The USA300 clone of methicillin-resistant Staphylococcus aureus causes concurrent epidemics of skin and soft tissue infections (SSTIs) and bloodstream infections (BSIs). Because USA300 SSTIs serve as a source for BSIs, strategies to control the USA300 SSTI epidemic may lessen the severity of the USA300 BSI epidemic.
Abstract
Background. Since its emergence in 2000, epidemic spread of the methicillin-resistant Staphylococcus aureus (MRSA) clone USA300 has led to a high burden of skin and soft tissue infections (SSTIs) in the United States, yet its impact on MRSA bloodstream infections (BSIs) is poorly characterized.
Methods. To assess clonality of the MRSA isolates causing SSTI and BSI during the epidemic period, a stratified, random sample of 1350 unique infection isolates (from a total of 7252) recovered at the Community Health Network of San Francisco from 2000 to 2008 were selected for genotyping. Risk factors and outcomes for 549 BSI cases caused by the USA300 epidemic clone and non-USA300 MRSA clones were assessed by retrospective review of patient medical records.
Results. From 2000 to 2008, secular trends of USA300 SSTI and USA300 BSI were strongly correlated (Pearson r = 0.953). USA300 accounted for 55% (304/549) of BSIs as it was the predominant MRSA clone that caused community-associated (115/160), healthcare-associated community-onset (125/207), and hospital-onset (64/182) BSIs. Length of hospitalization after BSI diagnosis and mortality rates for USA300 and non-USA300 were similar. Two independent risk factors for USA300 BSI were identified: concurrent SSTI (adjusted relative risk, 1.4 [95% confidence interval {CI}, 1.2–1.6]) and anti-MRSA antimicrobial use in the preceding 30 days (0.7 [95% CI, .6–.8]). Isolates from concurrent SSTI were indistinguishable genotypically from the USA300 isolates that caused BSI.
Conclusions. USA300 SSTIs serve as a source for BSI. Strategies to control the USA300 SSTI epidemic may lessen the severity of the concurrent USA300 BSI epidemic.
Since 2000, a dramatic increase in the number of methicillin-resistant Staphylococcus aureus (MRSA) infections has been observed in North America, mostly related to the emergence of the USA300 clone as a primary cause of community-associated disease [1–3]. More recent studies revealed that USA300 has spread from the community to hospitals where it has become the predominant clone isolated in various settings [4, 5]. Most USA300 infections manifest as skin and soft tissue infections (SSTIs) [6], although life-threatening infections have also been documented among persons with comorbid conditions as well as healthy community-dwelling individuals [7, 8]. Inasmuch as SSTIs can result in dissemination of staphylococci into the bloodstream, the impact of this focus of infection—especially in the context of the USA300 epidemic—on the burden and outcome of BSI has not been well studied.
Staphylococcus aureus is one of the most common etiologies of bloodstream infection (BSI) [9, 10]. MRSA BSI is a serious complication of MRSA colonization and/or infection, associated with substantial morbidity and mortality [11–14] and unresolved treatment issues [15, 16]. Bacteremia was present in 75% of invasive MRSA diseases in a population-based multicentric study performed in 2004–2005 in the United States [17], with an estimated standardized incidence rate of MRSA BSI at 23.8 per 100 000 inhabitants per year. In this study, results of molecular typing of a subset of isolates (11.3% of reported cases) suggested that USA300 accounted for 67% (100/150) of community-associated invasive MRSA disease, but only 16% (34/216) of healthcare-associated and 22% (108/485) of nosocomial invasive MRSA diseases [17], which led to the authors’ statement that “most invasive MRSA disease in the United States is still caused by MRSA strain of healthcare origin” [17]. However, this may not be the case in the most affected areas, such as San Francisco, where USA300 emerged in 2000 and dramatically changed MRSA epidemiology [18–20]. Indeed, it has been shown that the prevalence of USA300 strains among cases of healthcare-associated MRSA BSI may vary dramatically among geographically clustered hospitals [21]. The purpose of our study was to (1) assess temporal trends of MRSA BSI and (2) determine risk factors associated with BSI due to the USA300 clonal type from 2000 to 2008 in the Community Health Network of San Francisco, which includes San Francisco General Hospital, a public tertiary care center, and 13 citywide outpatient clinics.
METHODS
Study Design
As part of routine laboratory-based surveillance, we banked 9817 MRSA isolates cultured from infection sites of patients treated at San Francisco General Hospital from 1 January 2000 to 31 December 2008. The number of unique MRSA isolates (ie, 1 isolate per patient per year) was 7252 for the study period, excluding concurrent infection isolates as well as infecting isolates from the same patient within a 12-month period. A stratified random sample of 150 of these unique MRSA isolates per stratum-year was selected for genotyping to assess secular trends in MRSA clonal types and infection types.
