Abstract
Fibrinogen-like protein 2 (Fgl2, fibroleukin) is a leukocyte product that exhibits significant homology to secreted proteins of diverse function, including growth factors, lectins, and components of extracellular matrix. Prior studies found that Fgl2 is IFNγ-inducible, possesses direct coagulant activity, and inhibits T cell proliferation and dendritic cell maturation in vitro. Here, we demonstrate that Fgl2 expression is up-regulated during type 1 immunity in vivo and establish that such up-regulation is IFNγ-, signal transducer and activation of transcription protein 1-, and IFN response factor 1-dependent. To investigate functional roles for Fgl2 during type 1 immunity, we generated Fgl2-deficient mice. Those animals are born at predicted Mendelian frequencies, appear overtly healthy, and contain normal numbers and frequencies of lymphoid cells. Although Fgl2 is IFNγ-inducible and putatively regulates T cell activation/proliferation, we demonstrate that Fgl2-deficient and control mice exhibit similar degrees of T cell expansion, immunopathology, and/or pathogen burdens during protozoan (Toxoplasma gondii), bacterial (Yersinia enterocolitica, Listeria monocytogenes, and Mycobacterium tuberculosis), and viral (murine γ-herpesvirus-68 and Sendai) infections. Fgl2-deficient mice also reject allografts with similar kinetics as control mice. Moreover, despite prior reports that Fgl2 functions as a procoagulant enzyme, we demonstrate that Fgl2-deficient and control mice produce similar levels of fibrin, a product of the coagulation cascade, during T. gondii infection and allograft rejection. Together, our findings suggest that Fgl2, although highly conserved and IFNγ-inducible, is not a critical mediator of either type 1 immunity or immune-associated coagulant activity.
Fibrinogen-like protein 2 (Fgl2) is a leukocyte product whose physiologic functions remain to be established. Its sequence is highly conserved between mouse and human and exhibits 36% identity at the amino acid level to the β and γ chains of fibrinogen (1). Homology is greatest in a fibrinogen-related domain that is shared by a number of functionally unrelated proteins, including the coagulant molecule fibrinogen, the matrix-forming tenascins, the lectin-binding ficolins, and the angiopoietin growth factors. Like Fgl2 (2), each of those proteins can be secreted as homooligomeric structures.
Fgl2 was originally cloned as a gene selectively expressed by cytotoxic T lymphocytes (3) but was subsequently recloned as an IFNγ-inducible macrophage gene (4) and as the target of a mAb that suppressed a virally induced coagulopathy (5). Functional studies using neutralizing antisera suggested that Fgl2 may also contribute to coagulant activity associated with spontaneous abortion in mice (6). These observations led to a series of publications describing the direct coagulant activity of Fgl2 (5, 7, 8). In addition, recombinant Fgl2 was recently shown to suppress T cell proliferation and dendritic cell maturation in vitro (9). Together, these prior reports suggest that Fgl2 may regulate coagulation and/or immunity during type 1 responses.
To definitively evaluate roles for Fgl2 in type 1 immunity and immune-associated coagulant activity, we generated Fgl2-deficient mice. Despite demonstrating that Fgl2 is IFNγ-regulated in vivo, we report that a number of IFNγ-associated immune responses are not demonstrably perturbed in mice lacking the capacity to express Fgl2. We also demonstrate that Fgl2-deficient mice produce WT levels of fibrin in response to Toxoplasma gondii infection and transplantation. These findings suggest that Fgl2 does not function as a critical immunosuppressive coagulant during type 1 immunity. The Fgl2-deficient mice described herein should be valuable tools for defining both physiologic and pathologic functions of this highly conserved IFNγ-inducible protein.
Materials and Methods
Analyses of Fgl2 mRNA and Protein Levels. cDNA encompassing the entire coding region of murine Fgl2 was cloned by PCR, sequenced, and used to reprobe previously described Northern blots (10, 11). Real-time PCR-based quantitation of Fgl2 mRNA was performed and normalized to levels of β-2-microglobulin, as described (12). The Fgl2 primers (GGTCAACAGTTTGGATGGCAA, TTGAACCGGCTGTGACTGC) and probe (TTCCAAGTGTCCCAGCCAAGAACACA) span an intron and do not amplify genomic DNA.
Murine Fgl2 was expressed with a C-terminal histidine tag in Chinese hamster ovary cells and purified from supernatant using lens culinaris agglutinin-coupled agarose (Vector Laboratories) followed by nickel-chelated agarose (Invitrogen). A rat IgM mAb specific for mouse Fgl2 was obtained by immunizing Wistar rats with purified Fgl2, generating hybridomas, and screening for Fgl2 reactivity by ELISA and Western blotting.
Mice. IFNγ-, IFNγ receptor-(IFNγR), and IFN response factor 1- (Irf1) deficient mice were purchased from The Jackson Laboratory. Signal transducer and activation of transcription protein 1 (Stat1)-deficient and control 129/Sv and C57BL/6 mice were purchased from Taconic Farms. To produce gene-targeted Fgl2-deficient mice, C57BL/6 DNA flanking the mouse fgl2 gene was cloned into the pPNT gene-targeting vector (13). After transfection and drug selection of C57BL/6 embryonic stem cells, Southern blotting was used to identify clones possessing the desired homologous recombination. Chimeric mice were then generated and backcrossed to C57BL/6 mice. On germline transmission of the targeted allele, heterozygous Fgl2 mutant mice were intercrossed to produce fully C57BL/6-inbred Fgl2-deficient animals. Fgl2-deficient mice were genotyped by PCR using the following primers: Fgl2-5′, AATGGGATTTTCTGGGGCAC; Fgl2-3′, CCAGTGCTTTCAAGCATTCC; neo, TGAAGAACGAGATCAGCAGC.
