Abstract
Atraumatic application of attenuated SIVmac239Δnef vaccine to the tonsils of rhesus macaques provided protection against challenge 26 weeks later with infectious SIVmac251 applied through this route. Early events at the mucosal portal of entry of challenge virus were followed. Wild-type virus was detected in nonvaccinated controls by day 4, and then simian immunodeficiency virus (SIV) replicated vigorously at days 7 and 14. In contrast, a challenge of 10 of 10 vaccinees with SIV did not significantly raise RNA levels in the plasma or increase infected cells in lymphoid tissues, as assessed by single-cell labeling for viral RNA and nef protein. Vaccine virus was found in the tonsils of all vaccinees, but challenge virus was only detected at this portal of entry in 4 of 10 monkeys. In the tonsil, the challenge virus did not induce an expansion of perforin+ killer cells. However, there was a significant increase in γδ T cells and mature dendritic cells relative to unvaccinated controls. Therefore, during tonsillar SIVΔnef vaccination, infection is blocked early at the entry portal, which we propose is due in part to innate functions of γδ T and dendritic cells.
Simian immunodeficiency virus (SIV) infection of rhesus macaques provides a model for HIV infection in humans. It would be valuable to determine whether vaccination against SIV blocks infection at the site of viral entry, including mucosal surfaces. Most efforts have instead been directed to the long-term effects of the vaccine. Also, the extent of protection is generally monitored in blood, and more information is needed on protection in lymphoid tissues, where the virus replicates and immune responses are generated. We have considered these questions by analyzing early changes in rhesus macaques immunized with a live attenuated SIV vaccine, generated by deletion of the nef gene, and challenged with wild-type SIV through the tonsils. We realized that early events during the entry of a vaccine and challenge virus were readily amenable to analysis in tonsils, which are a model for transmission through mucosa-associated lymphoid tissue (MALT), e.g., in anal transmission by means of rectal MALT.
MALT is comprised of a lymphoepithelium equipped with antigen transporting M cells that allows infectious agents and vaccines to access lymphoid tissue lying beneath it. Each region of the MALT contributes to the pathogenesis of infections and protective vaccines (1). MALT provides sites for the replication of immunodeficiency viruses in vivo, both HIV in humans (2, 3) and SIV in monkeys (4). Beneath the epithelium are dendritic cells (DCs) (3, 5). In tissue culture, DCs transmit HIV (6-8) and SIV (9) for replication in T cells. DCs also present HIV (10-12) and SIV (13) antigens to specific T cells.
We have previously followed infectious (14) and vaccine (15) forms of SIV following their atraumatic application to lingual and palatine tonsils. The virus begins to replicate in oral MALT, primarily in T cells, and then spreads to distal lymphoid organs.
Here, we use the accessibility of the oral MALT to study its potential as a site for vaccination against immunodeficiency viruses. We will show that tonsillar vaccination with SIVmac239Δnef (15) protects against wild-type SIVmac251, much like the protection reported when vaccine is given i.v. Surprisingly, vaccinated animals rapidly and efficiently contain the challenge SIVmac251 at the tonsil portal of entry, even though the vaccine strain continues to replicate. In tissue sections, protection is accompanied by an expansion of mature DCs and γδ T cells but not by an expansion of cytotoxic cells that express perforin or granzyme B. We suggest that exposure to immunodeficiency viruses in the setting of vaccination allows DCs and γδ T cells, possibly by a release of β chemokines, to exert innate functions that resist challenge with infectious viruses.
Materials and Methods
Animals. Juvenile rhesus macaques (Macaca mulatta) of Indian origin were bred at the German Primate Center or imported from the Laboratory Animal Breeder & Services (Yemassee, NC) or China. Animal care and handling (16) was performed according to in-house guidelines and German Animal Protection Laws. Monkeys were of either sex, weighed 3.1-4.8 kg, and tested negative for antibodies to simian T lymphotrophic virus type 1, simian D-type retrovirus, and SIV. Viral inoculation, physical exams, and bleeding used ketamine anesthesia, whereas lymph node removal used combined ketamine, xylazine, and atropine.
Vaccination with Attenuated nef-Deleted SIV and Challenge with Pathogenic Wild-Type SIV. For vaccination, we used an SIVΔnef virus stock (15) attenuated by a 513-bp deletion in nef and the U3 region (SIVΔNU) (17). We injected 300 median tissue culture 50% infective dose (TCID50) i.v. into three animals and applied ≈105 TCID50 to palatine and lingual tonsils of 13 monkeys as described (14). To monitor long-term protection, five vaccinees (three immunized i.v and two by the tonsillar route 26 weeks before) were challenged, along with two untreated controls through the tonsils with 2-3 × 103 TCID50 of SIVmac251 provided by A. M. Aubertin (Institute of Virology, Medical Faculty, Strasbourg, France; ref. 14). Some vaccinees were observed for 56-57 weeks after challenge and are still alive. To monitor short-term protection at the portal of virus challenge, the other 10 SIVΔnef vaccinees were challenged at 26-27 weeks through the tonsils along, with 10 untreated controls. The challenge was 2-3 × 103 TCID50 of SIVmac251 but represented a further in vitro passage from A. M. Aubertin's virus stock in rhesus monkey peripheral blood mononuclear cells (PBMCs) (kindly provided by S. Norley, Robert-Koch-Institute, Berlin).
