Abstract
We hypothesized that ethanol (EtOH) might act through the endocannabinoid system to inhibit luteinizing hormone-releasing hormone (LHRH) release. Therefore, we examined the mechanism by which EtOH and anandamide (AEA), an endogenous cannabinoid, inhibit LHRH release from incubated medial basal hypothalamic explants. In previous work, we demonstrated that EtOH inhibits the N-methyl-d-aspartic acid-stimulated release of LHRH by increasing the release of two neurotransmitters: β-endorphin and γ-aminobutyric acid (GABA). In the present work, bicuculline, a GABAergic antagonist, completely prevented the inhibition of AEA (10-9M) on N-methyl-d-aspartic acid-induced LHRH release, but naltrexone, a μ-opioid receptor antagonist, had no effect. AEA also significantly increased GABA release but had no effect on β-endorphin release. Therefore, AEA could inhibit LHRH release by increasing GABA but not β-endorphin release. Because EtOH and AEA acted similarly to inhibit LHRH release, we investigated whether both substances would affect the adenylate cyclase activity acting through the same GTP-coupled receptors, the cannabinoid receptors 1 (CB1-rs). AEA and EtOH (10-1M) reduced the forskolin-stimulated accumulation of cAMP, but AM251, a specific antagonist of CB1-r, significantly blocked that inhibition. Additionally we investigated whether CB1-r is involved in the inhibition of LHRH by EtOH and AEA. AEA and EtOH reduced forskolin-stimulated LHRH release, but AM251 significantly blocked that inhibition. Also, we demonstrated that EtOH did not act by increasing AEA synthase activity to inhibit LHRH release in our experimental conditions. Therefore, our results indicate that EtOH inhibits the release of LHRH acting through the endocannabinoid system.
Keywords: anandamide, cannabinoid receptor 1, cyclic adenosine monophosphate, γ-amino butyric acid
It is well known that ethanol (EtOH) and Δ9-tetrahydrocannabinol (THC), the major active ingredient of marijuana, can suppress reproductive function in humans, monkeys, and small rodents, such as the rat (1-6). Because the actions of EtOH and THC on the hypothalamic-gonadotrophic axis are similar (6), and central cannabinoid receptors 1 (CB1-rs) are concentrated in the preoptic area and in the arcuate nucleus, important sites of releasing and inhibiting hormones, we hypothesized that the effects of EtOH might be mediated by the endocannabinoid system.
On the basis of in vitro experiments with incubation of medial basal hypothalamus (MBH), Lomniczi et al. (7) demonstrated that EtOH inhibited the N-methyl-d-aspartic acid (NMDA)-stimulated release of luteinizing hormone-releasing hormone (LHRH) by increasing the release of two neurotransmitters: β-endorphin and γ-aminobutyric acid (GABA). The inhibition was reversed by naltrexone, a μ-opioid receptor antagonist, and by bicuculline, a GABA receptor antagonist, suggesting that at least two inhibitory pathways regulate the release of LHRH (7). Additionally, it has been shown that nitric oxide activates cyclooxygenase, which increases the release of prostanoids (8). Prostaglandin E2, by activating adenylate cyclase (AC) with a consequent increase in cAMP, evokes exocytosis of LHRH granules by activation of protein kinase A (9). Furthermore, β-endorphin inhibits nitric oxide synthase activity and naltrexone increases basal nitric oxide levels (10), indicating a tonic inhibitory β-endorphin action suppressing LHRH release by using the pathway described above.
The primary action of GABA appears to be to inhibit the nitric oxidergic activation of cyclooxygenase (11). Therefore, lower levels of prostaglandins decrease AC activity with the consequent decrease of LHRH release. Also, THC decreases hormones that control sexual behavior in male rats (6). THC exerts its effects by binding to specific GTP-binding protein-coupled receptors, the CB1-r located mainly in the CNS (12-14) and the CB2-r located mainly in peripheral tissues (15). Both CB1-r and CB2-r are functionally linked to inhibition of AC (16). After the demonstration of the presence of cannabinoid receptors, endogenous substances that bind to these receptors were discovered. There are two main endocannabinoids, arachidonoyl ethanolamide [anandamide (AEA)] and 2-arachidonoyl glycerol, both derivatives of arachidonic acid (17). AEA binds with high affinity to CB1-r (18). Specific antagonists of these receptors, such as AM251, have been developed (19). The activation of the endocannabinoid system is in parallel with changes in the activity of several neurotransmitters, including GABA and dopamine (20). In vitro studies demonstrated that EtOH, as well as THC, increases GABA content in the MBH (21, 22). It has been shown that GABA inhibits the release of LHRH (11) and that stimulatory neurotransmitters such as norepinephrine act through cAMP to increase the release of LHRH (23). Because the mechanism of action of a cannabinoid acting on its receptors is by the inhibition of AC with a consequent decrease of cAMP, in the present study we investigated whether EtOH would use the same pathway used by endocannabinoids to inhibit the release of LHRH.
