Abstract
Cell based regenerative therapy has emerged as one of the most promising options of treatment for patients suffering from heart failure. Various adult stem cells types have undergone extensive clinical trials with limited success which is believed to be more of a cytokine effect rather than cell therapy. Pluripotent human embryonic stem cells (hESCs) have emerged as an attractive candidate stem cell source for obtaining cardiomyocytes (CMs) because of their tremendous capacity for expansion and unquestioned potential to differentiate into CMs. Studies carried out in animal models indicate that ES-derived CMs can partially remuscularize infarcted hearts and improve contractile function; however, the effect was not sustained over long follow up periods due to their limited capacity of cell division in vivo. Thus, the concept of transplanting multipotent cardiovascular progenitors derived from ES cells has emerged since the progenitors retain robust proliferative ability and multipotent nature enabling repopulation of other myocardial elements also in addition to CMs. Transplantation of CMs (progenitors) seeded in biodegradable scaffold and gel based engineered constructs has met with modest success due to issues like cell penetration, nutrient and oxygen availability and inflammation triggered during scaffold degradation inversely affecting the seeded cells. Recently cell sheet based tissue engineering involving culturing cells on ‘intelligent’ polymers has been evolved. Generation of a 3-D pulsatile myocardial tissue has been achieved. However, these advances have to be looked at with cautious optimism as many challenges need to be overcome before using these in clinical practice.
Keywords: Cardiomyocytes, infarct, progenitors, regeneration, stem cells
Introduction
The adult human myocardium has limited regenerative capacity and the muscle lost during infarction due to ischaemia, is replaced by non-contractile scar tissue ultimately initiating congestive heart failure. A typical human infarct involves the loss of a large number of cardiomyocytes. Although a broad array of treatment options are available, but conventional management of heart failure generally does not successfully replace lost cardiomyocyte mass or myocardial scar with new contractile cells. These issues have generated a lot of interest towards alternative treatment strategies.
Cell-based regenerative therapies have evolved quickly over the last decade and hold promise as a long term treatment strategy. Continuing research efforts are focused towards identifying endogenous or exogenous stem cells with the ability to differentiate into committed CMs and repopulate lost myocardium. As a result several cell types have been tested for cell based therapy including (Fig. 1) human foetal CMs, skeletal muscle myoblasts, smooth muscle cells, bone marrow-derived haematopoietic stem cells, mesenchymal stem cells, resident cardiac stem and progenitor cells, umbilical cord blood derived stem cells, adipose-derived stem cells, embryonic stem cells, induced pluripotent stem cells and recently very small embryonic like stem cells. Preclinical studies in animal disease models have indicated that some of these cardiogenic progenitors could contribute towards muscle replacement through endogenous reparative processes. The first clinical trial in patients produced some encouraging results, showing modest benefits mostly owing to favourable paracrine influence of stem cells on the disease microenvironment. A deeper analysis of the proposed paracrine effects revealed that the transplanted stem cells basically attenuated inflammation, increased angiogenesis, reduced apoptosis of the surrounding cells and promoted recovery of the injured tissue through wound healing1–9. However, there is a lot of disagreement regarding the ability of many of these cell types to truly differentiate into cardiomyocytes thus leading to controversies10,11.
Fig. 1.
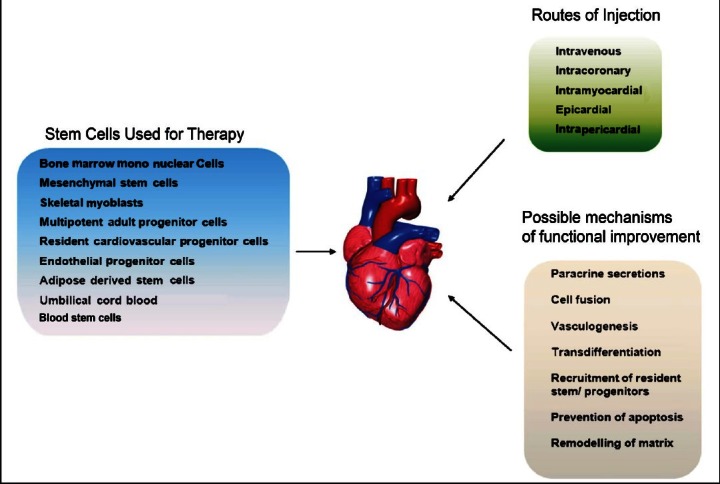
Schematic representation of the various adult stem cell types tested for cardiac regenerative abilities, their mode of action and the various routes of delivery.
Of all these cell types, bone marrow derived cells and skeletal myoblasts have been most extensively tried and tested in humans, but Phase I and II clinical trials to date have yielded mixed results12–18. Skeletal myocytes do not functionally integrate with the host cardiac tissue, are unable to form the appropriate gap junctions and arrhythmias have been induced when these cells are transplanted19. Though both these cell types were initially asserted to have cardiomyogenic potential, the preclinical studies have indicated otherwise20–23. In contrast human embryonic stem cells (hESCs) derived from the inner cell mass of spare blastocyst are pluripotent and can self-renew and differentiate into all cell types including CMs. Among various stem cell sources considered so far, hESCs are more promising for realizing cardiac regeneration through cardiomyocyte replacement because of their qualities. Firstly, the cardiomyogenic potential of hESCs is well established contrary to many adult stem cell types which have been pursued for cardiac regeneration24–26. Secondly, hESCs have the property of multi-lineage differentiation and thus can also be differentiated into smooth muscle cells and endothelial cells, enabling them to repopulate all myocardial tissue elements, lost during infarction not just CMs. Thirdly, well established protocols exist for isolation, maintenance and differentiation of hESCs and their scale up. hESC-derived CMs (hESC-CMs) exhibit robust proliferative capacity both in vitro25,27,28 and in vivo29, and transplanted hESC-CMs survive in experimental rodents models of infarction, form stable cardiac implants, attenuate ventricular remodelling and preserve contractile function30–32.
Cardiogenic differentiation of hESCs
The ability of hESCs to differentiate into spontaneously contracting cardiomyocytes has piqued the interest of many investigators. This led to a spurge of interest in studying the molecular embryogenesis of heart and identifying the key growth regulators and signaling pathways involved in heart formation. Three major approaches are widely used to derive CMs from hESCs, as reviewed elsewhere also33, without any genetic modification viz. (i) spontaneous differentiation by embryoid body (EB) formation, (ii) differentiation by co-culture with a visceral endoderm like cell line, and (iii) directed differentiation of cells in serum free/serum containing medium supplemented with various combinations of growth factors and small molecule inhibitors.
Embryoid body formation: CMs are one of the first cell types induced from pluripotent stem cells in EBs, where cell to cell interactions stimulate the expression of markers for mesodermal and early cardiac cell lineages34. Therefore, the first approach involves growing hESCs in suspension cultures or low adherent dishes or in hanging drops in a medium containing foetal bovine serum (FBS) resulting in formation of EBs which contract spontaneously post plating on gelatin coated dishes on around day 12 of culture24–26,35,36. The addition of growth factors and/or small molecules reducing oxygen tension, reducing insulin levels in the differentiation medium, electrical stimulation and aggregation in a novel v96 plate system are some of the approaches which have further enhanced directed differentiation of EBs to the cardiac lineage37–41.