Assays for Infection. Cell-associated viral loads were determined in a limiting dilution coculture assay (14, 16), and RNA loads by quantitative competitive PCR (18).
Quantitative Real-Time RT-PCR. As described (19), virus RNA (virus RNA isolation kit, Qiagen, Valencia, CA) in 10 μl per reaction was amplified by a one-tube real-time RT-PCR using a brilliant single-step QRT-PCR core kit (Stratagene). PCRs were run in a 40-μl reaction in duplicate with 4 μl of 10× core buffer, 5 mM MgCl2/0.8 mM dNTP mix/200 nM primers/150 nM fluorogenic TaqMan probe/1.25 units of Stratascript RT/0.025 units of TaqDNA-polymerase. To quantify viral loads, standard RNA templates were created from the p239Sp5′ plasmid (donated by R. M. Ruprecht, Dana-Farber Cancer Institute, Boston; ref. 19), with a detection limit of 25 viral copies per ml of plasma.
In Situ Hybridization. Virus replication in frozen and paraffin sections was detected with a 35S-antisense probe (Lofstrand Laboratories, Gaithersburg, MD; ref. 20), and the number of infected cells was recorded per mm2. To discriminate between cells producing vaccine and wild-type virus, we labeled sections with nef antibodies before in situ hybridization.
Characterization of Isolates by PCR. C8166 cells infected with isolates recovered from the lymphoid organs or PBMCs were lysed in buffer K (50 mM KCl/15 mM Tris·HCl, pH 7.5/2.5 mM MgCl2/0.5% Tween 20/100 μg of proteinase K per ml) for 0.5 h overnight before heat inactivation at 95 C for 10 min. The lysates were PCR-amplified with two different primer pairs, allowing us to differentiate between SIVΔnef virus and nef-containing challenge virus. By using the primers Sns and Sna (21), which flank the deletion in nef and the U3 region of SIVΔnef, a 163-bp fragment and a 672-bp fragment were obtained in the PCR for SIVΔnef and SIVmac251, respectively. To detect SIVmac251 but not SIVΔnef, the PCR was performed with the Sns primer and the primer Δna (21), which is complementary to the U3 region deleted in SIVΔnef, resulting in a 556-bp PCR product in the case of SIVmac251. Isolates were classified as vaccine virus (V) if the Sns+SnaPCR resulted in a 163-bp fragment, as challenge virus (C) if the Sns+Δna PCR resulted in a 556-bp fragment, and as vaccine and challenge virus (V+C) if both PCRs gave a product of the expected size. The PCR conditions were: 94°C for 2 min, 40 cycles of 40 sec at 94°C, 1 min at 61°C, and 1 min at 72°C.
Immunohistochemistry. Paraffin and frozen sections (fixed in 4% paraformaldehyde for 10 min) of tonsils were analyzed. Dewaxed paraffin sections were subjected to high-temperature antigen retrieval. Sections were incubated with antibodies to CD4 (NCL-CD4-1F6; Novocastra, Newcastle upon Tyne, U.K.), CD8 (C8/144B; Dakopatts, Hamburg, Germany), perforin (P1-8; Kamiya Biomedical Company, Seattle), granzyme B (GrB-7; Dakopatts), CD1a (Leu 6; BD Pharmingen, San Diego), Langerin (22), DC-LAMP (23), CD83 (HB15A; Immunotech, Marseille, France), anti-nef (ARP3092; Centralized Facility for AIDS Reagents, National Institute for Biological Standards and Control, Potters Bar, U.K.), and γδ T cells (B1.1; BD Pharmingen). Antibody binding was visualized with the alkaline-phosphatase anti-alkaline phosphatase method with New Fuchsin as a chromogen.
Statistical Analysis. Differences between control and vaccine values were analyzed by spss 9.0.1 or the graphpad prism software, and P values <0.05 in the unpaired Student's t test or Mann-Whitney U test for continuous variables, were scored as significant.
