Abstract
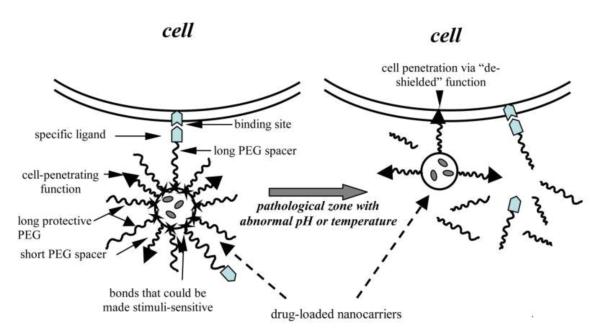
Cell-penetrating peptide (CPP)-mediated intracellular drug delivery system, often specifically termed as “the Trojan horse approach”, has become the “holy grail” in achieving effective delivery of macromolecular compounds such as proteins, DNA, siRNAs, and drug carriers. It is characterized by the unique cell- (or receptor-), temperature-, and payload-independent mechanisms, therefore offering potent means to improve poor cellular uptake of a variety of macromolecular drugs. Nevertheless, this “Trojan horse” approach also acts like a double-edged sword, causing serious safety and toxicity concerns to normal tissues or organs for in vivo application, due to lack of target selectivity of the powerful cell penetrating activity. To overcome this problem of potent yet non-selective penetration vs. targeting delivery, a number of “smart” strategies have been developed in recent years, including controllable CPP-based drug delivery systems based on various stimuli-responsive mechanisms. This review article provides a fundamental understanding of these smart systems, as well as a discussion of their real-time in vivo applicability.
Keywords: cell-penetrating peptide (CPP), drug delivery system, prodrug, targeted delivery, in vivo
1. Introduction
There is a philosophical story in ancient China about the Impenetrable Shield and All-piercing Spear. The man, wielding a claimed best spear and shield for sale, is touting that his spear is so sharp as to pierce any shield, while his praise for the shield is being strong enough against any weapon. A looker-on then suggests: “What would happen if the spear were used against the shield?”
Playing defense-and-offense together is a universal paradox not merely limited in war. In drug delivery field, scientists always struggle with the dilemma between the potency of drug penetration and shield of unwanted drug exposure. Cell-penetrating peptides (CPP), a.k.a. protein transduction domains (PTD), are arguably the most powerful “spear” to break through bio-barriers for mediating macromolecular drug delivery. The discovery of CPP was dated back to 1988 by two independent groups, who identified the Tat cell-penetrating sequence almost simultaneously [1, 2]. However, CPP has not started its magic journey in drug delivery until 1994, when Tat was reported to be capable of delivering its chemically linked macromolecular cargos into cells of all tissue/organ types [3]. Later, the findings from Dowdy’s group in 1999 set a milestone for CPP-based drug delivery, in which the recombinant Tat-β-galactosidase fusion protein was demonstrated to be able to diffuse through the formidable blood-brain barrier (BBB) following intraperitoneal injection [4]. The cell-penetrating ability of CPP was so overwhelming that CPP has become a new paradigm in the recent decade to overcome various bio-barriers including retina and neurons [5, 6], blood brain barrier [4, 7], intestine wall [8, 9], and skin [10-12]; all of which indeed have created a serious impediment for conventional delivery of macromolecular drugs. The CPP-based drug delivery strategies have recently been called as the “Trojan horse” approach [13]. Nevertheless, the nonselective in vivo penetration of CPP has caused growing pharmacological concerns. In fact, a thorny issue has been brought up for CPP-based therapy, i.e. Is the Trojan horse too wild to go only to Troy [14]? Hence, it has become a pressing need to shield the normal tissue or cells against the CPP-mediated penetration so as to prevent severe side effects, whereas aiming its desired function at the pathologic sites. In this review, we will focus on the discussion of various strategies established to shun the disadvantages of the CPP-mediated delivery and resolve the dilemma of potent yet non-selective penetration vs. targeting delivery. We hope to advance the fundamental understanding of real-time in vivo applicability of the remarkable “Trojan horse” delivery approach.
2. Approaches for CPP-mediated drug delivery
CPP-based drug delivery offers great potential for improving intracellular delivery of drugs with poor permeability; e.g. therapeutic proteins that are precluded from crossing cell membranes on account of their large size and high hydrophilicity. CPPs are capable of penetrating into a wide variety of mammalian cells independent of tissue or organism types [15], insect cells [16], and even plant cells [17, 18]. Moreover, some certain CPPs can facilitate targeted delivery to subcellular structure such as cell nucleus [19-22]. Cargos that have been successfully delivered by CPPs include small molecules, peptides, proteins, nucleic acids, quantum dots, polysaccharides, liposomes and nanoparticles [23-30]. Interestingly, the size of the payload is not an essential limiting factor for the CPPs-mediated cell delivery [14], although very large cargos may seem to be less effective in cell transduction [31]. Furthermore, most CPPs are relatively non-toxic [32], when compared with other polymeric type of transduction agents. The mechanisms of CPP-mediated cell transduction have not yet been fully elucidated. Four different approaches can be used to prepare CPP-assisted delivery systems, including: (1) genetic fusion of the protein drug with a CPP, (2) covalent linkage of the drug with a CPP, (3) formation of ionic complex between the cationic CPP and an anionic drug such as DNA or siRNAs, and (4) modification of drug-loaded nanoparticles with CPP.
2.1 Recombinant fusion protein
To construct a CPP-protein delivery system with recombinant methods, CPP is integrated into a functional protein as a transduction-enhancing motif. With CPP being selectively fused to a designated site, it offers distinct benefits in mass manufacturing, quality control, and homogenous products. Dowdy’s group first reported the synthesis of an in-frame TAT bacterial expression vector that could produce the cell-permeable recombinant protein chimeras [33, 34]. Since then, application of bacterial expression vectors has become a standard practice to fuse a CPP sequence into a therapeutic protein, and a general protocol has already been successfully established [35], providing great convenience for producing CPP-protein drugs. However, these recombinant methods are incompatible with non-protein molecules such as nucleic acids or polymers.
2.2 Covalent chemical linkage
Covalent chemical conjugation is a versatile means to link CPP with virtually all kinds of cargos. There are numerous methods to conjugate CPP with the cargos via either a stable or cleavable manner, such as thiol-maleimide, amide bond, disulfide and thioester linkages. Under many circumstance a cleavable bond is selected to ensure the release of the cargos so that they can fully execute their biological functions. For instance, disulfide bond is used to yield a reversible conjugation, which can subsequently be broken down under a cytosolic environment where abundant reducing agents such as glutathione or reductase are present
Yet, the major drawback of the covalent methods is the heterogeneous structure of the final products which, consequently, impose difficulties in quality control or characterization. Site-specific conjugation methods have been explored to overcome these problems, yet with very limited success thus far. In addition, these chemical conjugation methods require intensive labor and customized skills for individuals involved in such works.
Due to strong electrostatic interaction between CPP and nucleic acid drugs, it still remains a formidable challenge to create monomeric conjugates [36]. Therefore, to achieve success in the conjugation, it is essential to overcome the strong electrostatic interaction that tends to cause the formation of tightly associated aggregates instead of monomeric conjugates [37]. Of notice, a similar problem exists in preparing the CPP-insulin chemical conjugates, because of the anionic nature of insulin [38].
2.3 Non-covalent method
Owing to the lingering difficulties in preparing the chemical CPP-gene conjugates, non-covalent complexes formed via the electrostatic interaction are often employed for CPP-mediated gene delivery. The complexes thus prepared normally carry net positive charges by adding excessive amount of CPP to secure their binding with the negative proteoglycans on the cell surface and their subsequent cellular uptake. Aside from cell-internalization, another important function of CPP is to facilitate nuclear entry, which is essential for gene expression [39]. However, due to the small size of CPPs, their ability to bind and condense gene drugs is relatively poor, compared with that of the high molecular weight cationic polymers. Hence, the CPP/gene complexes usually exhibit a less compact structure that is less favorable for gene transduction.
CPPs were also effective in delivering quantum dots [40] and proteins into mammalian [41, 42] and plant cells [18, 43] via the non-covalent conjugation. This is probably due to the permeation enhancer-like functions of CPP, which leads to membrane destabilization and, consequently, enhanced permeability, but detailed information is unknown for the mechanisms. Nevertheless, intracellular drug delivery via this method is somewhat unpredictable and more like a case-by-case situation, pending on the nature of the cargos as well as the sequence of the CPP. It should be noted that synthetic amphipathic peptides, another class of CPPs that usually consist of both a hydrophobic and a hydrophilic domain, also displayed the ability to mediate intercellular protein delivery via the non-covalent conjugation method [44].
2.4 Surface modification of nanoparticle-based drug carriers
Incorporation of CPP would not only facilitate cellular uptake of the modified nanoparticle carrier, but also significantly enhance the efficiency of CPP in delivering a larger amount of the loaded drugs per se; in contrast to the CPP-drug conjugate that requires at least one CPP entity for the delivery of one single drug molecule. Additionally, nanoparticles can accommodate drugs of vastly different physiochemical properties, and many of those cannot be directly conjugated with the CPP. This approach offers the benefit of delivering a broad spectrum of different drugs. The CPP-modified nanoparticle systems include lipid- (e.g. liposomes) or polymer-based nanocarriers (e.g. micelles), as well as inorganic carriers such as silver-, iron-based nanoparticles, and quantum dots. We previously reported that CPPs could be successfully immobilized on these nanoparticle surface via either adsorption (typically via charge interaction) [10, 45], or chemical conjugation [46]. Both methods produced satisfactory outcomes of enhanced intracellular uptake.
In principle, nucleic acid drugs are not necessary to be encapsulated into the CPP-modified nanoparticles for their delivery. A commonly adopted method is to absorb the nucleic acid drugs onto the cationic surface of the CPP-modified nanoparticles via electrostatic interaction. Nearly 100-fold enhancement in gene expression was observed by using this method, compared to that achieved by the conventional CPP/gene complexation approach [47].
2.5 Pros and cons of CPP-mediated drug delivery
Since initial cellular studies were carried out under the stringently controlled in vitro conditions, the negative impact caused by non-selective uptake by other tissues was hardly noticed. Indeed, concerns of no specificity of this approach were largely muffled among the triumphant cheers after observing the unprecedented success in obtaining significant intracellular delivery. Along with the remarkable growth of the in vivo investigation in recent years, there is increased awareness of the side effects caused by the non-selective cell-penetration effects. For example, the non-specific and uncontrollable distribution of the CPP-linked cargos becomes a potential life threat in application of cytotoxins as therapeutic agents. Upon systemic administration of CPP, the body turns into the Wild West, and the “Trojan horse” is thus brought up. Therefore, the need for harnessing its natural power is pressing. The ultimate goal in further pursuit is to efficiently implement cellular transduction and improve treatment outcomes of the CPP-based therapies, while to restrict the undesired action on normal tissues and cells to the minimum.
