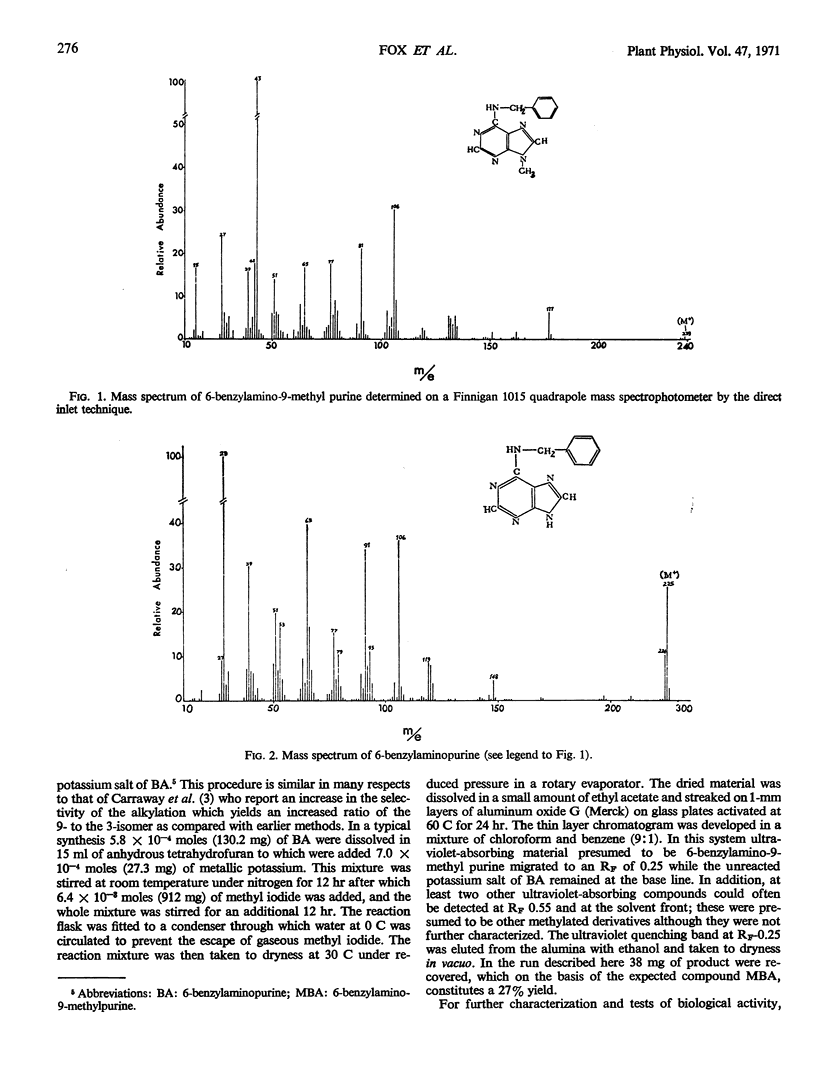
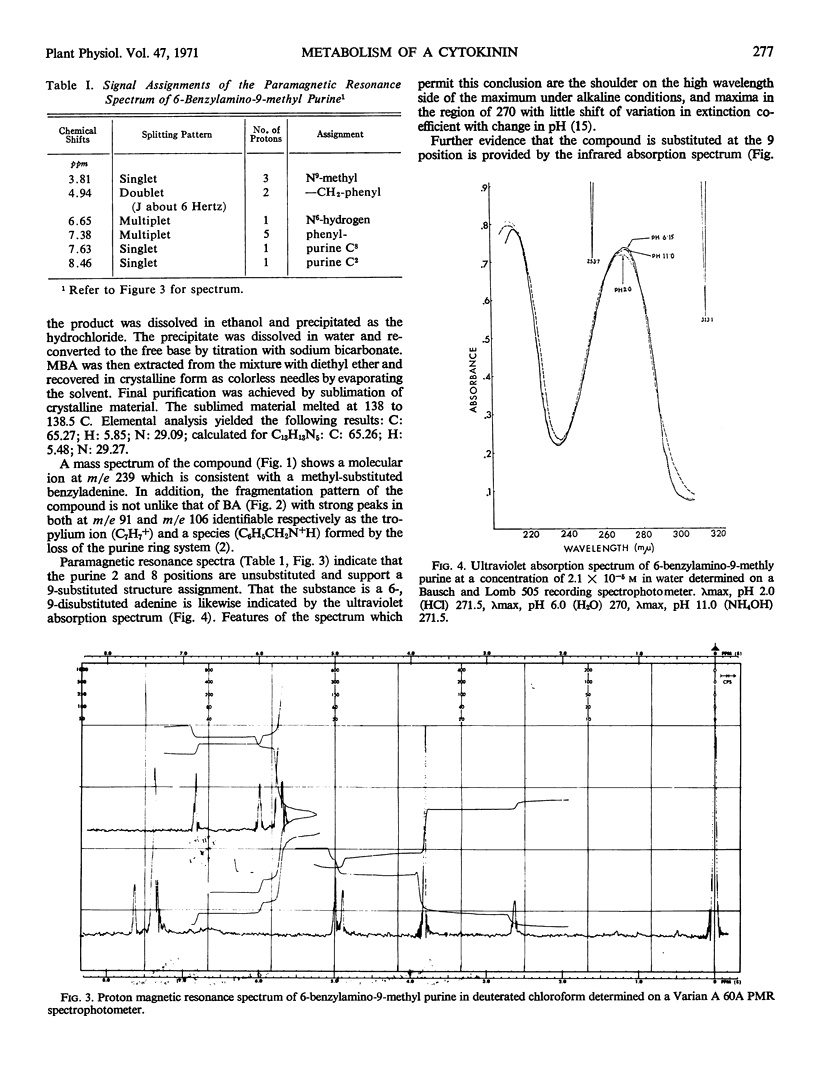
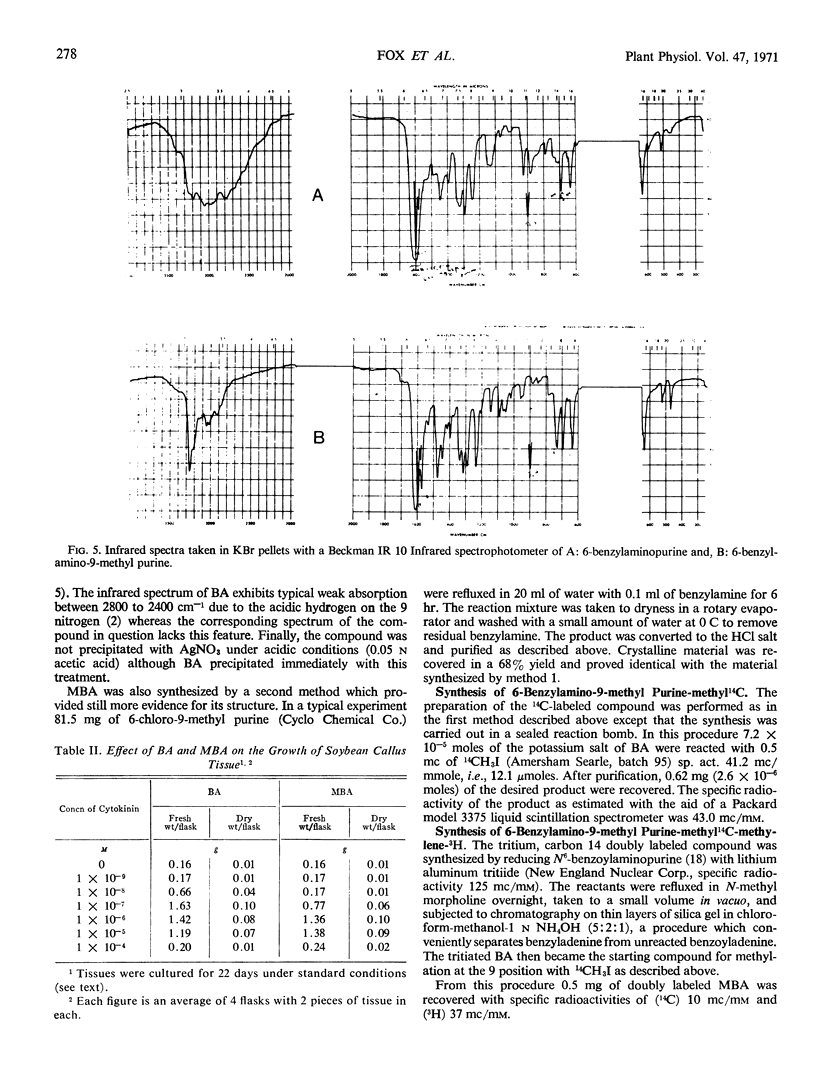
Abstract
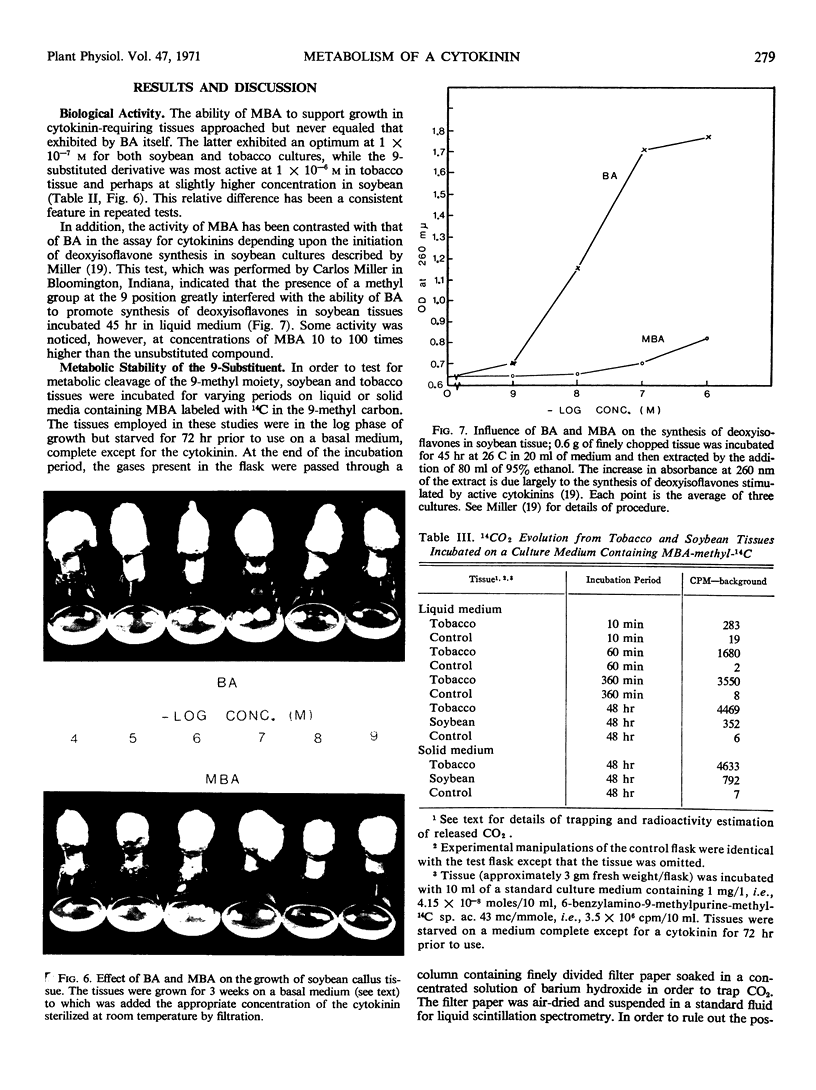
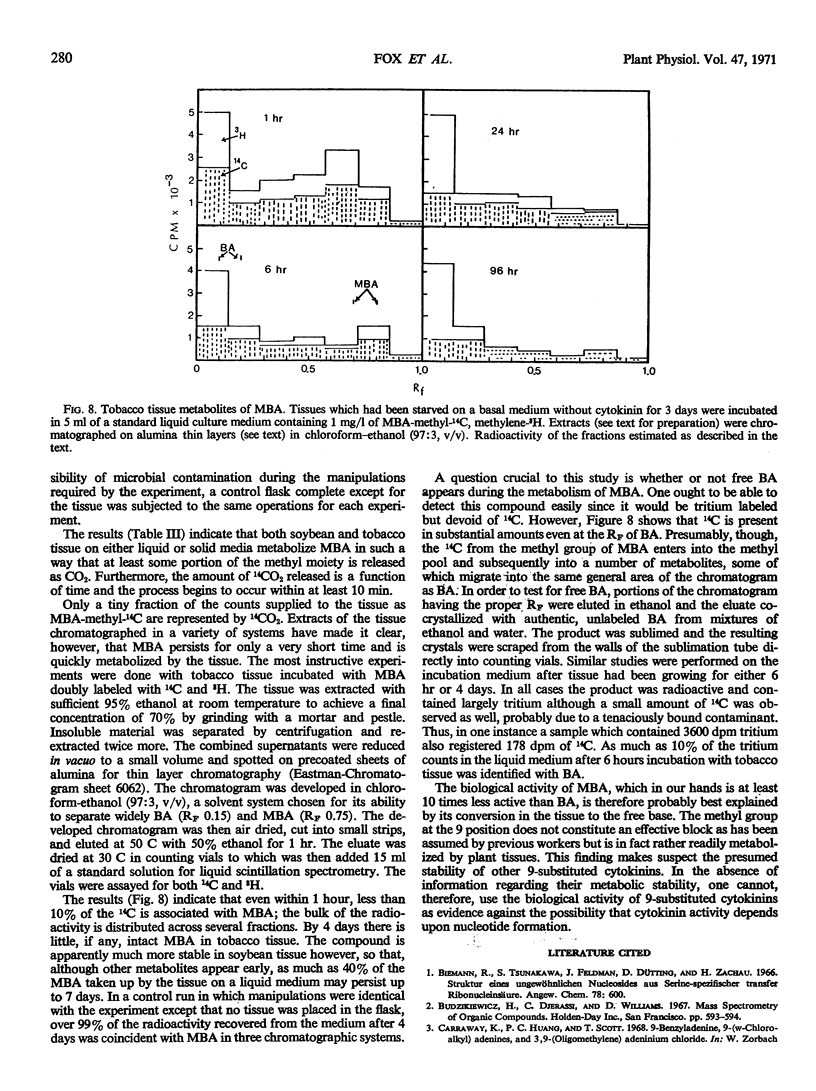
In order to test the metabolic stability of 9-substituted cytokinins, 6-benzylamino-9-methyl purine has been synthesized and labeled with 14C in the 9-methyl carbon or doubly labeled with 14C in the 9-methyl carbon and 3H in the methylene moiety of the