Abstract
Effects of the R- and S-isomers and racemate of 1-(α-methylbenzyl)-3-(3,4-dichlorophenyl)urea (MBPU) were measured on phosphorylation and electron transport in mung bean (Phaseolus aureus L.) mitochondria and spinach (Spinacia oleracea L.) chloroplasts.
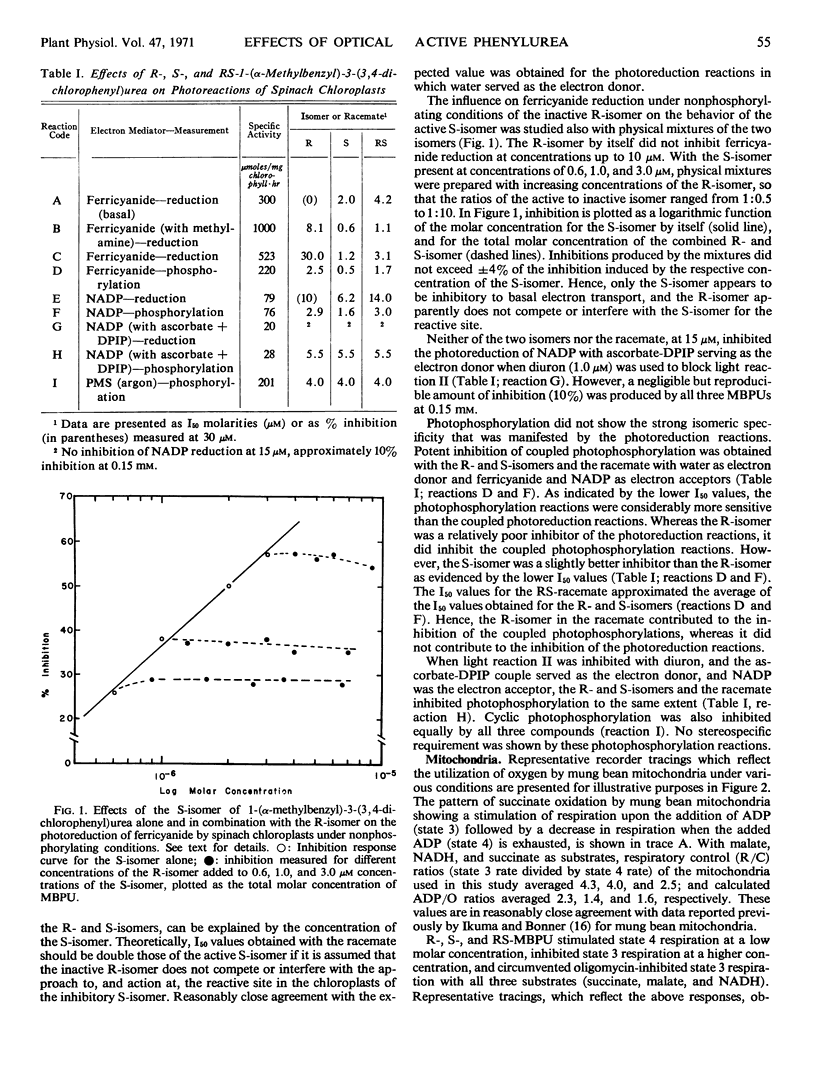
In chloroplasts, S-MBPU inhibited basal and methylamine-uncoupled electron transport with ferricyanide as the oxidant, both photoreduction and coupled photophosphorylation with water as the electron donor and with ferricyanide and nicotinamide adenine dinucleotide phosphate (NADP) as oxidants, and cyclic photophosphorylation with phenazine methosulfate as the electron mediator under an argon gas phase. With ascorbate 2,6-dichloro-phenolindophenol as the electron donor, phosphorylation coupled to NADP reduction was inhibited, but the reduction of NADP was not inhibited. The R-isomer of MBPU, like the S-isomer, inhibited all of the photophosphorylation reactions studied. However, unlike the S-isomer, the R-isomer either did not inhibit or was a very weak inhibitor of all photoreduction reactions. The effects of the MBPUs on the chloroplast reactions can be explained by action at two different sites: an optically specific site near photosystem II and the oxygen evolution pathway, and a second optically nonspecific site associated with the generation of ATP.