3. How to Curb the “Trojan Horse”
An ideal drug delivery system should act specifically at the desired targets while bypassing the normal tissues. The utmost objective is to maximize the drug concentration at the target sites while minimizing drug exposure to other organs. The “Trojan horse” helps deliver cargos through the otherwise impermeable bio-barriers, but with an indiscriminate nature in internalizing all kinds of tissues/cells. Therefore, the “Trojan horse” must be somewhat curbed for achieving both therapeutic efficacy and safety.
Prodrug strategies can improve the usability of the parent drugs which are inherited with poor solubility, chemical instability, inadequate absorption and tissue penetration, and the absence of drug targeting functions [48]. Previously, we presented an innovative prodrug concept for controlled delivery of CPP-linked macromolecules by masking the CPP activity during administration and then restoring its ability at the target tissues [49]. Through this strategy, the normal tissues are spared due to the poor cellular uptake of the CPP-masked drugs, but specific action on target tissues occurs when CPP is unmasked. Overall, the principle of such a prodrug system is based on a stimuli-responsive mechanism. In brief, a CPP-based delivery system is designed to be responsive to a certain microenvironment around the target organs or cells (e.g. the existence of a lowered pH or overexpressed proteases), or to an exogenous triggering agent that can be delivered to the target sites. By doing this, the CPP-based systems could execute the therapeutic or diagnostic actions exclusively on the desired regions.
A summary of various smart strategies is presented herein to provide an overview of how to achieve effective yet safe CPP-mediated drug delivery.
3.1. Prodrug strategy based on electrostatic interaction
3.1.1 ATTEMPTS for targeted delivery
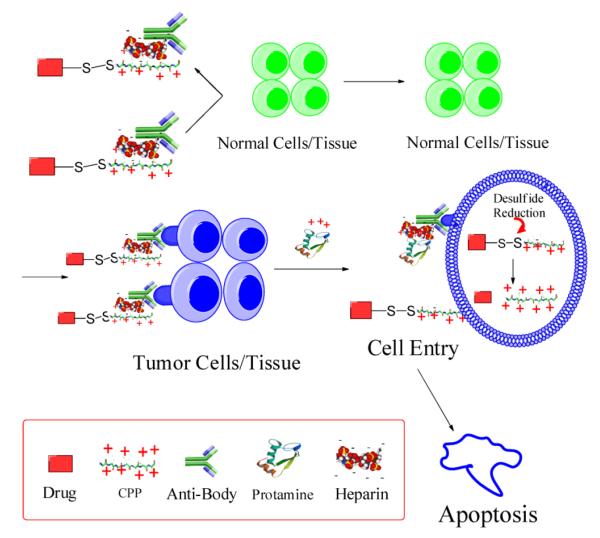
We developed a system for protein delivery termed “ATTEMPTS” (antibody targeted, [protamine] triggered, electrically modified prodrug-type strategy) [49, 50]. In brief, the mechanism responsible for the prodrug-type function is based on the charge neutralization of CPP by heparin and the competitive binding between CPP and protamine with heparin. The system consists of: (a) the drug compartment that contains a conjugate of the polycationic CPP and the protein drug, and (b) the targeting compartment that is made of a heparin-modified antibody (Fig. 1). The polyanionic heparin motif on the antibody performs strong electrostatic binding with the polycationic CPP, resulting in the formation of a polyelectrolyte Drug-CPP Hep-Ab prodrug-style complex possessing active targeting function due to the antibody. The selected macromolecular drug could be a cell-impermeable protein toxin, and its conjugation with a CPP endows the protein drug with the cell-penetration ability. Most critically, the formation of an electrostatic linkage between CPP on the drug and heparin on the antibody resolves the selectivity issue, because the CPP’s cell-internalization function is inhibited due to the binding of heparin.
Figure 1.
Schematic diagram of the ATTEMPTS delivery system. Redraw based on [50].
The naturally sourced low molecular weight protamine (LMWP), identified by our laboratory by proteolytic digestion of native protamine, is an ideal CPP for this ATTEMPTS system. The binding affinity of LMWP to heparin is strong, and disassociation does not take place unless it is treated with a high slat solution (0.94 M NaCl). Such heparin-binding strength of LMWP sits ideally within the range between that of antithrombin III (eluted from a heparin column at 0.6 M NaCl) and of protamine (eluted at 1.3 M NaCl) [51]. Therefore, the Drug-CPP Hep-Ab complexes would remain stable during targeting, ensuring no detachment of the two compartments caused by electrostatic competition by other endogenous components (e.g. antithrombin III) in the circulation. As a result, the CPP is steadily blocked, rendering the CPP-mediated nonspecific cell transduction completely sealed. On the other hand, release of the active CPP-drug conjugates is initiated by systemic injection of an appropriate dose of protamine, after a sufficient duration to allow site-specific accumulation of the CPP-Drug Ab-Hep complexes through antibody-mediated targeting or EPR-mediated passive targeting. Protamine is the natural antagonist used clinically for heparin reversal and is therefore a safe triggering agent. As shown in Fig. 1, owing to the stronger heparin-binding affinity by protamine over LMWP, the CPP-Drug conjugates would be released from the complexes due to an electrostatic substitution. Detailed information of this strategy can be found from a review article previously published by our laboratory [49].
3.1.2 Protease-activatable systems based on interaction of polyanionic peptide with CPP
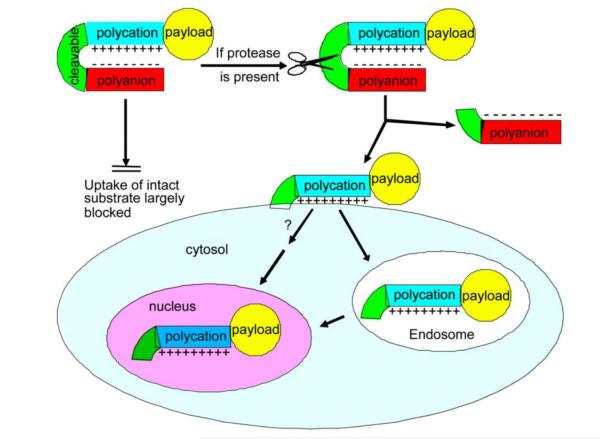
Other than the strong electrostatic interaction between CPP and heparin used in the ATTEMPTS that must be triggered by exogenous protamine, a relatively weak blocker (e.g. anionic peptide) of the CPP was also explored by Tsien’s group to create the prodrug feature [52]; as displayed in Fig. 2. However, binding of such a weak blocker with the CPP is not stable enough in vivo, and therefore insertion of a protease-substrate peptide linker between the blocker and CPP was attempted to create a fixed intramolecular hairpin structure. This peptide linkage was cleavable at the tumor target due to its specially designed substrate sequence responsive to the over-expressed proteases on the tumor. In systemic circulation, the hairpin structure remains intact and the CPP blocked, leaving the delivery system cell-impermeable and pharmacologically inactive. The hairpin would disassociate around the target tumor as a consequence of enzymatic cleavage of the peptide linker. The exposed CPP could then mediate its payload entering into the tumor cells.
Figure 2.
Schematic illustration of ACPPs. CPP is blocked by a polyanionic peptide (E9) via a hairpin structure hooked with an MMP2/9-cleavable linker. Cellular uptake of the payload is induced by the cleavage of the linker and subsequently the exposure of CPP. Reproduced with permission from [52].
Based on this strategy, Tsien’s group developed a system, named “activatable cell penetrating peptides” (ACPPs), for tumor imaging [52]. Matrix metalloproteinases 2 and 9 (MMP2/9) were specifically overexpressed in matrix of intercellular space in tumor, and played a key role in the migration of tumor cells and angiogenesis [53-55]. An MMP2/9 substrate peptide, XPLGLAG, was selected as the cleavable linker, and poly Glu (E9) was chosen as the inhibitory polyanionic moiety to interact with CPP. The CPP-bearing payload would not function until the linker was cleaved by MMP2/9. The hairpin structure based on a weak electrostatic interaction would then disassociate, leading to the restored functions of CPP and the specific delivery of payload into tumor cells. This ACPP system not only effectively reached tumor nodules, but also yielded the highest uptake at the tumor–stromal boundary. It provided sharp-contrast images that distinguished the high-uptake regions from those with low uptake; an pattern corresponded well to the MMP activity distribution [56].
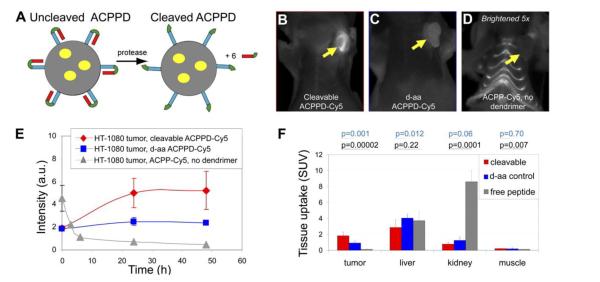
Tsien group further developed a modified system called “ACPP-conjugated dendrimers (ACPPD)” (Fig. 3) by adding a large-molecular-weight carrier (dendrimer, PAMAM) to the polyarginine CPP of the ACPP. Introduction of the carrier not only benefited tumor targeting via the EPR effect, but also provided amplifying signals because of the presence of more than one contrast agent per peptide [57]. The functional groups of macromolecules also offered great feasibility for synthesis of multimodality probes. In the ACPPD system, there were six ACPPs per nanoparticle, securing the activation and, consequently, increased cellular drug uptake. It was evident that the contrast between the tumor and adjacent tissues were significantly improved, due to an augmented accumulation of contrast agents inside the tumor and reduced uptake by adjacent tissues [56, 57].
Figure 3.
B> Cy5-labeled ACPPD for tumor imaging. (A) The structure of ACPPD. Each ACPPD consists of a dendrimer (gray circle) covalently linked with ACPPs segments (blue) (typically, about six CPP are linked to each dendrimer molecule). Tumor proteases such as MMP-2/9 cleaved linkers (green), leading to detachment of the polyanions (red) and exposure of the CPPs. These yellow ovals represent the payloads (contrast agents). (B–D) Fluorescence images in tumor at 48 h post-dose. (E) Time courses of tumor fluorescence. (F) Standardized tissue uptake values in samples of tumor, liver, kidney, and muscle, at 48 h post-dose. Reproduced with permission from [57].
Similar to the activation mechanism adopted by Tsien’s group, a protease cleavable linker (an MMP2/7 substrate peptide, PLGVR) was employed to conjugate the CPP with the inhibitory polyanionic domain (E9) for specific delivery of quantum dots (QD) triggered by MMP enzymes (Fig. 4) for tumor imaging purpose [58]. Furthermore, the distal end of E9 was also modified with PEG to aid passive tumor targeting via the EPR effect.
Figure 4.
Schematic illustration of MMP-activated quantum dots (QD). QD is coated with 5–10 molecules of streptavidin, producing stoichiometric conjugation with biotinylated CPP (transporter) based on the strong biotin-streptavidin interaction. The inhibitory polyanionic domain (E9) with the PEG chain (blocker) is linked to the E9 via a MMP 2/7 substrate peptide linker. Reproduced with permission from [58].
