Abstract
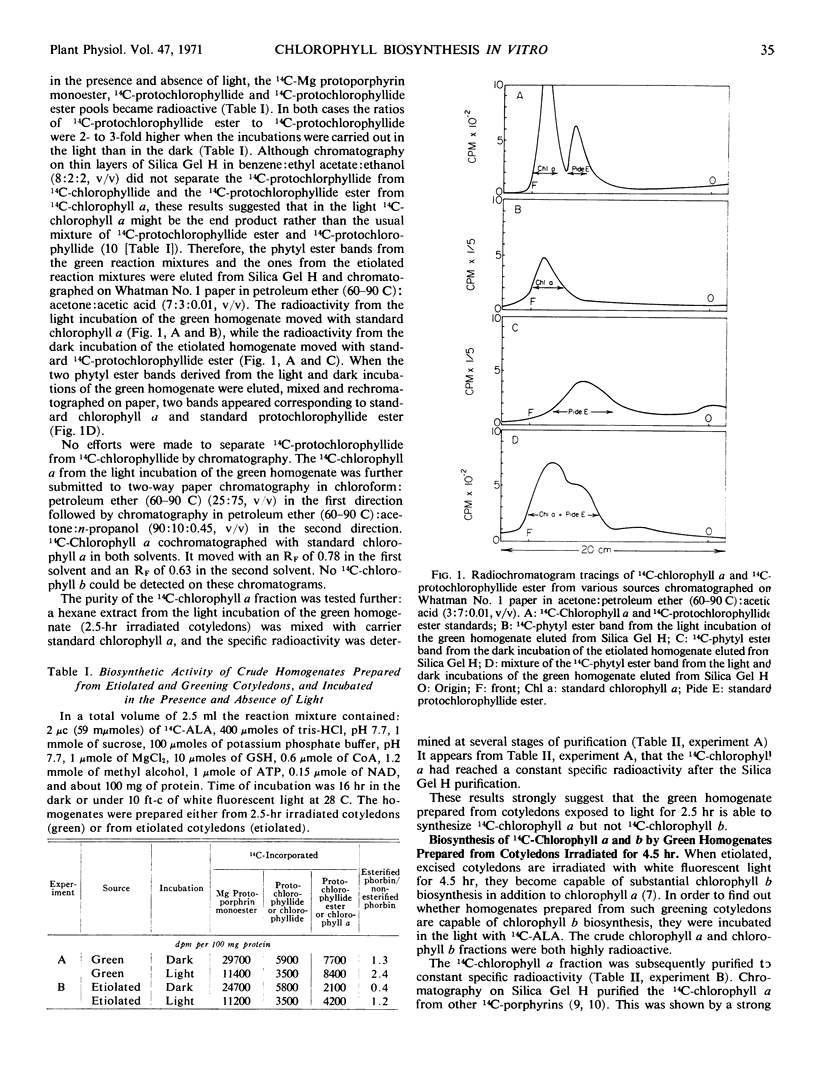
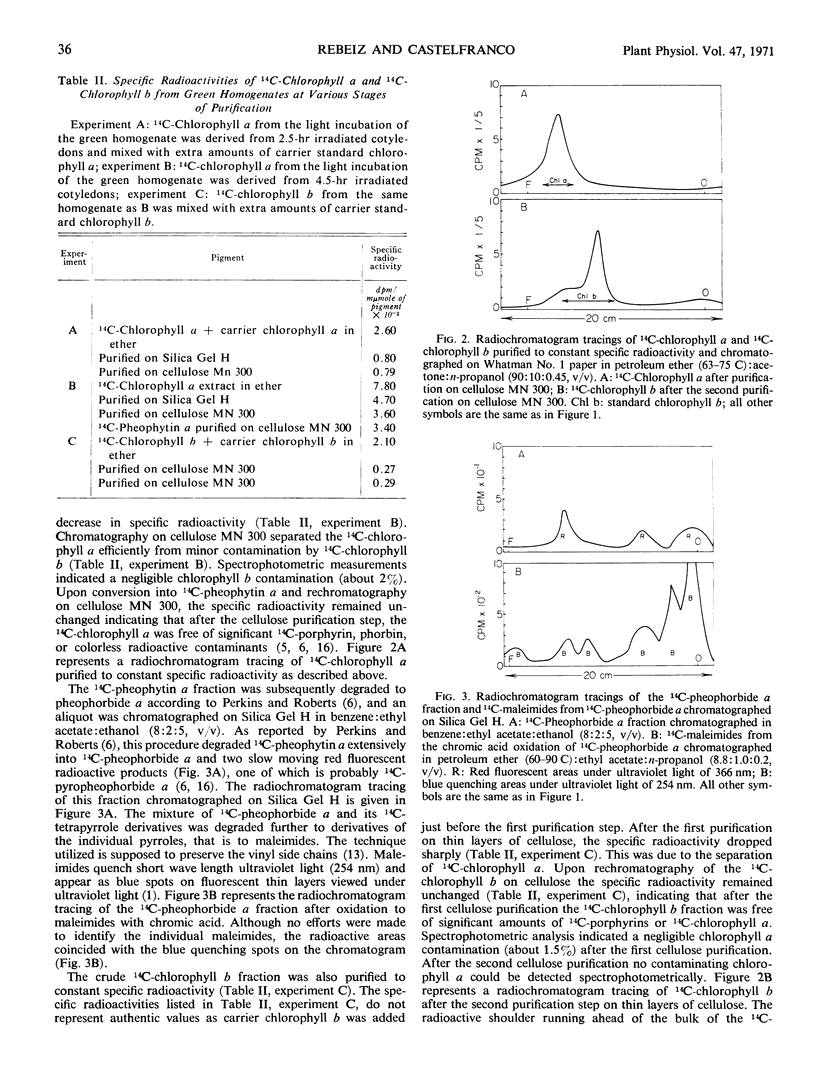
A crude homogenate obtained from greening cucumber (Cucumis sativus, L.) cotyledons in tris-sucrose, pH 7.7, containing coenzyme A, glutathione, potassium phosphate, pH 7.7, methyl alcohol, magnesium, nicotinamide adenine dinucleotide, and adenosine triphosphate, is able to incorporate 4-14C-δ-aminolevulinic acid into chlorophyll a and b in the presence of oxygen. If the homogenates are prepared from etiolated cotyledons which have been exposed to light for two and one-half hours, 14C-chlorophyll a is synthesized. However, when the homogenates are prepared from cotyledons illuminated for four and one-half hours, both 14C-chlorophyll a and b are produced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ellsworth R. K., Aronoff S. Investigations of the biogenesis of chlorophyll a. IV. Isolation and partial characterization of some biosynthetic intermediates between Mg-protoporphine IX monomethyl ester and Mg-vinylpheoporphine a5, obtained from Chlorella mutants. Arch Biochem Biophys. 1969 Mar;130(1):374–383. doi: 10.1016/0003-9861(69)90047-2. [DOI] [PubMed] [Google Scholar]
- Ellsworth R. K., Aronoff S. Investigations on the biogenesis of chlorophyll a. I. Purification and mass spectra of maleimides from the oxidation of chlorophyll and related compounds. Arch Biochem Biophys. 1968 Mar 20;124(1):358–364. doi: 10.1016/0003-9861(68)90338-x. [DOI] [PubMed] [Google Scholar]