Materials and Methods
Animals. Male rats of the Wistar strain (220-250 g) were kept in group cages in an animal room having a photoperiod of 14 h of light (0500 to 1900 hours) and room temperature of 22-24°C. Animals had free access to laboratory chow and tap water. The experimental procedures reported here were approved by the Animal Care Committee of the Center of Experimental Pharmacology and Botanicals of the National Council for Research of Argentina and carried out in accord with the Declaration of Helsinki.
In Vitro Studies. After decapitation and removal of the brain, the MBH was dissected by making frontal cuts just behind the optic chiasm, extending dorsally for 1.0 mm; a horizontal cut extended from this point caudally to just behind the pituitary stalk, where another frontal cut was made. Longitudinal cuts were made 1.0 mm lateral to the midline bilaterally. The hypothalami were preincubated in Krebs-Ringer bicarbonate-buffered medium (pH 7.4) containing 0.1% glucose for 15 min before replacement with fresh medium or medium containing the substances to be tested. The incubation was continued for 30 min followed by removal of the medium and storage of samples at -20°C before assays. All incubations were carried out in a Dubnoff shaker (50 cycles per min; 95% O2/5% CO2) at 37°C. In the experiments measuring cAMP, isobutylmethylxanthine (10-4 M), a phosphodiesterase inhibitor, was added to the buffer to prevent the degradation of cAMP.
RIAs. LHRH was measured by RIA by using a highly specific LHRH antiserum kindly provided by A. Barnea (University of Texas Southwestern Medical Center, Dallas). The sensitivity of the assay was 0.2 pg per tube, and the curve was linear up to 100 pg of LHRH. The intraassay coefficient of variation of the LHRH RIA ranged from 4% to 7.3%, and the interassay coefficient of variation was 8.9%. All samples were measured in duplicate. β-Endorphin also was measured by RIA by using a highly specific antiserum kindly provided by G. Chrousos (National Institute of Child Health and Human Development, Bethesda). Intra- and interassay coefficients of variation were 5% and 7%, respectively. cAMP was measured by RIA by using the Ab provided by the National Institute of Diabetes and Digestive and Kidney Diseases (Bethesda). Intra- and interassay coefficients of variation were 8.1% and 10.5%, respectively.
GABA Assay. For the determination of GABA concentrations, the media were heated for 10 min at 100°C and centrifuged at 10,000 × g for 10 min. The supernatants were stored at -20°C until GABA determination by the [H3]muscimol radioreceptor assay as described by Bernasconi et al. (ref. 24; sensitivity range, 12.5-200 pmol). This method measures the concentration of endogenous GABA receptor ligands metabolically related to GABA (25).
AEA Synthase Activity. Synthase activity was assayed as described by Paria et al. (26) with minor modifications. The MBH were homogenized in 200 μl of buffer [20 mM Tris·HCl/1 mM EDTA (pH 7.6)] and were centrifuged at 2,000 × g for 15 min. Supernatant protein (150-100 μg) was incubated in a total volume of 200 μl of 5 × 10-2 M Tris·HCl (pH 9.0) with 40 μM (0.1 μCi; 1 Ci = 37 GBq) of [1-14C]arachidonic acid (40-60 μCi/mmol, Perkin-Elmer) and 2 × 10-2 M ethanolamine during 5 min at 37°C. The reaction was terminated by the addition of 400 μl of chloroform/methanol (1:1) mixture. Two additional washes of the aqueous phase with 400 μl of chloroform were performed.