Tumor-associated proteases are characteristically overexpressed at the invasive front of solid tumors or at the sites of angiogenesis where they can be easily accessed via passive targeting using the EPR effect. Hence, proteases can be an ideal target for diagnosis and treatment of cancers [59]. However, soluble proteases, such as MMPs, are prone to leakage from the tumor tissues into the blood stream [52], and the activation of the prodrug-type systems might already occur before reaching the tumor. On one hand, the released proteases may favor activation of the prodrug molecules in the vasculature surrounding the tumor, and enhance the uptake of the activated CPP-based drugs across the tumor endothelium via transcytosis [60]. On the other hand, those activated drug molecules could also be brought into systemic circulation, resulting in unwanted drug distribution in normal tissues [61], or enhanced background signal that can reduce the contrast intensity in tumor diagnosis and imaging [52]. Hence, the tumor proteases and their substrate sequences need to be carefully selected to improve the targeting specificity.
3.1.3 Masking CPP with pH-sensitive inhibitory polyanionic group
In this strategy, the selected inhibitory polyanionic moiety for binding the CPP was poly sulfonaminde (PSD), characterized by its pH-dependent charge—negative at pH 7.4 and neutral below pH 7 (Fig. 5) [62] [63]. The extracellular environment of a tumor is acidic [64], owing to an increased lactic acidosis from the highly active aerobic glycolysis (i.e. Warburg effect). Hence, PSD could bind with CPP via charge interaction and block its cell-penetrating activity during systemic circulation and bypass its action on normal tissues. Once reaching the acidic tumor milieu, PSD would become neutral in charge and thus detach itself from the cationic CPP, reactivating the CPP-mediated cell penetration process [63].
Figure 5.
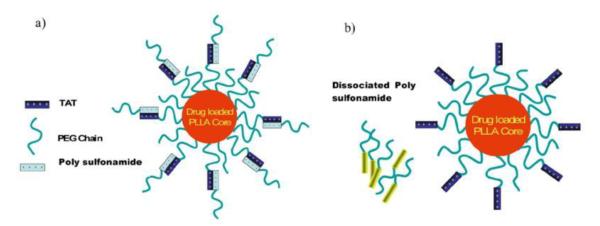
The pH-responsive blocking and de-blocking of CPP with polysulfonamide (PSD). The negatively charged PSD-b-PEG masks the polycationic TAT on the surface of the micelles by electrostatic interaction at normal pH. The extended PEG chains inhibit CPP-mediated cell internalization of the micelles, and also enhance tumor passive targeting via their produced long-circulation effect. PSD gradually loses its positive charges when approaching the tumor in response to the decreased pH, and its subsequent detachment at the tumor matrix releases its shielding effect on TAT. Reproduced with permission from [63].
3.1.4 Photolabile-caged CPP strategy
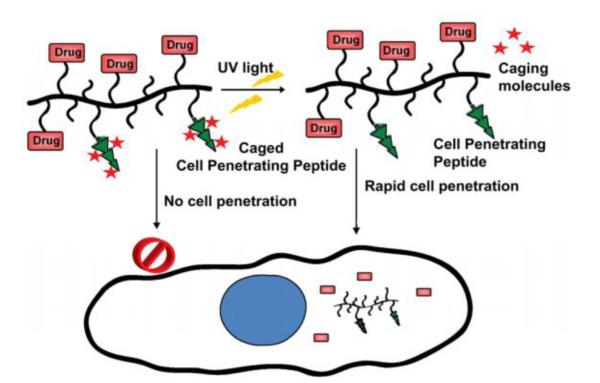
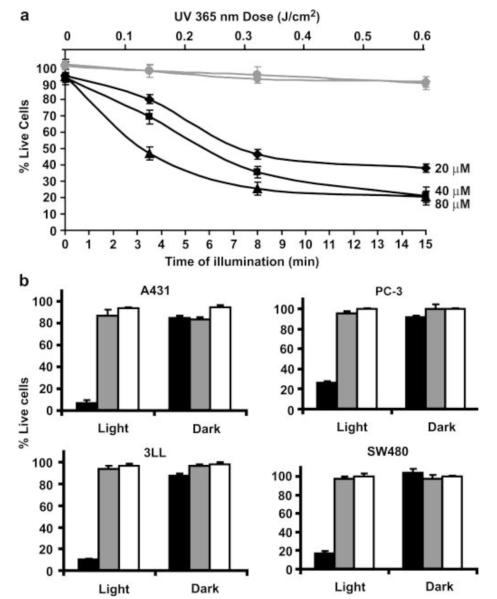
External physical triggers (e.g. ultrasound, magnetic field, and light) have been extensively investigated for stimuli-responsive delivery systems. The unique advantage is that drug release is highly controllable, owing to its prompt response to the on/off setting of the external triggering. An interesting idea was presented by Shamay and co-workers, in which CPP was modified with photolabile caged molecules, thereby providing light-dependent cell internalization [65]. As shown in Fig. 6, the positive charges of lysine residues on the CPP (52-RRMKWKK-58) were temporarily masked by photo-cleavable groups, Nvoc(6-nitroveratrylcarbonyl), thus forming the caged CPP (cCPP, Ac-KRRMKNvocWKNvocKNvoc). Once being illuminated with the UV light, the protecting groups (Nvoc) were cleaved, and the cationic Lys residual side chains were exposed. The CPP then regained sufficient positive charges and subsequently restored its cell transduction activity. It was found that 10-min light illumination was adequate to remove the caged molecules, inducing up to 80% cellular uptake of the pro-apoptotic peptide-loaded polymer-CPP conjugates. A high cytotoxic activity (i.e. causing 90% of cell death) was observed after 2 h of incubation (Fig. 7).
Figure 6.
Schematic illustration of light-induced tumor penetration of the polymer-cCPP conjugates. Under the dark, the cCPP is inactive due to the caged effect of Nvoc that masks the CPP. Once being illuminated with UV light, the activated cCPP then induces rapid intracellular transduction. Reproduced with permission from [65].
Figure 7.
Cytotoxicity due to light-induced delivery of P-(cCPP)-KLAK into cancer cells. (a) Viability of the PC-3 cells as a function of the illumination time and dose. (b) Light-induced cytotoxicity by P-(cCPP)-KLAK against various cancer cell lines. Reproduced with permission from [65].
In fact, the cCPP still contains positive charges on its two arginine residues. However, for achieving effective delivery, the amount of positive charged residues of the polyK- or polyR-rich peptides is required to no less than four [58]. Therefore, the cCPP could not take effect unless removal of its caging molecules.
A drawback of using this light-uncaged strategy lies in the poor tissue penetration of the short-wavelength UV light, limiting its application if the target site sits deeply inside the organs or body. Another obstacle is the risk of causing non-specific phototoxcity; on the contrary, this setback could be turned to advantage, because extensive UV light exposure could perform complete eradication of the infected tissues, including cancer and supporting endothelial or stromal cells [65].
3.2 Steric hindrance to block cell penetration functionality
PEG modification is the most effective method in creating long-circulation effect through steric hindrance. It can physically prevent the drug from degradation by circulating proteases or recognition by the host immune system [64]. PEGylation can prolong the circulation time of liposomes and other nanoparticles, augmenting their preferential accumulation in tumor via the EPR effect [66-68]. Nevertheless, PEG chains can also severely hinder tumor uptake [69, 70]. In addition, the hydrophilic PEG layer can impede diffusion of the drug molecules out of the carrier even at relatively low polymer concentrations [71, 72]. Yet, from a positive standpoint, this may also reduce the unwanted drug exposure to normal cells, and thereby alleviate drug-induced side effects. Hence, it is a potential solution to the nonselectivity issue of CPP-mediated cell entry by blocking CPP via the steric effect of PEG. Indeed, this strategy has been widely explored in recent years.
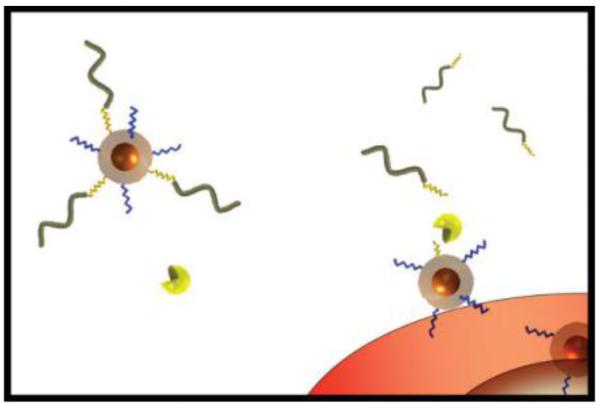
A general design is by following the “long and short chains” model, of which a long-chain PEG polymer is employed to create steric coating over the short-chain CPP. The PEG molecules, via circumstance-responsive bond, are linked to a CPP-modified carrier (e.g., liposomes and nanoparticles), thus shielding the CPP in non-targeted organs. PEG would be removed after the bond cleavage in the specific circumstance. The functionality of CPPs reinstalls as they fully expose on the surface of the carriers.
3.2.1 The pH-triggering dePEGylation
Acidic extracellular pH (pHe) ranges from 7.0 to 6.5 in animal tumor xenografts [73, 74], spontaneous tumors [75], and human tumors [76]. Hence, acidic pH-sensitive bonds have been widely used in drug delivery. Torchilin’s group developed a pH-sensitive, PEGylated TAT-liposome system (TATp-liposomes) for DNA delivery [77]. TAT was anchored on the liposome surface via a short-chain PEG, while long-chain PEG was linked to liposome via pH-sensitive hydrazone bond to create a steric layer over the TAT molecules. DNA plasmid encoding for the green fluorescent protein (pGFP) was selected as a model drug and loaded into the liposomes. The pGFP-loaded TATp-liposomes were then administered intra-tumorally into mice, and the efficiency of tumor cell transduction was examined after 72 h by monitoring GFP expression. The pGFP-TATp-liposomes with the low pH-detachable PEG yielded significantly enhanced transfection in tumor cells. In contrast, the non-cleavable PEGylated TAT-liposomes displayed a minimal pGFP transfection due to the steric hindrance of PEG.
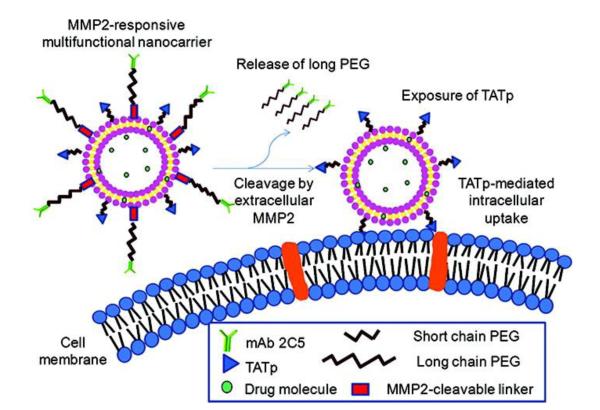
Torchilin’s group also developed a more advanced system—a double-targeted, long-circulating PEGylated liposomal system via a mechanism involving: (1) a specific myosin antibody (2G4) was positioned onto the surface via a cleavable long spacer of PEG to achieve receptor-mediated active targeting; (2) the pH-detachable PEG shielded TAT-liposomes to prevent nonselective cell penetration and prolong systemic circulation; (3) tumor acidic microenvironment triggered the removal of PEG and exposure of TAT (Fig. 8) [78]. At normal pH, TAT molecules were shielded by the PEG and the attached antibody. This liposomal system presented a high tumor binding specificity due to the 2G4 antibody ligand (myosin) but very limited cellular uptake. Brief exposure (15–30 min) to acidic condition (pH 5.0–6.0) led to the removal of PEG protection, and CPP molecules were exposed, and activated cellular uptake of the liposomes. This approach shed light of the possibility of synergizing the antibody-mediated tumor targeting with TAT-mediated cellular transduction.