Results
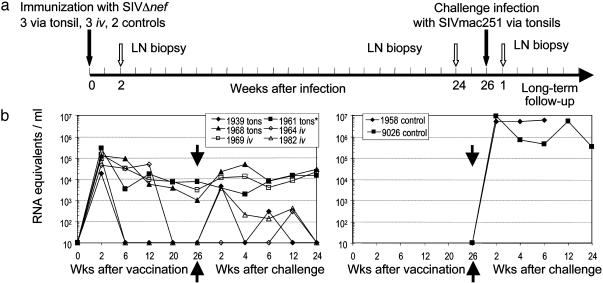
Long-Term Protection with SIVΔnef Vaccination by Tonsillar and i.v. Routes. SIVΔnef (from SIVmac239) was applied to the tonsils (15) to compare its long-term protection with i.v. vaccination (24). Three animals were infected i.v. with 300 TCID50 SIVΔnef, three others received 105 TCID50 SIVΔnef applied atraumatically to the tonsils, and two animals served as unvaccinated controls (Fig. 1a). Twenty-six weeks later, all were challenged at the tonsil with 2-3 × 103 TCID50 SIVmac251. Cell-associated viral loads were assessed in limiting dilution assays (Fig. 6, which is published as supporting information on the PNAS web site) and plasma viral RNA measured (Fig. 1b). Following challenge, there was little or no increase in prevaccination viral loads of <102 infectious units per106 PBMC and 101 to 104 RNA equivalents in all five vaccinees (Figs. 1b and 6). In contrast, both controls reached levels of >103 infectious units per 106 PBMC and >106 RNA copies per ml (Fig. 1b) Therefore, vaccination with SIVmac239Δnef, by either i.v. or tonsillar routes, provides long-term protection against mucosal challenge with SIVmac251.
Fig. 1.
Long-term protection after SIVΔnef vaccination. (a) Study design whereby monkeys were vaccinated through the tonsils or i.v., challenged with SIVmac251 through the tonsil, and studied for 56 weeks. (b) Plasma viral RNA before and after challenge with SIVmac251. Animal 1961 was not challenged due to high viral load.
Protection by Tonsillar SIVΔnef Is Manifest in the Blood Early After Challenge. To study early events at the virus entry portal, we vaccinated 10 monkeys 26 weeks earlier with 105 TCID50 SIVΔnef on the tonsils and challenged these animals and 10 unvaccinated controls with 2-3 × 103 TCID50 SIVmac251 through the tonsil (Fig. 2a). We then studied four animals from each group at days 4 and 14/15, and two at days 7/8. With the exception of monkey 10140, the mean viremia of the vaccinated animals was 770 ± 250 RNA equivalents per ml at weed 26 before challenge, and after challenge, the mean viremia for all three time points (day 4, 1 week, and 2 weeks) was 590 ± 230 per ml (Fig. 2b). For the controls, the mean viremia after challenge rose to 2.3 × 105 ± 0.2 × 105 RNA equivalents per ml for two monkeys at days 7/8 and 2.1 × 106 ± 1.2 × 106 for four animals at days 14/15 (P < 0.0001 relative to vaccinated challenged monkeys). Similarly, the vaccinated animals showed little or no boost in infectious units in PBMC (Fig. 7, which is published as supporting information on the PNAS web site). Therefore, all vaccinees were protected at early times against the challenge virus.
Fig. 2.
Early protection of monkeys after SIVΔnef tonsillar vaccination. (a) Study design with 10 monkeys vaccinated through the tonsil and 10 controls. At 26 weeks, all were challenged with SIVmac251 through the tonsil (gray arrow). Four animals from each group were killed at days 4 and 14/15, and two per group at 7-8 days after challenge (blue arrows), to look for protection at the entry portal. (b) Plasma RNA of vaccinated and control animals before and after challenge.
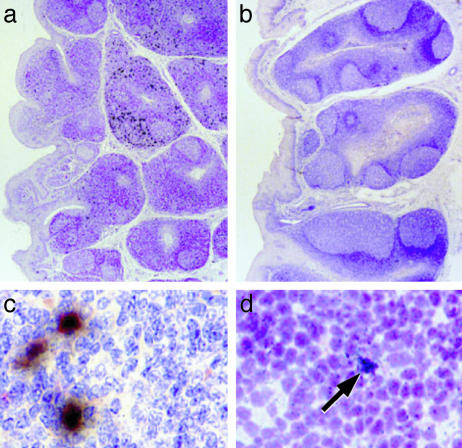
Protection by Tonsillar SIVΔnef Is Manifest in Lymphoid Tissues Early After Challenge. We monitored SIV in individual lymphoid organs in all animals with two assays. First, we measured infectious units in cell suspensions cocultured with a permissive cell line. In the controls, infectious virus became detectable at day 4 in the tonsil and draining retropharyngeal node and rose to >103 infectious units per 106 cells in most lymphoid organs by days 7/8 and 14/15. In contrast, infectious virus remained at <10-100 infectious units per 106 cells in vaccinated and challenged monkeys, except for 10140 (Tables 1, 2, 3). Virus isolates were characterized by PCR to distinguish the challenge nef-containing virus from the vaccine strain (Materials and Methods). In the controls, the challenge virus was already detected by PCR at day 4 in the tonsil, but in 5 of 6 vaccinees, only the SIVΔnef vaccine and not the challenge virus was observed at 1 week after challenge (Tables 1, 2, 3). Second, we looked for productively infected cells by in situ hybridization of sections with 35S antisense SIV RNA and by immunolabeling for nef protein. The early protection against challenge virus was clearly evident by both approaches (Fig. 3). RNA+ cells were rare in the tonsils of vaccinees challenged with wild-type SIV, in contrast to abundant infected cells in controls (Fig. 3 a vs. b). The nef protein was also readily identified in cells of the controls but not the vaccinees; when double labeling was carried out (Fig. 3c), most RNA+ cells in the control monkeys were also nef+. Together, the results in Tables 1, 2, 3 and Fig. 3 indicate that challenge virus rapidly took hold in control monkeys and spread vigorously in a systemic manner. In contrast, vaccinated monkeys quickly and efficiently resisted the same challenge at the tonsillar portal of SIV entry.