Organic phases were evaporated to dryness under nitrogen gas and redissolved in 40 μl of chloroform/methanol (1:1) mixture. Samples and authentic standards were applied on Silica Gel 60 plates with Concentration Zone (Merck). The synthesized [14C]AEA was resolved by using the organic layer of an ethyl acetate/hexane/acetic acid/water (100:50:20:100) mixture. The plate was exposed to x-ray film at -70°C. After autoradiography, distribution of radioactivity on the plate was counted in a scintillation counter by scraping off the corresponding spots of the plate. The retardation factor values of AEA and arachidonic acid were 0.33 and 0.78, respectively.
Chemicals. LHRH and cAMP for iodination and standard were purchased from Peninsula Laboratories. Iodine-125 for iodination was purchased from New England Nuclear. Naltrexone, bicuculline, NMDA, forskolin (FRSK), AEA, and isobutylmethylxanthine were from Sigma Aldrich. AM251 [N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide] was from Tocris (Ellisville, MO), and EtOH was from Merck.
Statistics. All data are expressed as means plus 1 SEM. Comparisons between groups were performed by using one-way ANOVA followed by the Student-Newman-Keuls multiple comparison test for unequal replicates. Student's t test was used when comparing two groups. Differences with P values <0.05 were considered significant.
Results
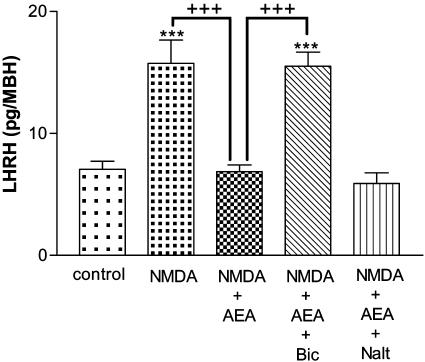
Effect of AEA on NMDA-Stimulated LHRH Release. LHRH release was dramatically increased by NMDA (2 × 10-2 M, P < 0.001), and this release was highly significantly inhibited (P < 0.001) by AEA (10-9 M; Fig. 1). To investigate whether this inhibition was caused by GABA and/or β-endorphin, bicuculline (10-4 M), a GABAergic antagonist, and naltrexone (10-6 M), an opioid antagonist, were separately added to the media. Bicuculline completely prevented the inhibition of AEA on NMDA-induced LHRH release (P < 0.001), but naltrexone had no effect. These results suggest that GABA but not β-endorphin is involved in the AEA/LHRH release-inhibiting pathway (Fig. 1).
Fig. 1.
Effect of NMDA (2 × 10-2 M), AEA (10-9 M), bicuculline (Bic) (10-4 M), and naltrexone (Nalt) (10-6 M) on LHRH release from MBH incubated in vitro. Here, and in subsequent figures, the height of the column represents the mean and the vertical line represents SEM. ***, P < 0.001 vs. control; +++, P < 0.001 vs. NMDA plus AEA.
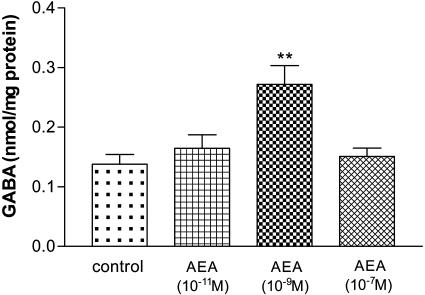
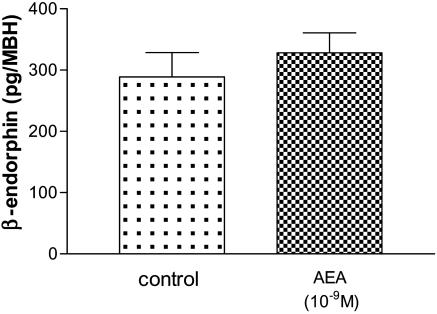
Effect of AEA on GABA and β-Endorphin Release. Incubation of MBH with AEA (10-9 M) significantly increased GABA release (P < 0.01), whereas other doses of AEA (10-11 and 10-7 M) had no significant effect (Fig. 2). We found no effect of AEA (10-9 M) on β-endorphin release (Fig. 3). These results reinforced the hypothesis that GABA but not β-endorphin is involved in the AEA/LHRH release-inhibiting pathway.
Fig. 2.
Effect of different doses of AEA on GABA release from MBH. AEA (10-9 M) significantly increased GABA release. **, P < 0.01 vs. control.
Fig. 3.
Effect of AEA (10-9 M) on β-endorphin release from MBH incubated in vitro.