Figure 8.
Schematic illustration of the multifunctional stimuli-responsive nano-carrier system for achieving selective and enhanced uptake by target cells. Specific internalization into the target cells was observed, due to the subsequent low-pH-responding removal of PEG and mAb-PEG moieties. Reproduced with permission from [78].
More recently, a similar system (pH-sensitive PEGylated long-circulating liposomes modified with TAT and nucleosome-specific antibody (mAb 2C5)) was reported by the same group; but a modification was that hydrazone pH-sensitive bond was only applied to conjugation of PEG coating while the mAb 2C5 attached to the liposomes in a noncleavable manner [79]. The mAb 2C5 could not only mediated active targeting to tumor, but also enhance the interaction with the tumor cells, displaying synergistic effect with the deshielded TAT.
The above-mentioned strategy involves a “shielding and exposure” process: the CPP is shielded by PEG, and then becomes exposed and activated following detachment of PEG. There is a different strategy based on the “pop-up” mechanism [80]. This “pop-up” system was prepared via a self-assembly process involving two block copolymers: PLA3kD-b-PEG2kD-b-poly His2kD-TAT and polyHis5kD-b-PEG3.4kD (Fig. 9). The hydrophobic parts (PLA3kD and polyHis5kD blocks) of these two polymers formed the core of the nanoparticle, surrounded by a hydrophilic corona of the PEG2kD and PEG3.4kD blocks. The polyHis2kD block was non-ionized at neutral pH, thus enabling the hydrophobic binding of the conjugated CPP molecules closely onto the surface of these nanoparticles. The CPP was thus masked by the long-chain PEG3.4kD blocks which were positioned outside the surface. Since the ionization process was pH-dependent, the shell became positively charged at acidic pH. Consequently, the electrostatic repulsion force overcame the hydrophobic interaction, and caused the polyHis2kD-TAT conjugates to pop out of the surface. This feature enables pH-triggered TAT de-shielding in tumor, and specific uptake by targeted tumor cells. Of note, the long polyHis5kD blocks became ionized in the endosome due to the lower pH, and their acquired positive charges then facilitated endosome escape and the release of drugs into the cytoplasm. At pH 7.4, poor uptake of the micelles was observed in MCF-7 cells. The uptake was increased by 30-fold at pH 7.0 and further up to 70-fold at pH 6.8, due to Cli to ck on zoom image the of pop-up TAT molecules on the surface of these micelles.
Figure 9.
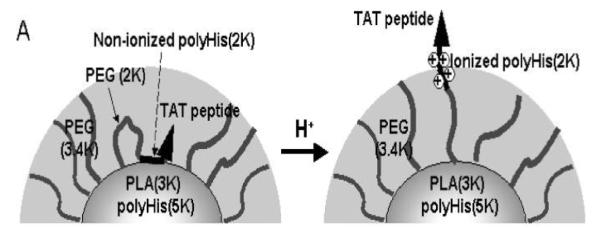
Schematic illustration of the low pH-induced “pop-up” targeting mechanism of the TAT-conjugated micelles. Reproduced with permission from [80].
3.2.2 Exogenous cysteine-triggered dePEGylation
He’s group prepared the liposomes, featured by the thiolytic cleavable disulfide bridge linker between PEG and DOPE (DOPE-S-S-mPEG5000) (Fig. 10) [81]. TAT linked with a short-chain PEG (DOPE-PEG1600-TAT) was also immobilized on the surface of these liposomes, but was shielded by the long-chain PEG5000 blocks. Due to the EPR effect, the PEGylated liposomes would preferentially accumulate in tumor. The disulfide bond of DOPE-S-S-mPEG5000 was cleaved by giving an exogenous cleaving reagent of L-cysteine (L-Cys). The detachment of PEG5000 would expose TAT molecules. Results showed that cellular uptake was inhibited by the PEG steric effect, but with addition of exogenous L-Cys, the uptake was increased by 3 fold, implying the success of cysteine-triggered dePEGylation. The liposomes were injected intratumorally into H22 tumor bearing mice, followed by injection of L-Cys. The uptake in tumor of the cleavable PEGylated liposomes was significantly higher than that of the TAT-liposomes modified by the non-cleavable PEG, indicating that this system could selectively deliver payload to tumor in a controllable manners.
Figure 10.
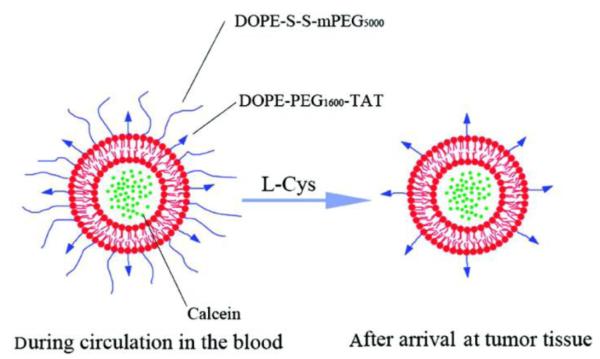
Schematic illustration of the cleavable-PEG and TAT co-modified liposomes. The liposomes are calcein-loaded and rhodamine B-phospholipid (Rh-PE) labeled. Reproduced with permission from [81].
Cleavage of the disulfide bond could also occur gradually during systemic circulation, owing to its relatively instable behavior. However, this event was rather limited, because the slow rate of cleavage and minor loss of the shielding PEG would not significantly impair the steric effect. In addition, gradual reduction of the disulfide bonds could be offset by the increase in DOPE-S-S-mPEG5000 doses. According to the report, 8% of the cleavable PEG employed in the surface modification was already sufficient to produce an effective protection for in vivo application [81].
3.2.3 Enzymatic dePEGylation based on protease-sensitive linker
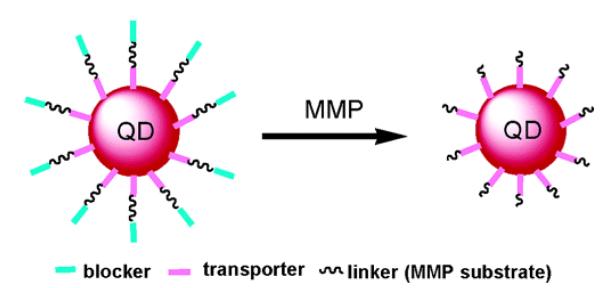
Expression of MMPs is up-regulated in a variety of tumors, and thus MMPs would be a better target than antigen molecules that are only over-expressed in a subset of cancers for broad-spectrum diagnostic applications. Moreover, MMPs are localized in tumor extracellular matrix and possess amplification effect via enzymatic catalysis, rendering MMPs an ideal target for detecting small, invasive and metastatic tumors that might otherwise undetectable via routine radiological examination [57].
Bhatia’s group applied an MMP-2 substrate peptide (GPLGVRGC) as the cleavable linker [59]. The magnetofluorescent dextran-coated iron oxide nanoparticles were modified with long-chain PEG via the MMP-2 cleavable spacer to mask CPP (Fig. 11). Linkage of the MMP2-responsive PEG was able to remain stable in serum, thus avoiding the nonspecific interaction. In vivo studies showed that the PEGylated nanoparticles were able to selectively accumulate at the xenograft tumor via EPR effect, and PEG was subsequently detached due to cleavage of the linker by endogenous MMP-2. As a consequence, CPP-mediated cell penetration of the iron oxide nanoparticles was activated, as demonstrated by both fluorescent and MRI imaging.
Figure 11.
The MMP2-dependent dePEGylation of a CPP-based imaging system. Removable PEG (wavy-gray) was immobilized on the surface of a magnetofluorescent nanoparticle via an MMP2-substrate peptide (jagged-yellow) to shield the activity of CPP (jagged-blue). DePEGylation occurred in the tumor microenvironment following MMP-2 cleavage, and the CPP were exposed. Reproduced with permission from [59].
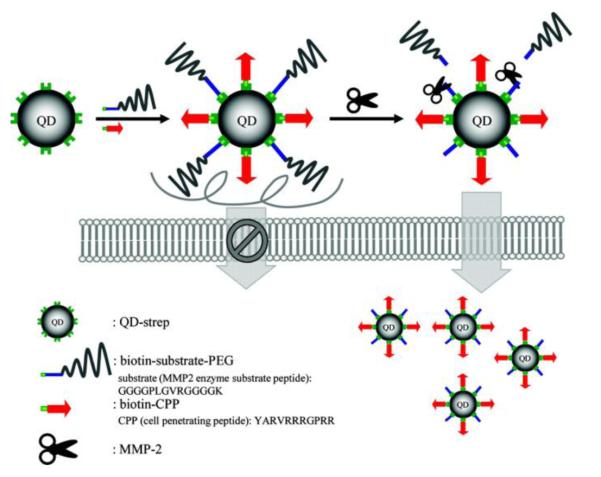
Similarly, Mok and co-workers also applied the enzyme-cleavage strategy for cancer diagnosis, by using the MMP2-senstive, PEG- and CPP-modified quantum dots (QD) [82]. As illustrated in Fig. 12, the streptavidin-modified QD (QD-strep) was linked with biotin-CPP and biotin-peptide-PEG conjugates via the specific biotin-streptavidin interaction; while the peptide linker was a substrate for the MMP-2 enzyme. The number of PEG chains on the surface was examined to evaluate its effect on shielding the CPP. Nine PEG chains on each 12-nm QD were at least required in order to inhibit approximately 80% of the cellular uptake. By treatment of exogenous MMP-2 (10 μg/ml), the cellular uptake was enhanced by 4 fold (from 21.9 to 79.1%).
Figure 12.
Schematic illustration of MMP2-specific dePEGylation and intracellular delivery of quantum dots (QDs). Reproduced with permission from [82].
As aforementioned (Section 3.1.2), the proteases leaked out into vasculature would result in premature trigger of the exposure of CPP and unwanted penetration. Recently, a multifunctional drug delivery strategy was proposed to enhance the targetability to the MMP2 overexpressed tumor, featured by the PEGylated immunoliposomes containing MMP2-cleavable bonds and TAT (Fig. 13) [83]. The PEG-induced passive targeting and antibody-mediated active targeting provided increased tumor accumulation of the liposomes, while the antibody-PEG motif would be cleaved in tumor due to the MMP2 substrate linker, and the TAT thus deshielded.
Figure 13.
MMP2-cleavable PEGylated immunoliposomes and the activation of TAT-mediated intracellular delivery in tumor. Reproduced with permission from [83].