Of the 7252 unique MRSA isolates, 549 originated from patients’ blood cultures. The medical records of the corresponding 549 patients were reviewed to confirm MRSA BSI according to Centers for Disease Control and Prevention criteria [22]. Within 7 days preceding the BSI episode, 119 (22%) of these patients also had MRSA isolated from another infected site, for which the non-BSI isolate is also available for genotyping. All 549 MRSA BSI isolates and 119 concurrent MRSA isolates were genotyped as described below.
The following data were collected using a standardized instrument from each patient's electronic medical record: age, sex, preadmission location(s), hospitalization dates, housing, alcohol abuse, injection drug use, comorbidities, and risk factors for MRSA infection including recent surgery, wounds, catheter, and antistaphylococcal antibiotic use over the last 30 days. Hospital-onset BSI was defined by a first positive blood culture obtained for patients admitted for ≥48 hours [23]. Healthcare-associated, community-onset BSI was defined by a positive blood culture within 48 hours of admission if the patient fulfilled any of the following criteria: (1) received intravenous therapy or wound care at home in the 30 days before BSI, (2) attended a hospital or hemodialysis clinic or received intravenous chemotherapy in the 30 days before BSI, (3) had central venous catheters or urinary catheters in the 30 days before BSI, (4) was hospitalized in an acute care hospital or underwent surgery in the 90 days before BSI, or (5) resided in a nursing home or a long-term care facility. Community-associated BSI was defined by a positive blood culture obtained within the 48 hours after admission for patients who did not fit the criteria for healthcare-associated BSI.
Data collection was approved by the Committee on Human Research, Office of Research Administration, at the University of California, San Francisco. Informed consent was waived because the study involved retrospective chart reviews.
Genotypic Characterization
All MRSA isolates were genotyped by spa typing of the polymorphic repeat regions of protein A [24], and presence or absence of the arginine catabolic mobile element (ACME) and Panton-Valentine leukocidin genes (lukF-PV and lukS-PV) using polymerase chain reaction (PCR)–based assays [25] as previously described. The USA300 clone was defined as having spa type t008 (YHGFMBQBLO) and was PCR positive for ACME and Panton-Valentine leukocidin genes [25, 26]. Pulsed-field gel electrophoresis (PFGE) after SmaI-macrorestriction digest of chromosomal DNA [27] was performed on 570 (28%) isolates to validate clonality as defined by spa and PCR-based typing methods. PFGE patterns were designated according to the nomenclature described by McDougal et al [28] (eg, USA100, USA300, USA500). Multilocus sequence typing of fragments of 7 housekeeping genes [29] was performed on 20 isolates from different PFGE clonal groups.
Statistical Methods
For the stratified random samples, the survey data analysis function of the Stata statistical package (version 9.1, College Station, Texas) was used to compute estimates for each stratum-year and for the entire strain population, with appropriate weighting to restore the original proportions. Pearson r correlation was used to evaluate secular trends. For the BSI cross-sectional study, 2-tailed Fisher exact tests were used to evaluate associations between USA300 BSI and demographic or clinical characteristics. Unadjusted relative risks and 95% confidence intervals (CIs) were calculated using Stata. For multivariable analysis, we used Cochran-Mantel-Haenszel procedure and included all variables that proved to be statistically significant in bivariate analysis; backward elimination of statistically nonsignificant (P > .05) variables yielded a final multivariate model and estimates of adjusted relative risks.
RESULTS
Concurrent SSTI and BSI USA300 Epidemics
The number of unique MRSA infections treated at the Community Health Network of San Francisco increased from 341 cases in 2000 to 1323 cases in 2004, falling to 777 cases in 2008 (Figure 1A). Molecular genotyping of a stratified random sample of 150 MRSA isolates per year allowed for assessment of changes in secular trends of the infecting MRSA strain types (Figure 1A). The MRSA epidemic, which peaked in 2004–2005, was due almost entirely to the emergence of the USA300 clonal type, which also peaked in 2004 when this clone caused an estimated 1021 cases, declining to 650 cases by 2008. During the epidemic period, the number of infections due to non-USA300 strain types—USA100, USA500, USA1000, and USA1100 and other low-frequency strain types—declined gradually from a peak of an estimated 394 cases in 2002 to 127 cases in 2008 (Figure 1A).
Figure 1.