Co-culture with END2 cells: This approach is based on co-culture of hESCs with a mouse visceral endodermal like cell line END-2 initially reported by Mummery et al42. During early embryonic development, it is known that the anterior endoderm secretes signaling molecules which guide the formation of the cardiogenic mesoderm in vivo and on similar premise co-culture of hESCs with END-2 cells induces beating within 12 days of co-culture even in cell lines which do not undergo cardiogenesis spontaneously42. Further studies have improved the efficiency of END-2 associated differentiation by omitting serum from the cultures43, adding small molecule inhibitors44 and by eliminating insulin from the co-culture medium45.
Directed differentiation: This approach involves treating high-density cultures of undifferentiated hESCs sequentially with cardiogenic mesoderm inducers activin A/nodal, bone morphogenetic protein like BMP2 and BMP4, members of the canonical and non-canonical wnt signaling cascade, members of the fibroblast growth factor (FGF) family and inhibitors of certain pathways in a stage specific manner in a defined culture medium. Laflamme et al30 reported directed differentiation of hESCs in both suspension and monolayer cultures by sequential treatment with activin A and BMP4 in a serum free medium. This method has proved to be much more efficient than EB-based methods that has yielded >30 per cent CMs30. Later on Yang and colleagues46 improved the efficacy of this approach (40 - 50%) using stage specific addition of activin A, BMP4, FGF-2, vascular endothelial growth factor (VEGF) and the wnt signaling inhibitor dickkopf homolog 1(Dkk-1). This led to the gradual transition of hES cells through the primitive streak stage, nascent mesoderm, cardiogenic mesoderm eventually to form early multipotent cardiovascular progenitors (MCPs). The MCPs on clonal expansion in culture gave rise to CMs, endothelial cells and smooth muscle cells. Various other groups have used similar protocols with different combinations of cytokines to achieve cardiac differentiation47–49. A recent study by Parsons et al50 reports nicotinamide (NAM) alone to be sufficient to induce the specification of cardio-mesoderm directly from pluripotent hESCs that further progressed to cardioblasts which in turn generated human beating cardiomyocytes with high efficiency. Several growth factors and small molecule inhibitors have been studied for their effects on cardiogenesis and consequently, human cardiomyogenesis in vitro is now becoming a process which, to a certain extent, can be effectively manipulated and directed.
Cardiomyocytes obtained by differentiating hES cells have been extensively characterized by transcriptional, immunocytochemical, ultrastructural, and functional endpoints. Phenotypically hESC-CMs exhibit, spherical, triangular or multi-angular, spindle shaped or rod shaped morphologies with randomly organized sarcomeres and intercalated discs resembling foetal CMs and are for most part elongated, striated cells, demonstrating intercalated Z discs and tight junctions between adjacent cells24–26,51. Various groups have carried out transcriptome analysis of the hESCs differentiating into CMs52–56 and one study has analysed the molecular signature of hESC-CM clusters57. hESC-CMs express a number of cardiac markers, including cardiac-specific transcription factors (Nkx2.5, Tbx5,Tbx 20,Mesp1 GATA4, MEF2c, and Isl1) sarcomeric proteins (cTNI, CTNT, sarcomeric MHCs and actins), and chamber-specific proteins (MLC2V and MLC2A and ANP) and ion channel genes24–26,58. These exhibit spontaneous beating activity, cardiac ionic currents specific to various developmental stages and nodal, atrial, and ventricular-like action potentials and generate a functional syncytium which has stable spontaneous pace making activity and synchronous action-potential propagation27,59–63.
We have two in-house derived hES cell lines KIND1 and KIND264, which show good propensity to differentiate into endoderm and mesoderm, respectively65. We have successfully differentiated KIND2 to yield tripotent cardiovascular progenitors using all the three differentiation approaches. The results indicate that as compared to spontaneous and END2 associated differentiation, directed differentiation of feeder-free KIND2 hES cells led to several folds higher expression of cardiac transcripts by quantitative-PCR (q-PCR66) and was therefore, the most efficient.
Cardiac regeneration using hESCs
First generation cell therapy: Pioneering preclinical studies were performed using mouse embryonic stem cells and these have laid the foundation for similar studies using hESCs67–74. Preclinical studies undertaken in both small and large animal disease models to assess the efficacy of hESC-CMs have been reviewed in the following sections. However, over the time it has become evident that since hESC-CMs are terminally differentiated, their beneficial effects on heart function were short-lived. The heart contains diverse muscle and non-muscle cell types and, therefore, regeneration of myocardium requires not just repopulation of functional CMs but also the development of a network of blood vessels (arterial and venous smooth muscle cells and endothelial cells) to support and nourish these newly formed CMs. This led to the hunt for a more primitive cell type which was multipotent and could proliferate more robustly. As a result, hESCs have been differentiated into early stage MCPs which have been shown to give rise to endothelial cells, smooth muscle cells and CMs both in vitro and in vivo post transplantation. The contributions of both these cell types towards cardiac regeneration have been discussed below (Fig. 2):
Fig. 2.
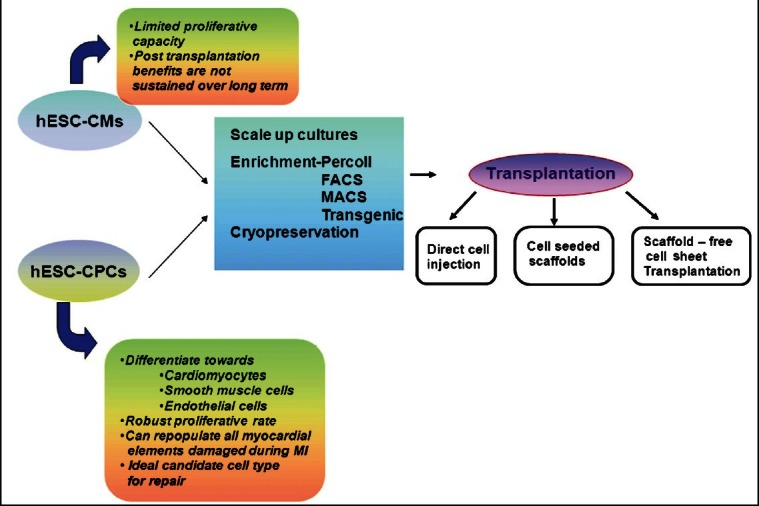
Work flow (thin arrows) and associated issues (big curved arrows) with hES cells derived cardiomyocytes (hESC-CMs) and cardiac progenitors (hESC-CPCs) during cell therapy. hESC-CMs or hESC-MCPs need to be expanded in culture, enriched and either cryopreserved for future use or directly used for transplantation by various routes as indicated. Enrichment strategies include density based Percoll gradient centrifugation, FACS / MACS using specific early cardiac markers or by transgenic overexpression of cardiac specific genes.
Preclinical studies using terminally differentiated hESC-CMs: Most of the initial cardiac transplantation experiments were performed in the uninjured heart. Klug and colleagues75 were the first to show that CMs derived from mouse ESCs could be purified using genetic selection and could form stable grafts in the mouse heart. Similarly when hESC-CMs were transplanted into the uninjured hearts of immunocompromised mice, rats, and pigs, these formed grafts of human myocardium29,76–78. Initial studies demonstrated that hESC-CMs could survive and integrate functionally following transplantation into pig hearts. The only setback was that the cells did not appear to mature considerably in vivo at the transplantation site. Transplantation of hESC-CMs to uninjured nude rat hearts showed that the cells survived, proliferated, and formed myocardial tissue78. The cells matured over the 4-week study period, and notably, non-cardiac cells present in the xenografts were preferentially cleared from the rat hearts78.