Nevertheless, there is a common problem of the protease-triggered de-PEGylation strategy. On one hand, sufficient number of PEG chains is required to yield an effective steric layer for inhibiting CPP-mediated penetration and prolonging the circulation time. Yet, on the other hand, PEG can also act as a barrier against the access of tumor proteases and subsequently their-mediated cleavage of the substrate linkers. Therefore, the density of the PEG coating must be carefully optimized to reach a balanced act between steric protection and the accessibility to the linker for proteases, so that the removal of PEG and exposure of CPP can be achieved simultaneously.
If the CPP-based nanoparticle or macromolecular systems are activated in tumor blood vessel lumen, extravasation of these drug carriers from blood vessel lumen into the tumor would then become the rate-limiting step [59]. In this case, PEGylation can prolong the circulation time of these carrier cargos, and thereby augment their preferential accumulation in the tumor interstitial space via the EPR effect [84]. Moreover, releasable PEG chains can be used to provide a reversible masking effect on the CPP molecules, which could alleviate their unwanted side reactions with normal tissues but only reactivate the CPP’s function in tumor. To this regard, releasable-PEG modification can be adopted as a common strategy to establish a CPP-based smart drug delivery system. However, the immunogenicity of PEG is often ignored in most studies. Repeated injection of PEGylated liposomes has been reported to elicit PEG-specific antibodies [85], as well as to induce rapid elimination and hepatic uptake of the PEGylated liposomes [86, 87]. Such accelerated blood clearance could impose a barrier to obtaining the most effective pharmacokinetic (PK) and pharmacodynamic (PD) profiles [84]. Information is not sufficient yet to detail the structure-antigenecity relationship of PEG, and hence further investigation on this subject is greatly warranted. Although an alternative polymer, poly(hydroxyethyl- L-asparagine), has been proposed to solve this problem, more investigations are still needed to further confirm its effectiveness in vivo [88].
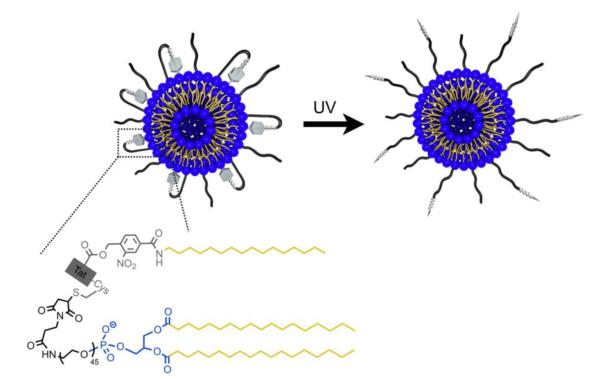
3.2.4 UV activation of PEGylated lipid-TAT-lipid looped liposomes
Hansen and coworkers developed a constrained and UV-activatable cell-penetrating liposomal system with a PEGylated lipid-TAT-lipid anchored loop structure (“n” shape) on its surface (Fig. 14) [89]. An alkyl anchor was conjugated to the N-terminus the TAT and a PEG-phospholipid at the C-terminus. The TAT was constrained in the loop anchored on the liposomes and overclouded by the PEG. Between TAT and its N-terminal alkyl chain was a UV-cleavable linker. Upon the target cells and with application of UV irradiation, the linker was cleaved and the loop opened, transforming into TAT-PEGylated liposomes with revived cell penetration ability.
Figure 14.
Schematic illustration of the UV-activatable looped TAT-liposomes. Reproduced with permission from [89].
3.3 Thermo-sensitive systems
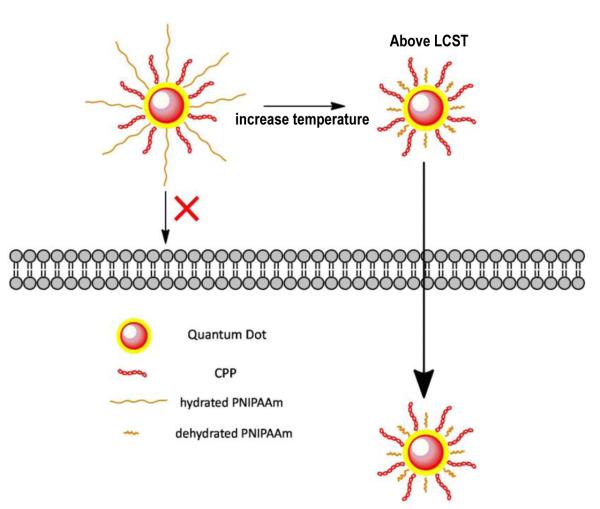
Other than application of the chemical stimuli, the approach of utilizing thermo-sensitive CPP-modified QDs was also examined [90]. As demonstrated in Fig. 15, biotinylated CPPs was immobilized on the streptavidin-modified QDs. In addition, the biotinylated, thermo-sensitive poly(N-iso-propylacrylamide) (PNIPAAm, Mw 11.5K) was co-immobilized to shield the action of CPP. The chain length of PNIPAAm can be regulated by adjusting the temperature based on its lower critical solution temperature (LCST). Below the LSCT, the PNIPAAm chains stretch out of the surface thus shielding the CPP, whereas above the LSCT, the polymer chains shrink thus exposing the CPP. This strategy that is responsive to physical stimulus facilitates “smart” drug delivery via a mechanism of temperature-controlled “shielding/deshielding” of CPP. Results showed that after incubation for 1 h at 37 °C (above LCST), 86% of cellular uptake was observed due to the deshielding of CPP, compared to a value of 52% at 25 °C. However, the basal level of uptake was relatively high, and according to the authors this phenomenon could be accounted for nonspecific endocytosis. The nonspecificity indicated that the steric layer created by the PNIPAAm chains was less effective than PEG. Moreover, further modification of the polymer structure is required in order to achieve the suitable LCST for in vivo application.
Figure 15.
Schematic illustration of the thermally triggered cellular uptake of QDs. Above the LCST, the shielded CPP molecules were exposed on the surface of the micelles due to dehydration and collapse of the PNIPAAm chains, and CPP became active thereby resulting in enhanced cell penetration of QDs.
3.4 Targeting fusion protein (post-control pattern)
As noted, CPP-mediated uptake is nonselective and independent upon cell types. However, the intracellular microenvironments (e.g. proteases) vary between normal and abnormal cells, and thus this differentiation could be used as a targeting strategy. A post-control type of delivery systems was therefore established, in which the CPP-based fusion protein would take action only on certain cell types, despite its nonselective nature in cell transduction. Described below are two examples of these intracellular post-control strategies:
3.4.1 Selective intracellular activation strategy in targeted cells
Dowdy’s group developed a selective intracellular-activation strategy based on a prodrug-type fusion protein chimera [91]. The apoptosis-promoting caspase-3 protein was fused with TAT peptide. The TAT-Casp3 chimera, despite being able to indiscriminately transduce into all of the exposed cells, would remain inactive in normal cells, similar to the behavior of a prodrug. Nevertheless, the TAT-Casp3 chimera can be cleaved by the HIV protease, release the activated Casp3, and trigger apoptosis of solely the HIV-infected cell in a selective manner (Fig. 16).
Figure 16.
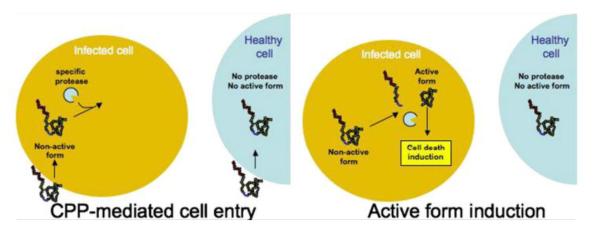
Schematic illustration of the targeting mechanism of the prodrug-type TAT–Casp3. TAT–Casp3 was specifically activated by the HIV protease that was present in infected cells and, consequently, resulted in cell apoptosis. On the contrary, TAT–Casp3 remained as the inactive zymogen in healthy cells. Reproduced with permission from [92].
The Casp3 zymogen (upper panel in Fig. 17) contains: (1) a N-terminal Pro domain with a caspase cleavage recognition site, (2) the p17 domain with catalytic Cys residue followed by a second caspase cleavage site, and (3) the p12 domain. The zymogen is pharmacologically inactive. However, once being exposed to the intracellular upstream caspases (e.g., caspase-8), the Casp3 zymogen would lose the Pro domain, transform into the active p17:p12 hetero-tetramer, and induce selective cell apoptosis. For construction of a cell-permeable Casp3 zymogen, TAT was strategically selected to substitute the Pro domain using recombinant method. Furthermore, the two caspase cleavage sequences were also replaced by the HIV proteolytic cleavage sequences p7-p1(D) and p17-p24(A) for HIV therapy. The TAT-Casp3 protein thus prepared displayed the ability in transducing both HIV-infected and uninfected cells. However, due to substitution of the HIV proteolytic sites, the prodrug-type TAT-Casp3 was only activated by HIV protease inside the infected cells, resulting in selective apoptosis. In contrast, TAT-Casp3 would remain inactive in the normal, uninfected cells, therefore avoiding side effects caused by the nonspecific transduction.
Figure 17.
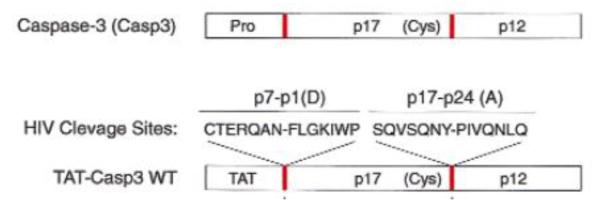
Structure of TAT-Casp3 WT (nether) and Casp3 (zymogen, upper). Comparing with the Casp3 zymogen, the Pro domain was substituted with TAT, and the endogenous two cleavage sites (red bars, upper) were substituted with the HIV proteolytic cleavage sites, p7-p1(D) and p17-p24(A) (red bars, nether). Reproduced with permission from [91].
3.4.2 Selective degradation in non-target cells
A different tumor-specific, CPP-mediated therapeutic strategy was developed by Kizaka’s and Kondoh’s groups based on removal of the off-targeting drugs via their selective degradation in normal tissues [93-95]. An oxygen-dependent degradation domain (ODD) originating from HIF-1α was used as the linker to conjugate procaspase-3 (Casp3 zymogen) with TAT (termed TOP3) for targeting hypoxia cancer cells. The ODD would be selectively degraded via the ubiquitin-proteasome pathway inside the normal cells under normal oxygen concentrations, while being stable in the hypoxic tumor cells (Fig. 18). Therefore, although the TOP3 was characterized with a nonspecific but enhanced intracellular transduction due to the presence of TAT, the ODD-mediated degradation of TOP3 would occur once entering the oxygenated normal cells. On the contrary, TOP3 in the hypoxic cancer cells would remain stable, where the procaspase-3 moiety was activated into an active caspase-3. Consequently, the caspases-3 activates apoptotic pathways in the tumor cells, even in those apoptosis-resistant cells [95, 96], thereby achieving targeted cancer therapy. Such a strategy was able to improve the survival time of mice harboring invasive and metastatic pancreatic cancer cells [97]. Interestingly, these researchers also applied this method to in vivo imaging by using near-infrared fluorescence-labeled recombinant protein containing both CPP and ODD domains [98].