Because EtOH and AEA acted similarly to inhibit LHRH release, we investigated whether both substances would affect AC activity through the same G protein-coupled receptor, CB1-r. Additionally we investigated whether CB1-r is involved in the inhibition of LHRH by EtOH and AEA. We performed the following experiments with MBH incubated in the presence of AEA and EtOH with or without the presence of AM251, the specific CB1-r antagonist.
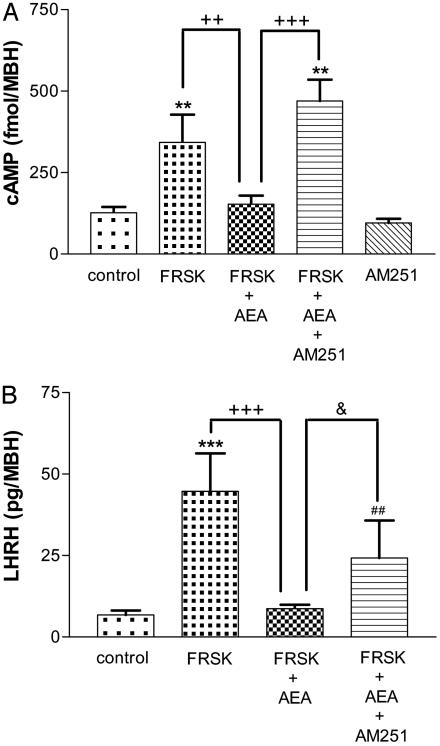
Effect of AEA on FRSK-Stimulated cAMP Content and LHRH Release. The cAMP content was increased (P < 0.01) by FRSK (7.6 × 10-5 M), an activator of AC, and it was significantly reduced (P < 0.001) by AEA (10-9 M; Fig. 4A). To investigate whether this inhibition was mediated by CB1-r, AM251, a specific antagonist of CB1-r, was added to the media. AM251 (10-5 M) completely blocked (P < 0.001) the inhibition of AEA on FRSK-stimulated cAMP content. LHRH release was dramatically increased (P < 0.001) by FRSK (7.6 × 10-5 M) and significantly reduced (P < 0.001) by AEA (10-9 M; Fig. 4B). AM251 (10-5 M) partially blocked (P < 0.05) the inhibition of AEA on FRSK-stimulated LHRH release.
Fig. 4.
(A) Effect of FRSK (7.6 × 10-5 M), AEA (10-9 M), and AM251 (10-5 M) on cAMP content from MBH incubated in vitro. **, P < 0.01 vs. control; +++, P < 0.001; ++, P < 0.01 vs. FRSK plus AEA. (B) Effect of FRSK (7.6 × 10-5 M), AEA (10-9 M), and AM251 (10-5 M) on LHRH release from MBH incubated in vitro.***, P < 0.001 vs. control; +++, P < 0.001 vs. FRSK plus AEA (ANOVA); ##, P < 0.01 vs. control; &, P < 0.05 vs. FRSK plus AEA (Student's t test).
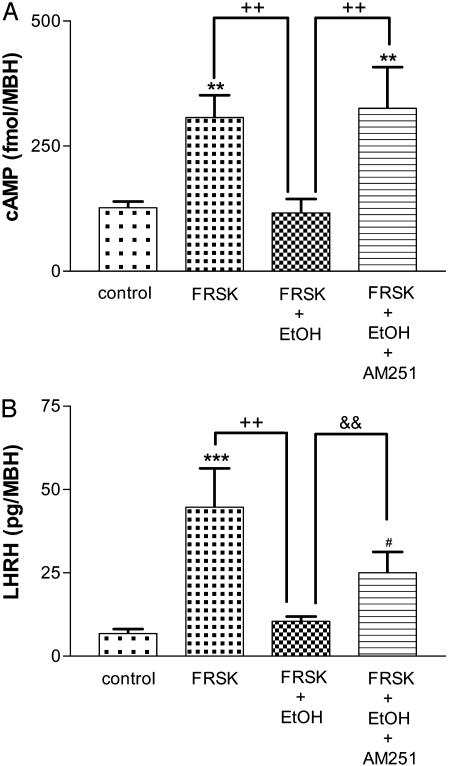
Effect of EtOH on FRSK-Stimulated cAMP Content and LHRH Release. As before, cAMP concentration was increased (P < 0.01) by FRSK (7.6 × 10-5 M) and it was significantly decreased (P < 0.01) by EtOH (10-1 M; Fig. 5A). To investigate whether this inhibition was mediated by CB1-r, AM251 (10-5 M) was added to the media. AM251 completely blocked (P < 0.01) the inhibition of EtOH on FRSK-stimulated cAMP content. LHRH release was dramatically increased (P < 0.001) by FRSK (7.6 × 10-5 M) and significantly inhibited (P < 0.01) by EtOH (10-1 M; Fig. 5B). AM251 (10-5 M) partially blocked (P < 0.01) the inhibition of EtOH on FRSK-stimulated LHRH release.