Six- to 10-week old mice were used for all experiments, except for bone marrow chimeras, which were irradiated (1,000 rad) and reconstituted (1 × 107 bone marrow cells per mouse) at 6 weeks of age and then infected 7 weeks later. All animals were housed in a specific pathogen-free facility and cared for according to the Trudeau Institute Animal Care and Use Committee guidelines.
Measurements of Fibrin and Immune Parameters. Fibrin levels within tissue samples were measured quantitatively by Western blotting or qualitatively by immunohistochemistry, as described (12, 14). Hematocrits and platelet counts were determined using a Coulter Counter (Beckman Coulter). IFNγ protein levels in sera were determined by sandwich ELISA (BD Pharmingen), and tissue levels of various mRNA were measured by real-time PCR and normalized to levels of β-2-microglobulin, as described (12).
Infections. T. gondii strain ME49 cysts were obtained from brains of chronically infected C57BL/6 mice, and infections were initiated by peroral administration of 10 cysts, as described (12). Parasite numbers in infected animals were determined by real-time PCR (12).
Stocks of Yersinia enterocolitica (strain WA; American Type Culture Collection no. 27729) and Listeria monocytogenes (strain EGD, supplied by R. North, Trudeau Institute) were prepared after passage through C57BL/6 mice (15, 16). Mice were infected with the indicated dosages and analyzed 5 days after infection, at which time bacterial burdens are near their peak.
Mycobacterium tuberculosis H37Rv was grown from laboratory stocks (supplied by R. North) in Proskauer-Beck liquid media to midlogarithmic phase, aliquoted, and frozen at -70°C. Mice were infected with ≈100 bacteria by using a Middlebrook Airborne Infection apparatus (Middlebrook, Terre Haute, IN) (17). Alternatively, mice were infected i.v. via the lateral tail vein with 2 × 105 bacteria suspended in saline (17). Numbers of viable bacteria in organs and numbers of IFNγ-producing CD4 T cells were determined as described (18).
Murine γ-herpesvirus (MHV)-68 (WUMS strain) was propagated in NIH 3T3 fibroblasts, and mice were anesthetized and intranasally infected with 400 plaque-forming units of virus (19). Titers of lytic virus were determined by plaque assay, titers of latent virus were determined by an in vitro reactivation assay, and numbers of virus-specific T cells were quantified by flow cytometry by using MHC class I tetramer reagents produced by the Trudeau Institute Molecular Biology Core Facility (19). Infections with Sendai virus were performed and analyzed as described (20).
Transplant Studies. Heterotopic cardiac allografting (BALB/c > C57BL/6) was performed with anastomoses to the abdominal aorta and vena cava (21). In some experiments, costimulation blockade using CD154 mAb MR1 was undertaken, as described (22); control allograft recipients in these studies were treated with hamster IgG. Graft function was monitored by daily palpation, and rejection was confirmed by laparotomy and histologic examination. Skin grafting was performed as described (23, 24).
Statistics. Statistical analyses were performed with instat 3.0 (GraphPad, San Diego) and χ2, Mann-Whitney, or Student's t tests, as appropriate.
Results
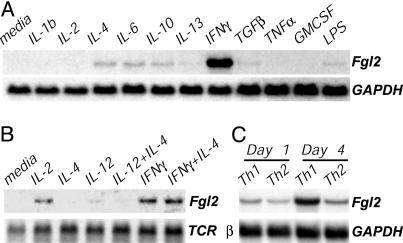
Fgl2 Is IFNγ-Inducible in Vitro Using Northern analysis, we found that exposure to IFNγ markedly and specifically up-regulated expression of Fgl2 in the RAW263.7 murine macrophage cell line (Fig. 1A). Primary mouse lymphocytes also expressed detectable levels of Fgl2 mRNA after 24 h of culture in the presence of IFNγ or IL-2 but not IL-12 or IL-4 (Fig. 1B). Polyclonal T cell activation with anti-CD3 mAb up-regulated Fgl2 expression as well (not shown). Consistent with this IFNγ and IL-2 inducibility, mouse lymphocytes preferentially up-regulated Fgl2 expression when cultured under conditions that favored the development of type 1 cells, in comparison to those that favored the development of type 2 cells (Fig. 1C). Thus, in agreement with prior reports (2, 4), we found that Fgl2 is IFNγ-inducible in vitro.
Fig. 1.
IFNγ-inducible and T helper (Th)1-biased expression of Fgl2 in vitro. Northern blots are shown. (A) RAW264.7 mouse macrophages stimulated for 6 h with the indicated cytokines (5 ng/ml) or lipopolysaccharide (10 μg/ml). (B) C57BL/6 lymph node cells cultured for 24 h in the presence of the indicated cytokines. (C) C57BL/6 lymph node cells stimulated with plate-bound anti-CD3 in the presence of IL-12 and anti-IL-4 (Th1 conditions), or IL-4 and anti-IFNγ (Th2 conditions).
