target Genes.
Because of the finding that p38 MAPK regulates CAR-mediated CYP2B6 induction, we then examined the role of p38 MAPK in the CAR-mediated regulations of genes encoding other drug-metabolizing enzymes (CYP2C9, CYP2A7, CYP3A4, and UGT1A1). As shown in Fig. 2D, activation of CAR significantly increased CYP2C9 and CYP2A7 mRNA level only in the presence of anisomycin. In contrast to CYP2B6, CYP2C9, and CYP2A7, activation of CAR increased CYP3A4 and UGT1A1 mRNA level regardless of the presence of anisomycin. These results indicate that p38 MAPK selectively regulates the CAR-mediated induction of CYP2B6, CYP2C9, and CYP2A7 but not CYP3A4 or UGT1A1.
Discussion
CAR-mediated induction of CYP2B6 mRNA was strongly potentiated by the activation of p38 MAPK in HepG2 cells as effectively as that in human primary hepatocytes. In addition, p38 MAPK is constitutively activated in human primary hepatocytes but not in human hepatoma cell lines including HepG2 cells. Thus, p38 MAPK may play a role in the CAR-mediated CYP2B6 induction in human primary hepatocytes. Only one set of CAR-regulated genes requires p38 MAPK for their activation: this set includes genes such as CYP2A7 and CYP2C9, in addition to CYP2B6. In contrast, p38 MAPK plays no role in CAR activation of the CYP3A4 or UGT1A1 genes. Various cell signaling has been shown to regulate activation of CAR and CAR-mediated transcription of a target gene. Growth factor–activated extracellular signal-related kinase 1/2 signaling represses CAR activation and nuclear translocation (Koike et al., 2007). cAMP and early growth response 1 synergize CAR-mediated transcription of CYP2B6 genes in HepG2 cells (Ding et al., 2006; Inoue and Negishi, 2008). AKT and NFκB signaling represses the CYP2B6 gene (Assenat et al., 2004; Kodama et al., 2004). However, those previous studies were limited only to the CYP2B gene. Therefore, to our knowledge, our study is the first to demonstrate that cell signaling, such as p38 MAPK, not only regulates CAR-mediated transcriptions but also determines the specificity for which genes are activated by CAR.
Diabetes is known to up-regulate p38 MAPK activation in mouse liver (Qiao et al., 2006). Phenobarbital induction of CYP2B1 is stimulated in the liver of streptozotocin-induced diabetic rats (Yoshida et al., 1996). On the other hand, p38 MAPK is down-regulated in mouse liver tumors (Iyoda et al., 2003). Phenobarbital induction is attenuated in tumor-developing rat livers (Numazawa et al., 2005). These observations are consistent with our hypothesis that p38 MAPK signaling plays a critical role in the regulation of CAR-mediated activation of genes. Therefore, the outcome of drug metabolism (both degree and specificity of induction) after therapeutic treatment should be affected by physiologic and disease conditions that alter cell signaling, such as p38 MAPK. To expand our finding of p38 MAPK and to implicate it in drug therapy, the molecular mechanism by which p38 MAPK promotes CAR-mediated transcription of the CYP2B6 gene needs to be investigated in the future.
In conclusion, we characterized p38 MAPK as essential cell signaling that regulates CAR-mediated transcriptions. Thus, p38 MAPK activity, which is altered by physiologic and pathophysiological conditions, should be considered when the outcome of drug metabolism is projected and/or is evaluated after treatment.
Supplementary Material
Acknowledgments
The authors thank Dr. S. Nagamori for providing FLC7 cells, and Life Technologies for providing human primary hepatocytes.
Abbreviations
- CAR
constitutive active/androstane receptor
- CITCO
6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde O-(3,4-dichlorobenzyl)oxime
- MAPK
mitogen activated protein kinase
- MAPKAPK2
MAPK activated protein kinase 2
- PCR
polymerase chain reaction
- TCPOBOP
1,4-Bis-[2-(3,5-dichloropyridyloxy)]benzene, 3,3′,4,4′-Tetrachloro-1,4-bis(pyridyloxy)benzene
- UGT
UDP-glucuronosyltransferase
Authorship Contributions
Participated in research design: Saito, Negishi.