Figure 18.
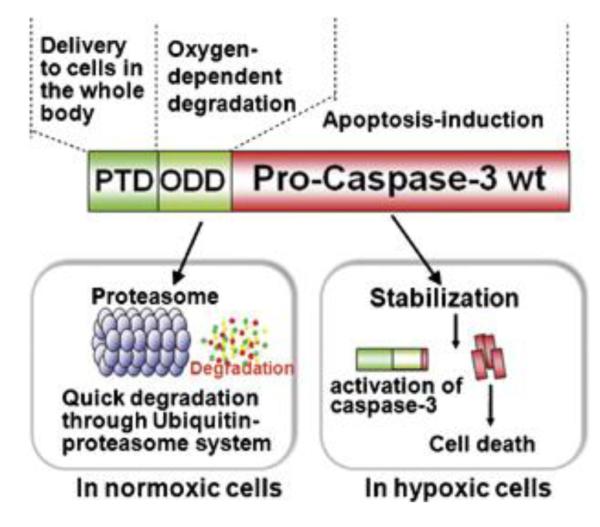
Structure of TOP3 and its HIF-activated process. TOP3 consists of three domains: TAT protein transduction domain, the ODD of HIF-1, and procaspase-3 domain. The stability of TOP3 was regulated by the ODD. Only in hypoxic cells, the TOP3 would remain stable. The procaspase-3 domain was activated under hypoxic conditions and consequently induced cell apoptosis. Reproduced with permission from [95].
A similar system was also reported by Yu and co-workers, of which a recombinant cell-permeable p53 fusion protein containing ODD was shown to remain stable in hypoxia cells and inhibited tumor cell growth [99].
It should be noted that due to the rapid but nonspecific cell transduction mediated by CPP, the drug molecules would preferentially accumulate in the early-reaching regions, which often were not the targeted destination. This distribution pattern raises the concern that a high and uneconomical dose is required in order to reach an effective drug concentration at the targeted tissues. Hence, preferential drug accumulation at the tumor target is needed in resolving this problem and improving the treatment efficacy. Potential solutions include encapsulation of the drug into a nanoparticle, or modification of the drug or the carrier with an antibody or targeting ligand.
4. Perspectives on different “curb” schemes
A drug delivery system must overcome multiple bio-barriers before it can reach the final destination. For instance, there are at least three obstacles for tumor targeted drug delivery: (1) selective drug accumulation at the tumor site after systemic administration, (2) diffusion of the drug into tumor tissues, and (3) penetration through tumor cell membrane [100]. Although CPP can assist the attached cargo to penetrate through vascular endothelium and cell membrane, it is usually accompanied with toxic side effects, due to the lack of tissue-selectivity of the CPP. Therefore, a number of strategies have been attempted to regulate the penetration function of CPP, so that both the in vivo efficacy and selectivity in cell-transduction can be concomitantly obtained. Hence, the selection of a proper “curb” strategy is imperative to achieve a clinically useful CPP-mediated drug delivery.
A general “curb” design is based on “shielding and deshielding” schemes for resolving the dilemma of potent and comprehensive penetration vs. targeting delivery. Although use of CPP-protein conjugates or fusion proteins is a simple means, a marriage between CPP methodology and nanotechnology should represent great promise in achieving clinical breakthrough. Application of appropriate nanocarriers can provide significant benefits, such as improving the pharmacokinetic (PK) profiles and targeting efficiency. Especially for cancer therapy, by using the nanoparticulate systems, preferential drug accumulation at the tumor target can be achieved via the EPR effect. Moreover, incorporation of active targeting ligands or antibodies can further increase accumulation in tumor and reduce undesired drug exposure to normal tissues.
Additionally, a CPP-based nanocarrier is necessary for those drugs that are difficult to conjugate with the CPP directly, thus expanding the use of CPP method to virtually all drugs regardless of their inherited nature. The CPP-based nanotechnology is also preferred under an efficiency/cost concern that each CPP “Trojan horse” is used to deliver only ONE single small molecule drug. Furthermore, the great availability of functional groups on the nanoparticles is advantageous for functional modification of targeting ligands and antibodies.
Since the regulation of CPP’s activity is based on various stimuli-responsive mechanisms, the behavior of the responsive factors significantly affects the efficacy of treatment. The intensity of responsive factors (e.g. pH and hypoxia) increases gradually towards the center of the tumor regions [96, 101]. As a result, insufficient activation (deshielding of CPP) may occur at the margin of the targeted tissues. More critically, inactivated CPP-based systems diffuse poorly towards the center of a tumor, rendering ineffective drug concentrations in the deeper regions of the tumor. Although CPP was reported to possess the ability in facilitating intra-tumor penetration [46], so far there has been no in-depth investigation in tumors, of which the interior is heterogeneous and notoriously hard to reach by drug molecules, due to the exceedingly high interstitial fluid pressure (IFP). Nevertheless, using CPP as a tool to enhance intratumoral drug delivery is worth further investigation.
With use of exogenous agents to de-protect the CPP, safety of the de-protecting agents is a concern, and an ideal trigger should be safe and potent for in vivo action. For instance, the triggering agent used in our ATTEMPTS system, protamine, is an FDA-approved drug and therefore can be applied safely to unmask the CPP.
Lastly, although topical application is not a focus in this review, it is of great value for clinical practice because drug exposure can be strictly limited to the targeted tissues, thereby avoiding CPP-associated nonspecific uptake. As a case in point, transcutaneous immunization is to target to the epidermis where is rich in antigen-presenting cells. The feasibility of CPP-facilitated topical antigen delivery was demonstrated in our previous studies [10, 11]. In addition, CPP-conjugated epidermal growth factor was reported for its topical wound healing function [102]. Furthermore, nose-to-brain delivery mediated by CPP was also explored, and we found that the intranasal CPP-modified nanoparticles was able to penetrate into brain [45]. Such observation was echoed with the brain targeting results from other groups [103].
In conclusion, smart strategies are based on mechanisms of controlling the action of CPP on specifically the target cells offer a promising solution for effective yet safe CPP-based therapy. More prospectively, fundamental understanding of how to curb the Trojan challenges seems to be essential in translating this start-of-art technique into real-time clinical application.
Scheme 1.
The Impenetrable Shield and All-piercing Spear (illustration comes as the courtesy of the Chinese Savvy)
Acknowledgments
We thank the support from NSFC, China (91029743, 81172996), and National Basic Research Program of China (973 Program 2013CB932503), and Shanghai Pu-jiang Scholar Program, as well as School of Pharmacy, Fudan University & The Open Project Program of Key Lab of Smart Drug Delivery (Fudan University), MOE & PLA, China (SDD2011-02). This work was also supported in part by NIH R01 Grant CA114612 and by Grant R31-2008-000-10103-01 from the World Class University (WCU) project of the MEST and NRF of South Korea. Victor C. Yang is currently a Participating Faculty in the Department of Molecular Medicine and Biopharmaceutical Sciences, College of Medicine & College of Pharmacy, Seoul National University, South Korea.
We are also grateful for the invaluable comments from Dr. Feng Li (Hampton University).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- [2].Green M, Loewenstein PM. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- [3].Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-Mediated Delivery of Heterologous Proteins into Cells. Proc. Natl. Acad. Sci. U. S. A. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: Delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- [5].Dietz GPH, Kilic E, Bahr M. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-Mediated protein transduction. Mol. Cell. Neurosci. 2002;21:29–37. doi: 10.1006/mcne.2002.1165. [DOI] [PubMed] [Google Scholar]
- [6].Barnett EM, Elangovan B, Bullok KE, Piwnica-Worms D. Selective cell uptake of modified Tat peptide-fluorophore conjugates in rat retina in ex vivo and in vivo models. Invest. Ophthalmol. Vis. Sci. 2006;47:2589–2595. doi: 10.1167/iovs.05-1470. [DOI] [PubMed] [Google Scholar]
- [7].Dietz GP, Bahr M. Peptide-enhanced cellular internalization of proteins in neuroscience. Brain Res. Bull. 2005;68:103–114. doi: 10.1016/j.brainresbull.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [8].Kamei N, Morishita M, Eda Y, Ida N, Nishio R, Takayama K. Usefulness of cell-penetrating peptides to improve intestinal insulin absorption. J. Control Release. 2008;132:21–25. doi: 10.1016/j.jconrel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- [9].Kamei N, Morishita M, Takayama K. Importance of intermolecular interaction on the improvement of intestinal therapeutic peptide/protein absorption using cell-penetrating peptides. J. Control Release. 2009;136:179–186. doi: 10.1016/j.jconrel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- [10].Yang YX, Jiang YF, Wang Z, Liu JH, Yan L, Ye JX, Huang YZ. Skin-permeable quaternary nanoparticles with layer-by-layer structure enabling improved gene delivery. J Mater Chem. 2012;22:10029–10034. [Google Scholar]
- [11].Huang Y, Park YS, Moon C, David AE, Chung HS, Yang VC. Synthetic skin-permeable proteins enabling needleless immunization. Angew. Chem. Int. Ed. Engl. 2010;49:2724–2727. doi: 10.1002/anie.200906153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lopes LB, Brophy CM, Furnish E, Flynn CR, Sparks O, Komalavilas P, Joshi L, Panitch A, Bentley MV. Comparative study of the skin penetration of protein transduction domains and a conjugated peptide. Pharmaceutical research. 2005;22:750–757. doi: 10.1007/s11095-005-2591-x. [DOI] [PubMed] [Google Scholar]
- [13].Dietz GP, Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell. Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [14].Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?”. J. Control Release. 2005;109:77–85. doi: 10.1016/j.jconrel.2005.09.032. [DOI] [PubMed] [Google Scholar]
- [15].Mano M, Teodosio C, Paiva A, Simoes S, Pedroso de Lima MC. On the mechanisms of the internalization of S4(13)-PV cell-penetrating peptide. Biochem. J. 2005;390:603–612. doi: 10.1042/BJ20050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chen YJ, Liu BR, Dai YH, Lee CY, Chan MH, Chen HH, Chiang HJ, Lee HJ. A gene delivery system for insect cells mediated by arginine-rich cell-penetrating peptides. Gene. 2012;493:201–210. doi: 10.1016/j.gene.2011.11.060. [DOI] [PubMed] [Google Scholar]