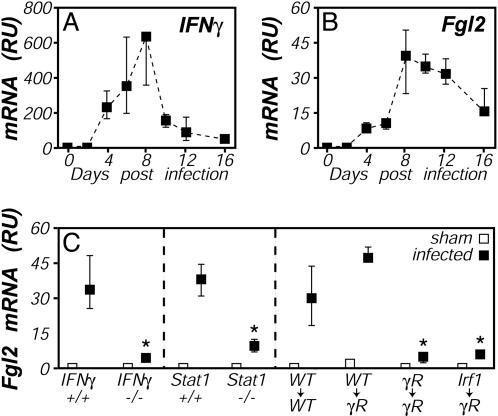
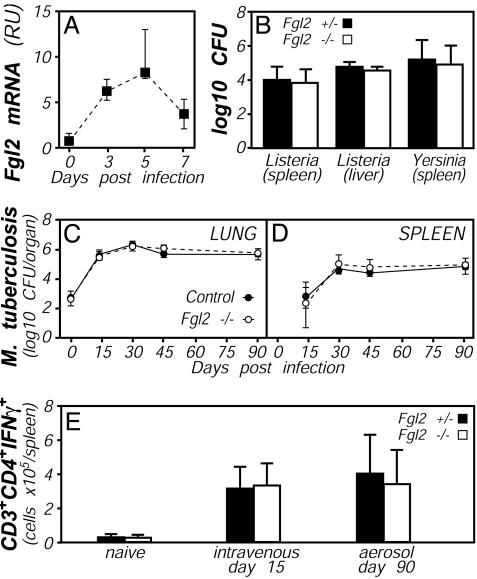
Fgl2 Is IFNγ-Inducible in Vivo In mouse models, infection by the protozoan parasite T. gondii elicits a robust type 1 immune response (25). Using real-time PCR, we observed that hepatic expression of Fgl2 mRNA was up-regulated ≈40-fold during the acute phase of T. gondii infection (Fig. 2B), correlating kinetically with peak levels of IFNγ (Fig. 2A) and fibrin (12). This Fgl2 induction largely depended on IFNγ, because levels of Fgl2 mRNA were dramatically suppressed in mice lacking IFNγ or Stat1 (Fig. 2C), a primary transducer of IFNγ signaling (26). Analyses of reciprocal bone marrow chimeras, created by using WT and IFNγR deficient mice, established that IFNγR expression by bone marrow derived cells is important for this in vivo up-regulation of Fgl2 expression (Fig. 2C). A parallel chimera study also revealed an important role for Irf1 in the T. gondii-stimulated Fgl2 induction (Fig. 2C). Notably, we could not directly evaluate roles for Irf1 by using fully Irf1-deficient mice, because those animals succumbed to T. gondii infection before peak Fgl2 induction (not shown). Thus, Fgl2 is inducible in vivo during infection via a mechanism that involves IFNγ and recognized components of IFNγ signaling pathways, including the IFNγR, Stat1, and Irf1.
Fig. 2.
IFNγ-, Stat1-, and Irf-dependent up-regulation of Fgl2 expression during T. gondii infection. (A and B) Mice were perorally infected with 10 ME49 T. gondii cysts, and tissues were harvested on the indicated days. Relative levels of mRNA encoding IFNγ (A) and Fgl2 (B) were determined by real-time PCR. (C) Mice were infected with T. gondii (filled symbols) or were sham-infected (open symbols). Tissues were harvested on day 8 after infection, and relative levels of Fgl2 mRNA were determined by real-time PCR. (Right) Shown is an experiment using bone marrow chimeras in which WT, IFNγR-deficient, or Irf1-deficient bone marrow was used to reconstitute lethally irradiated WT or IFNγR-deficient hosts. Throughout, data depict the median and range of four to five mice per time point. *, P < 0.05.
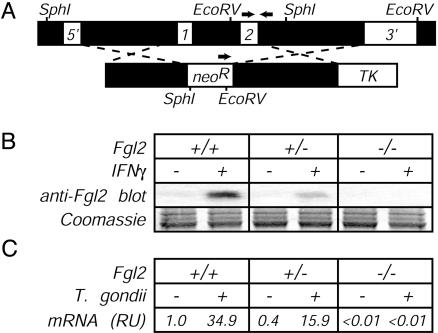
Fgl2-Deficient Mice. To evaluate the function of Fgl2 in vivo, we generated Fgl2-deficient mice. Using conventional gene-targeting methods, we replaced both fgl2 exons in C57BL/6-derived embryonic stem cells with a neomycin resistance cassette (Fig. 3A) and then used those stem cells to generate fully C57BL/6-inbred Fgl2-deficient animals. Lack of Fgl2 did not significantly impair embryonic development, because offspring derived from heterozygote by homozygote matings yielded a nearly perfect Mendelian distribution of fgl2 genotypes (29% female heterozygote, 25% female homozygote, 24% male heterozygote, and 22% male homozygote; n = 568). The slight reduction in homozygote frequencies was not significant (P > 0.05 by χ2 analysis). Consistent with successful disruption of the fgl2 gene, peritoneal macrophages from Fgl2-deficient mice, in contrast to those derived from littermate controls, failed to secrete detectable Fgl2 protein on treatment with IFNγ (Fig. 3B). Likewise, Fgl2 mRNA was readily detected in T. gondii-infected WT and Fgl2-heterozygous mice but not in Fgl2-deficient mice (Fig. 3C). Adult mice lacking Fgl2 were overtly healthy and possessed normal numbers and frequencies of B cells, CD4 T cells, CD8 T cells, and macrophages in lymphoid organs (not shown). Basal levels of all Ig isotypes were also indistinguishable between WT and Fgl2-deficient mice (not shown).