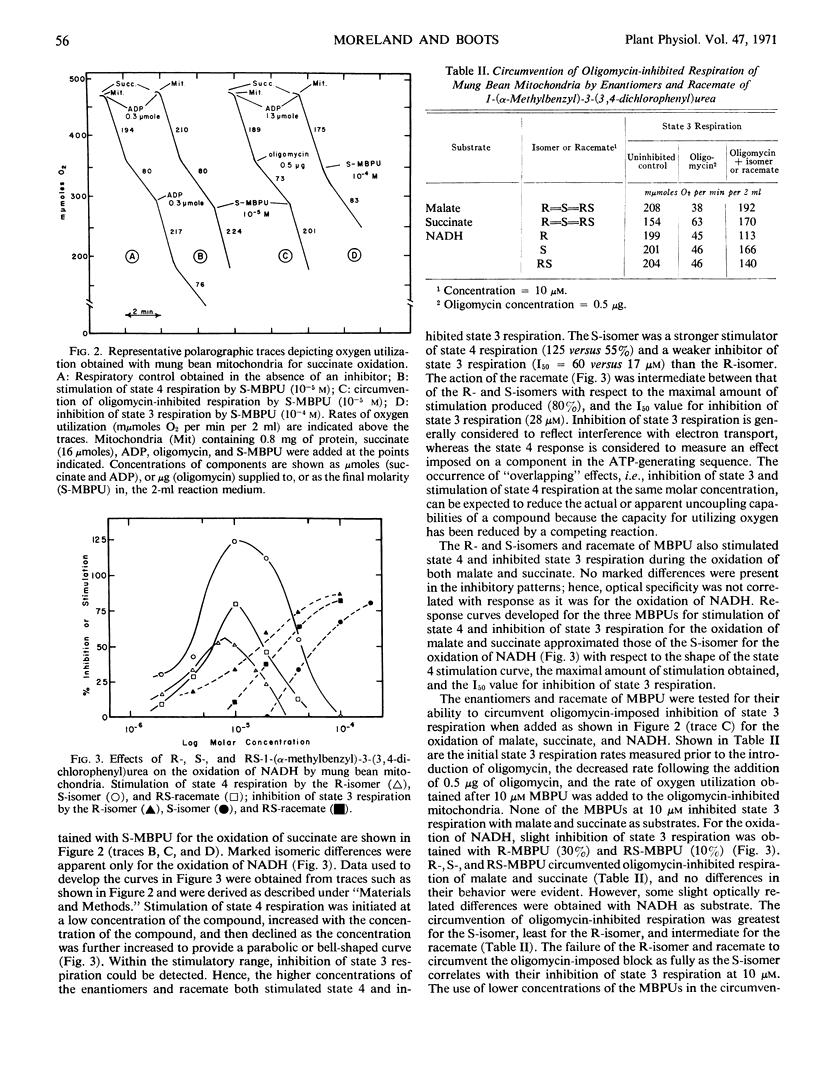
In mitochondria, both the R- and S-isomers stimulated state 4 respiration, inhibited state 3 respiration, and released oligomycin-inhibited respiration with malate, succinate, and NADH as substrates. Both enantiomers were equally active in all studies with malate and succinate as substrates. However, with NADH as substrate, R-MBPU was a stronger inhibitor of state 3 respiration and a weaker stimulator of state 4 respiration than S-MBPU.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J. E., Lieberman M. Cytochrome Components & Electron Transfer in Sweet Potato Mitochondria. Plant Physiol. 1962 Jan;37(1):90–97. doi: 10.1104/pp.37.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955 Nov;217(1):409–427. [PubMed] [Google Scholar]
- Cunningham W. P. Oxidation of Externally Added NADH by Isolated Corn Root Mitochondria. Plant Physiol. 1964 Jul;39(4):699–703. doi: 10.1104/pp.39.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari T. E., Moreland D. E. Effects of 3,5-Dihalogenated-4-hydroxybenzonitriles on the Activity of Mitochondria From White Potato Tubers. Plant Physiol. 1969 Mar;44(3):429–434. doi: 10.1104/pp.44.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E. Inhibitors of the Hill reaction. Plant Physiol. 1961 Nov;36(6):788–803. doi: 10.1104/pp.36.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromet-Elhanan Z. Energy-transfer inhibitors and electron transport inhibitors in chloroplasts. Arch Biochem Biophys. 1968 Mar 11;123(3):447–456. doi: 10.1016/0003-9861(68)90165-3. [DOI] [PubMed] [Google Scholar]
- HUMPHREYS T. E., CONN E. E. The oxidation of reduced diphosphopyridine nucleotide by lupine mitochondria. Arch Biochem Biophys. 1956 Jan;60(1):226–243. doi: 10.1016/0003-9861(56)90413-1. [DOI] [PubMed] [Google Scholar]
- Hackett D. P. Effects of salts on DPNH oxidase activity & structure of sweet potato mitochondria. Plant Physiol. 1961 Jul;36(4):445–452. doi: 10.1104/pp.36.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Bonner W. D. Properties of Higher Plant Mitochondria. I. Isolation and Some Characteristics of Tightly-coupled Mitochondria from Dark-grown Mung Bean Hypocotyls. Plant Physiol. 1967 Jan;42(1):67–75. doi: 10.1104/pp.42.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S., Good N. E. Effect of Salts and Electron Transport on the Conformation of Isolated Chloroplasts. II. Electron Microscopy. Plant Physiol. 1966 Mar;41(3):544–552. doi: 10.1104/pp.41.3.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. S. Chlorotri-n-butylin. An inhibitor of photophosphorylation in isolated chloroplasts. Biochim Biophys Acta. 1968 Jan 15;153(1):203–210. doi: 10.1016/0005-2728(68)90161-8. [DOI] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Inhibitory effect of salicylaldoxime on chloroplast photooxidation-reduction reactions. Biochem Biophys Res Commun. 1966 Sep 22;24(6):903–908. doi: 10.1016/0006-291x(66)90335-4. [DOI] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Renger G., Vater J., Witt H. T. Effect of salicylaldoxime on the complete electron transport system of photosynthesis and on the isolated reaction cycle II. Biochem Biophys Res Commun. 1967 Feb 21;26(4):477–480. doi: 10.1016/0006-291x(67)90572-4. [DOI] [PubMed] [Google Scholar]
- TAUSSKY H. H., SHORR E. A microcolorimetric method for the determination of inorganic phosphorus. J Biol Chem. 1953 Jun;202(2):675–685. [PubMed] [Google Scholar]
- Weinbach E. C., Garbus J. Mechanism of action of reagents that uncouple oxidative phosphorylation. Nature. 1969 Mar 15;221(5185):1016–1018. doi: 10.1038/2211016a0. [DOI] [PubMed] [Google Scholar]
- Wilson R. H., Hanson J. B. The effect of respiratory inhibitors on NADH, succinate and malate oxidation in corn mitochondria. Plant Physiol. 1969 Sep;44(9):1335–1341. doi: 10.1104/pp.44.9.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt H. T., Döring G., Rumberg B., Schmidt-Mende P., Siggel U., Stiehl H. H. Electron transport in photosynthesis. Brookhaven Symp Biol. 1966;19:161–168. [PubMed] [Google Scholar]