Fig. 5.
(A) Effect of FRSK (7.6 × 10-5 M), EtOH (10-1 M), and AM251 (10-5 M) on cAMP content from MBH incubated in vitro.**, P < 0.01 vs. control; ++, P < 0.01 vs. FRSK plus EtOH. (B) Effect of FRSK (7.6 × 10-5M), EtOH (10-1 M), and AM251 (10-5 M) on LHRH release from MBH incubated in vitro. ***, P < 0.001 vs. control; ++, P < 0.01 vs. FRSK plus EtOH (ANOVA); #, P < 0.05 vs. control; &&, P < 0.01 vs. FRSK plus EtOH (Student's t test).
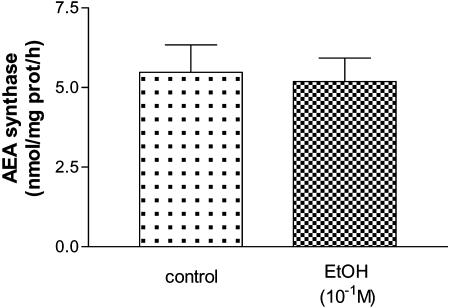
Effect of EtOH on AEA Synthase Activity. To examine the possibility that EtOH acted by synthesis of AEA that was released and activated the CB1-r on GABA neurons which then inhibited LHRH release from LHRH neurons, we measured the effect of EtOH on AEA synthase activity in our preparation and experimental conditions. No significant difference was found between MBH incubated for 30 min with EtOH (10-1 M) and the control group (Fig. 6), suggesting that EtOH did not affect AEA synthase activity in vitro.
Fig. 6.
Effect of EtOH (10-1 M) on AEA synthase activity in MBH incubated for 30 min.
Discussion
Much evidence exists that EtOH exerts its pharmacological effects in the CNS by modulating the function of intracellular signal transduction pathways by acting on several receptors (27). To understand the mechanism of action of AEA and EtOH by means of CB1-r, it is necessary to put the findings into the context of previous work (7). Those experiments indicated that EtOH inhibits the NMDA-stimulated release of LHRH by increasing the release of two neurotransmitters: β-endorphin and GABA. The same experimental model was used to investigate the effect of bicuculline and naltrexone on the AEA-induced decrease of NMDA-stimulated LHRH release. Bicuculline reversed the action of AEA, but naltrexone was without effect, demonstrating that GABA, but not β-endorphin, is involved in the AEA-induced inhibition of LHRH release. In fact, AEA did not affect β-endorphin release. In vitro studies demonstrated that EtOH and THC increased GABA content in MBH (21, 22). In a previous work (7), we demonstrated that EtOH increased GABA release, and in the present work we demonstrated that there was a bell-shaped curve of AEA-stimulated GABA release with highly significant release only at 10-9 M AEA. Additionally, in situ hybridizations performed by Hohmann and Herkenham (28) demonstrated that CB1-r colocalized with GABAergic neurons of the corpus striatum. Furthermore, a localization of GABAergic neurons with CB1-r was also seen in the hypothalamus (personal communication, M. Herkenham, Section of Functional Neuroanatomy National Institutes of Mental Health, Bethesda). Therefore, our data indicate that alcohol, as well as AEA, stimulates GABA release by acting through CB1-r.
To determine whether EtOH and AEA would both inhibit FRSK activation of AC by acting through CB1-r, we investigated their effects on MBH cAMP content. The FRSK-induced increase of cAMP content was blocked by alcohol and AEA, and these inhibitions were prevented by AM251, the CB1-r antagonist. These results also indicate that both alcohol and AEA act by means of CB1-r in the MBH.