- Hardy S. I., Castelfranco P. A., Rebeiz C. A. Effect of the hypocotyl hook on greening in etiolated cucumber cotyledons. Plant Physiol. 1970 Nov;46(5):705–707. doi: 10.1104/pp.46.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEFFREY S. W. Purification and properties of chlorophyll c from Sargassum flavicans. Biochem J. 1963 Feb;86:313–318. doi: 10.1042/bj0860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. J., ROBERTS D. W. Purification of chlorophylls, pheophytins and pheophorbides for specific activity determinations. Biochim Biophys Acta. 1962 Apr 23;58:486–498. doi: 10.1016/0006-3002(62)90059-8. [DOI] [PubMed] [Google Scholar]
- REBEIZ C. A., CASTELFRANCO P., ENGELBRECHT A. H. FRACTIONATION AND PROPERTIES OF AN EXTRA-MITOCHONDRIAL ENZYME SYSTEM FROM PEANUTS CATALYZING THE BETA-OXIDATION OF PALMITIC ACID. Plant Physiol. 1965 Mar;40:281–286. doi: 10.1104/pp.40.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Castelfranco P. A. Protochlorophyll biosynthesis in a cell-free system from higher plants. Plant Physiol. 1971 Jan;47(1):24–32. doi: 10.1104/pp.47.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Haidar M. A., Yaghi M. Porphyrin Biosynthesis in Cell-free Homogenates from Higher Plants. Plant Physiol. 1970 Oct;46(4):543–549. doi: 10.1104/pp.46.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiz C. A., Yaghi M., Abou-Haidar M. Photochlorophyll Biosynthesis in Cucumber (Cucumis sativus, L.) Cotyledons. Plant Physiol. 1970 Jul;46(1):57–63. doi: 10.1104/pp.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüdiger W., O Carra P., O hEocha C. Structure of phycoerythrobilin and phycocyanobilin. Nature. 1967 Sep 30;215(5109):1477–1478. doi: 10.1038/2151477a0. [DOI] [PubMed] [Google Scholar]