Fig. 3.
Production of Fgl2-deficient mice. (A Upper) Schematic of the Fgl2 gene, indicating (i) locations of the two Fgl2 exons, (ii) restriction enzyme sites and probes used for confirming successful disruption of the fgl2 gene by Southern blotting, and (iii) locations of PCR primers (arrows) used to routinely screen for the targeted mutation. (Lower) Schematic of the targeting construct, indicating regions flanking the fgl2 exons that were cloned into the targeting vector and the relative placement of selectable elements. neoR, neomycin resistance gene; TK, thymidine kinase gene. (B) Impaired secretion of Fgl2 protein by Fgl2-deficient cells. Thioglycollate-elicited peritoneal macrophages were isolated from littermate mice of the indicated fgl2 genotype. After 24 h of culture in the presence or absence of IFNγ (20 ng/ml), cell-free supernatants were assayed for Fgl2 expression by Western blotting with Fgl2-specific mAb. Coomassie blue staining of a gel run in parallel documented equivalent sample loading in all lanes. (C) Impaired production of Fgl2 mRNA by T. gondii-infected Fgl2-deficient mice. As in Fig. 2, mice were infected with T. gondii, liver tissue was harvested on day 8 after infection, and levels of Fgl2 mRNA were determined by real-time PCR. Data depict the average of four to five mice per group after normalization to uninfected WT mice.
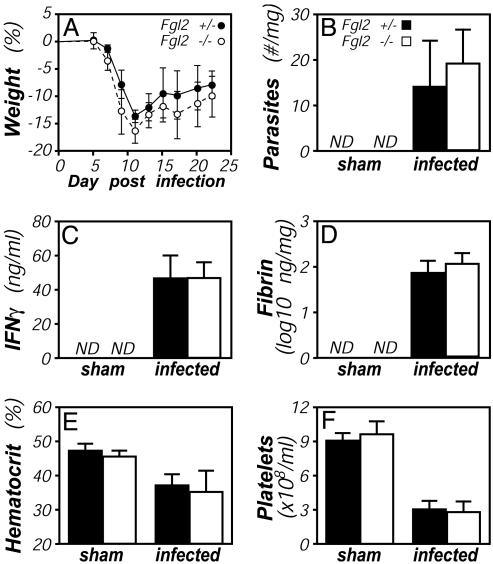
Responses to T. gondii in Fgl2-Deficient Mice. The capacity of C57BL/6 mice to survive the acute phase of infection by strain ME49 T. gondii depends critically on their ability to produce IFNγ and fibrin (12, 27). Given that Fgl2 is IFNγ-inducible (Figs. 1 and 2) and putatively contributes to fibrin formation (5, 7, 8), we evaluated T. gondii infection in Fgl2-deficient mice. In contrast to IFNγ- or fibrin-deficient mice, which succumb within 2 weeks of infection (12, 27), Fgl2-deficient mice survived acute infection by strain ME49 T. gondii. Indeed, the severity of the acute infection, as measured by weight loss, was not significantly different between Fgl2-deficient and littermate control mice (Fig. 4A). We performed more extensive analyses at day 8 after infection, when Fgl2 expression is at its peak (Fig. 2B). At that time, parasite numbers, immunity (IFNγ, tumor necrosis factor α, and nitric oxide levels), fibrin deposition, and pathology (hematocrit reductions and thrombocytopenia) were comparable in Fgl2-deficient and littermate control mice (Fig. 4 B-F and data not shown). Hepatic mRNA levels of a number of IFNγ-inducible genes (inducible nitric oxide synthase, monokine induced by IFNγ, indoleamine 2,3-dioxygenase, and IFNγ-inducible protein 10) also did not appreciably differ between T. gondii-infected Fgl2-deficient and control mice (not shown). Thus, despite its IFNγ inducibility (Figs. 1 and 2) and putative procoagulant functions (5, 7, 8), Fgl2 expression is not required to survive the acute phase of T. gondii infection in mice, a model in which both IFNγ and coagulation play critical protective roles (12, 27).
Fig. 4.
T. gondii infection of Fgl2-deficient mice. Fgl2-deficient (open bars) and littermate control Fgl2-heterozygous (filled bars) mice were infected with T. gondii, as in Fig. 2. (A) All mice survived the acute phase of the infection, and there were no significant differences in weight loss/recovery (n = 5, P > 0.05 at all time points). At day 8 after infection, cohorts of five mice were assayed for hepatic parasite burdens by real-time PCR (B), levels of IFNγ protein in plasma by ELISA (C), and levels of hepatic fibrin by quantitative Western blotting (D). In addition, hematocrits (E) and platelet numbers (F) were quantified by Coulter Counter. None of the measured parameters differed significantly between Fgl2-deficient and heterozygous control mice.