Previously, we demonstrated that EtOH increased GABA release and inhibited LHRH release (7). In the present work, we significantly reversed AEA and EtOH inhibition on LHRH release by the CB1-r antagonist, AM251, which indicates that both acted by means of CB1-r. Moreover, AEA did so by stimulating GABA release because bicuculline reversed it, just as we described for EtOH, confirming our previous results (7). Therefore, our evidence indicates that alcohol and AEA act through CB1-r inhibiting AC activity, preventing the inhibition of basal GABA release by cAMP. Furthermore we found that AEA stimulated GABA release in our experimental conditions. Obrietan and van den Pol (29) suggested that cAMP, the second messenger linked to cannabinoid receptors, could inhibit GABA release. The inhibition of LHRH release by AEA and EtOH from the hypothalamus is mediated in part by CB1-r inhibiting AC. AM251 did not completely reverse the inhibition of LHRH release by EtOH, so this inhibition could be due to the presence of a second inhibitory pathway, such as the opioid system, because EtOH increases the release of β-endorphin, which also can inhibit LHRH release (7). In the case of AEA, the partial reversal of the inhibition of LHRH release by AM251 is more difficult to explain, but recent research suggests the presence of a new central endocannabinoid receptor in mouse brain, CB3-r (30), which could bind AEA but not AM251. Although CB1-rs were distributed in the hypothalamic area where LHRH neurons are found, in a previous work we did not observe colocalization of CB1-r with LHRH neurons when using immunohistochemical techniques (31). Therefore, AEA and EtOH might act through CB1-r located on GABAergic neurons to inhibit LHRH release. Furthermore, it has been shown that GABA type A receptors colocalize with LHRH neurons in the hypothalamus (32).
Basavarajappa et al. (33-35) demonstrated that chronic EtOH exposure increases the extracellular levels of endocannabinoids in human neuroblastoma cells and in primary cultures of cerebellar granular neurons. Therefore, we had to determine the effect of EtOH on the levels of AEA after 30 min of incubation in our in vitro experimental model. We did not find any change in the activity of AEA synthase after exposure to EtOH in MBH. Our experimental procedure is a short-term incubation (30 min). Therefore, our results are not in conflict with the synthesis of AEA found after long-term exposure as shown in cell culture studies.
In a previous work (36), we showed that AEA inhibits prolactin release from the pituitary of male rats by acting on CB1-r on dopaminergic neurons in the MBH, activating dopamine release into the portal vessels that inhibits prolactin release. Moreover, Wang et al. (37) and Hungund et al. (27) have found that cannabinoids and alcohol activate the same reward pathways. Indeed, both cannabinoids and alcohol cause the release of dopamine in the nucleus accumbens of CB1-r+/+ mice. CB1-r-/- (knockout for CB1-r) mice exhibited a complete lack of alcohol-induced dopamine release and alcohol craving as compared with WT mice (38). Interference with dopamine-mediated neurotransmission could underlie some of the effects of AEA on the hypothalamic-pituitary axis. These effects consist mainly of the enhancement or inhibition of hypothalamic hormones, which leads to an increase in adrenocorticotrophic hormone release (39) and the inhibition of growth hormone, luteinizing hormone, and prolactin (40, 41).
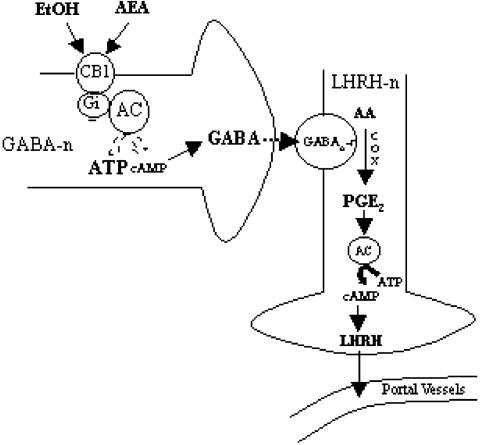
In summary, in the present work, we demonstrated that AEA and EtOH inhibit LHRH release acting through CB1-r located on GABAergic neurons by inhibiting AC and therefore stimulating the release of GABA that binds to GABA type A receptors located on LHRH neurons as illustrated in Fig. 7.
Fig. 7.
Diagram of the postulated mechanism of action of alcohol and AEA to suppress LHRH release acting through CB1-r. For explanation, see Discussion. GABA-n, GABA neuron; GABAAr, GABA type A receptor; LHRH-n, LHRH neuronal terminal; AA, arachidonic acid; Gi, GTP inhibitory binding protein; PGE2, prostaglandin E2; COX, cyclooxygenase. Solid arrows indicate stimulation. Dashed arrows indicate inhibition.
