Abstract
A cell free system prepared from etiolated cucumber (Cucumis sativus, L) in tris-sucrose buffer is able to incorporate δ-aminolevulinic acid-4- 14C into the two components of protochlorophyll: protochlorophyllide and protochlorophyllide ester. The activity is associated with the etioplasts. Optimal incorporation is obtained at pH 7.7. For the formation of protochlorphyllide ester, oxygen, reduced glutathione, methyl alcohol, magnesium, inorganic phosphate, and nicotinamide adenine dinucleotide are required. For the formation of 14C-protochlorophyllide, adenosine triphosphate, and coenzyme A are required in addition to the above. The requirement for methyl alcohol is highly specific, and the methyl group appears to be incorporated into the protochlorophyll molecules. A biosynthetic scheme resulting in the parallel production of 14C-protochlorophyllide and 14C-protochlorophyllide ester from 14C-Mg protoporphyrin monoester is presented.
Full text
PDF

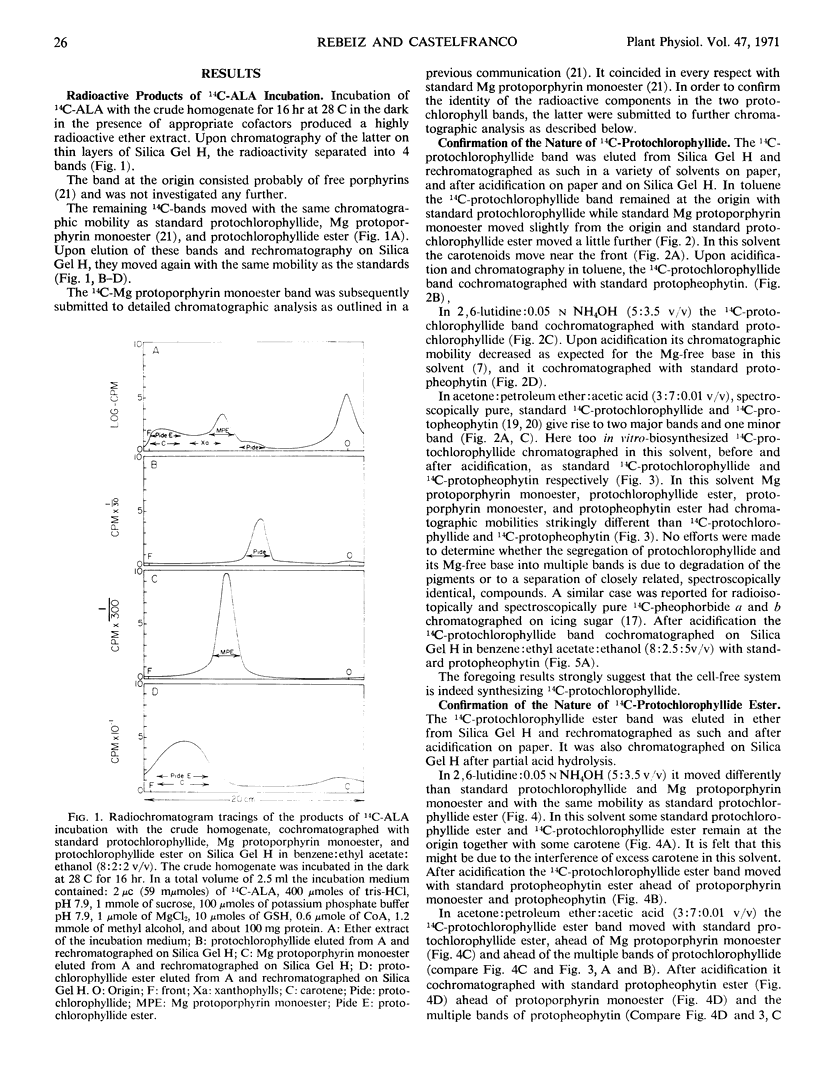
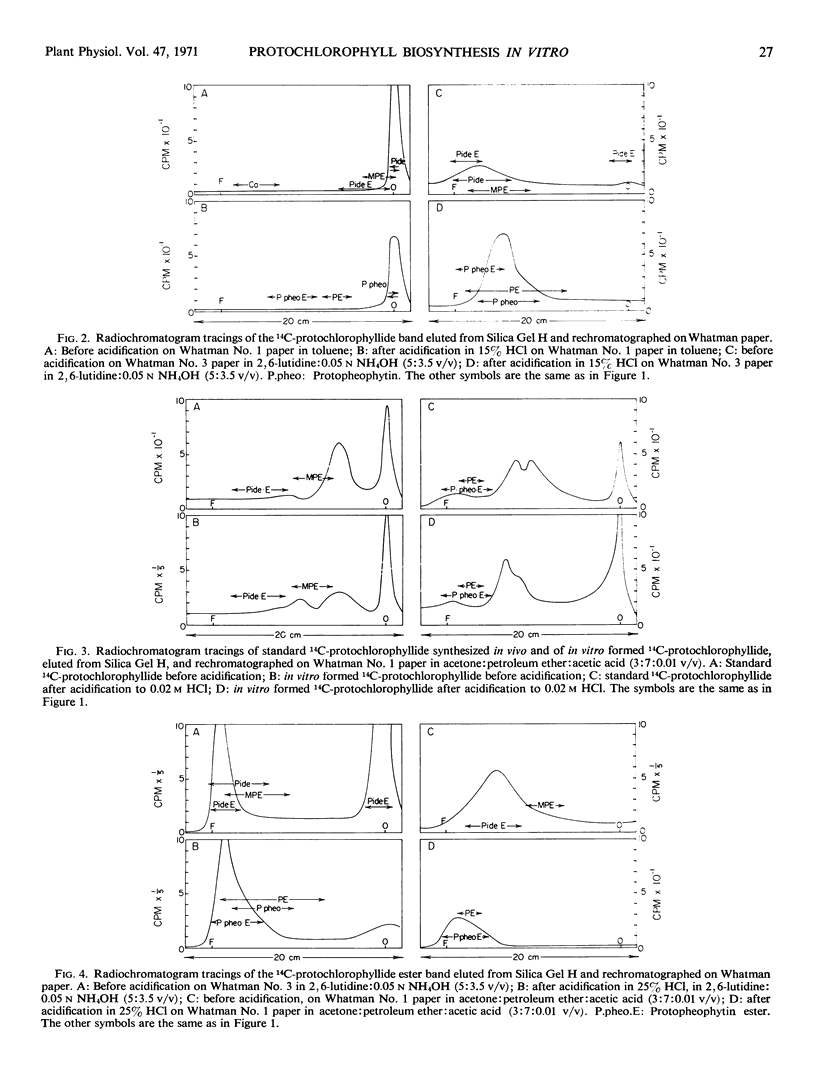
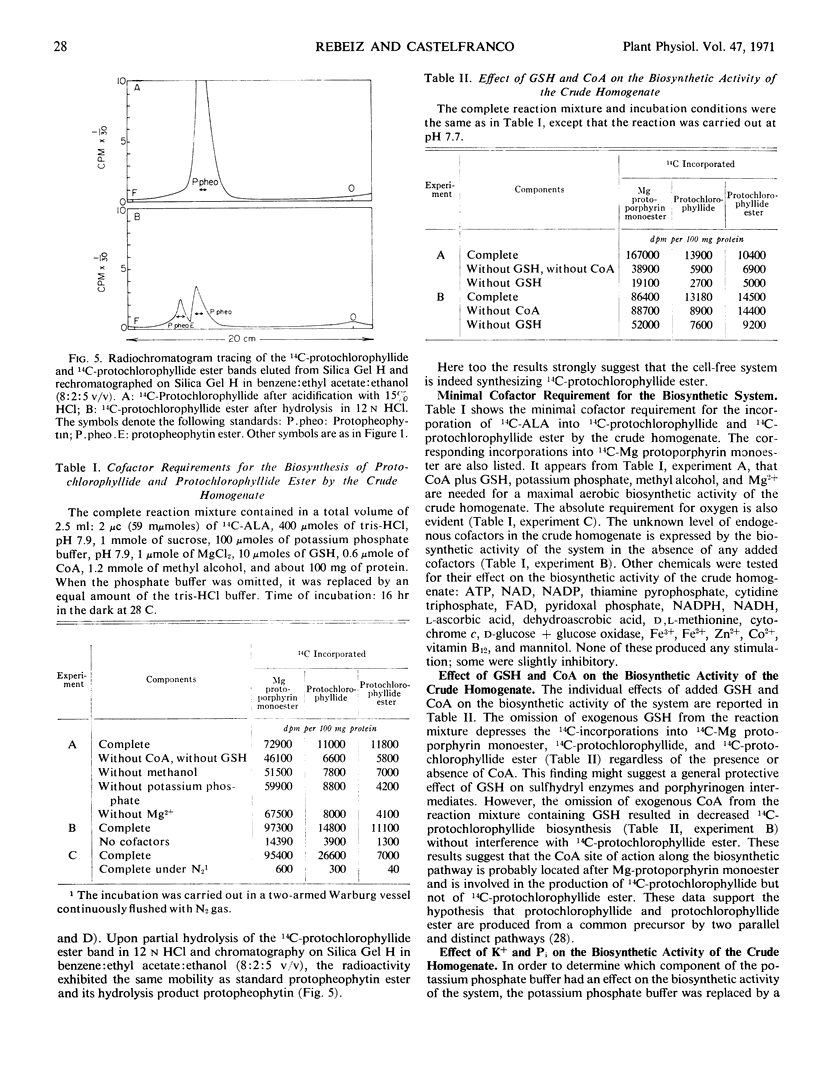
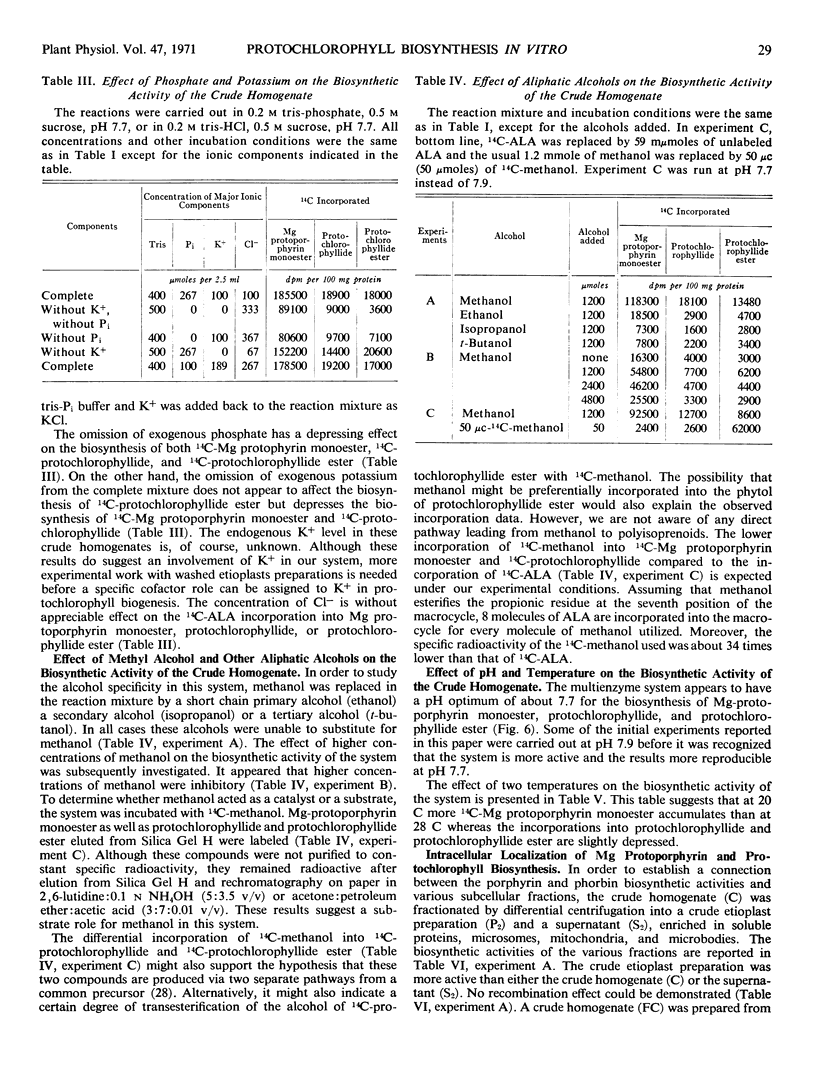



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARELL E. F., KAHN J. S. SYNTHESIS OF PORPHYRINS BY ISOLATED CHLOROPLASTS OF EUGLENA. Arch Biochem Biophys. 1964 Oct;108:1–6. doi: 10.1016/0003-9861(64)90347-9. [DOI] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations of the biogenesis of chlorophyll a. IV. Isolation and partial characterization of some biosynthetic intermediates between Mg-protoporphine IX monomethyl ester and Mg-vinylpheoporphine a5, obtained from Chlorella mutants. Arch Biochem Biophys. 