- [17].Chugh A, Eudes F. Cellular uptake of cell-penetrating peptides pVEC and transportan in plants. J Pept Sci. 2008;14:477–481. doi: 10.1002/psc.937. [DOI] [PubMed] [Google Scholar]
- [18].Chang M, Chou JC, Chen CP, Liu BR, Lee HJ. Noncovalent protein transduction in plant cells by macropinocytosis. New phytol. 2007;174:46–56. doi: 10.1111/j.1469-8137.2007.01977.x. [DOI] [PubMed] [Google Scholar]
- [19].Niedzielski MF, Hopewell R, Ismail Z, Estable MC. MCEF is localized to the nucleus by protein sequences encoded within three distinct exons, where it represses HIV-1 Tat-transactivation of LTR-directed transcription. Int J Biol Sci. 2007;3:225–236. doi: 10.7150/ijbs.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].de la Fuente JM, Berry CC. Tat peptide as an efficient molecule to translocate gold nanoparticles into the cell nucleus. Bioconjug. Chem. 2005;16:1176–1180. doi: 10.1021/bc050033+. [DOI] [PubMed] [Google Scholar]
- [21].Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- [22].Braddock M, Chambers A, Wilson W, Esnouf MP, Adams SE, Kingsman AJ, Kingsman SM. HIV-1 TAT “activates” presynthesized RNA in the nucleus. Cell. 1989;58:269–279. doi: 10.1016/0092-8674(89)90841-6. [DOI] [PubMed] [Google Scholar]
- [23].Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- [24].Wadia JS, Dowdy SF. Transmembrane delivery of protein and peptide drugs by TAT-mediated transduction in the treatment of cancer. Adv. Drug Deliv. Rev. 2005;57:579–596. doi: 10.1016/j.addr.2004.10.005. [DOI] [PubMed] [Google Scholar]
- [25].Deshayes S, Morris MC, Divita G, Heitz F. Cell-penetrating peptides: tools for intracellular delivery of therapeutics. Cell. Mol. Life Sci. 2005;62:1839–1849. doi: 10.1007/s00018-005-5109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Walther C, Meyer K, Rennert R, Neundorf I. Quantum dot-carrier peptide conjugates suitable for imaging and delivery applications. Bioconjug. Chem. 2008;19:2346–2356. doi: 10.1021/bc800172q. [DOI] [PubMed] [Google Scholar]
- [27].Henriques ST, Costa J, Castanho MA. Translocation of beta-galactosidase mediated by the cell-penetrating peptide pep-1 into lipid vesicles and human HeLa cells is driven by membrane electrostatic potential. Biochemistry (Mosc) 2005;44:10189–10198. doi: 10.1021/bi0502644. [DOI] [PubMed] [Google Scholar]
- [28].Torchilin VP. Cell penetrating peptide-modified pharmaceutical nanocarriers for intracellular drug and gene delivery. Biopolymers. 2008;90:604–610. doi: 10.1002/bip.20989. [DOI] [PubMed] [Google Scholar]
- [29].Torchilin VP. Tatp-mediated intracellular delivery of pharmaceutical nanocarriers. Biochem. Soc. Trans. 2007;35:816–820. doi: 10.1042/BST0350816. [DOI] [PubMed] [Google Scholar]
- [30].Snyder EL, Dowdy SF. Cell penetrating peptides in drug delivery. Pharm. Res. 2004;21:389–393. doi: 10.1023/B:PHAM.0000019289.61978.f5. [DOI] [PubMed] [Google Scholar]
- [31].Zorko M, Langel U. Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Deliv. Rev. 2005;57:529–545. doi: 10.1016/j.addr.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [32].Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur. Biophys. J. 2011;40:387–397. doi: 10.1007/s00249-011-0682-7. [DOI] [PubMed] [Google Scholar]
- [33].Ezhevsky SA, Nagahara H, Vocero-Akbani AM, Gius DR, Wei MC, Dowdy SF. Hypo-phosphorylation of the retinoblastoma protein (pRb) by cyclin D:Cdk4/6 complexes results in active pRb. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10699–10704. doi: 10.1073/pnas.94.20.10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27(Kip1) induces cell migration. Nat. Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- [35].Dietz GP, Bahr M. Synthesis of cell-penetrating peptides and their application in neurobiology. Methods Mol. Biol. 2007;399:181–198. doi: 10.1007/978-1-59745-504-6_13. [DOI] [PubMed] [Google Scholar]
- [36].Meade BR, Dowdy SF. Exogenous siRNA delivery using peptide transduction domains/cell penetrating peptides. Adv. Drug Deliv. Rev. 2007;59:134–140. doi: 10.1016/j.addr.2007.03.004. [DOI] [PubMed] [Google Scholar]
- [37].Park YS, Huang Y, Park YJ, David AE, White L, He H, Chung HS, Yang VC. Specific down regulation of 3T3-L1 adipocyte differentiation by cell-permeable antisense HIF1alpha-oligonucleotide. J. Control Release. 2010;144:82–90. doi: 10.1016/j.jconrel.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].He HN, David AE, Zhang J, Park YS, Wang JX, Huang YZ, Wang JK, Yang VC. Low molecular weight protamine/insulin formulation with potential to attenuate protamine-masqueraded insulin allergy. Macromol Res. 2011;19:1224–1226. [Google Scholar]
- [39].Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9:E18–29. doi: 10.1208/aapsj0901003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu BR, Huang YW, Winiarz JG, Chiang HJ, Lee HJ. Intracellular delivery of quantum dots mediated by a histidine- and arginine-rich HR9 cell-penetrating peptide through the direct membrane translocation mechanism. Biomaterials. 2011;32:3520–3537. doi: 10.1016/j.biomaterials.2011.01.041. [DOI] [PubMed] [Google Scholar]
- [41].Hu JW, Liu BR, Wu CY, Lu SW, Lee HJ. Protein transport in human cells mediated by covalently and noncovalently conjugated arginine-rich intracellular delivery peptides. Peptides. 2009;30:1669–1678. doi: 10.1016/j.peptides.2009.06.006. [DOI] [PubMed] [Google Scholar]
- [42].Hou YW, Chan MH, Hsu HR, Liu BR, Chen CP, Chen HH, Lee HJ. Transdermal delivery of proteins mediated by non-covalently associated arginine-rich intracellular delivery peptides. Exp. Dermatol. 2007;16:999–1006. doi: 10.1111/j.1600-0625.2007.00622.x. [DOI] [PubMed] [Google Scholar]
- [43].Lu SW, Hu JW, Liu BR, Lee CY, Li JF, Chou JC, Lee HJ. Arginine-rich intracellular delivery peptides synchronously deliver covalently and noncovalently linked proteins into plant cells. J. Agric. Food Chem. 2010;58:2288–2294. doi: 10.1021/jf903039j. [DOI] [PubMed] [Google Scholar]
- [44].Lo SL, Wang S. Intracellular Protein Delivery Systems Formed by Noncovalent Bonding Interactions between Amphipathic Peptide Carriers and Protein Cargos. Macromol. rapid commun. 2010;31:1134–1141. doi: 10.1002/marc.200900934. [DOI] [PubMed] [Google Scholar]
- [45].Yan L, Wang HY, Jiang YF, Wang Z, Liu JH, Yang YX, Huang SW, Huang YZ. Cell-penetrating Peptide-modified PLGA Nanoparticles for Enhanced Nose-to-brain Macromolecular Delivery. Macromol Res. 2012 DOI 10.1007/s13233-013-1029-2. [Google Scholar]
- [46].Liu J, Zhao Y, Guo Q, Wang Z, Wang H, Yang Y, Huang Y. TAT-modified nanosilver for combating multidrug-resistant cancer. Biomaterials. 2012;33:6155–6161. doi: 10.1016/j.biomaterials.2012.05.035. [DOI] [PubMed] [Google Scholar]
- [47].Rudolph C, Schillinger U, Ortiz A, Tabatt K, Plank C, Muller RH, Rosenecker J. Application of novel solid lipid nanoparticle (SLN)-gene vector formulations based on a dimeric HIV-1 TAT-peptide in vitro and in vivo. Pharm. Res. 2004;21:1662–1669. doi: 10.1023/b:pham.0000041463.56768.ec. [DOI] [PubMed] [Google Scholar]
- [48].Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Jarvinen T, Savolainen J. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- [49].Huang Y, Park YS, Wang J, Moon C, Kwon YM, Chung HS, Park YJ, Yang VC. ATTEMPTS system: a macromolecular prodrug strategy for cancer drug delivery. Curr. Pharm. Des. 2010;16:2369–2376. doi: 10.2174/138161210791920441. [DOI] [PubMed] [Google Scholar]
- [50].Kwon YM, Li YT, Liang JF, Park YJ, Chang LC, Yang VC. PTD-modified ATTEMPTS system for enhanced asparaginase therapy: a proof-of-concept investigation. J. Control Release. 2008;130:252–258. doi: 10.1016/j.jconrel.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Yang VC, Naik SS, Song H, Dombkowski AA, Crippen G, Liang JF. Construction and characterization of a t-PA mutant for use in ATTEMPTS: a drug delivery system for achieving targeted thrombolysis. J. Control Release. 2005;110:164–176. doi: 10.1016/j.jconrel.2005.09.027. [DOI] [PubMed] [Google Scholar]
- [52].Jiang T, Olson ES, Nguyen QT, Roy M, Jennings PA, Tsien RY. Tumor imaging by means of proteolytic activation of cell-penetrating peptides. Proc. Natl. Acad. Sci. U. S. A. 2004;101:17867–17872. doi: 10.1073/pnas.0408191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Davidson B, Goldberg I, Kopolovic J, Lerner-Geva L, Gotlieb WH, Ben-Baruch G, Reich R. MMP-2 and TIMP-2 expression correlates with poor prognosis in cervical carcinoma - A clinicopathologic study using immunohistochemistry and mRNA in situ hybridization. Gynecol. Oncol. 1999;73:372–382. doi: 10.1006/gyno.1999.5381. [DOI] [PubMed] [Google Scholar]
- [54].Fang JM, Shing Y, Wiederschain D, Yan L, Butterfield C, Jackson G, Harper J, Tamvakopoulos G, Moses MA. Matrix metalloproteinase-2 is required for the switch to the angiogenic phenotype in a tumor model. Proc. Natl. Acad. Sci. U. S. A. 2000;97:3884–3889. doi: 10.1073/pnas.97.8.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Turpeenniemi-Hujanen T. Gelatinases (MMP-2 and -9) and their natural inhibitors as prognostic indicators in solid cancers. Biochimie. 2005;87:287–297. doi: 10.1016/j.biochi.2005.01.014. [DOI] [PubMed] [Google Scholar]
- [56].Olson ES, Aguilera TA, Jiang T, Ellies LG, Nguyen QT, Wong EH, Gross LA, Tsien RY. In vivo characterization of activatable cell penetrating peptides for targeting protease activity in cancer. Integr. Biol (Camb) 2009;1:382–393. doi: 10.1039/b904890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Olson ES, Jiang T, Aguilera TA, Nguyen QT, Ellies LG, Scadeng M, Tsien RY. Activatable cell penetrating peptides linked to nanoparticles as dual probes for in vivo fluorescence and MR imaging of proteases. Proc. Natl. Acad. Sci. U. S. A. 2010;107:4311–4316. doi: 10.1073/pnas.0910283107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang Y, So MK, Rao J. Protease-modulated cellular uptake of quantum dots. Nano Lett. 2006;6:1988–1992. doi: 10.1021/nl0611586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Harris TJ, von Maltzahn G, Lord ME, Park JH, Agrawal A, Min DH, Sailor MJ, Bhatia SN. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4:1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Mazza M, Uchegbu IF, Schatzlein AG. Cancer and the blood-brain barrier: ‘Trojan horses’ for courses? Br. J. Pharmacol. 2008;155:149–151. doi: 10.1038/bjp.2008.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].van Duijnhoven SM, Robillard MS, Nicolay K, Grull H. Tumor targeting of MMP-2/9 activatable cell-penetrating imaging probes is caused by tumor-independent activation. J. Nucl. Med. 2011;52:279–286. doi: 10.2967/jnumed.110.082503. [DOI] [PubMed] [Google Scholar]