Acknowledgments
We thank Ana Ines Casella for her administrative assistance. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Argentina, Grant PICT 995-6117, Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina, and National Institutes of Mental Health Grant MH51853 (to S.M.M.).
Abbreviations: EtOH, ethanol; LHRH, luteinizing hormone-releasing hormone; AEA, anandamide; NMDA, N-methyl-d-aspartic acid; GABA, γ-aminobutyric acid; THC, Δ9-tetrahydrocannabinol; CBn-r, cannabinoid receptor n; MBH, medial basal hypothalamus; AC, adenylate cyclase; FRSK, forskolin.
References
- 1.Cicero, T. J. & Badger, T. (1977) J. Pharmacol. Exp. Ther. 201, 427-433. [PubMed] [Google Scholar]
- 2.Cicero, T. J., Nexman, K. S., Gerrity, M., Scumoker, P. F. & Bell, R. D. (1982) Life Sci. 31, 1587-1596. [DOI] [PubMed] [Google Scholar]
- 3.Mendelson, J. H., Mello, N. K. & Ellingboe, J. (1977) Exp. Ther. 202, 676-682. [PubMed] [Google Scholar]
- 4.Dees, W. L., Rettori, V., Kozlowski, G. P. & McCann, S. M. (1985) Alcohol 2, 641-646. [DOI] [PubMed] [Google Scholar]
- 5.Murphy, L., Gher, J., Steger, R. & Bartke, A. (1994) Pharmacol. Biochem. Behav. 48, 1011-1017. [DOI] [PubMed] [Google Scholar]
- 6.Rettori, V., Wenger, T., Snyder, G., Dalterio, S. & McCann, S. M. (1987) Neuroendocrinology 46, 488-493. [DOI] [PubMed] [Google Scholar]
- 7.Lomniczi, A., Mastronardi, C. A., Falleti, A., Seilicovich, A., De Laurentiis, A., McCann, S. M. & Rettori, V. (2000) Proc. Natl. Acad. Sci. USA 97, 2337-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rettori, V., Gimeno, M., Lyson, K. & McCann, S. M. (1992) Proc. Natl. Acad. Sci. USA 89, 11543-11546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Negro-Vilar, A., Ojeda, S. M. & McCann, S. M. (1979) Endocrinology 104, 1749-1757. [DOI] [PubMed] [Google Scholar]
- 10.Faletti, A. G., Mastronardi, C. A., Lomniczi, A., Seilicovich, A., Gimeno, M., McCann, S. M. & Rettori, V. (1999) Proc. Natl. Acad. Sci. USA 96, 1722-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seilicovich, A., Duvilanski, B., Pisera, D., Theas, S., Gimeno, M., Rettori, V. & McCann, S. M. (1995) Proc. Natl. Acad. Sci. USA 92, 3421-3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devane, W. A., Dysarz, F. A., Johnson, M., Melvin, L. S. & Howlett, A. C. (1988) Mol. Pharmacol. 34, 605-613. [PubMed] [Google Scholar]
- 13.Herkenham, M., Lynn, A. B., Little, M. D., Johnson, M. R., Melvin, L. S., De Costa, B. R. & Rice, K. C. (1990) Proc. Natl. Acad. Sci. USA 87, 1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pertwee, R. G., ed. (1995) in Cannabinoid Receptors (Academic, London), pp. 1-34.