1969 Mar;130(1):374–383. doi: 10.1016/0003-9861(69)90047-2. [DOI] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations on the biogenesis of chlorophyll a. 3. Biosynthesis of Mg-vinylpheoporphine a5 methylester from Mg-protoporphine IX monomethylester as observed in Chlorella mutants. Arch Biochem Biophys. 1968 Apr;125(1):269–277. doi: 10.1016/0003-9861(68)90661-9. [DOI] [PubMed] [Google Scholar]
- GRANICK S. Magnesium protoporphyrin monoester and protoporphyrin monomethyl ester in chlorophyll biosynthesis. J Biol Chem. 1961 Apr;236:1168–1172. [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines G. D., Ellsworth R. K. Methyl chlorophyllide a as a probable intermediate in the chlorophyll a pathway. Plant Physiol. 1969 Dec;44(12):1742–1744. doi: 10.1104/pp.44.12.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton D., Stumpf P. K. Fat Metabolism in Higher Plants. XXXVII. Characterization of the beta-Oxidation Systems From Maturing and Germinating Castor Bean Seeds. Plant Physiol. 1969 Apr;44(4):508–516. doi: 10.1104/pp.44.4.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEFFREY S. W. Purification and properties of chlorophyll c from Sargassum flavicans. Biochem J. 1963 Feb;86:313–318. doi: 10.1042/bj0860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES O. T. MAGNESIUM 2,4-DIVINYLPHAEOPORPHYRIN A5 MONOMETHYL ESTER, A PROTOCHLOROPHYLL-LIKE PIGMENT PRODUCED BY RHODOPSEUDOMONAS