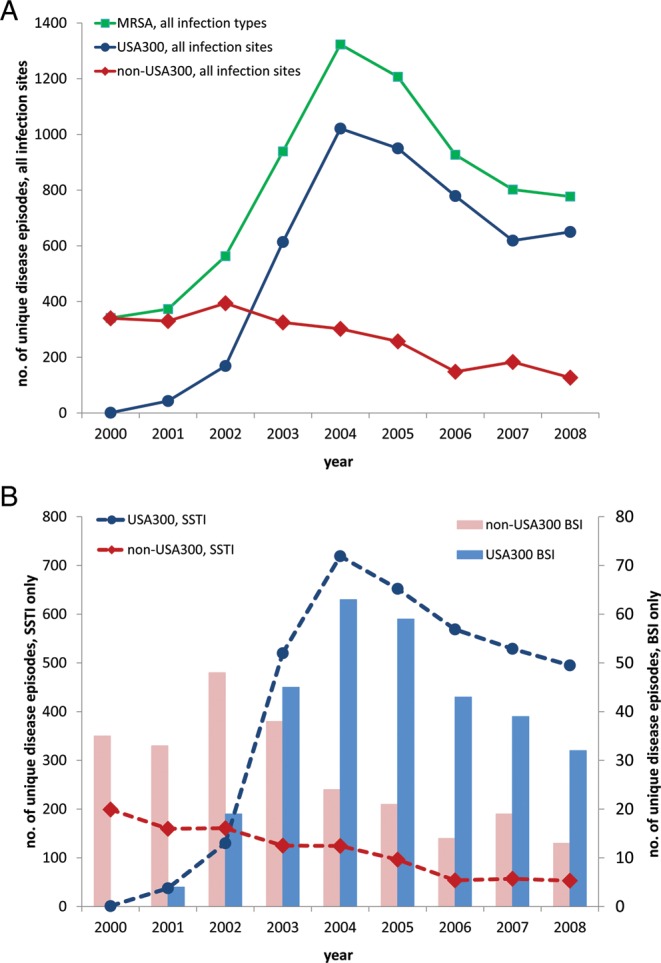
Secular trends of methicillin-resistant Staphylococcus aureus infection (A), including skin and soft tissue infection and bloodstream infection (B), according to USA300 and non-USA300 genotypes. Abbreviations: BSI, bloodstream infection; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infection.
The MRSA epidemic was driven by concurrent epidemics of USA300 SSTI and USA300 BSI (Figure 1B). At the peak of the epidemic in 2004, 64% (843/1323) of infections were SSTIs, of which 85% (719/843) were estimated to be caused by USA300. The USA300 SSTI epidemic was paralleled by a concurrent USA300 BSI epidemic, as indicated a strong correlation between the secular trends of USA300 SSTI and USA300 BSI from 2000 to 2008 (Pearson r = 0.953, P = .0001) (Figure 1B). USA300 accounted for 55% (304/549) of BSI cases overall and was responsible for 72% (63/87) of BSI cases at the height of the MRSA epidemic in 2004 (Figure 1B). USA100 accounted for 17% (n = 95), USA1000 for 13% (n = 72), and USA500 for 9% (n = 49) of BSIs (Table 1). USA300 had an increased propensity to cause SSTI compared with non-USA300 (75% [3653/4846] vs 43% [1029/2406], P < .0001). Although USA300 was associated with BSI in a lower proportion of cases than non-USA300 (6.3% [304/4846] vs 10.2% [245/2406], P < .0001), USA300 alone caused more BSIs than all non-USA300 MRSA clones combined.
Table 1.
Genotypic Characteristics of Methicillin-Resistant Staphylococcus aureus (MRSA) Isolates Cultured From Blood in Patients With Community-Associated, Healthcare-Associated Community-Onset, and Hospital-Onset MRSA Bloodstream Infections
| MRSA Strain Types, PFGE (MLST ACME-PCR PVL-PCR) | Community-Associated | Healthcare-Associated Community-Onset No. (%) | Hospital-Onset | Total |
|---|---|---|---|---|
| USA300 (ST8 ACME+ PVL+) | 115 (72) | 125 (60) | 64 (35) | 304 (55) |
| USA100 (ST5 ACME− PVL−) | 7 (4) | 30 (14) | 58 (32) | 95 (17) |
| USA1000 (ST59 ACME− PVL−) | 19 (12) | 26 (13) | 27 (15) | 72 (13) |
| USA500 (ST8 ACME− PVL−) | 13 (8) | 16 (8) | 20 (11) | 49 (9) |
| USA1100 (ST30 ACME− PVL+) | 4 (3) | 4 (2) | 2 (1) | 10 (2) |
| Low-frequency types | 2 (1) | 6 (3) | 11 (6) | 19 (3) |
Abbreviations: ACME, arginine catabolic mobile element; MLST, multilocus sequence typing; MRSA, methicillin-resistant Staphylococcus aureus; PCR, polymerase chain reaction; PFGE, pulsed-field gel electrophoresis; PVL, Panton-Valentine leukocidin.