Table 1. Cell-associated viral load and detection of vaccine and challenge forms of SIV by PCR 4 days after challenge.
| SIVΔnef-vaccinated monkeys
|
Control nonvaccinated monkeys
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Tissue examined | 10121 | 11106 | 11111 | 10118 | 10659 | 10662 | 10655 | 10656 |
| PBMC | 0.33(V) | — | 8(nd) | 2(V) | 2(C) | 1(C) | 0.33 | 2 |
| Palatine tonsil | 2(V) | — | 8(V) | 32(V) | 8 | 32 | 32(C) | 64(C) |
| Spleen | 0.33(V) | — | 32(V) | 64(V) | 4(C) | 4 | 2 | 0.33 |
| LN axillary | 4(V) | 0.33(V) | 32(V) | 32(V) | — | 28 | 2 | 0.33 |
| LN mesenteric | 0.33(V) | — | 2(V) | 0.33(nd) | 0.33 | — | 2 | — |
| LN submandibular | 0.33(V) | — | 4(V) | 64(V) | 4 | 2(C) | 1 | — |
| LN retropharyngeal | 0.33(V) | 1(V/C) | 4(V) | 16(V) | 16 | 16 | 2 | 1 |
| Thymus | — | — | — | — | — | — | — | — |
Cell-associated viral load expressed as infectious units per 106 mononuclear cells. Characterization of reisolates by PCR are shown in parentheses.—, virus isolation negative; V, vaccine virus; C, challenge virus; nd, not done. In the control animals, we performed PCR on only one to two reisolates per monkey, as indicated, to verify the presence of wild-type challenge virus.
Table 2. Cell-associated viral load and detection of vaccine and challenge forms of SIV by PCR 7-8 days after challenge.
| SIVΔnef-infected monkeys
|
Control nonvaccinated monkeys
|
|||
|---|---|---|---|---|
| Tissue examined | 10124 | 10130 | 10674 | 10678 |
| PBMC | 1(V) | — | 256 | 256 |
| Palatine tonsil | 4(V/C) | 4(V) | 4,096(C) | 4,096(C) |
| Spleen | 16(V) | 64(V) | 4,096 | 1,024 |
| LN axillary | 32(V) | 32(V) | 128 | 1024 |
| LN mesenteric | 8(V) | 8(V) | 4096 | 256 |
| LN submandibular | 8(V) | 128(V) | 1,024 | 1,024 |
| LN retropharyngeal | 2(C) | 16(V) | 4,096 | 1,024 |
| Thymus | — | 1(V) | 128 | 2 |
Cell-associated viral load expressed as infectious units per 106 mononuclear cells. Characterization of reisolates by PCR are shown in parentheses.—, virus isolation negative; V, vaccine virus; C, challenge virus; nd, not done. In the control animals, we performed PCR on only one to two reisolates per monkey, as indicated, to verify the presence of wild type challenge virus.
Table 3. Cell-associated viral load and detection of vaccine and challenge forms of SIV by PCR 14-15 days after challenge.