In experimental animal models of acute myocardial infarction (MI), beneficial effects on heart function have been reported after transplantation of hESC-CMs to the injury site. However, the risk of teratoma formation was highlighted and the implanted cells, in one study, did not integrate with the host tissue and signs of reactive fibrosis were documented79. Caspi and colleagues31 demonstrated surviving hESC-CM grafts in infarcted rat hearts for as long as 8 wk post transplantation, as well as improved left ventricular dimensions and function in cardiomyocyte recipients versus controls at 4 and 8 wk post transplantation by echocardiography. They also showed that transplantation of undifferentiated hESCs resulted in the formation of teratomas and that predifferentiating the hESCs to CMs ex vivo significantly reduces teratoma formation upon transplantation in infarcted rat hearts. To enhance graft survival, Laflamme and colleagues30 developed a pro-survival cocktail containing various anti-death factors, extracellular matrix - matrigel and survival factors which significantly improved survival of hESC- CMs in infarcted heart of nude rats. The grafts survived and were present even after 4 wk post transplantation and had remuscularized around 11 per cent of the infarct zone with the recipient hearts showing preserved global contractile function, regional wall thickening and left ventricular dimension versus the control. Van Laake et al32 examined the transplantation of hESC-CMs in a murine model i.e. acutely infarcted immunodeficient (NOD-SCID mice) and reported the survival of hESC-CM graft cells for as long as 12 wk post-transplantation. Magnetic resonance imaging (MRI) performed at 4 wk post transplantation indicated beneficial functional effects on left ventricular dimensions and function. However, the authors reported that these functional benefits were not observed at 12 wk79.
In conclusion, although studies with hESC-CMs have shown functional improvement post transplantation into an injured heart, these benefits are not sustained during long term follow up studies32,79. The potential pitfalls among others include (i) the lack of electrical coupling between the graft and the recipient tissue, (ii) inability of the hESC-CMs to keep pace with the rapid rodent heart rate (~450 beats/min for the rat), (iii) terminally differentiated CMs have a limited capacity of cell division due to which the benefits start diminishing with time, and lastly (iv) repopulating the CMs alone would not help as supporting cells and vasculature would also be required to nourish the graft in the hostile environment of the infarct.
Preclinical studies using hESC derived MCPs: From a clinical perspective, the ideal cardiac progenitor cells are those that can proliferate, survive and differentiate into multiple mature cardiac cell types when transplanted into normal or diseased heart80. Thus, MCPs are an attractive alternative due to their dual capacities of giving rise to multiple cell types in the heart and robust rate of proliferation. Once transplanted into the infarcted myocardium, the in vivo cues support further differentiation and maturation. Lineage tracing studies have uncovered different pools of cardiovascular progenitors in the early mouse, chick, drosophila and mammalian embryos81–84. Taking clues from these studies, several groups have now reported the isolation of MCPs during differentiation of both mouse and human ESCs using varied markers. Wu et al85 isolated Nkx2.5+ progenitors from developing transgenic mouse embryos and differentiating mouse ESC cultures. These progenitors were bipotential with the ability to form cardiomyocytes and smooth muscle cells. Several groups86–88 identified another population of MCPs marked by the second heart field markers Isl1 in mouse, rat and human myocardium and in differentiating human ESCs. These tripotent progenitors could give rise to cardiomyocytes, smooth muscle cells and endothelial cells. Kattman and colleagues89 isolated another tripotent cardiovascular progenitor population marked by brachyury (Bry) and VEGF receptor Flk-1 (foetal liver kinase-1), from mouse ESC derived EBs. This population appeared later and was distinct from the Bry+/ Flk-1+ hemangioblast population and gave rise to cardiomyocytes, smooth muscle cells and endothelial cells. Yang and colleagues46 have reported isolation of KDRlow/c-kit(CD117)neg MCPs during cardiovascular differentiation of ESCs, which retain the capacity to differentiate into three major cell types of the heart. Blin and colleagues90 identified a very early SSEA-1+ cardiac progenitor population derived from primate (human and monkey) ES and induced pluripotent stem cells (iPSCs) which appear even earlier than Flk1+ and other previously isolated MCPs. The cells could be induced to differentiate into any one of the three major cardiac lineages by treatment with specific growth factors like PDGF (platelet derived growth factor) for smooth muscle cells, VEGF for endothelial cells and conditioned medium of both fibroblasts and CMs for ventricular-like CMs.
Studies in a rat model of MI showed that injection of MCPs in the proximity of healed infarcts or stimulation of endogenous MCPs led to the replacement of approximately 42 per cent of the scar with newly-formed myocardium, attenuated ventricular dilation and prevented the chronic decline in function of the infarcted heart91. Smits and group92 isolated a sca+ cardiomyocyte progenitor population from human foetal hearts and stimulated its in vitro expansion by 5-azacytidine and transforming growth factor β (TGFβ) treatment. They compared the effects of transplantation of human cardiomyocyte progenitors versus those of cardiomyocytes derived from these progenitors in infarcted mouse hearts. The authors concluded that transplantation of both cell types resulted in a higher ejection fraction and reduced the extent of left ventricular remodelling up to 3 months after MI when compared with control animals. Both cell types also generated new cardiac tissue consisting of CMs and blood vessels and therefore, this excludes the need for in vitro predifferentiation of these progenitor cells into cardiomyocytes before transplantation. Blin et al90 transplanted rhesus monkey ES cells derived SSEA-1+ MCPs into an immunosuppressed rhesus monkey model of MI and reported their successful engraftment and differentiation into ventricular myocytes which reconstituted 20 per cent of the scar tissue. Christoforou and colleagues93 sorted green fluorescent protein (GFP) tagged MCPs under control of Nkx2.5 enhancer and differentiated these over 7-30 days before transplantation into infarcted mouse hearts. MCPs engrafted long-term in the infarct zone and surrounding myocardium without causing teratomas or arrhythmias and differentiated into cross-striated CMs forming gap junctions with the host cells, while also contributing to neovascularization. MCP transplantation also preserved the functional output of the infarcted heart.
Second generation cell therapy: Results from various clinical trials have indicated that cell based therapies involving direct injection of CMs lead to significant cell loss due to physical strain, primary hypoxia or cell wash-out. Time-course quantification using TUNEL assay has demonstrated that most of graft cells die within a few days in rat model94. To overcome this obstacle, tissue engineered myocardial patch transplantation has been examined as the second generation cell therapy. Delivery of cells in tissue-like structures that preserve cellular attachments could increase cell delivery efficiency and reduce cell death.
Scaffold based tissue engineering: The most popular approach of tissue engineering is to seed cells into 3-D biodegradable scaffolds as alternatives to extracellular matrix (ECM). It is hoped that the scaffold degrades over time; cells can proliferate and fill the space formerly occupied by the polymer resulting in tissue formation95. The use of scaffolds for tissue engineering is supportive for myocardial regeneration. Li et al96 were the first to demonstrate therapeutic potentials of myocardial patch using gelatin sponges. Leor and group97 reported that bioengineered heart grafts using porous alginate scaffolds attenuated left ventricular dilatation and heart function deterioration in MI model. Clinical trials of collagen-based myocardial patch with bone marrow cells and vicryl mesh-based patch seeded with fibroblasts are now undergoing98,99. Zimmermann et al100 have developed another tissue engineering technology by forming a gel of cell and collagen solution mixture. Their constructs also prevented further dilatation, induced systolic wall thickening of the left ventricular infarcted area, and improved fractional shortening of damaged hearts in a rat MI model101. Some researchers have reported that direct injection of cell and matrix solution mixture improved damaged heart function102.