SPHEROIDES. Biochem J. 1963 Nov;89:182–189. doi: 10.1042/bj0890182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A. B. A procedure for isolation of proplastids from etiolated maize leaves. J Cell Biol. 1968 Jul;38(1):238–244. doi: 10.1083/jcb.38.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. T. A protein-protochlorophyll complex obtained from inner seed coats of Cucurbita pepo. The resolution of its two pigment groups into true protochlorophyll and a pigment related to bacterial protochlorophyll. Biochem J. 1966 Oct;101(1):153–160. doi: 10.1042/bj1010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NICHOLAS R. E. H., RIMINGTON C. Paper chromatography of porphyrins; some hitherto unrecognized porphyrins and further notes on the method. Biochem J. 1951 Mar;48(3):306–309. doi: 10.1042/bj0480306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. J., ROBERTS D. W. Purification of chlorophylls, pheophytins and pheophorbides for specific activity determinations. Biochim Biophys Acta. 1962 Apr 23;58:486–498. doi: 10.1016/0006-3002(62)90059-8. [DOI] [PubMed] [Google Scholar]
- REBEIZ C. A., CASTELFRANCO P., ENGELBRECHT A. H. FRACTIONATION AND PROPERTIES OF AN EXTRA-MITOCHONDRIAL ENZYME SYSTEM FROM PEANUTS CATALYZING THE BETA-OXIDATION OF PALMITIC ACID. Plant Physiol. 1965 Mar;40:281–286. doi: 10.1104/pp.40.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBEIZ C., CASTELFRANCO P., BREIDENBACH R. W. ACTIVATION AND OXIDATION OF ACETIC ACID-1-C14 BY CELL FREE HOMOGENATES OF GERMINATING PEANUT COTYLEDONS. Plant Physiol. 