In the pre-epidemic year of 2000, the incidence of non-USA300 BSIs was 161 per 100 000 hospitalizations within inpatient wards, whereas it was 31 per 100 000 visits in the emergency department (Figure 2). These data are consistent with non-USA300 MRSA (eg, the prototypical hospital-associated MRSA strain USA100) causing predominantly nosocomial infection among patients with vascular access devices (central or peripheral venous catheter) or infected surgical wounds. At the peak of the epidemic in 2004–2005, a paradigm shift occurred in which USA300 was responsible for a high incidence of 96 BSI cases per 100 000 visits in the emergency department (Figure 2). USA300 also accounted for 149 BSI cases per 100 000 hospitalizations within inpatient wards in 2005 (Figure 2).
Figure 2.
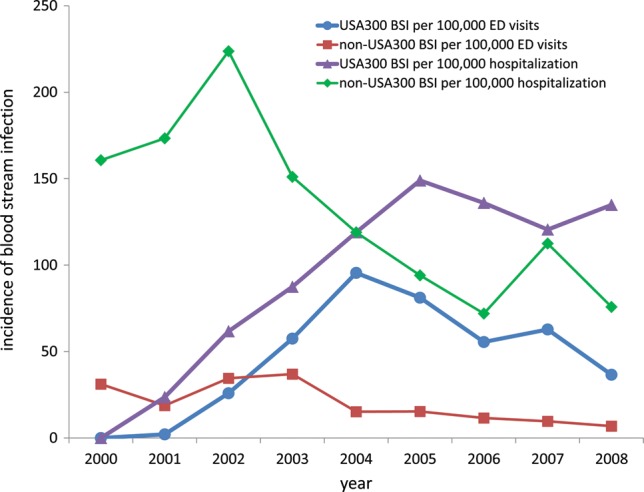
Incidence rates of USA300 and non-USA300 methicillin-resistant Staphylococcus aureus bloodstream infections diagnosed in the emergency department (ED; expressed per 100 000 ED visits) or within inpatient wards (expressed as per 100 000 hospitalizations). Abbreviations: BSI, bloodstream infections; ED, emergency department.
USA300 was the predominant cause of both community-associated BSI (72% [115/160]) and healthcare-associated, community onset BSI (60% [125/207]). Although USA300 was also the predominant cause of hospital-onset BSI (35% [64/182]), the prototypical hospital-associated MRSA strain type USA100 was still an important cause of hospital-onset infections (32% [58/182]).
Patient Characteristics and Risk Factors for USA300 BSI
In bivariate analysis, patients ≥60 years of age were significantly less likely to have USA300 BSI than those <60 years of age (40% [34/86] vs 58% [270/463], P = .001) (Table 2). USA300 BSIs were significantly more likely to be diagnosed in the emergency department than in other hospital locations (69% [182/264] vs 43% [122/285], P < .001). Increased risk of USA300 BSI was also significantly associated with intravenous drug use (P = .001), being homeless or marginally housed (P = .005), hepatitis C infection (P = .006), human immunodeficiency virus (HIV) infection (P = .002), and prior SSTI in the preceding 12 months (P = .002). Patients who were treated with antimicrobial agents active against MRSA in the preceding 30 days were significantly less likely to have USA300 BSI than those who were not treated (40% [61/151] vs 61% [243/398], P < .001).
Table 2.