Thus, tissue engineered myocardial patch transplantation may increase graft cell survival and improve damaged heart function more than isolated cell injection, spurring significant interest as second generation cell therapy for severe heart failure.
Cell sheet based tissue engineering: Scaffold-based myocardial patches have some disadvantages, such as insufficient cell migration into pre-fabricated scaffolds and inflammatory reactions due to polymer biodegradation. To overcome these issues, Shimizu et al103 established scaffold- free cell sheet-based tissue engineering. Cell sheets include pure cells without any ECM alternative materials and are transplantable onto damaged heart directly as a myocardial patch. The cells are cultured on an intelligent surface made of the temperature responsive polymer, poly (N-isopropylacrylamide) (PIPAAm). This surface has the unique property of being hydrophobic and cell adhesive under normal culture conditions at 37°C and hydrophilic and non-cell adhesive at temperatures lower than 32°C due to rapid hydration and swelling of the polymer. This property allows spontaneous detachment of the cells as continuous sheets with all the adjacent cell-to-cell junctions, ECM contacts and adhesive proteins intact just by simply lowering the temperature and negates the use of denaturing enzymes like trypsin. This system is highly advantageous as opposed to scaffold based culture system which requires the proteolytic degradation of the matrix in turn affecting cell-to-cell junctions and adhesive proteins. Cell sheets are directly transplanted onto heart surface without suture and cells can be effectively delivered as thin, but large-area cell-dense constructs without cell loss.
Zimmerman et al100 created beating rings which they termed “engineered heart tissue” by mixing murine CMs, with liquid collagen type I, matrigel, and serum-containing culture medium. The further development of these constructs to large, force generating grafts of multiple rings led to a remarkable improvement in ventricular function once transplanted on an infarcted heart101. Several studies have demonstrated cardiac function improvement by cell sheet transplantation104. Neonatal rat cardiac cell sheets transplanted onto infarcted rat hearts communicated with host myocardium both morphologically and functionally, resulting in significant improvement of left ventricular ejection fraction105.
Resident cardiac stem cells, mesenchymal stem cells, ES cells and a variety of other cell types have been used as sources for sheet fabrication. Implantation of these sheets onto the injured myocardium has shown modest benefits. As a step further, Shimizu and coworkers106 have proposed 2-D cell sheet stacking to create cell-dense 3-D myocardial tissues which may pulsate synchronously and evoke native contraction power. Long-term observation has showed one year survival of beating grafts and revealed that their size, conduction velocity, and contractile force increased in proportion to the host growth, indicating in vivo permanent survival of engineered tissues. Stacked cardiomyocyte sheets transplanted into nude rat subcutaneous tissues also pulsated and surface electrogram originating from the graft was detectable apart from host electrocardiogram. Morphological analyses showed characteristic structures of heart tissue including elongated CMs, well-differentiated sarcomeres, gap junctions and multiple blood vessels107. Another group108 has created a bioartificial heart by detergent based decellularization of rat hearts and repopulation with neonatal cardiac cells or rat aortic endothelial cells. These recellularized constructs were cultured under simulated physiological conditions for organ maturation. Ultimately, chronic coronary perfusion, pulsatile left ventricular load and synchronized left ventricular stimulation led to the formation of contractile myocardium that performed stroke work.
Transplantation of three layered grafts made of endothelial cell sheets and cardiomyocyte sheets also enhanced neovascularization and improved cardiac function rather than CM sheets alone109. Stevens et al110 designed “tri-cell” cardiac patches called cardio-HUVEC-MEF patches containing hESC-CMs, human umbilical vein endothelial cells (HUVECs), and mouse embryonic fibroblasts (MEFs) in 1:1:0.5 ratios, respectively. Transplantation of these tri-cell patches into nude rats resulted in 10-fold larger cell grafts as compared to patches composed only of CMs. The authors concluded that inclusion of vascular and stromal elements enhanced the in vitro performance of engineered human myocardium. Bel and colleagues111 developed a composite cell sheet made of adipose derived stromal cell seeded with ES derived MCPs which when transplanted in rhesus monkeys was able to differentiate into CMs and reconstitute up to 20 per cent of the scar tissue. There was robust engraftment of autologous adipose derived stromal cells which were transplanted in sheet form along with the MCP sheet and increased angiogenesis compared to sham animals. Zakharova et al112 showed high efficiency of engraftment with cell sheet grafts containing cardiomyocyte progenitors and cardiac stromal cells. The transplanted cells effectively rescued myocardium function after infarction by promoting not only neovascularization but also inducing a significant level of cardiomyogenesis.
Emerging trends
Induced pluripotent stem cells (iPSCs) for cardiovascular repair: iPSCs are novel pluripotent stem cells generated from adult somatic tissues by reprogramming originally with transduction of a few defined transcription factors, such as Oct4, Sox2, Klf4, and c-myc113. Recent studies have demonstrated that human iPSCs may prove to be an additional source of cardiomyocytes which share similarities with hESC-CMs114–117. It has been shown that iPSCs are similar to hESCs in terms of their morphology, proliferation, feeder dependence, surface markers, gene expression, epigenetic status, formation of EBs in vitro, promoter activities, telomerase activities, and in vivo teratoma formation118. Technically, iPSCs are cultured under conditions virtually identical to those for hESCs. Using the same cardiac differentiation protocol originally developed for hESCs, iPSCs can be similarly differentiated into CMs119. Human iPSC-CMs have been implanted in mouse models of MI, and these regenerated myocardium, smooth muscle, and endothelial tissue, restoring post-ischaemic contractility performance and electric stability120. Mauritz et al121 have compared functional benefits of iPSC-derived Flk-1+ve MCPs vs Flk-1-ve cells by injection into a mouse model of acute MI. The Flk-1+ve cells showed better engraftment and exerted superior functional benefits as compared to Flk-1-ve cells. iPSC derived MCPs cells thus represent a suitable autologous cell source for myocardial regeneration as these are committed, have the capability to form myocardial cells and contribute to revascularization. However, clinical trials in gene therapy have shown that the integration of viral vectors in the genome may promote malignancy; besides some of the virus-encoded genes can act as oncogenes. Further, comprehensive analyses of various ESC and iPSC lines showed that iPSCs differ from ESCs at the molecular level when comparing gene expression signatures80,81. When multiple transcriptional datasets from human fibroblasts were compared with ESCs and iPSCs derived from fibroblasts, it was found that iPSCs shared four times more genes in common with fibroblasts than ESCs122. Besides differences at the level of mRNA expression, microRNA expression also differs between iPSCs and ESCs, with some cancer related microRNAs highly expressed in iPSCs123–125. Thus, the clinical application of iPSC derived cardiomyocytes appears to be a distant possibility at present.