Responses to Bacteria in Fgl2-Deficient Mice. We next assessed roles for Fgl2 during other type 1 immune responses. Immune control of the Gram-positive bacteria L. monocytogenes also requires expression of IFNγ (28). As with T. gondii infection, we found that Fgl2 expression is up-regulated during L. monocytogenes infection, with kinetics mirroring that of IFNγ (Fig. 5A and data not shown). Nevertheless, when Fgl2-deficient and littermate control mice were infected with L. monocytogenes, bacterial burdens in liver and spleen were not significantly different (Fig. 5B). In a parallel study, no mice succumbed to infection over a 28-day period (not shown). Thus, Fgl2 does not play a critical role in determining bacterial burdens or survival of mice infected with L. monocytogenes. In a subsequent experiment, we compared the susceptibility of Fgl2-deficient and littermate control mice to infection by Y. enterocolitica, a Gram-negative bacteria whose control in C57BL/6 mice requires host production of IFNγ (29). Five days after infection with Y. enterocolitica, despite a 7-fold induction of hepatic Fgl2 expression in WT mice (not shown), bacterial burdens were indistinguishable between Fgl2-deficient and littermate control mice (Fig. 5B). Subsequent infections over a range of Y. enterocolitica doses likewise failed to reveal significant differences in susceptibility between Fgl2-deficient and control mice (not shown). Thus, expression of Fgl2 is dispensable for IFNγ-dependent protection against infection by prototypical Gram-positive and -negative bacteria.
Fig. 5.
Bacterial responses in Fgl2-deficient mice. (A) Up-regulation of Fgl2 expression during bacterial infection. WT C57BL/6 mice were i.p. infected with 1 × 105 L. monocytogenes, and levels of Fgl2 mRNA levels were quantified by real-time PCR. (B) Similar bacterial burdens in Fgl2-deficient (open bars) and control heterozygous mice (filled bars) after bacterial infection. Colony-forming units in the indicated organs were determined at day 5 after i.p. infection with 1 × 105 L. monocytogenes or i.v. infection with 5 × 103 Y. enterocolitica. In each case, data depict average and standard deviation of five mice per group, and no statistically significant differences were observed. (C-E) Similar bacterial burdens and T cell responses in Fgl2-deficient (open symbols) and control mice (filled symbols) after mycobacterial infection. CFUs in lung (C) or spleen (D) were determined on the indicated days after aerosol infection with M. tuberculosis. (E) Numbers of splenic CD3+CD4+ cells that could produce IFNγ on in vitro reactivation were quantified by flow cytometry 15 days after i.v. infection or 90 days after aerosol infection with M. tuberculosis. For C-E, data depict the average and standard deviation of four mice per group, except that only two Fgl2-deficient mice were analyzed at day 45.
Pulmonary infection of C57BL/6 mice with M. tuberculosis elicits type 1 immunity, and IFNγ production by activated T cells is critical for the control of mycobacterial burdens (30). We evaluated roles for Fgl2 during persistent mycobacterial infection by infecting Fgl2-deficient mice with aerosolized M. tuberculosis. As shown in Fig. 5 C and D, mycobacterial burdens were similar in the lungs and spleens of Fgl2-deficient and control mice. Moreover, equivalent numbers of activated T cells accumulated and persisted in M. tuberculosis-infected Fgl2-deficient and control mice (Fig. 5E). Thus, unlike IFNγ, Fgl2 does not function critically in either the control of bacterial burdens or the expansion/persistence of activated T cells during M. tuberculosis infection.
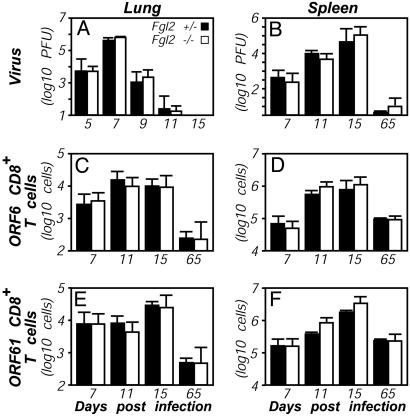
Responses to Viral Infections in Fgl2-Deficient Mice. To further evaluate roles for Fgl2 in the activation and persistence of T cells in vivo, we turned to viral models. MHV-68 is a DNA virus that elicits type 1 immunity in mice and is widely used as a model for human γ-herpesvirus infection (31). T cells play critical roles in clearing and controlling the lytic and latent infections, respectively. In C57BL/6 mice, T cells recognizing MHV-68 can be quantitatively tracked by using well characterized MHC tetramer reagents (32). Thus, we intranasally infected Fgl2-deficient mice with MHV-68, and at times thereafter, we evaluated lytic viral titers in the lung, latent virus in the spleen, and numbers of antigen-specific T cells in the lung airways and spleen. As shown in Fig. 6, Fgl2 deficiency did not significantly affect viral titers or numbers of CD8 T cells specific for two distinct viral antigens. In a subsequent study, we similarly evaluated the expansion and persistence of antigen-specific antiviral T cells after infecting mice with Sendai virus, an RNA virus. In this case, we quantified antigen-specific T cells in the spleen, lung parenchyma, bronchoalveolar fluid, and draining mediastinal lymph nodes at days 10 and 50 after infection (20). As with MHV-68 infection, Fgl2-deficient and control mice infected with Sendai virus exhibited similar numbers of antiviral T cells (data not shown). Thus, despite reports that Fgl2 regulates T cell activation and expansion in vitro (9), expression of Fgl2 does not appreciably regulate the activation, expansion, or persistence of antiviral antigen-specific T cells in vivo.
Fig. 6.
Antiviral responses in Fgl2-deficient mice. (A and B) Similar viral titers in Fgl2-deficient (open bars) and heterozygous control (filled bars) mice. Mice were intranasally infected with 400 plaque-forming units (pfus) MHV-68. Viral titers in the lung (A) and spleen (B) were determined at the indicated times after infection. The detection limit of the plaque assay was 1 pfu per 1.2 × 107 spleen cells. Data shown are representative of two independent experiments. (C-F) Similar numbers of virus-specific T cells in Fgl2-deficient (open bars) and heterozygous control (filled bars) mice. MHV-68-specific CD8+ T cells in lung airways (C and E) and the spleen (D and F) were quantified by flow cytometry after staining with MHC class I tetramer ORF6487-495/Db (C and D) or ORF61524-531/Kb (E and F). Data depict the average and standard deviation of three mice per time point.