Risk Factors for Methicillin-Resistant Staphylococcus aureus USA300 Bloodstream Infections
| Variable | USA300 Infections Among Patients With Variable Present, No. (%) | USA300 Infections Among Patients With Variable Absent, No. (%) | Relative Risk (95% CI) | P Value* |
|---|---|---|---|---|
| Hospital-onset | 64/182 (35) | 240/367 (65) | 0.5 (.4–.7) | <.001 |
| Community-associated | 115/160 (72) | 189/389 (49) | 1.5 (1.3–1.7) | <.001 |
| Healthcare-associated community-onset | 125/207 (60) | 179/342 (52) | 1.2 (1.0–1.3) | .066 |
| Hospitalization | 111/190 (58) | 14/17 (82) | 0.7 (.6–.9) | .053 |
| Hemodialysis | 29/54 (54) | 96/153 (63) | 0.9 (.6–1.1) | .24 |
| Prior surgery | 42/62 (68) | 83/145 (57) | 1.2 (.9–1.5) | .157 |
| Central venous catheter | 29/56 (52) | 96/151 (64) | 0.8 (.6–1.1) | .123 |
| Age (years) | ||||
| 5–17 | 8/13 (62) | 296/536 (55) | 1.1 (.7–1.7) | .65 |
| 18–29 | 38/58 (66) | 266/491 (54) | 1.2 (1.0–1.5) | .100 |
| 30–39 | 52/85 (61) | 252/464 (54) | 1.1 (.9–1.4) | .24 |
| 40–49 | 83/146 (57) | 221/403 (55) | 1.0 (.9–1.2) | .68 |
| 50–59 | 89/161 (55) | 215/388 (55) | 1.0 (.8–1.2) | .98 |
| ≥60 | 34/86 (40) | 270/463 (58) | 0.7 (.5–.9) | .001 |
| Race | ||||
| White | 144/242 (60) | 157/293 (54) | 1.1 (1.0–1.2) | .169 |
| Black | 104/169 (62) | 197/366 (54) | 1.1 (1.0–1.3) | .095 |
| Hispanic | 33/72 (46) | 268/463 (58) | 0.8 (.6–1.0) | .055 |
| Other | 20/52 (38) | 281/483 (58) | 0.7 (.5–.9) | .007 |
| Male sex | 220/398 (55) | 84/151 (56) | 1.0 (.8–1.2) | .94 |
| IVDU | 164/261 (63) | 133/278 (48) | 1.3 (1.1–1.5) | .001 |
| Homeless/marginally housed | 168/274 (61) | 127/258 (49) | 1.2 (1.1–1.5) | .005 |
| Alcohol abuse | 86/158 (54) | 207/377 (55) | 1.0 (.8–1.2) | .92 |
| Diabetes | 245/429 (57) | 59/119 (50) | 1.2 (.9–1.4) | .144 |
| Renal insufficiency | 82/147 (56) | 222/402 (55) | 1.0 (.9–1.2) | .91 |
| Congestive heart failure | 39/79 (49) | 265/470 (56) | 0.9 (.7–1.1) | .25 |
| HCV infection | 170/278 (61) | 134/271 (49) | 1.2 (1.1–1.4) | .006 |
| COPD | 22/43 (51) | 279/499 (56) | 0.9 (.7–1.2) | .55 |
| Immunosuppressive therapy | 8/21 (38) | 294/526 (56) | 0.7 (.4–1.2) | .108 |
| HIV infection | 69/101 (68) | 208/410 (51) | 1.3 (1.1–1.6) | .002 |
| <0.35 × 109 CD4+ cells/L | 49/73 (67) | 13/17 (76) | 0.9 (.6–1.2) | .45 |
| SSTI in past 12 months | 96/145 (66) | 208/404 (51) | 1.3 (1.1–1.5) | .002 |
| Antibiotic use in past 30 daysa | 61/151 (40) | 243/398 (61) | 0.7 (.5–.8) | <.001 |
| BSI diagnosis in ED | 182/264 (69) | 122/285 (43) | 1.6 (1.4–1.9) | <.001 |
*Numbers in boldface indicate statistical significance (P <.05).
Abbreviations: BSI, bloodstream infection; CI, confidence interval; COPD, chronic obstructive pulmonary disease; ED, emergency department; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IVDU, intravenous drug use; MRSA, methicillin-resistant Staphylococcus aureus; SSTI, skin and soft tissue infection.
aOnly agents usually active on MRSA were collected. Data were missing for 13 (2.4%) on race, 10 (1.8%) on IVDU, 17 (3.1%) on homeless/marginally housed status, 13 (2.4%) on alcohol abuse, and 37 (6.7%) on HIV infection. Of the 101 patients with HIV infection, 11 (11%) had missing data on CD4 count in the preceding 6 months.
Concurrent Sites of Infections
The vast majority (524/549) of patients had concurrent infection sites (Table 3), which could serve as a point source for seeding of staphylococci into the blood. USA300 BSI was more frequently found in patients with MRSA BSI associated with SSTI (69% [137/199] vs 48% [167/350], P < .001), osteomyelitis (65% [60/92] vs 53% [244/457], P = .037), and endocarditis (68% [52/77] vs 53% [252/472], P = .021). USA300 BSI was less frequent in patients with MRSA BSI and intravascular device infection (42% [44/106] vs 59% [260/443], P = .001), or surgical site infection (36% [9/25] vs 56% [296/524], P = .046).
Table 3.