| SIVΔnef-vaccinated monkeys
|
Control nonvaccinated monkeys
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 10138 | 10140 | 11112 | 11118 | 10685 | 10686 | 10931 | 10932 | |
| PBMC | C | 64(nd) | — | 2(C) | 8,192 | 512 | 128(C) | 128(C) |
| Palatine tonsil | 16(V) | 4,096(V/C) | 32(V/C) | 32(V/C) | 8,192(C) | 4,096(C) | 512 | 32 |
| Spleen | 0.33(V) | 4,096(V) | 8(V) | 4(V) | 8,192 | 8,192 | 2,048(C) | 256 |
| LN axillary | 4(V/C) | 512(V/C) | 8(V) | 8(V) | 4,096 | 8,192 | 8192 | 1,024 |
| LN mesenteric | — | 1,024(V/C) | — | 4(C) | 4,096 | 8,192 | 4096 | 8,192 |
| LN submandibular | 4(V) | 1,024(V/C) | 4(V) | 32(V/C) | 1,024 | 8,192 | 256 | 512 |
| LN retropharyngeal | 0.33(V) | 1,024(V/C) | 4(C) | 16(nd) | 4,096 | 8,192 | 4096 | 512 |
| Thymus | — | 0.33(V/C) | — | 0.33(C) | NA | 64 | 1,024 | 1,024 |
Cell-associated viral load expressed as infectious units per 106 mononuclear cells. Characterization of reisolates by PCR are shown in parentheses.—, virus isolation negative; V, vaccine virus; C, challenge virus; nd, not done; NA, reisolate not available due to bacterial contamination. In the control animals, we performed PCR on only one to two reisolates per monkey, as indicated, to verify the presence of wild-type challenge virus.
Fig. 3.
Early protection against SIV challenge at the tonsillar entry portal. (a and b) In situ with 35S-antisense SIV RNA in sections of the lingual tonsil, 7 days after challenge of unvaccinated (a) and SIVΔnef-vaccinated (b) animals. Each black deposit is an infected cell. (c and d) Wild-type SIVmac251 was detected in infected cells (black) by immunolabeling (red) for viral nef protein in the unvaccinated controls (c) but not SIVΔnef vaccinees (d; arrow to a nef--infected cell).
Initial Studies of Protection Mechanisms at the Portal of SIV Entry. We assessed three changes:
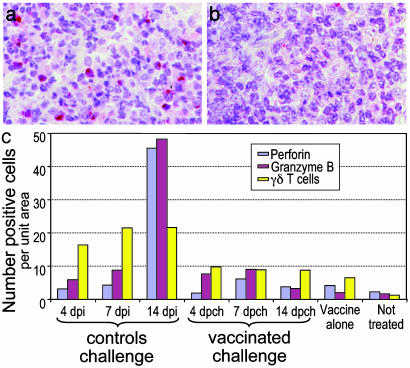
SIV challenge does not expand cells with cytotoxic granules: To identify potential killer cells (CD8+ cytolytic T lymphocytes and natural killer cells), we stained tonsil sections for perforin and granzyme B. In the controls, killer cells expanded 1 week after challenge (Fig. 4a), similar to prior work during exposure to SIVΔnef vaccine; these included both CD8+ T cells and CD3- natural killer cells (15). In contrast, when vaccinated animals were challenged (Fig. 4b), granzyme B+ cells increased a little and perforin+ cells not at all. Within the limits of assay sensitivity, this implies that killer cells did not expand during the time SIV challenge was being resisted at the portal of entry.
Increased γδ T-cells in the tonsils from vaccinees: γδ T cells have innate roles (25). Recent studies showed that HIV and SIV immunization expanded these cells, which can produce β-chemokines and IFN-γ (26, 27). We found that control tonsils contained only few γδ T cells (1.3 ± 0.6 cells per mm2), mainly in crypt epithelium and T-dependent zones. In contrast, there was ≈5-fold elevation (6.5± 2.6 γδ T cells per field; P < 0.0001) in vaccinated animals before and after SIV challenge (Fig. 4c). Unvaccinated animals, possibly because of greater viral loads, showed even larger increases in γδ T cells after challenge (mean 19.3 ± 1.5 cells per field for all three time points examined; Fig. 4c). Therefore, both infection and vaccination expands γδ T cells at the entry portal.
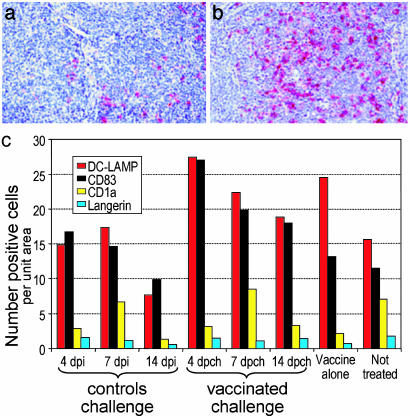
Mature but not immature DCs expand in the tonsils of vaccinated macaques. We also observed an increase in mature DCs in the tonsils of vaccinees. In the case of the DC-LAMP marker (23), increases were seen from 16 ± 1.2 in controls to 22 ± 2.0 in vaccinated animals (P = 0.02), whereas in the case of CD83, DCs increased from 12 ± 2.0 in controls to 22 ± 1.5 for all vaccinated challenged animals (P < 0.0001; Fig. 5 a and b). In contrast, immature DCs, (CD1a+ or Langerin+; ref. 22), did not increase (Fig. 5c). Therefore, a distinct feature of the portal of entry in vaccinees is an expansion of mature DCs.