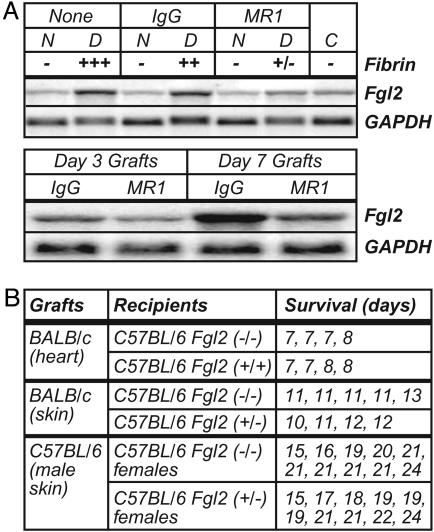
Transplant Responses in Fgl2-Deficient Mice. IFNγ expression and fibrin deposition also accompany graft rejection. Using a heter-otopic cardiac allograft model, we found up-regulated expression of Fgl2 mRNA within transplanted tissue (Fig. 7A). Interestingly, this Fgl2 up-regulation was suppressed by costimulation blockade with CD154 mAb (Fig. 7A), a regimen that suppresses allograft rejection and associated intragraft fibrin deposition (22). In this model, levels of Fgl2 expression positively correlated with levels of intragraft fibrin deposition (Fig. 7A).
Fig. 7.
Expression and function of Fgl2 during graft rejection. (A) Expression of Fgl2 mRNA correlates with fibrin deposition and allograft rejection. (Upper) Heterotopic BALB/c cardiac allografts were transplanted to C57BL/6 mice. Northern blots were prepared from day 3 donor (D) BALB/c allograft hearts, native (N) C57BL/6 hearts from the allograft recipients, and control (C) nontransplanted BALB/c hearts. Blots were successively hybridized with the indicated probes. Graft recipients received no treatment (None), donor spleen cell transfusion plus IgG, or donor spleen cell transfusion plus anti-CD40 ligand mAb MR1, as indicated. Fibrin was measured semiquantitatively by immunohistochemistry. (Lower) A second Northern blot was prepared from an independent series of day 3 and day 7 graft samples. (B) Similar kinetics of graft rejection in Fgl2-deficient and heterozygous control mice. As indicated, cardiac or skin grafts were transplanted to Fgl2-deficient or control mice, and times to rejection were recorded.
To evaluate the cellular distribution of transplant-associated Fgl2 expression, we performed in situ hybridization studies. Numerous graft-infiltrating leukocytes expressed Fgl2 mRNA, and staining adjacent sections with probes for T cell (CD3) and macrophage (CD11b) genes suggested that both cell types expressed Fgl2 transcripts (not shown). Immunohistochemistry using Fgl2-specific mAb confirmed readily detectable Fgl2 protein expression by graft-infiltrating leukocytes (not shown).
To functionally evaluate roles for Fgl2 during allograft rejection, we transplanted BALB/c hearts into C57BL/6 Fgl2-deficient mice. The Fgl2-deficient mice rejected cardiac allografts at the same rate as littermate control mice, and allografts in Fgl2-deficient and control mice exhibited indistinguishable levels of fibrin deposition (Fig. 7B and data not shown). In a follow-up study, we performed analogous skin grafting experiments. Again, Fgl2-deficient mice rejected allografts at a rate that was comparable to control mice (Fig. 7B). Analyses of skin graft rejection across a minor histocompatibility barrier (i.e., male to female grafts) also failed to reveal statistical differences in rates of graft rejection (Fig. 7B).
Discussion
This study evaluated the regulation and function of Fgl2. Consistent with prior reports (3, 4), we found that Fgl2 is IFNγ-inducible in cultured leukocytes (Fig. 1). We also established that type 1 lymphocytes preferentially express Fgl2 (Fig. 1), and that Fgl2 is IFNγ-inducible in vivo during infection (Fig. 2). Consistent with the accepted biology of IFNγ signaling pathways (26), we found that Stat1 and Irf1 play critical roles in the in vivo up-regulation of Fgl2 expression.
Prior reports suggested that Fgl2 may be a physiologically significant coagulant molecule. While studying a lethal coagulopathy induced by hepatitis virus in certain strains of mice, Levy and coworkers (5) identified Fgl2 as the target of a mAb that suppressed the coagulopathy. Follow-up in vitro studies suggested that Fgl2 possesses direct coagulant activity (8). Fgl2-specific antibodies were also found to suppress the coagulation-associated spontaneous abortion that characterizes certain mouse strain breeding combinations (6). However, the notion that Fgl2 possesses coagulant activity is controversial, because others failed to replicate that finding (33).
We recently found that coagulation performs a critical protective function during T. gondii infection (12). Apparently, the IFNγ produced during that infection causes clinically significant hemorrhage, which in turn necessitates a protective coagulative response (12). In contrast to fibrin-deficient mice (12), Fgl2-deficient mice did not succumb acutely to T. gondii infection (Fig. 4). Moreover, levels of fibrin deposition were indistinguishable between Fgl2-deficient and control mice (Fig. 4). We conclude that Fgl2 is not a critical mediator of the coagulative response that functions protectively during T. gondii infection.