Concurrent Infections Among Patients With Methicillin-Resistant Staphylococcus aureus USA300 Bloodstream Infections
| Variable | USA300 Infections Among Patients With Variable Present, No. (%) | USA300 Infections Among Patients With Variable Absent, No. (%) | Relative Risk (95% CI) | P Value* |
|---|---|---|---|---|
| SSTI | 137/199 (69) | 167/350 (48) | 1.4 (1.2–1.7) | <.001 |
| Osteomyelitis | 60/92 (65) | 244/457 (53) | 1.2 (1.0–1.5) | .037 |
| Endocarditis | 52/77 (68) | 252/472 (53) | 1.3 (1.1–1.5) | .021 |
| Pneumonia | 56/95 (59) | 248/454 (55) | 1.1 (.9–1.3) | .44 |
| Septic arthritis | 18/39 (46) | 286/510 (56) | 0.8 (.6–1.2) | .23 |
| Septic shock | 16/30 (53) | 288/519 (55) | 1.0 (.7–1.4) | .82 |
| Intravascular device infection | 44/106 (42) | 260/443 (59) | 0.7 (.6–.9) | .001 |
| Surgical site infection | 9/25 (36) | 295/524 (56) | 0.6 (.4–1.1) | .046 |
| No concurrent infection | 8/25 (32) | 296/524 (56) | 0.6 (.3–1.0) | .016 |
*Numbers in boldface indicate statistical significance (P <.05).
Abbreviations: CI, confidence interval; SSTI, skin and soft tissue infection.
A multivariate analysis of those risk factors that were significantly associated with USA300 BSI yielded only 2 independent risk factors: concurrent SSTI (adjusted relative risk, 1.4 [95% CI, 1.2–1.6]) and anti-staphylococcal antimicrobial use in the preceding 30 days (adjusted relative risk, 0.7 [95% CI, .6–.8]) (Table 4).
Table 4.
Adjusted Relative Risk for Methicillin-Resistant Staphylococcus aureus USA300 Bloodstream Infections
| Variable | Adjusted Relative Risk (95% Confidence Interval) | P Value |
|---|---|---|
| Concurrent skin and soft tissue infection | 1.4 (1.2–1.7) | <.001 |
| Antibiotic use in past 30 days | 0.7 (.6–.8) | <.001 |
Results are shown for the most stable multivariable analysis. Addition to the analysis of variables for bloodstream infection diagnosis in emergency department, hospital-onset methicillin-resistant Staphylococcus aureus (MRSA) infection status, community-associated MRSA infection status, human immunodeficiency virus infection, hepatitis C virus infection, age >60 years, skin and soft tissue infection in past 12 months, intravenous drug use, and homeless/marginally housed led to spurious estimates of relative risks.
Outcomes of USA300 BSI
The length of stay after diagnosis of BSI was virtually identical for patients with BSI caused by USA300 compared with non-USA300 (median [interquartile range], 14 [8–24] days vs 14 [8–25] days, P = .627), suggesting that USA300 does not cause more severe or more difficult-to-treat infections. The total length of stay was shorter for patients with BSI caused by USA300 compared with non-USA300 (16 [9–27] days vs 22 [12–38] days, P < .001), reflecting the fact that USA300 BSIs were typically diagnosed prior to, or shortly after, hospital admission. Mortality that was directly attributable to MRSA BSI was similar for patients with USA300 compared with non-USA300 (8% [24/304] vs 9% [23/245], P = .534).
Strain-Relatedness of MRSA From Concurrent Infection Sites
From 2000 to 2008, 119 of 549 (22%) of patients also had available MRSA isolates that originated from a concurrent body site within 7 days preceding the original BSI episode. Of those, 98% (117/119) of the strain pairs were genetically indistinguishable from one another. All (59/59) of the USA300 BSI isolates were genetically indistinguishable from concurrent non-BSI isolates, including all (29/29) of the concurrent SSTI isolates.
DISCUSSION
The current study demonstrates that MRSA BSI is predominantly a community disease in an area highly affected by the USA300 epidemic. Although this is in line with a study showing that USA300 was the most common pathogen responsible for SSTI infections diagnosed in the emergency departments of 11 US cities in 2004 [30], others have questioned the ability of this clone to be a significant cause of invasive disease [17, 31]. Indeed, others have found that the vast majority of community-associated MRSA infections were SSTIs and rarely involved other body sites; only 0.3% of cases were bacteremic [32]. Our study demonstrates that, in the Community Health Network of San Francisco, USA300 BSI emerged in parallel with the dramatic increase in USA300 SSTI: (1) both epidemics followed similar patterns, emerging in 2001, rapidly increasing until 2004, and declining somewhat thereafter; (2) predisposing conditions were essentially the same (including being homeless, intravenous drug user, HIV- and/or hepatitis C virus–infected); (3) almost half of patients with USA300 BSI presented with concurrent SSTI, which appeared as an independent risk factor for USA300 BSI in multivariate analysis; (4) in a subset of patients for whom MRSA was isolated from SSTI prior to MRSA BSI diagnosis, both MRSA isolates shared indistinguishable genotypes. Moreover, in patients with MRSA BSI, prior use of systemic antibacterial agents active against community-associated MRSA strains was negatively associated with the diagnosis of USA300 BSI on multivariate analysis. These data collectively suggest that BSI is a frequent complication of SSTI caused by USA300. This may be due to a number of factors, such as enhanced propensity of USA300 for invasive disease. Social factors could also play a role. For example, USA300 occurred, as it often does, in a high-risk population with limited access to basic hygiene and standard care (eg, 57% of patients with USA300 BSI were homeless); delayed access to healthcare may also contribute.