Very small embryonic like stem cells (VSELs) for cardiovascular repair: VSELs are a unique population of rare Oct-4+CXCR4+ CD133+lin-CD45- pluripotent stem cells found in human tissues including cord blood and bone marrow126 that are deposited during early embryogenesis in developing organs/tissues as a reserve population of pluripotent stem cells to serve as a backup pool of stem cells responsible to maintain life-long tissue homeostasis. These were initially identified by Kucia et al127 in adult murine bone marrow (BM) as a rare population of stem cells with embryonic characteristics. Wojakowski et al128 have reported an ex vivo expansion and differentiation protocol for differentiation of BM-derived VSELs into CMs. These stem cells get mobilized into blood in patients with acute coronary syndromes and stroke129,130.
Dawn and colleagues130 have shown that direct intramyocardial injection of VSELs 48 h after coronary artery occlusion in mice had promising effects. Administration of VSELs led to improved contractility and reduced remodelling as well as reduced myocyte hypertrophy after 35 days of follow up. In mice that received HSCs no such effects occurred. Beneficial effects were observed despite use of only small number (10,000 cells) of VSELs whereas a much higher number of hematopoietic cells (100,000 cells) were not effective. Predifferentiation of expanded VSELs allowed the use of larger numbers of cells resulted in a much improved left ventricular ejection fraction, myocardial systolic thickening and attenuated remodelling sustained over the 6 month follow up131,132.
VSELs share pluripotent characteristics with ES cells but do not form teratoma and can be isolated from an autologus source133. Thus both the major drawbacks associated with ES cells like immune-rejection and risk of teratoma formation can be overcome by use of VSELs. These cells have the potential but much remains to be done to exploit these in the field of regenerative medicine.
Conclusions
Although adult stem cells, such as bone marrow, skeletal myoblast and mesenchymal stem cells are the most common cell source for cardiac regeneration in clinical trials as these have no risks of immune rejection with autologous transplantation, these are non-tumourigenic, and have no ethical obstacle; these are restricted in their renewal and differentiation potential to become functional CMs. Cardiomyogenic potential of iPS cells is well established both in vitro and in vivo. However, significant challenges, such as risk of viral vector and low efficacy during reprogramming as well as tumour formation remain to be overcome before the translation of iPS cell technology into clinical practice. Human ES cells and VSELs are being extensively pursued based on positive data from preliminary studies in small animal disease models. These represent an ideal cell source with the potential of unlimited expansion, differentiation into desired cell types in a controlled manner and well established functional benefits upon transplantation. Issues like improving efficacy of cardiac differentiation protocols, large scale generation of pure populations of MCPs or CMs, appropriate time of transplantation post MI, the right cell number to be transplanted, site of transplantation, low oxygen and nutrient penetration in thick stacked cell sheets, immune rejection and the like warrant further and careful studies in preclinical models.
References
- 1.Kupatt C, Horstkotte J, Vlastos GA, Pfosser A, Lebherz C, Semisch M, et al. Embryonic endothelial progenitor cells expressing a broad range of pro-angiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J. 2005;19:1576–8. doi: 10.1096/fj.04-3282fje. [DOI] [PubMed] [Google Scholar]
- 2.Kupatt C, Hinkel R, Lamparter M, von Brühl M-L, Pohl T, Horstkotte J, et al. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: Role of PI3-Kinase/AKT. Circulation. 2005;112(Suppl 9):117–22. doi: 10.1161/CIRCULATIONAHA.104.524801. [DOI] [PubMed] [Google Scholar]
- 3.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11:367–8. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 4.Uemura R, Xu M, Ahmad N, Ashraf M. Bone marrow stem cells prevent left ventricular remodeling of ischemic heart through paracrine signaling. Circ Res. 2006;98:1414–21. doi: 10.1161/01.RES.0000225952.61196.39. [DOI] [PubMed] [Google Scholar]
- 5.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirotsou M, Zhang Z, Deb A, Zhang L, Gnecchi M, Noiseux N, et al. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;104:1643–8. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinkel R, El-Aouni C, Olson T, Horstkotte J, Mayer S, Müller S, et al. Thymosin β4 is an essential paracrine factor of embryonic endothelial progenitor cell mediated cardioprotection. Circulation. 2008;117:2232–40. doi: 10.1161/CIRCULATIONAHA.107.758904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alfaro MP, Pagni M, Vincent A, Atkinson J, Hill MF, Cates J, et al. The Wnt modulator sFRP2 enhances mesenchymal stem cell engraftment, granulation tissue formation and myocardial repair. Proc Natl Acad Sci USA. 2008;105:18366–71. doi: 10.1073/pnas.0803437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boudoulas KD, Hatzopoulos AK. Cardiac repair and regeneration: The Rubik's cube of cell therapy for heart disease. Dis Model Mech. 2009;2:344–58. doi: 10.1242/dmm.000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vieyra DS, Jackson KA, Goodell MA. Plasticity and tissue regenerative potential of bone marrow-derived cells. Stem Cell Rev. 2005;1:65–9. doi: 10.1385/SCR:1:1:065. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 13.Menasche P. Skeletal myoblasts as a therapeutic agent. Prog Cardiovasc Dis. 2007;50:7–17. doi: 10.1016/j.pcad.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Schuleri KH, Boyle AJ, Hare JM. Mesenchymal stem cells for cardiac regenerative therapy. Handb Exp Pharmacol. 2007;180:195–218. doi: 10.1007/978-3-540-68976-8_9. [DOI] [PubMed] [Google Scholar]
- 15.Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, et al. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113–21. doi: 10.1016/S0140-6736(05)67861-0. [DOI] [PubMed] [Google Scholar]
- 16.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 17.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance STelevation infarct regeneration) trial. Circulation. 2006;113:1287–94. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 18.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 19.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. J Cell Biol. 2000;149:731–40. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–9. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 21.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, et al. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–8. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 22.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 23.Nygren JM, Jovinge S, Breitbach M, Säwén P, Röll W, Hescheler J, et al. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10:494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 24.Kehat I, Kenyagin-Karsenti D, Snir M, Segev H, Amit M, Gepstein A, et al. Human embryonic stem cells can differentiate into myocytes with structural and functional properties of cardiomyocytes. J Clin Invest. 2001;108:407–14. doi: 10.1172/JCI12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 26.Mummery C, Ward D, van den Brink CE, Bird SD, Doevendans PA, Opthof T, et al. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–42. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, et al. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–63. doi: 10.1152/ajpheart.00020.2003. [DOI] [PubMed] [Google Scholar]