- [62].Kang SI, Bae YH. pH-induced solubility transition of sulfonamide-based polymers. J. Control Release. 2002;80:145–155. doi: 10.1016/s0168-3659(02)00021-4. [DOI] [PubMed] [Google Scholar]
- [63].Sethuraman VA, Bae YH. TAT peptide-based micelle system for potential active targeting of anti-cancer agents to acidic solid tumors. J. Control Release. 2007;118:216–224. doi: 10.1016/j.jconrel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Martin GR, Jain RK. Noninvasive measurement of interstitial pH profiles in normal and neoplastic tissue using fluorescence ratio imaging microscopy. Cancer Res. 1994;54:5670–5674. [PubMed] [Google Scholar]
- [65].Shamay Y, Adar L, Ashkenasy G, David A. Light induced drug delivery into cancer cells. Biomaterials. 2011;32:1377–1386. doi: 10.1016/j.biomaterials.2010.10.029. [DOI] [PubMed] [Google Scholar]
- [66].Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- [67].Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv. Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- [68].Greish K. Enhanced permeability and retention of macromolecular drugs in solid tumors: a royal gate for targeted anticancer nanomedicines. J. Drug Target. 2007;15:457–464. doi: 10.1080/10611860701539584. [DOI] [PubMed] [Google Scholar]
- [69].Cui J, Li C, Guo W, Li Y, Wang C, Zhang L, Zhang L, Hao Y, Wang Y. Direct comparison of two pegylated liposomal doxorubicin formulations: is AUC predictive for toxicity and efficacy? J. Control Release. 2007;118:204–215. doi: 10.1016/j.jconrel.2006.12.002. [DOI] [PubMed] [Google Scholar]
- [70].Gullotti E, Yeo Y. Extracellularly activated nanocarriers: a new paradigm of tumor targeted drug delivery. Mol. Pharm. 2009;6:1041–1051. doi: 10.1021/mp900090z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Gabizon A, Papahadjopoulos D. The Role of Surface-Charge and Hydrophilic Groups on Liposome Clearance Invivo. Biochim. Biophys. Acta. 1992;1103:94–100. doi: 10.1016/0005-2736(92)90061-p. [DOI] [PubMed] [Google Scholar]
- [72].Torchilin VP, Omelyanenko VG, Papisov MI, Bogdanov AA, Jr., Trubetskoy VS, Herron JN, Gentry CA. Poly(ethylene glycol) on the liposome surface: on the mechanism of polymer-coated liposome longevity. Biochim. Biophys. Acta. 1994;1195:11–20. doi: 10.1016/0005-2736(94)90003-5. [DOI] [PubMed] [Google Scholar]
- [73].Lee ES, Na K, Bae YH. Super pH-sensitive multifunctional polymeric micelle. Nano Lett. 2005;5:325–329. doi: 10.1021/nl0479987. [DOI] [PubMed] [Google Scholar]
- [74].Mohajer G, Lee ES, Bae YH. Enhanced intercellular retention activity of novel pH-sensitive polymeric micelles in wild and multidrug resistant MCF-7 cells. Pharm. Res. 2007;24:1618–1627. doi: 10.1007/s11095-007-9277-5. [DOI] [PubMed] [Google Scholar]
- [75].Prescott DM, Charles HC, Poulson JM, Page RL, Thrall DE, Vujaskovic Z, Dewhirst MW. The relationship between intracellular and extracellular pH in spontaneous canine tumors. Clin. Cancer Res. 2000;6:2501–2505. [PubMed] [Google Scholar]
- [76].Engin K, Leeper DB, Cater JR, Thistlethwaite AJ, Tupchong L, McFarlane JD. Extracellular pH distribution in human tumours. Int. J. Hyperthermia. 1995;11:211–216. doi: 10.3109/02656739509022457. [DOI] [PubMed] [Google Scholar]
- [77].Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J. Drug Target. 2007;15:538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug. Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Koren E, Apte A, Jani A, Torchilin VP. Multifunctional PEGylated 2C5-immunoliposomes containing pH-sensitive bonds and TAT peptide for enhanced tumor cell internalization and cytotoxicity. J. Control Release. 2012;160:264–273. doi: 10.1016/j.jconrel.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lee ES, Gao Z, Kim D, Park K, Kwon IC, Bae YH. Super pH-sensitive multifunctional polymeric micelle for tumor pH(e) specific TAT exposure and multidrug resistance. J. Control Release. 2008;129:228–236. doi: 10.1016/j.jconrel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Kuai R, Yuan W, Qin Y, Chen H, Tang J, Yuan M, Zhang Z, He Q. Efficient Delivery of Payload into Tumor Cells in a Controlled Manner by TAT and Thiolytic Cleavable PEG Co-Modified Liposomes. Mol. Pharm. 2010;7:1816–1826. doi: 10.1021/mp100171c. [DOI] [PubMed] [Google Scholar]
- [82].Mok H, Bae KH, Ahn CH, Park TG. PEGylated and MMP-2 specifically dePEGylated quantum dots: comparative evaluation of cellular uptake. Langmuir. 2009;25:1645–1650. doi: 10.1021/la803542v. [DOI] [PubMed] [Google Scholar]
- [83].Zhu L, Kate P, Torchilin VP. Matrix metalloprotease 2-responsive multifunctional liposomal nanocarrier for enhanced tumor targeting. ACS nano. 2012;6:3491–3498. doi: 10.1021/nn300524f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fang J, Nakamura H, Maeda H. The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 2011;63:136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- [85].Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J. Control Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- [86].Ishida T, Masuda K, Ichikawa T, Ichihara M, Irimura K, Kiwada H. Accelerated clearance of a second injection of PEGylated liposomes in mice. Int. J. Pharm. 2003;255:167–174. doi: 10.1016/s0378-5173(03)00085-1. [DOI] [PubMed] [Google Scholar]
- [87].Ishida T, Atobe K, Wang X, Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high-dose first injection. J. Control Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- [88].Romberg B, Oussoren C, Snel CJ, Carstens MG, Hennink WE, Storm G. Pharmacokinetics of poly(hydroxyethyl-l-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administration. Biochim. Biophys. Acta. 2007;1768:737–743. doi: 10.1016/j.bbamem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- [89].Hansen MB, van Gaal E, Minten I, Storm G, van Hest JC, Lowik DW. Constrained and UV-activatable cell-penetrating peptides for intracellular delivery of liposomes. J. Control Release. 2012 doi: 10.1016/j.jconrel.2012.10.008. DOI: 10.1016/j.jconrel.2012.10.008. [DOI] [PubMed] [Google Scholar]
- [90].Kim C, Lee Y, Kim JS, Jeong JH, Park TG. Thermally triggered cellular uptake of quantum dots immobilized with poly(N-isopropylacrylamide) and cell penetrating peptide. Langmuir. 2010;26:14965–14969. doi: 10.1021/la102632m. [DOI] [PubMed] [Google Scholar]
- [91].Vocero-Akbani AM, Heyden NV, Lissy NA, Ratner L, Dowdy SF. Killing HIV-infected cells by transduction with an HIV protease-activated caspase-3 protein. Nat. Med. 1999;5:29–33. doi: 10.1038/4710. [DOI] [PubMed] [Google Scholar]
- [92].Vives E, Schmidt J, Pelegrin A. Cell-penetrating and cell-targeting peptides in drug delivery. Biochim. Biophys. Acta. 2008;1786:126–138. doi: 10.1016/j.bbcan.2008.03.001. [DOI] [PubMed] [Google Scholar]
- [93].Harada H, Hiraoka M, Kizaka-Kondoh S. Antitumor effect of TAT-oxygen-dependent degradation-caspase-3 fusion protein specifically stabilized and activated in hypoxic tumor cells. Cancer Res. 2002;62:2013–2018. [PubMed] [Google Scholar]
- [94].Inoue M, Mukai M, Hamanaka Y, Tatsuta M, Hiraoka M, Kizaka-Kondoh S. Targeting hypoxic cancer cells with a protein prodrug is effective in experimental malignant ascites. Int. J. Oncol. 2004;25:713–720. [PubMed] [Google Scholar]
- [95].Harada H, Kizaka-Kondoh S, Li G, Itasaka S, Shibuya K, Inoue M, Hiraoka M. Significance of HIF-1-active cells in angiogenesis and radioresistance. Oncogene. 2007;26:7508–7516. doi: 10.1038/sj.onc.1210556. [DOI] [PubMed] [Google Scholar]
- [96].Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer Sci. 2003;94:1021–1028. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kizaka-Kondoh S, Itasaka S, Zeng L, Tanaka S, Zhao T, Takahashi Y, Shibuya K, Hirota K, Semenza GL, Hiraoka M. Selective killing of hypoxia-inducible factor-1-active cells improves survival in a mouse model of invasive and metastatic pancreatic cancer. Clin. Cancer Res. 2009;15:3433–3441. doi: 10.1158/1078-0432.CCR-08-2267. [DOI] [PubMed] [Google Scholar]
- [98].Kuchimaru T, Kadonosono T, Tanaka S, Ushiki T, Hiraoka M, Kizaka-Kondoh S. In vivo imaging of HIF-active tumors by an oxygen-dependent degradation protein probe with an interchangeable labeling system. PloS one. 2010;5:e15736. doi: 10.1371/journal.pone.0015736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Yu Z, Wu J, Wu S, Jia P, Tong Y, Wu X, Wang Y. A recombinant cell-permeable p53 fusion protein is selectively stabilized under hypoxia and inhibits tumor cell growth. Cancer Lett. 2009;279:101–107. doi: 10.1016/j.canlet.2009.01.030. [DOI] [PubMed] [Google Scholar]
- [100].Murray JC, Carmichael J. Targeting Solid Tumors - Challenges, Disappointments, and Opportunities. Adv. Drug Deliv. Rev. 1995;17:117–127. [Google Scholar]
- [101].Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- [102].Choi JK, Jang JH, Jang WH, Kim J, Bae IH, Bae J, Park YH, Kim BJ, Lim KM, Park JW. The effect of epidermal growth factor (EGF) conjugated with low-molecular-weight protamine (LMWP) on wound healing of the skin. Biomaterials. 2012;33:8579–8590. doi: 10.1016/j.biomaterials.2012.07.061. [DOI] [PubMed] [Google Scholar]
- [103].Xia H, Gao X, Gu G, Liu Z, Zeng N, Hu Q, Song Q, Yao L, Pang Z, Jiang X, Chen J, Chen H. Low molecular weight protamine-functionalized nanoparticles for drug delivery to the brain after intranasal administration. Biomaterials. 2011;32:9888–9898. doi: 10.1016/j.biomaterials.2011.09.004. [DOI] [PubMed] [Google Scholar]