The emergence of epidemic MRSA BSI at our institution stands in contrast to decreases in the incidence of hospital-onset MRSA central line–associated BSI in most intensive care units in the United States during the same time period [33]. We should note that the incidence of BSIs caused by non-USA300, which are predominantly hospital-associated MRSA strains, has been similarly in the decline since 2003 at our institution (Figure 2). Nonetheless, the dramatic increase in incidence of USA300 BSI in both outpatient and inpatient settings had been driving the overall MRSA BSI epidemic (Figure 2). Incidence of USA300 BSI has been in decline from its peak in 2004. The decline of USA300 epidemic may be due to increased public awareness and better treatment, including incision and drainage for SSTI and appropriate antimicrobial use.
The outcome of USA300 MRSA BSI in our study was not significantly different than that of non-USA300 MRSA BSI in terms of length of stay in the hospital after MRSA BSI diagnosis and attributable mortality. This is in agreement with a recent cohort study performed in 4 Veterans Administration hospitals which showed that USA300 MRSA BSI was associated with an increased risk of severe sepsis, and septic shock, but there were no significant differences in mortality [34]. Results of another case-control study performed in Chicago suggested that patients with community-onset USA300 MRSA infections are less likely to require intensive care unit admission, or to die within 7 days after admission, than matched patients with community-onset non-USA300 MRSA infections [35].
Study limitations include the characteristics of the population served by the Community Health Network of San Francisco, with a high prevalence of homelessness and intravenous drug use; thus, our findings may not apply to other population groups. Indeed, the epidemiology of S. aureus BSI is highly dependent on the patient population served by the facility. In addition, as San Francisco has been an epicenter of the USA300 epidemic in the United States, which remains highly heterogeneous over the country, MRSA BSI patterns described herein are not generalizable at the country level. Moreover, because all data were derived from a single institution, the applicability to other facilities is limited. Last, patients with MRSA BSI who either did not have blood cultures drawn or received appropriate antibiotics before blood cultures were drawn could have been missed. However, this study clearly suggests that USA300 BSI emerged as a consequence of the USA300 SSTI epidemic in the poor urban area of San Francisco, and that MRSA BSI is now predominantly a community disease. These findings have implications for the prevention of MRSA BSI in the San Francisco General Hospital, as infection control interventions within the hospital based on previous experiences with traditional, hospital-acquired MRSA will probably have little impact. Indeed, as most patients with MRSA BSI are currently diagnosed upon admission in the emergency department, prevention efforts have to concentrate on prior events, including appropriate treatment of SSTI, and better access to standard hygiene and care. In addition, the fact that most cases of MRSA BSI present in the emergency department has implications for the selection of empirical antibacterial treatment for patients with suspected community-onset BSI, as MRSA coverage is clearly necessary in this setting.
