Abstract
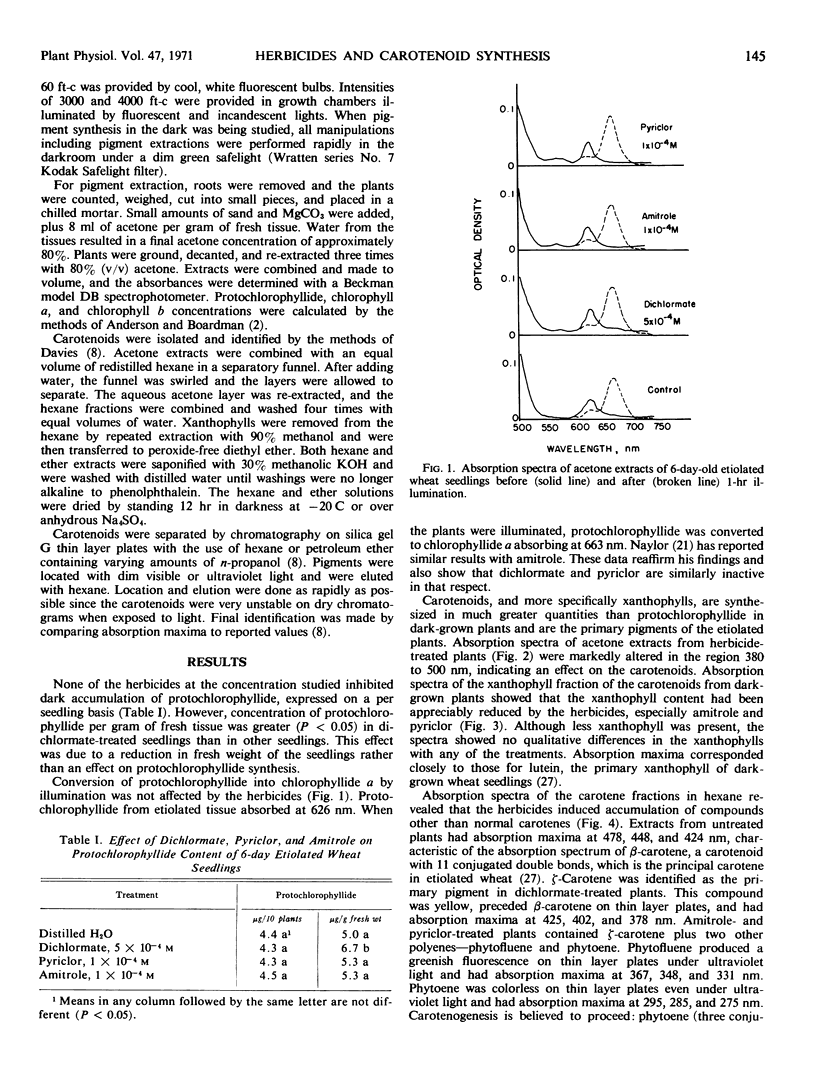
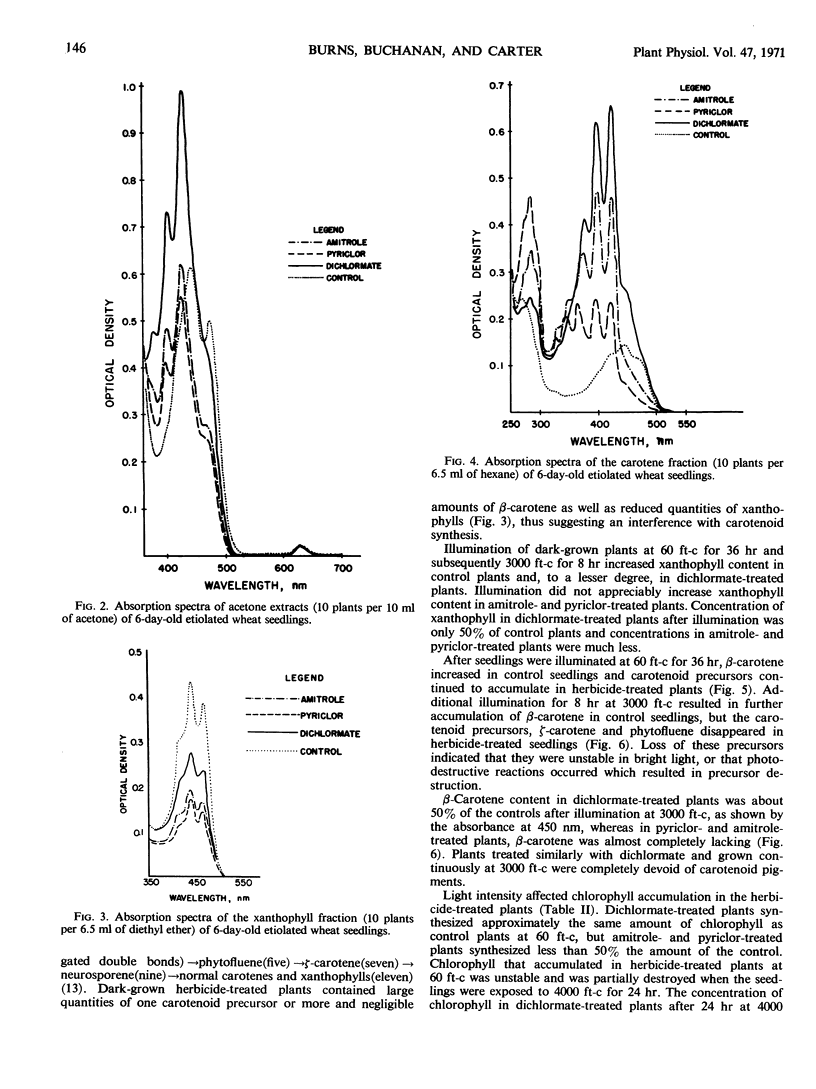
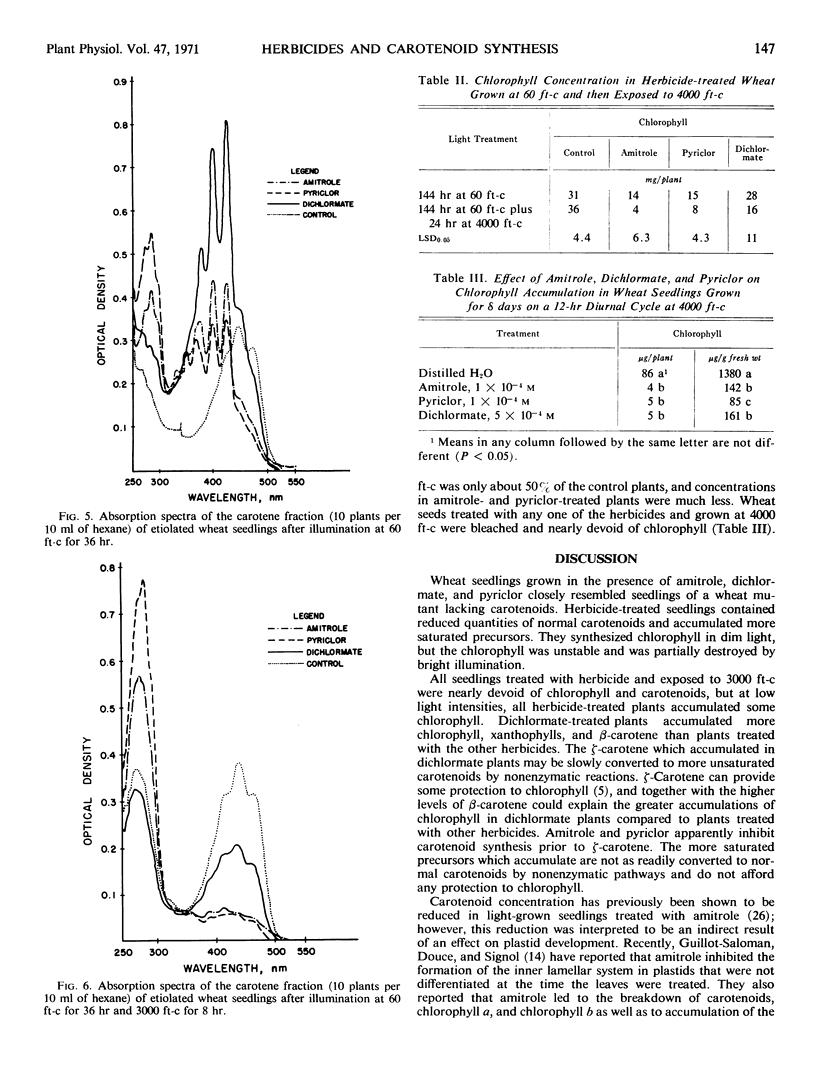
Amitrole (3-amino-s-triazole), dichlormate (3,4-dichlorobenzyl methylcarbamate), and pyriclor (2,3,5-trichloro-4-pyridinol) inhibited normal carotenogenesis in etiolated wheat (Triticum aestivum L. var. Coker 65-20) seedlings. Carotenoid precursors accumulated in treated plants. In dichlormate-treated plants, ζ-carotene accumulated, whereas phytofluene, phytoene, and ζ-carotene accumulated in amitrole- and pyriclor-treated plants. None of the herbicides interfered with protochlorophyllide synthesis or its conversion to chlorophyllide when etiolated plants were illuminated. Chlorophyll accumulated in treated plants exposed to light at 60 foot candles, but was unstable and partially destroyed by illumination at 4000 foot candles. These data suggest that the phytotoxicity of amitrole, pyriclor, and dichlormate is due to inhibition of the synthesis of carotenoids and to the consequent photodestruction of chlorophyll and chloroplast disruption.