Fig. 4.
Lack of expansion of perforin+ killer cells in vaccinated animals challenged with wild-type SIVmac251. Cytotoxic cells were identified by immunolabeling (red) for perforin 2 weeks after challenge with SIV in unvaccinated (a) and SIVΔnef-vaccinated (b) monkeys. The results at different time points after challenge are shown in c. γδ cells were also enumerated, and means of each group are shown (P < 0.0001 for the differences in γδ cells between controls and SIVΔnef-vaccinated animals, and between controls and SIV-challenged animals).
Fig. 5.
Expansion of DC-LAMP+ DCs in vaccinees challenged with SIVmac251. (a and b) DCs immunolabeled (red) for the mature DC marker, DC-LAMP, in palatine tonsils from unvaccinated (a) and SIVΔnef-vaccinated (b) monkeys. (c) Quantitative data and means from each group (P = 0.01 for DC-LAMP; P = 0.001 for CD83).
Discussion
Here, we evaluated a tonsillar mode of attenuated SIVΔnef vaccination. Virus was applied to palatine and lingual tonsils with a cotton swab. Some virus might have spilled over to other parts of the oral cavity and gastrointestinal tract, but spillage might not be large, given a lack of infection with either SIV or SIVΔnef in gastric lymph nodes (14, 15). We chose the tonsil route for two reasons. First, this could be valuable for vaccination, as in the lingual application of attenuated Sabin polio vaccine; second, this route facilitated the study of early events at a MALT site of entry. Comparison of set point viral load levels in monkeys infected with the same SIVΔnef virus by the tonsillar route (n = 16; this study and ref. 15) or i.v. (n = 7; this study and ref. 17) did not show reduced pathogenicity of tonsillar infection, also in relation to other studies with nef-deletion mutants of SIV (24, 28, 29). We found that tonsil vaccinees were protected against a tonsillar challenge with pathogenic SIVmac251. A distinct feature was the early protection provided by vaccination at the site of virus entry.
Once long-term protection was observed in vaccinated monkeys, we considered some mechanistic aspects at the entry portal. Importantly, the challenge virus was resisted at the same time that vaccine virus retained its infectivity in the oral MALT and distal lymphoid organs. Possibly, protection was due to neutralizing antibodies. Passive transfer of neutralizing antibody before infection can provide protection at a mucosal surface (30), but if such antibodies were present in the vaccinees, how would they account for resistance to a high dose of challenge virus but allow for replication of vaccine virus? Possibly, killer lymphocytes were fully active and able to kill cells infected with challenge virus (31). CD8+ T cells lytic for SIV-infected targets help to control virus replication during primary infection (32). However, we did not see a rapid expansion of perforin+ killer cells after challenge, as assessed by immunolabeling of sections. This finding is in agreement with recent data showing that live attenuated SIV does not yield a vigorous T cell response during protection to virus challenge (33).
In contrast, γδ T cells may have been provided some protection. SIVΔnef tonsillar vaccination elevated γδ T cells, which may be important in vaccine immunity (26, 27); e.g., ex vivo-depletion of γδ T cells from PBMCs of immunized macaques significantly decreases CD8+ suppressor activity for SIV replication and the production of RANTES, MIP-1α, and MIP-1β (26). Also, γδ T cells from individuals vaccinated with recombinant canary pox HIV produce IFN-γ (27). γδ T cells can promote the maturation of cultured monocyte-derived DCs, which present antigen better to naive T cells (34). Thus, γδ T cells can contribute to innate and adaptive arms of immunity.
We also observed increases in mature DCs at the site of entry of the challenge virus. This increase had not been studied previously in other vaccine settings. DCs might produce cytokines like IL-12 and IFNs, and chemokines like MIP-1α (CCL4), MIP-1β (CCL5), and RANTES (CCL6). Immunization with live-attenuated SIV vaccine leads to production of CCL 4, 5, and 6 (35), which can block viral entry (36). These chemokines are also induced when DCs encounter a maturation stimulus, such as CD40L (37) on immune CD4+ T cells. Therefore, we would suggest that immune T cells stimulate DCs to produce large amounts of chemokines, in tandem with those produced by γδ T cells (above), and these quickly block the take and spread of SIVmac251. Perhaps these mechanisms also prevent superinfection in naturally infected individuals.
Supplementary Material
Acknowledgments
We thank Gudrun Groβschupff, Birgit Raschdorff, Petra Meier, and Klaus Sure for technical assistance. This work was supported by European Community Contract QLK2-CT-1999-01215 and National Institutes of Health Grant AI40874. The monoclonal antibody to SIVnef from Dr. K. Kent and Ms. C. Arnold was obtained through the Centralized Facility for AIDS Reagents supported by the European Union Program European, Vaccine against AIDS (Contract QLK2-CT-1999-00609), and the U.K. Medical Research Council.