- 28.McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39:865–73. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laflamme MA, Gold J, Xu C, Hassanipour M, Rosler E, Police S, et al. Formation of human myocardium in the rat heart from human embryonic stem cells. Am J Pathol. 2005;167:663–71. doi: 10.1016/S0002-9440(10)62041-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 31.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, et al. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 32.van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Res. 2007;1:9–24. doi: 10.1016/j.scr.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Vidarsson H, Hyllner J, Sartipy P. Differentiation of human embryonic stem cells to cardiomyocytes for in vitro and in vivo applications. Stem Cell Rev. 2010;6:108–20. doi: 10.1007/s12015-010-9113-x. [DOI] [PubMed] [Google Scholar]
- 34.Rajala K, Pekkanen-Mattila M, Aalto-Setälä K. Cardiac differentiation of pluripotent stem cells. 2011; 2011: 383790. Stem Cells Int. 2011;912 doi: 10.4061/2011/383709. 2011: 383790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- 36.Segev H, Kenyagin-Karsenti D, Fishman B, Gerecht-Nir S, Ziskind A, Amit M, et al. Molecular analysis of cardiomyocytes derived from human embryonic stem cells. Dev Growth Differ. 2005;47:295–306. doi: 10.1111/j.1440-169X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 37.Tomescot A, Leschik J, Bellamy V, Dubois G, Messas E, Bruneval P, et al. Differentiation in vivo of cardiac committed human embryonic stem cells in post myocardial infarcted rats. Stem Cells. 2007;25:2200–5. doi: 10.1634/stemcells.2007-0133. [DOI] [PubMed] [Google Scholar]
- 38.Burridge PW, Anderson D, Priddle H, Barbadillo Muñoz MD, Chamberlain S, Allegrucci C, et al. Improved human embryonic stem cell embryoid body homogeneity and cardiomyocyte differentiation from a novel V-96 plate aggregation system highlights interline variability. Stem Cells. 2007;25:929–38. doi: 10.1634/stemcells.2006-0598. [DOI] [PubMed] [Google Scholar]
- 39.Niebruegge S, Bauwens CL, Peerani R, Thavandiran N, Masse S, Sevaptisidis E, et al. Generation of human embryonic stem cell-derived mesoderm and cardiac cells using size-specified aggregates in an oxygen-controlled bioreactor. Biotechnol Bioeng. 2009;102:493–507. doi: 10.1002/bit.22065. [DOI] [PubMed] [Google Scholar]
- 40.Serena E, Figallo E, Tandon N, Cannizzaro C, Gerecht S, Elvassore N, et al. Electrical stimulation of human embryonic stem cells: cardiac differentiation and the generation of reactive oxygen species. Exp Cell Res. 2009;315:3611–9. doi: 10.1016/j.yexcr.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, et al. A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability. PLoS One. 2011;6(4):e18293. doi: 10.1371/journal.pone.0018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mummery C, Ward D, van den Brink CE, Bird SD, Doevendans PA, Opthof T, et al. Cardiomyocyte differentiation of mouse and human embryonic stem cells. J Anat. 2002;200:233–42. doi: 10.1046/j.1469-7580.2002.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passier R, Oostwaard DW, Snapper J, Kloots J, Hassink RJ, Kuijk E, et al. Increased cardiomyocyte differentiation from human embryonic stem cells in serum-free cultures. Stem Cells. 2005;23:772–80. doi: 10.1634/stemcells.2004-0184. [DOI] [PubMed] [Google Scholar]
- 44.Graichen R, Xu X, Braam SR, Balakrishnan T, Norfiza S, Sieh S, et al. Enhanced cardiomyogenesis of human embryonic stem cells by a small molecular inhibitor of p38 MAPK. Differentiation. 2008;76:357–70. doi: 10.1111/j.1432-0436.2007.00236.x. [DOI] [PubMed] [Google Scholar]
- 45.Freund C, Ward-van Oostwaard D, Monshouwer-Kloots J, van den Brink S, van Rooijen M, Xu X, et al. Insulin redirects differentiation from cardiogenic mesoderm and endoderm to neuroectoderm in differentiating human embryonic stem cells. Stem Cells. 2008;26:724–33. doi: 10.1634/stemcells.2007-0617. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 47.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H. Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development. 2008;135:2969–79. doi: 10.1242/dev.021121. [DOI] [PubMed] [Google Scholar]
- 48.Nakanishi M, Kurisaki A, Hayashi Y, Warashina M, Ishiura S, Kusuda-Furue M, et al. Directed induction of anterior and posterior primitive streak by Wnt from embryonic stem cells cultured in a chemically defined serum-free medium. FASEB J. 2009;23:114–22. doi: 10.1096/fj.08-111203. [DOI] [PubMed] [Google Scholar]
- 49.Paige SL, Osugi T, Afanasiev OK, Pabon L, Reinecke H, Murry CE. Endogenous Wnt/β-catenin signaling is required for cardiac differentiation in human embryonic stem cells. PLoS One. 2010;5(6):e11134. doi: 10.1371/journal.pone.0011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parsons XH, Teng YD, Parsons JF, Snyder EY, Smotrich DB, Moore DA. Efficient derivation of human cardiac precursors and cardiomyocytes from pluripotent human embryonic stem cells with small molecule induction. J Vis Exp. 2011;57:e3274. doi: 10.3791/3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norström A, Akesson K, Hardarson T, Hamberger L, Björquist P, Sartipy P. Molecular and pharmacological properties of human embryonic stem cell-derived cardiomyocytes. Exp Biol Med (Maywood) 2006;231:1753–62. doi: 10.1177/153537020623101113. [DOI] [PubMed] [Google Scholar]
- 52.Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D, Yanuka O, et al. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum Reprod. 2004;19:2875–83. doi: 10.1093/humrep/deh529. [DOI] [PubMed] [Google Scholar]
- 53.Beqqali A, Kloots J, Ward-van Oostwaard D, Mummery C, Passier R. Genome-wide transcriptional profiling of human embryonic stem cells differentiating to cardiomyocytes. Stem Cells. 2006;24:1956–67. doi: 10.1634/stemcells.2006-0054. [DOI] [PubMed] [Google Scholar]
- 54.Synnergren J, Adak S, Englund MC, Giesler TL, Noaksson K, Lindahl A, et al. Cardiomyogenic gene expression profiling of differentiating human embryonic stem cells. J Biotechnol. 2008;134:162–70. doi: 10.1016/j.jbiotec.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, et al. Transcriptional and functional profilling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu XQ, Soo SY, Sun W, Zweigerdt R. Global expression profile of highly enriched cardiomyocytes derived from human embryonic stem cells. Stem Cells. 2009;27:2163–74. doi: 10.1002/stem.166. [DOI] [PubMed] [Google Scholar]
- 57.Tran TH, Wang X, Browne C, Zhang Y, Schinke M, Izumo S, et al. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells. 2009;27:1869–78. doi: 10.1002/stem.95. [DOI] [PubMed] [Google Scholar]
- 58.Segev H, Kenyagin-Karsenti D, Fishman B, Gerecht-Nir S, Ziskind A, Amit M, et al. Molecular analysis of cardiomyocytes derived from human embryonic stem cells. Dev Growth Differ. 2005;47:295–306. doi: 10.1111/j.1440-169X.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- 59.Maltsev VA, Rohwedel J, Hescheler J, Wobus AM. Embryonic stem cells differentiate in vitro into cardiomyocytes representing sinusnodal, atrial and ventricular cell types. Mech Dev. 1993;44:41–50. doi: 10.1016/0925-4773(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 60.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 61.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 62.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Hzhaki I, et al. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 64.Kumar N, Hinduja I, Nagvenkar P, Pillai L, Zaveri K, Mukadam L, et al. Derivation and characterization of two genetically unique human embryonic stem cell lines on in-house-derived human feeders. Stem Cells Dev. 2009;18:435–45. doi: 10.1089/scd.2008.0234. [DOI] [PubMed] [Google Scholar]