Fibrin deposition also accompanies bacterial infections. In an extension of our T. gondii work, we have found that fibrin also plays critical protective roles during bacterial infections. Specifically, we found that fibrin-deficient mice rapidly succumb to otherwise sublethal doses of L. monocytogenes or Y. enterocolitica (S.T.S., unpublished work). However, we do not detect significant differences in bacterial burdens, susceptibility, or levels of fibrin deposition in Fgl2-deficient and control mice infected with L. monocytogenes or Y. enterocolitica (Fig. 5 and data not shown). Thus, Fgl2 is not a critical mediator of the protective coagulative responses that accompany those bacterial infections.
As mentioned, prior studies suggested Fgl2 stimulates pathological fibrin deposition during infection by mouse hepatitis virus (5). Recently, Levy and coworkers (34) reported their independent generation of Fgl2-deficient mice and provided evidence that those animals are indeed less susceptible to mouse hepatitis virus-mediated pathology. They also reported that cells from Fgl2-deficient mice are impaired in their capacity to stimulate coagulant activity on infection by mouse hepatitis virus, although coagulant activity was induced to WT levels in response to endotoxin (34). In combination with our findings, those studies raise the interesting possibility that Fgl2 contributes to pathologically significant coagulative responses under certain conditions but does not critically contribute to protective coagulative responses during type 1 immunity.
Levy and colleagues (34) also reported that 40% of homozygote Fgl2-deficient mice were lost during embryogenesis. In striking contrast, our analyses of >500 offspring reveal no significant deficiency in the birth of Fgl2-deficient mice. Although the reason for this discrepancy is unclear, we note that the mice produced by Levy and colleagues contain a LacZ insertion (34). Thus, it is formally possible that forced expression of β-galactosidase during embryogenesis could lead to the premature death of their Fgl2-deficient mice.
Because recombinant Fgl2 was recently shown to suppress T cell proliferation and dendritic cell maturation in vitro (9), we also quantitatively evaluated T cell responses in Fgl2-deficient mice. We did not discern significant suppressive roles for Fgl2 during M. tuberculosis and viral infections, where we used state-of-the-art flow cytometric methods to quantitatively assess the expansion, activation, and persistence of antigen-specific T cells. We also did not discern measurable roles for Fgl2 during the rejection of grafts across either major or minor histocompatibility barriers (Fig. 7), processes that are highly T cell-dependent (35). Together, these data suggest that Fgl2 does not critically regulate T cell numbers during immunity.
Conclusion
This study establishes that Fgl2 is IFNγ-inducible in vivo and suggests that Stat1 and Irf1 mediate the IFNγ-dependent up-regulation of Fgl2. In addition, this study suggests that Fgl2 does not play a critical role in many types of IFNγ-dependent immunity and/or immune-associated coagulation. It certainly remains possible that Fgl2 plays important roles that can be compensated for by other molecules, and/or that Fgl2 functions critically during responses that have yet to be evaluated. The Fgl2-deficient mice reported herein will enable decisive evaluations of the in vivo function of this highly conserved IFNγ-inducible protein.
Acknowledgments
We thank Paula Lanthier for technical assistance and Pamela Scott Adams and Troy Randall for guidance with real-time PCR and bone marrow chimeras, respectively. We also thank Dennis Huszar and Yubin Qiu (Millennium Pharmaceuticals, Cambridge, MA) for enabling the production of Fgl2-deficient mice. We are indebted to the employees of the Trudeau Institute Animal Breeding and Maintenance Facilities for dedicated care of our mice. This work was supported by funds from Trudeau Institute and National Institutes of Health Grants HL72937 (to S.T.S.) and AI46571 (to L.L.J.).
Abbreviations: Irf1, IFN response factor 1; MHV, murine γ-herpesvirus; Stat1, signal transducer and activation of transcription protein 1; Fgl2, fibrinogen-like protein 2; IFNγR, IFNγ receptor.