Notes
Financial support. This work was supported by a grant from the Association pour la Formation et la Recherche en Réanimation et en Infectiologie and by the Pontchaillou University Hospital, Rennes, France (to P. T.), and US Public Health Service grants National Institute of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) R01 AI070289 (to H. F. C.) and NIH NIAID R01 AI087674 (to B. A. D.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Carleton HA, Diep BA, Charlebois ED, Sensabaugh GF, Perdreau-Remington F. Community-adapted methicillin-resistant Staphylococcus aureus (MRSA): population dynamics of an expanding community reservoir of MRSA. J Infect Dis. 2004;190:1730–8. doi: 10.1086/425019. [DOI] [PubMed] [Google Scholar]
- 2.Chambers HF. The changing epidemiology of Staphylococcus aureus? Emerg Infect Dis. 2001;7:178–82. doi: 10.3201/eid0702.010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deleo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557–68. doi: 10.1016/S0140-6736(09)61999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–56. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- 5.Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(suppl 5):S344–9. doi: 10.1086/533590. [DOI] [PubMed] [Google Scholar]
- 6.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med. 2006;144:309–17. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 7.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 8.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis. 2009;48(suppl 4):S231–7. doi: 10.1086/598189. [DOI] [PubMed] [Google Scholar]
- 10.Corey GR. Staphylococcus aureus bloodstream infections: definitions and treatment. Clin Infect Dis. 2009;48(suppl 4):S254–9. doi: 10.1086/598186. [DOI] [PubMed] [Google Scholar]
- 11.Kaye KS, Anderson DJ, Choi Y, Link K, Thacker P, Sexton DJ. The deadly toll of invasive methicillin-resistant Staphylococcus aureus infection in community hospitals. Clin Infect Dis. 2008;46:1568–77. doi: 10.1086/587673. [DOI] [PubMed] [Google Scholar]
- 12.Fowler VG, Jr., Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med. 2003;163:2066–72. doi: 10.1001/archinte.163.17.2066. [DOI] [PubMed] [Google Scholar]
- 13.Marchaim D, Kaye KS, Fowler VG, et al. Case-control study to identify factors associated with mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect. 2010;16:747–52. doi: 10.1111/j.1469-0691.2009.02934.x. [DOI] [PubMed] [Google Scholar]
- 14.Laupland KB, Ross T, Gregson DB. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis. 2008;198:336–43. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–92. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 16.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–41. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–71. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 18.Liu C, Graber CJ, Karr M, et al. A population-based study of the incidence and molecular epidemiology of methicillin-resistant Staphylococcus aureus disease in San Francisco, 2004–2005. Clin Infect Dis. 2008;46:1637–46. doi: 10.1086/587893. [DOI] [PubMed] [Google Scholar]
- 19.Tattevin P, Diep BA, Jula M, Perdreau-Remington F. Long-term follow-up of methicillin-resistant Staphylococcus aureus molecular epidemiology after emergence of clone USA300 in San Francisco jail populations. J Clin Microbiol. 2008;46:4056–7. doi: 10.1128/JCM.01372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tattevin P, Diep BA, Jula M, Perdreau-Remington F. Methicillin-resistant Staphylococcus aureus USA300 clone in long-term care facility. Emerg Infect Dis. 2009;15:953–5. doi: 10.3201/eid1506.080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins TC, McCollister BD, Sharma R, et al. Epidemiology of healthcare-associated bloodstream infection caused by USA300 strains of methicillin-resistant Staphylococcus aureus in 3 affiliated hospitals. Infect Control Hosp Epidemiol. 2009;30:233–41. doi: 10.1086/595963. [DOI] [PubMed] [Google Scholar]
- 22.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: CG Mayhall , editor. Hospital epidemiology and infection control. Philadelphia:: Lippincott Williams and Wilkins; 2004. pp. 1672–89. [Google Scholar]
- 23.Friedman ND, Kaye KS, Stout JE, et al. Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 24.Shopsin B, Gomez M, Montgomery SO, et al. Evaluation of protein A gene polymorphic region DNA sequencing for typing of Staphylococcus aureus strains. J Clin Microbiol. 1999;37:3556–63. doi: 10.1128/jcm.37.11.3556-3563.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–9. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- 26.Diep BA, Chambers HF, Graber CJ, et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–57. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- 27.Tenover FC, McDougal LK, Goering RV, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–18. doi: 10.1128/JCM.44.1.108-118.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol. 2003;41:5113–20. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. 2000;38:1008–15. doi: 10.1128/jcm.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–74. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 31.Bancroft EA. Antimicrobial resistance: it's not just for hospitals. JAMA. 2007;298:1803–4. doi: 10.1001/jama.298.15.1803. [DOI] [PubMed] [Google Scholar]
- 32.Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943–51. doi: 10.1016/j.amjmed.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Burton DC, Edwards JR, Horan TC, Jernigan JA, Fridkin SK. Methicillin-resistant Staphylococcus aureus central line-associated bloodstream infections in US intensive care units, 1997–2007. JAMA. 2009;301:727–36. doi: 10.1001/jama.2009.153. [DOI] [PubMed] [Google Scholar]
- 34.Kreisel KM, Stine OC, Johnson JK, et al. USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn Microbiol Infect Dis. 2011;70:285–90. doi: 10.1016/j.diagmicrobio.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hota B, Lyles R, Rim J, et al. Predictors of clinical virulence in community-onset methicillin-resistant Staphylococcus aureus infections: the importance of USA300 and pneumonia. Clin Infect Dis. 2011;53:757–65. doi: 10.1093/cid/cir472. [DOI] [PubMed] [Google Scholar]