Abbreviations: DC, dendritic cell; SIV, simian immunodeficiency virus; MALT, mucosa-associated lymphoid tissue; TCID50, tissue culture 50% infective dose; PBMC, peripheral blood mononuclear cell.
References
- 1.Lehner, T., Bergmeier, L., Wang, Y., Tao, L. & Mitchell, E. (1999) Immunol. Rev. 170, 183-196. [DOI] [PubMed] [Google Scholar]
- 2.Tenner-Racz, K., von Stemm, A. M., Guhlk, B., Schmitz, J. & Racz, P. (1994) Res. Virol. 145, 177-182. [DOI] [PubMed] [Google Scholar]
- 3.Frankel, S. S., Wenig, B. M., Burke, A. P., Mannan, P., Thompson, L. D. R., Abbondanzo, S. L., Nelson, A. M., Pope, M. & Steinman, R. M. (1996) Science 272, 115-117. [DOI] [PubMed] [Google Scholar]
- 4.Veazey, R. S., DeMaria, M., Chalifoux, L. V., Shvetz, D. E., Pauley, D. R., Knight, H. L., Rosenzweig, M., Johnson, R. P., Desrosiers, R. C. & Lackner, A. A. (1998) Science 280, 427-431. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki, A. & Kelsall, B. L. (2000) J. Exp. Med. 191, 1381-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron, P. U., Freudenthal, P. S., Barker, J. M., Gezelter, S., Inaba, K. & Steinman, R. M. (1992) Science 257, 383-387. [DOI] [PubMed] [Google Scholar]
- 7.Granelli-Piperno, A., Finkel, V., Delgado, E. & Steinman, R. M. (1999) Curr. Biol. 9, 21-29. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek, T. B. H., Krooshoop, D. J. E. B., Bleijs, D., van Vliet, S. J., van Duijnhoven, G. C. F., Grabovsky, V., Alon, R., Figdor, C. G. & van Kooyk, Y. (2000) Nat. Immunol. 1, 353-357. [DOI] [PubMed] [Google Scholar]
- 9.Ignatius, R., Isdell, F., O'Doherty, U. & Pope, M. (1998) J. Med. Primatol. 27, 121-128. [DOI] [PubMed] [Google Scholar]
- 10.Buseyne, F., Gall, S. L., Boccaccio, C., Abastado, J. P., Lifson, J. D., Arthur, L. O., Riviere, Y., Heard, J. M. & Schwartz, O. (2001) Nat. Med. 7, 344-349. [DOI] [PubMed] [Google Scholar]
- 11.Granelli-Piperno, A., Zhong, L., Haslett, P., Jacobson, J. & Steinman, R. M. (2000) J. Immunol. 165, 6620-6626. [DOI] [PubMed] [Google Scholar]
- 12.Weissman, D., Ni, H., Scales, D., Dude, A., Capodici, J., McGibney, K., Abdool, A., Isaacs, S. N., Cannon, G. & Kariko, K. (2000) J. Immunol. 165, 4710-4717. [DOI] [PubMed] [Google Scholar]
- 13.Zhong, L., Granelli-Piperno, A., Pope, M., Ignatius, R., Lewis, M., Frankel, S. S. & Steinman, R. M. (2000) Eur. J. Immunol. 30, 3281-3290. [DOI] [PubMed] [Google Scholar]
- 14.Stahl-Hennig, C., Steinman, R. M., Tenner-Racz, K., Pope, M., Stolte, N., Matz-Rensing, K., Grobschupff, G., Raschdorff, B., Hunsmann, G. & Racz, P. (1999) Science 285, 1261-1265. [DOI] [PubMed] [Google Scholar]
- 15.Stahl-Hennig, C., Steinman, R. M., Ten Haaft, P., Uberla, K., Stolte, N., Saeland, S., Tenner-Racz, K. & Racz, P. (2002) J. Virol. 76, 688-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl-Hennig, C., Dittmer, U., Nisslein, T., Petry, H., Jurkiewicz, E., Fuchs, D., Wachter, H., Maetz-Rensing, K., Kuhn, E.-M., Kaup, F.-J., et al. (1996) J. Gen. Virol. 77, 2969-2981. [DOI] [PubMed] [Google Scholar]
- 17.Gundlach, B. R., Linhart, H., Dittmer, U., Sopper, S., Reiprich, S., Fuchs, D., Fleckenstein, B., Hunsmann, G., Stahl-Hennig, C. & Uberla, K. (1997) J. Virol. 71, 2225-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ten Haaft, P., Verstrepen, B., Uberla, K., Rosenwirth, B. & Heeney, J. (1998) J. Virol. 72, 10281-10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann-Lehmann, R., Swenerton, R. K., Liska, V., Leutenegger, C. M., Lutz, H., McClure, H. M. & Ruprecht, R. M. (2000) AIDS Res. Hum. Retroviruses 16, 1247-1257. [DOI] [PubMed] [Google Scholar]