- 65.Nagvenkar P, Pethe P, Pawani H, Telang J, Kumar N, Hinduja I, et al. Evaluating differentiation propensity of in-house derived human embryonic stem cell lines KIND-1 and KIND-2. In Vitro Cell Dev Biol Anim. 2011;47:406–19. doi: 10.1007/s11626-011-9420-9. [DOI] [PubMed] [Google Scholar]
- 66.Pawani H, Nagvenkar P, Pethe P, Bhartiya D. Differentiation of human ES cell line KIND2 to yield tripotent cardiovascular progenitors. In vitro Cell Dev Biol Anim. 2013;49:82–93. doi: 10.1007/s11626-012-9558-0. [DOI] [PubMed] [Google Scholar]
- 67.Min JY, Yang Y, Converso KL, Liu L, Huang Q, Morgan JP, et al. Transplantation of embryonic stem cells improves cardiac function in post infarcted rats. J Appl Physiol. 2002;92:288–96. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- 68.Min JY, Yang Y, Sullivan MF, Ke Q, Converso KL, Chen Y, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–9. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 69.Ménard C, Hagège AA, Agbulut O, Barro M, Morichetti MC, Brasselet C, et al. Transplantation of cardiac-committed mouse embryonic stem cells to infarcted sheep myocardium: a preclinical study. Lancet. 2005;366:1005–12. doi: 10.1016/S0140-6736(05)67380-1. [DOI] [PubMed] [Google Scholar]
- 70.Naito H, Nishizaki K, Yoshikawa M, Yamada T, Satoh H, Nagasaka S, et al. Xenogeneic embryonic stem cell-derived cardiomyocyte transplantation. Transplant Proc. 2004;36:2507–8. doi: 10.1016/j.transproceed.2004.06.031. [DOI] [PubMed] [Google Scholar]
- 71.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to theinfarcted myocardium. J Exp Med. 2006;203:2315–27. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hodgson DM, Behfar A, Zingman LV, Kane GC, Perez-Terzic C, Alekseev AE, et al. Stable benefit of embryonic stem cell therapy in myocardial infarction. Am J Physiol Heart Circ Physiol. 2004;287:H471–9. doi: 10.1152/ajpheart.01247.2003. [DOI] [PubMed] [Google Scholar]
- 73.Cho SW, Gwak SJ, Kim IK, Cho MC, Hwang KK, Kwon JS, et al. Granulocyte colony-stimulating factor treatment enhances the efficacy of cellular cardiomyoplasty with transplantation of embryonic stem cell-derived cardiomyocytes in infarcted myocardium. Biochem Biophys Res Commun. 2006;340:573–82. doi: 10.1016/j.bbrc.2005.12.044. [DOI] [PubMed] [Google Scholar]
- 74.Shiba Y, Hauch KD, Laflamme MA. Cardiac applications for human pluripotent stem cells. Curr Pharm Des. 2009;15:2791–806. doi: 10.2174/138161209788923804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Klug MG, Soonpaa MH, Koh GY, Field LJ. Genetically selected cardiomyocytes from differentiating embryonic stem cells form stable intracardiac grafts. J Clin Invest. 1996;98:216–24. doi: 10.1172/JCI118769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, et al. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 77.Dai W, Field LJ, Rubart M, Reuter S, Hale SL, Zweigerdt R, et al. Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts. J Mol Cell Cardiol. 2007;43:504–51. doi: 10.1016/j.yjmcc.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, et al. Human embryonic stem cell transplantation to repair the infarcted myocardium. Heart. 2007;93:1278–84. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Laake LW, Passier R, Monshouwer-Kloots J, Nederhoff MG, Ward-van Oostwaard D, Field LJ, et al. Monitoring of cell therapy and assessment of cardiac function using magnetic resonance imaging in a mouse model of myocardial infarction. Nat Protoc. 2007;2:2551–67. doi: 10.1038/nprot.2007.371. [DOI] [PubMed] [Google Scholar]
- 80.Qian L, Srivastava D. Monkeying around with cardiac progenitors: hope for the future. J Clin Invest. 2010;120:1034–6. doi: 10.1172/JCI42643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Srivastava D, Ivey KN. Potential of stem-cell-based therapies for heart disease. Nature. 2006;441:1097–9. doi: 10.1038/nature04961. [DOI] [PubMed] [Google Scholar]
- 82.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 83.Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature. 2008;453:322–9. doi: 10.1038/nature07040. [DOI] [PubMed] [Google Scholar]
- 84.Yi BA, Wernet O, Chien KR. Pregenerative medicine: developmental paradigms in the biology of cardiovascular regeneration. J Clin Invest. 2010;120:20–8. doi: 10.1172/JCI40820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 86.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, et al. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 87.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, et al. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bu L, Jiang X, Martin-Puig S, Caron L, Zhu S, Shao Y, et al. Human ISL1 heart progenitors generate diverse multipotent cardiovascular cell lineages. Nature. 2009;460:113–7. doi: 10.1038/nature08191. [DOI] [PubMed] [Google Scholar]
- 89.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell. 2006;11:723–32. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 90.Blin G, Nury D, Stefanovic S, Neri T, Guillevic O, Brinon B, et al. A purified population of multipotent cardiovascular progenitors derived from primate pluripotent stem cells engrafts in postmyocardial infarcted nonhuman primates. J Clin Invest. 2010;120:1125–39. doi: 10.1172/JCI40120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, et al. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res. 2008;103:107–16. doi: 10.1161/CIRCRESAHA.108.178525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smits AM, van Laake LW, den Ouden K, Schreurs C, Szuhai K, van Echteld CJ, et al. Human cardiomyocyte progenitor cell transplantation preserves long-term function of the infarcted mouse myocardium. Cardiovasc Res. 2009;83:527–35. doi: 10.1093/cvr/cvp146. [DOI] [PubMed] [Google Scholar]
- 93.Christoforou N, Oskouei BN, Esteso P, Hill CM, Zimmet JM, Bian W, et al. Implantation of mouse embryonic stem cell-derived cardiac progenitor cells preserves function of infarcted murine hearts. PLoS One. 2010;5:e11536. doi: 10.1371/journal.pone.0011536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang M, Methot D, Poppa V, Fujio Y, Walsh K, Murry CE. Cardiomyocyte grafting for cardiac repair: graft cell death and anti death strategies. J Mol Cell Cardiol. 2001;33:907–21. doi: 10.1006/jmcc.2001.1367. [DOI] [PubMed] [Google Scholar]
- 95.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 96.Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM. Survival and function of bioengineered cardiac grafts. Circulation. 1999;100(19 Suppl):II63–9. doi: 10.1161/01.cir.100.suppl_2.ii-63. [DOI] [PubMed] [Google Scholar]
- 97.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, et al. Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium? Circulation. 2000;102(19 Suppl 3):III56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 98.Chachques JC, Trainini JC, Lago N, Masoli OH, Barisani JL, Cortes-Morichetti M, et al. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): one year follow-up. Cell Transplant. 2007;16:927–34. doi: 10.3727/096368907783338217. [DOI] [PubMed] [Google Scholar]
- 99.Mirsadraee S, Wilcox HE, Korossis SA, Kearney JN, Watterson KG, Fisher J, et al. Development and characterization of an acellular human pericardial matrix for tissue engineering. Tissue Eng. 2006;12:763–73. doi: 10.1089/ten.2006.12.763. [DOI] [PubMed] [Google Scholar]