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Robertson D. S. Role of Carotenoids in Protecting Chlorophyll From Photodestruction. Plant Physiol. 1960 Jul;35(4):531–534. doi: 10.1104/pp.35.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Matsuda K., Siegel A., Weier T. E. Chloroplastic ribosome formation: inhibition by 3-amino-1,2,4-triazole. Plant Physiol. 1967 May;42(5):736–741. doi: 10.1104/pp.42.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels P. G., Pegelow E. J., Jr The action of sirmate (3,4-dichlorobenzyl methylcarbamate) on chloroplast ribosomes of Triticum vulgare L. seedlings. J Cell Biol. 1968 May;37(2):C1–C6. doi: 10.1083/jcb.37.2.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAES H. [Transfer of energy from excited chlorophyll to C40-polyenes with various chromophore groups]. Z Naturforsch B. 1961 Jul;16B:445–454. [PubMed] [Google Scholar]
- COHEN-BAZIRE G., STANIER R. Y. Specific inhibition of carotenoid synthesis in a photosynthetic bacterium and its physiological consequences. Nature. 1958 Jan 24;181(4604):250–252. doi: 10.1038/181250a0. [DOI] [PubMed] [Google Scholar]
- KOSKI V. M., SMITH J. H. C. Chlorophyll formation in a mutant, white seedling-3. Arch Biochem Biophys. 1951 Nov;34(1):189–195. doi: 10.1016/s0003-9861(51)80024-9. [DOI] [PubMed] [Google Scholar]
- SHIMIZU N., HAMURO Y. Deposition of glycogen and changes in some enzymes in brain wounds. Nature. 1958 Mar 15;181(4611):781–782. doi: 10.1038/181781a0. [DOI] [PubMed] [Google Scholar]
- Sander C., Laber L. J., Bell W. D., Hamilton R. H. Light sensitivity of plastids and plastid pigments present in the albescent maize mutant. Plant Physiol. 1968 May;43(5):693–697. doi: 10.1104/pp.43.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf F. T. Effects of Light and Darkness on Biosynthesis of Carotenoid Pigments in Wheat Seedlings. Plant Physiol. 1963 Nov;38(6):649–652. doi: 10.1104/pp.38.6.649. [DOI] [PMC free article] [PubMed] [Google Scholar]