- 20.Tenner-Racz, K., Stellbrink, H.-J., van Lunzen, J., Schneider, C., Jacobs, J.-P., Raschodorff, B., Grobschupff, G., Steinman, R. M. & Racz, P. (1998) J. Exp. Med. 187, 949-959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gundlach, B. R., Lewis, M. G., Sopper, S., Schnell, T., Sodroski, J., Stahl-Hennig, C. & Uberla, K. (2000) J. Virol. 74, 3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valladeau, J., Duvert-Frances, V., Pin, J. J., Dezutter-Dambuyant, C., Vincent, C., Massacrier, C., Vincent, J., Yoneda, K., Banchereau, J., Caux, C., et al. (1999) Eur. J. Immunol. 29, 2695-2704. [DOI] [PubMed] [Google Scholar]
- 23.de Saint-Vis, B., Vincent, J., Vandenabeele, S., Vanbervliet, B., Pin, J.-J., Ait-Yahia, S., Patel, S., Mattei, M.-G., Banchereau, J., Zurawski, S., et al. (1998) Immunity 9, 325-336. [DOI] [PubMed] [Google Scholar]
- 24.Daniel, M. D., Kirchhoff, F., Czajak, S. C., Sehgal, P. K. & Desrosiers, R. C. (1992) Science 258, 1938-1941. [DOI] [PubMed] [Google Scholar]
- 25.Carding, S. R. & Egan, P. J. (2002) Nat. Rev. Immunol. 2, 336-345. [DOI] [PubMed] [Google Scholar]
- 26.Lehner, T., Mitchell, E., Bergmeier, L., Singh, M., Spallek, R., Cranage, M., Hall, G., Dennis, M., Villinger, F. & Wang, Y. (2000) Eur. J. Immunol. 30, 2245-2256. [DOI] [PubMed] [Google Scholar]
- 27.Worku, S., Gorse, G. J., Belshe, R. B. & Hoft, D. F. (2001) J. Infect. Dis. 184, 525-532. [DOI] [PubMed] [Google Scholar]
- 28.Desrosiers, R. C., Lifson, J. D., Gibbs, J. S., Czajak, S. C., Howe, A. Y., Arthur, L. O. & Johnson, R. P. (1988) J. Virol. 72, 1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson, R. P. (1999) Nat. Med. 5, 154-155. [DOI] [PubMed] [Google Scholar]
- 30.Mascola, J. R., Stiegler, G., VanCott, T. C., Katinger, H., Carpenter, C. B., Hanson, C. E., Beary, H., Hayes, D., Frankel, S. S., Birx, D. L. & Lewis, M. G. (2000) Nat. Med. 6, 207-210. [DOI] [PubMed] [Google Scholar]
- 31.Metzner, K. J., Jin, X., Lee, F. V., Gettie, A., Bauer, D. E., Di Mascio, M., Perelson, A. S., Marx, P. A., Ho, D. D., Kostrikis, L. G. & Connor, R. I. (2000) J. Exp. Med. 191, 1921-1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz, J. E., Kuroda, M. J., Santra, S., Sasseville, V. G., Simon, M. A., Lifton, M. A., Racz, P., Tenner-Racz, K., Dalesandro, M., Scallon, B. J., et al. (1999) Science 283, 857-860. [DOI] [PubMed] [Google Scholar]
- 33.Stebbings, R. J., Almond, N. M., Stott, E. J., Berry, N., Wade-Evans, A. M., Hull, R., Lines, J., Silvera, P., Sangster, R., Corcoran, T., et al. (2002) Virol. 296, 338-353. [DOI] [PubMed] [Google Scholar]
- 34.Leslie, D. S., Vincent, M. S., Spada, F. M., Das, H., Sugita, M., Morita, C. T. & Brenner, M. B. (2002) J. Exp. Med. 196, 1575-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gauduin, M. C., Glickman, R. L., Ahmad, S., Yilma, T. & Johnson, R. P. (1999) Proc. Natl. Acad. Sci. USA 96, 14031-14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cocchi, F., DeVico, A. L., Garzino-Demo, A., Arya, S. K., Gallo, R. C. & Lusso, P. (1995) Science 270, 1811-1815. [DOI] [PubMed] [Google Scholar]
- 37.Sallusto, F., Palermo, B., Lenig, D., Miettinen, M., Matikainen, S., Julkunen, I., Forster, R., Burgstahler, R., Lipp, M. & Lanzavecchia, A. (1999) Eur. J. Immunol. 29, 1617-1625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.