1965 Mar;40:286–289. doi: 10.1104/pp.40.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Bogorad L. (Minus) S-adenosyl-L-methionine-magnesium protoporphyrin methyltransferase, an enzyme in the biosynthetic pathway of chlorophyll in Zea mays. Plant Physiol. 1967 Mar;42(3):463–465. doi: 10.1104/pp.42.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Chlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):33–37. doi: 10.1104/pp.47.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Haidar M. A., Yaghi M. Porphyrin Biosynthesis in Cell-free Homogenates from Higher Plants. Plant Physiol. 1970 Oct;46(4):543–549. doi: 10.1104/pp.46.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Yaghi M., Abou-Haidar M. Photochlorophyll Biosynthesis in Cucumber (Cucumis sativus, L.) Cotyledons. Plant Physiol. 1970 Jul;46(1):57–63. doi: 10.1104/pp.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C., Castelfranco P. An Extra-Mitochondrial Enzyme System from Peanuts Catalyzing the beta-Oxidation of Fatty Acids. Plant Physiol. 1964 Nov;39(6):932–938. doi: 10.1104/pp.39.6.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANO S., GRANICK S. Mitochondrial coproporphyrinogen oxidase and protoporphyrin formation. J Biol Chem. 1961 Apr;236:1173–1180. [PubMed] [Google Scholar]
- Yamada M., Stumpf P. K. Fat metabolism in higher plants. XXIV. A soluble beta-oxidative system from germinating seeds of Ricinus communis. Plant Physiol. 1965 Jul;40(4):653–658. doi: 10.1104/pp.40.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]