- 15.Munro, S., Thomas, K. L. & Abu-Shaar, M. (1993) Nature 365, 61-65. [DOI] [PubMed] [Google Scholar]
- 16.Howlett A. & Fleming, R. (1984) Mol. Pharmacol. 26, 532-538. [PubMed] [Google Scholar]
- 17.Mechoulam, R., Fride, E. & Di Marzo, V. (1998) Eur. J. Pharmacol. 359, 1-18. [DOI] [PubMed] [Google Scholar]
- 18.Devane, W. A., Hanus, L., Breuer, A., Pertwee, R. G., Stevenson, L. A., Griffin, G., Gibson, D., Mandelbaum, A., Etinger, A. & Mechoulam, R. (1992) Science 258, 1946-1949. [DOI] [PubMed] [Google Scholar]
- 19.Gatley, S., Gifford, A., Volkow, R., Ruoxi, L. & Makriyannis, A. (1996) Eur. J. Pharmacol. 307, 331-338. [DOI] [PubMed] [Google Scholar]
- 20.Pistis, M., Ferraro, L., Pira, L., Flore, G., Tanganelli, S., Gessa, G. L. & Devoto, P. (2002) Brain Res. 948, 155-158. [DOI] [PubMed] [Google Scholar]
- 21.de Miguel, R., Romero, J., Muñoz, R. M., García-Gil, L., González, S., Villanua, M. A., Makriyannis, A., Ramos, J. A. & Fernandez-Ruiz, J. J. (1998) Biochem. Pharmacol. 56, 1331-1338. [DOI] [PubMed] [Google Scholar]
- 22.Noto, T. & Myers, R. (1984) Neurochem. Res. 9, 1653-1665. [DOI] [PubMed] [Google Scholar]
- 23.Ojeda, S., Negro-Vilar, A. & McCann, S. M. (1979) Endocrinology 104, 617-624. [DOI] [PubMed] [Google Scholar]
- 24.Bernasconi, R., Bittiger, H. & Heid, J. (1980) J. Neurochem. 34, 614-618. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell, R., Grieve, G., Dow, R. & Fink, G. (1983) Neuroendocrinology 37, 169-176. [DOI] [PubMed] [Google Scholar]
- 26.Paria, B., Deutsch, D. & Dey, S. (1996) Mol. Reprod. Dev. 45, 183-192. [DOI] [PubMed] [Google Scholar]
- 27.Basavarajappa, B., Cooper, T. & Hungund, B. (1998) Brain. Res. 793, 212-218. [DOI] [PubMed] [Google Scholar]
- 28.Hohmann, A. & Herkenham, M. (2000) Synapse 37, 71-80. [DOI] [PubMed] [Google Scholar]
- 29.Obrietan, K. & van den Pol, A. N. (1997) J. Neurosci. 17, 4785-4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Breivogel, C., Griffin, G., Di Marzo, V. & Martín, B. (2001) Mol. Pharmacol. 60, 155-163. [PubMed] [Google Scholar]
- 31.Rettori, V., Lomniczi, A., Mohn, C., Scorticati, C., Vissio, P., Lasaga, M., Franchi, A. & McCann, S. M. (2002) Prog. Brain Res. 141, 175-181. [DOI] [PubMed] [Google Scholar]
- 32.Spergel, D. J., Kruth, U., Hanley, D., Sprengel, R. & Seeburg, P. (1999) J. Neurosci. 19, 2037-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basavarajappa, B. & Hungund, B. (1999) J. Neurochem. 72, 522-528. [DOI] [PubMed] [Google Scholar]
- 34.Basavarajappa, B., Saito, M., Cooper, T. & Hungund, B. (2000) Biochem. Biophys. Acta 1535, 78-86. [DOI] [PubMed] [Google Scholar]
- 35.Basavarajappa, B., Saito, M., Cooper, T. & Hungund, B. (2003) Eur. J. Pharmacol. 466, 73-83. [DOI] [PubMed] [Google Scholar]
- 36.Scorticati, C., Mohn, C., De Laurentiis, A., Vissio, P., Fernández-Solari, J., Seilicovich, A., McCann, S. M. & Rettori, V. (2003) Proc. Natl. Acad. Sci. USA 100, 2134-2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, L., Liu, J., Harvey-White, J., Zimmer, A. & Kunos, G. (2003) Proc. Natl. Acad. Sci. USA 100, 1393-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hungund, B. L., Szakall, I., Adam, A., Basavarajappa, B. S. & Vadasz, C. (2003) J. Neurochem. 84, 698-704. [DOI] [PubMed] [Google Scholar]
- 39.Weidenfeld, J., Feldman, S. & Mechoulam, R. (1994) Neuroendocrinology 59, 110-112. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez-Ruiz, J. J., Muñoz, R. M., Romero, J., Villanua, M. A., Makriyannis, A. & Ramos, J. A. (1997) Biochem. Pharmacol. 53, 1919-1927. [DOI] [PubMed] [Google Scholar]
- 41.Wenger, T., Jamali, K. A., Juaneda, C., Leonardelli, J. & Tramu, G. (1997) Biochem. Biophys. Res. Commun. 237, 724-728. [DOI] [PubMed] [Google Scholar]