References
- 1.Ruegg, C. & Pytela, R. (1995) Gene 160, 257-262. [DOI] [PubMed] [Google Scholar]
- 2.Marazzi, S., Blum, S., Hartmann, R., Gundersen, D., Schreyer, M., Argraves, S., von Fliedner, V., Pytela, R. & Ruegg, C. (1998) J. Immunol. 161, 138-147. [PubMed] [Google Scholar]
- 3.Koyama, T., Hall, L. R., Haser, W. G., Tonegawa, S. & Saito, H. (1987) Proc. Natl. Acad. Sci. USA 84, 1609-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lafuse, W. P., Castle, L., Brown, D. & Zwilling, B. S. (1995) Cell. Immunol. 163, 187-190. [DOI] [PubMed] [Google Scholar]
- 5.Parr, R. L., Fung, L., Reneker, J., Myers-Mason, N., Leibowitz, J. L. & Levy, G. (1995) J. Virol. 69, 5033-5038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark, D. A., Chaouat, G., Arck, P. C., Mittruecker, H. W. & Levy, G. A. (1998) J. Immunol. 160, 545-549. [PubMed] [Google Scholar]
- 7.Levy, G. A., Liu, M., Ding, J., Yuwaraj, S., Leibowitz, J., Marsden, P. A., Ning, Q., Kovalinka, A. & Phillips, M. J. (2000) Am. J. Pathol. 156, 1217-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan, C. W., Chan, M. W., Liu, M., Fung, L., Cole, E. H., Leibowitz, J. L., Marsden, P. A., Clark, D. A. & Levy, G. A. (2002) J. Immunol. 168, 5170-5177. [DOI] [PubMed] [Google Scholar]
- 9.Chan, C. W., Kay, L. S., Khadaroo, R. G., Chan, M. W., Lakatoo, S., Young, K. J., Zhang, L., Gorczynski, R. M., Cattral, M., Rotstein, O., et al. (2003) J. Immunol. 170, 4036-4044. [DOI] [PubMed] [Google Scholar]
- 10.Smiley, S. T., Boyer, S. N., Heeb, M. J., Griffin, J. H. & Grusby, M. J. (1997) Proc. Natl. Acad. Sci. USA 94, 11484-11489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao, W., Faia, K. L., Csizmadia, V., Smiley, S. T., Soler, D., King, J. A., Danoff, T. M. & Hancock, W. W. (2001) Transplantation 72, 1199-1205. [DOI] [PubMed] [Google Scholar]
- 12.Johnson, L. L., Berggren, K. N., Szaba, F. M., Chen, W. & Smiley, S. T. (2003) J. Exp. Med. 197, 801-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tybulewicz, V. L., Crawford, C. E., Jackson, P. K., Bronson, R. T. & Mulligan, R. C. (1991) Cell 65, 1153-1163. [DOI] [PubMed] [Google Scholar]
- 14.Healy, A. M., Hancock, W. W., Christie, P. D., Rayburn, H. B. & Rosenberg, R. D. (1998) Blood 92, 4188-4197. [PubMed] [Google Scholar]
- 15.Carter, P. B. (1975) Infect. Immun. 11, 164-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackaness, G. B. (1969) J. Exp. Med. 129, 973-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberts, A., Cooper, A., Belisle, J., Turner, J., Gonzalez-Juarerro, M. & Orme, I. (2002) in Methods in Microbiology, eds. Kaufman, S. & Kabelitz, D. (Academic, London), Vol. 32, pp. 433-462. [Google Scholar]
- 18.Cooper, A. M., Kipnis, A., Turner, J., Magram, J., Ferrante, J. & Orme, I. M. (2002) J. Immunol. 168, 1322-1327. [DOI] [PubMed] [Google Scholar]
- 19.Kim, I. J., Flano, E., Woodland, D. L. & Blackman, M. A. (2002) J. Immunol. 168, 3958-3964. [DOI] [PubMed] [Google Scholar]
- 20.Hogan, R. J., Cauley, L. S., Ely, K. H., Cookenham, T., Roberts, A. D., Brennan, J. W., Monard, S. & Woodland, D. L. (2002) J. Immunol. 169, 4976-4981. [DOI] [PubMed] [Google Scholar]
- 21.Gao, W., Topham, P. S., King, J. A., Smiley, S. T., Csizmadia, V., Lu, B., Gerard, C. J. & Hancock, W. W. (2000) J. Clin. Invest. 105, 35-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hancock, W. W., Sayegh, M. H., Zheng, X. G., Peach, R., Linsley, P. S. & Turka, L. A. (1996) Proc. Natl. Acad. Sci. USA 93, 13967-13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bailey, D. W. & Usama, B. (1960) Transplant. Bull. 7, 424-425. [DOI] [PubMed] [Google Scholar]
- 24.Johnson, L. L., Bailey, D. W. & Mobraaten, L. E. (1980) Immunogenetics 11, 363-372. [DOI] [PubMed] [Google Scholar]
- 25.Denkers, E. Y. & Gazzinelli, R. T. (1998) Clin. Microbiol. Rev. 11, 569-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stark, G. R., Kerr, I. M., Williams, B. R., Silverman, R. H. & Schreiber, R. D. (1998) Annu. Rev. Biochem. 67, 227-264. [DOI] [PubMed] [Google Scholar]
- 27.Suzuki, Y., Orellana, M. A., Schreiber, R. D. & Remington, J. S. (1988) Science 240, 516-518. [DOI] [PubMed] [Google Scholar]
- 28.Buchmeier, N. A. & Schreiber, R. D. (1985) Proc. Natl. Acad. Sci. USA 82, 7404-7408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Autenrieth, I. B., Beer, M., Bohn, E., Kaufmann, S. H. & Heesemann, J. (1994) Infect. Immun. 62, 2590-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flynn, J. L. & Chan, J. (2001) Annu. Rev. Immunol. 19, 93-129. [DOI] [PubMed] [Google Scholar]
- 31.Virgin, H. W. & Speck, S. H. (1999) Curr. Opin. Immunol. 11, 371-379. [DOI] [PubMed] [Google Scholar]
- 32.Doherty, P. C. & Christensen, J. P. (2000) Annu. Rev. Immunol. 18, 561-592. [DOI] [PubMed] [Google Scholar]
- 33.Zhang, J. & Broze, G. (2002) Thromb. Haemostasis 88, 872-874. [PubMed] [Google Scholar]
- 34.Marsden, P. A., Ning, Q., Fung, L. S., Luo, X., Chen, Y., Mendicino, M., Ghanekar, A., Scott, J. A., Miller, T., Chan, C. W., et al. (2003) J. Clin. Invest. 112, 58-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg, A. S. & Singer, A. (1992) Annu. Rev. Immunol. 10, 333-358. [DOI] [PubMed] [Google Scholar]