- 100.Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, et al. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–30. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 101.Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 102.Kofidis T, de Bruin JL, Hoyt G, Lebl DR, Tanaka M, Yamane T, et al. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J Thorac Cardiovasc Surg. 2004;128:571–8. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 103.Shimizu T, Yamato M, Kikuchi A, Okano T. Cell sheet engineering for myocardial tissue reconstruction. Biomaterials. 2003;24:2309–16. doi: 10.1016/s0142-9612(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 104.Masuda S, Shimizu T, Yamato M, Okano T. Cell sheet engineering for heart tissue repair. Adv Drug Deliv Rev. 2008;60:277–85. doi: 10.1016/j.addr.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 105.Miyagawa S, Sawa Y, Sakakida S, Taketani S, Kondoh H, Memon IA, et al. Tissue cardiomyoplasty using bioengineered contractile cardiomyocyte sheets to repair damaged myocardium: their integration with recipient myocardium. Transplantation. 2005;80:1586–95. doi: 10.1097/01.tp.0000181163.69108.dd. [DOI] [PubMed] [Google Scholar]
- 106.Shimizu T, Sekine H, Isoi Y, Yamato M, Kikuchi A, Okano T. Long-term survival and growth of pulsatile myocardial tissue grafts engineered by the layering of cardiomyocyte sheets. Tissue Eng. 2006;12:499–507. doi: 10.1089/ten.2006.12.499. [DOI] [PubMed] [Google Scholar]
- 107.Furuta A, Miyoshi S, Itabashi Y, Shimizu T, Kira S, Hayakawa K, et al. Pulsatile cardiac tissue grafts using a novel three-dimensional cell sheet manipulation technique functionally integrates with the host heart, in vivo. Circ Res. 2006;98:705–12. doi: 10.1161/01.RES.0000209515.59115.70. [DOI] [PubMed] [Google Scholar]
- 108.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 109.Sekine H, Shimizu T, Hobo K, Sekiya S, Yang J, Yamato M, et al. Endothelial cell co-culture within tissue-engineered cardiomyocyte sheets enhances neovascularization and improves cardiac function of ischemic hearts. Circulation. 2008;118(14 Suppl):S145–52. doi: 10.1161/CIRCULATIONAHA.107.757286. [DOI] [PubMed] [Google Scholar]
- 110.Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, et al. Physiological function and transplantation of scaffold-free and vascularized human cardiac muscle tissue. Proc Natl Acad Sci USA. 2009;106:16568–73. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bel A, Planat-Bernard V, Saito A, Bonnevie L, Bellamy V, Sabbah L, et al. Composite cell sheets: a further step toward safe and effective myocardial regeneration by cardiac progenitors derived from embryonic stem cells. Circulation. 2010;122(11 Suppl):S118–23. doi: 10.1161/CIRCULATIONAHA.109.927293. [DOI] [PubMed] [Google Scholar]
- 112.Zakharova L, Mastroeni D, Mutlu N, Molina M, Goldman S, Diethrich E, et al. Transplantation of cardiac progenitor cell sheet onto infarcted heart promotes cardiogenesis and improves function. Cardiovasc Res. 2010;87:40–9. doi: 10.1093/cvr/cvq027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 114.Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, et al. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30–41. doi: 10.1161/CIRCRESAHA.108.192237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chiriac A, Nelson TJ, Faustino RS, Behfar A, Terzic A. Cardiogenic induction of pluripotent stem cells streamlined through a conserved SDF-1/VEGF/BMP2 integrated network. PLoS One. 2010;5:e9943. doi: 10.1371/journal.pone.0009943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zwi L, Caspi O, Arbel G, Huber I, Gepstein A, Park IH, et al. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation. 2009;120:1513–23. doi: 10.1161/CIRCULATIONAHA.109.868885. [DOI] [PubMed] [Google Scholar]
- 117.Haase A, Olmer R, Schwanke K, Wunderlich S, Merkert S, Hess C, et al. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell. 2009;5:434–41. doi: 10.1016/j.stem.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 118.Kong CW, Akar FG, Li RA. Translational potential of human embryonic and induced pluripotent stem cells for myocardial repair: Insights from experimental models. Thromb Haemost. 2010;104:30–8. doi: 10.1160/TH10-03-0189. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 120.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mauritz C, Martens A, Rojas SV, Schnick T, Rathert C, Schecker N, et al. Induced pluripotent stem cell (iPSC)-derived Flk-1 progenitor cells engraft, differentiate, and improve heart function in a mouse model of acute myocardial infarction. Eur Heart J. 2011;32:2634–41. doi: 10.1093/eurheartj/ehr166. [DOI] [PubMed] [Google Scholar]
- 122.Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PloS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chin MH, Mason MJ, Xie W, Volinia S, Singer M, Peterson C, et al. Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell. 2009;5:111–2353. doi: 10.1016/j.stem.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Mah N, Prigione A, Wolfrum K, Andrade-Navarro MA, Adjaye J. A transcriptional roadmap to the induction of pluripotency in somatic cells. Stem Cell Rev. 2010;6:282–96. doi: 10.1007/s12015-010-9137-2. [DOI] [PubMed] [Google Scholar]
- 125.Malchenko S, Galat V, Seftor EA, Vanin EF, Costa FF, Seftor RE, et al. Cancer hallmarks in induced pluripotent cells: New insights. J Cell Physiol. 2010;225:390–3. doi: 10.1002/jcp.22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bhartiya D, Shaikh A, Nagvenkar P, Kasiviswanathan S, Pethe P, Pawani H, et al. Very small embryonic-like stem cells with maximum regenerative potential get discarded during cord blood banking and bone marrow processing for autologous stem cell therapy. Stem Cells Dev. 2012;21:1–6. doi: 10.1089/scd.2011.0311. [DOI] [PubMed] [Google Scholar]
- 127.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4 (+) SSEA-1 (+) Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 128.Wojakowski W, Tendera M, Kucia M, Zuba-Surma E, Milewski K, Wallace-Bradley D, et al. Cardiomyocyte differentiation of bone marrow-derived Oct-4+ CXCR4+SSEA-1+ very small embryonic-like stem cells. Int J Oncol. 2010;37:237–47. doi: 10.3892/ijo_00000671. [DOI] [PubMed] [Google Scholar]
- 129.Kucia M, Dawn B, Hunt G, Guo Y, Wysoczynski M, Majka M, et al. Cells expressing early cardiac markers reside in the bone marrow and are mobilized into the peripheral blood after myocardial infarction. Circ Res. 2004;95:1191–9. doi: 10.1161/01.RES.0000150856.47324.5b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dawn B, Tiwari S, Kucia MJ, Zuba-Surma EK, Guo Y, Sanganalmath SK, et al. Transplantation of bone marrow-derived very small embryonic-like stem cells attenuates left ventricular dysfunction and remodeling after myocardial infarction. Stem Cells. 2008;26:1646–55. doi: 10.1634/stemcells.2007-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wojakowski W, Ratajczak MZ, Tendera M. Mobilization of very small embryonic-like stem cells in acute coronary syndromes and stroke. Herz. 2010;35:467–72. doi: 10.1007/s00059-010-3389-0. [DOI] [PubMed] [Google Scholar]
- 132.Zuba-Surma EK, Guo Y, Taher H, Sanganalmath SK, Hunt G, Vincent RJ, et al. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodeling after myocardial infarction. J Cell Mol Med. 2011;15:1319–28. doi: 10.1111/j.1582-4934.2010.01126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhartiya D. Pluripotent VSELs get discarded during cord blood & bone marrow processing. Stem Cells Dev. 2012;21:2563–4. doi: 10.1089/scd.2011.0311. [DOI] [PubMed] [Google Scholar]