Conducted experiments: Saito, Moore.
Performed data analysis: Saito.
Wrote or contributed to the writing of the manuscript: Saito, Negishi.
Footnotes
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences Intramural Research Program [Grant Z01ES1005-01].
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre JM, Maurel P, Vilarem MJ, Pascussi JM. (2004) Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology 40:951–960 [DOI] [PubMed] [Google Scholar]
- Ding X, Lichti K, Kim I, Gonzalez FJ, Staudinger JL. (2006) Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. J Biol Chem 281:26540–26551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlaar E, Brown Z. (1999) p38 MAPK signalling cascades in inflammatory disease. Mol Med Today 5:439–447 [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. (1998) The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol 18:5652–5658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue K, Negishi M. (2008) Nuclear receptor CAR requires early growth response 1 to activate the human cytochrome P450 2B6 gene. J Biol Chem 283:10425–10432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyoda K, Sasaki Y, Horimoto M, Toyama T, Yakushijin T, Sakakibara M, Takehara T, Fujimoto J, Hori M, Wands JR, et al. (2003) Involvement of the p38 mitogen-activated protein kinase cascade in hepatocellular carcinoma. Cancer 97:3017–3026 [DOI] [PubMed] [Google Scholar]
- Joannard F, Rissel M, Gilot D, Anderson A, Orfila-Lefeuvre L, Guillouzo A, Atfi A, Lagadic-Gossmann D. (2006) Role for mitogen-activated protein kinases in phenobarbital-induced expression of cytochrome P450 2B in primary cultures of rat hepatocytes. Toxicol Lett 161:61–72 [DOI] [PubMed] [Google Scholar]
- Kawada M, Nagamori S, Aizaki H, Fukaya K, Niiya M, Matsuura T, Sujino H, Hasumura S, Yashida H, Mizutani S, et al. (1998) Massive culture of human liver cancer cells in a newly developed radial flow bioreactor system: ultrafine structure of functionally enhanced hepatocarcinoma cell lines. In Vitro Cell Dev Biol Anim 34:109–115 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19:6318–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Yamanaka Y, Iwazaki N, Nakajo I, Hosokawa M, Negishi M, Chiba K. (2005) Identification of HMG-CoA reductase inhibitors as activators for human, mouse and rat constitutive androstane receptor. Drug Metab Dispos 33:924–929 [DOI] [PubMed] [Google Scholar]
- Kodama S, Koike C, Negishi M, Yamamoto Y. (2004) Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24:7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. (2007) Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol 71:1217–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numazawa S, Shindo S, Maruyama K, Chibana F, Kawahara Y, Ashino T, Tanaka S, Yoshida T. (2005) Impaired nuclear translocation of CAR in hepatic preneoplastic lesions: association with an attenuated CYP2B induction by phenobarbital. FEBS Lett 579:3560–3564 [DOI] [PubMed] [Google Scholar]
- Obata T, Brown GE, and Yaffe MB (2000) MAP kinase pathways activated by stress: the p38 MAPK pathway. Crit Care Med 28:N67–77. [DOI] [PubMed]
- Qiao L, MacDougald OA, Shao J. (2006) CCAAT/enhancer-binding protein alpha mediates induction of hepatic phosphoenolpyruvate carboxykinase by p38 mitogen-activated protein kinase. J Biol Chem 281:24390–24397 [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. (1999) The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem 274:6043–6046 [DOI] [PubMed] [Google Scholar]
- Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T. (2001) The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 33:1232–1238 [DOI] [PubMed] [Google Scholar]
- Swales K, Kakizaki S, Yamamoto Y, Inoue K, Kobayashi K, Negishi M. (2005) Novel CAR-mediated mechanism for synergistic activation of two distinct elements within the human cytochrome P450 2B6 gene in HepG2 cells. J Biol Chem 280:3458–3466 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Kimura N, Oda H, Kakinuma A. (1996) Insulin suppresses the induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem Biophys Res Commun 229:182–188 [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M. (2003) Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett 548:17–20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.