side chain. Although the 6-benzylamino-9-methylpurine is chemically stable, cytokinin-requiring tissues begin removing the 9-substituent in as little as 10 minutes. Among the various metabolic products is free benzylaminopurine. Thus, the biological activity of 9-substituted cytokinins could be accounted for by their conversion to the free base.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Doctor B. P., Loebel J. E., Sodd M. A., Winter D. B. Nucleotide sequence of Escherichia coli tyrosine transfer ribonucleic acid. Science. 1969 Feb 14;163(3868):693–695. doi: 10.1126/science.163.3868.693. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Chen C. Characterization of labeled ribonucleic acid from tissue grown on 14C-containing cytokinins. J Biol Chem. 1967 Oct 10;242(19):4490–4494. [PubMed] [Google Scholar]
- Fox J. E. Incorporation of a kinin, N, 6-benzyladenine into soluble RNA. Plant Physiol. 1966 Jan;41(1):75–82. doi: 10.1104/pp.41.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Tavares J. E. On the significance of cytokinin incorporation into RNA. Plant Physiol. 1968 Aug;43(8):1244–1248. doi: 10.1104/pp.43.8.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madison J. T., Everett G. A., Kung H. Nucleotide sequence of a yeast tyrosine transfer RNA. Science. 1966 Jul 29;153(3735):531–534. doi: 10.1126/science.153.3735.531. [DOI] [PubMed] [Google Scholar]