Abstract
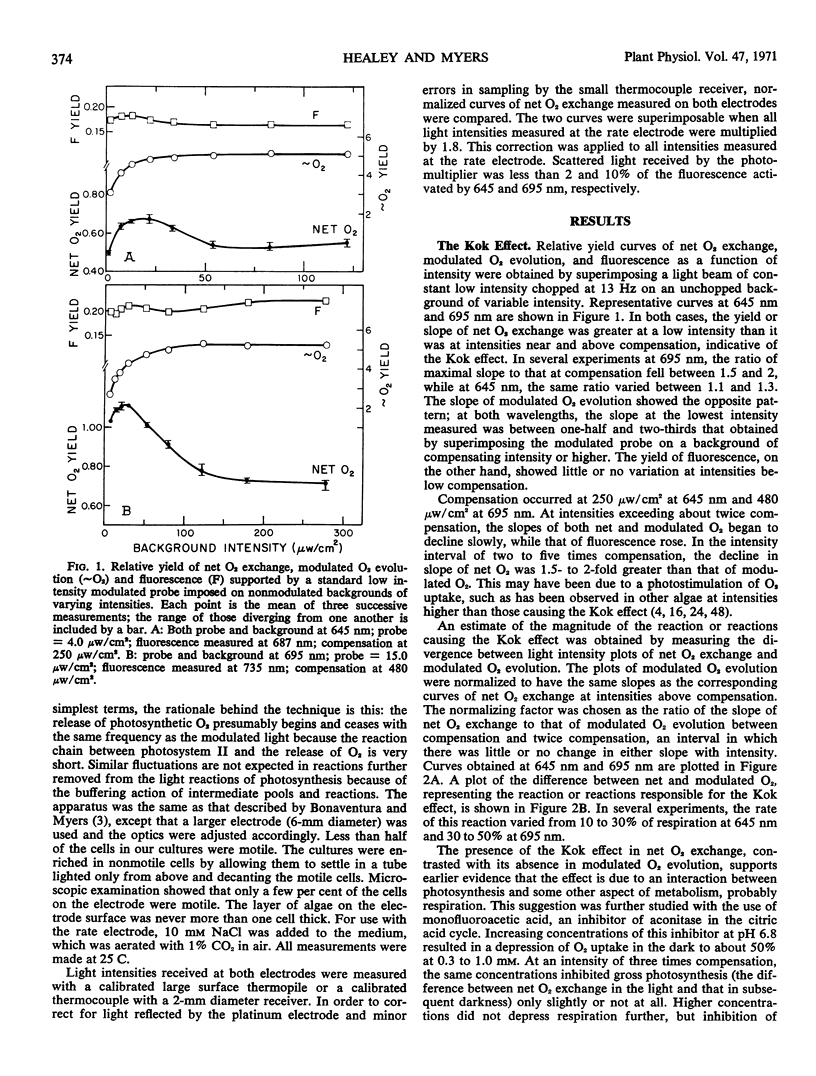
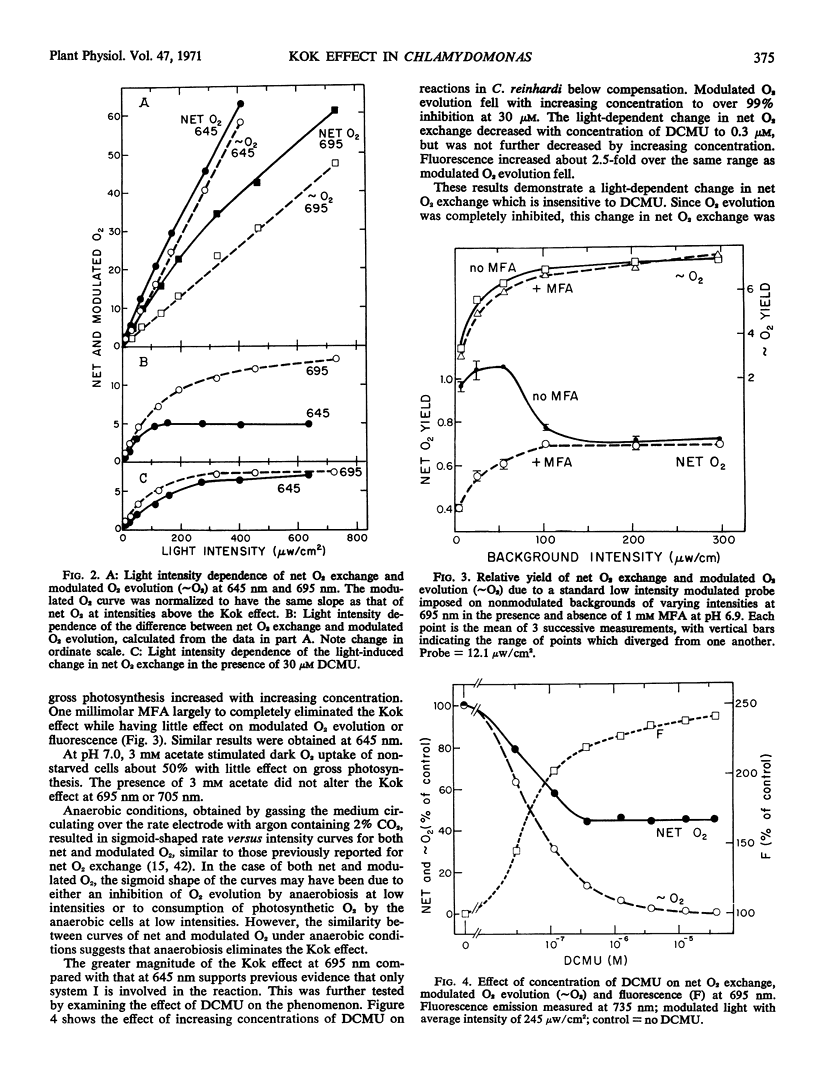
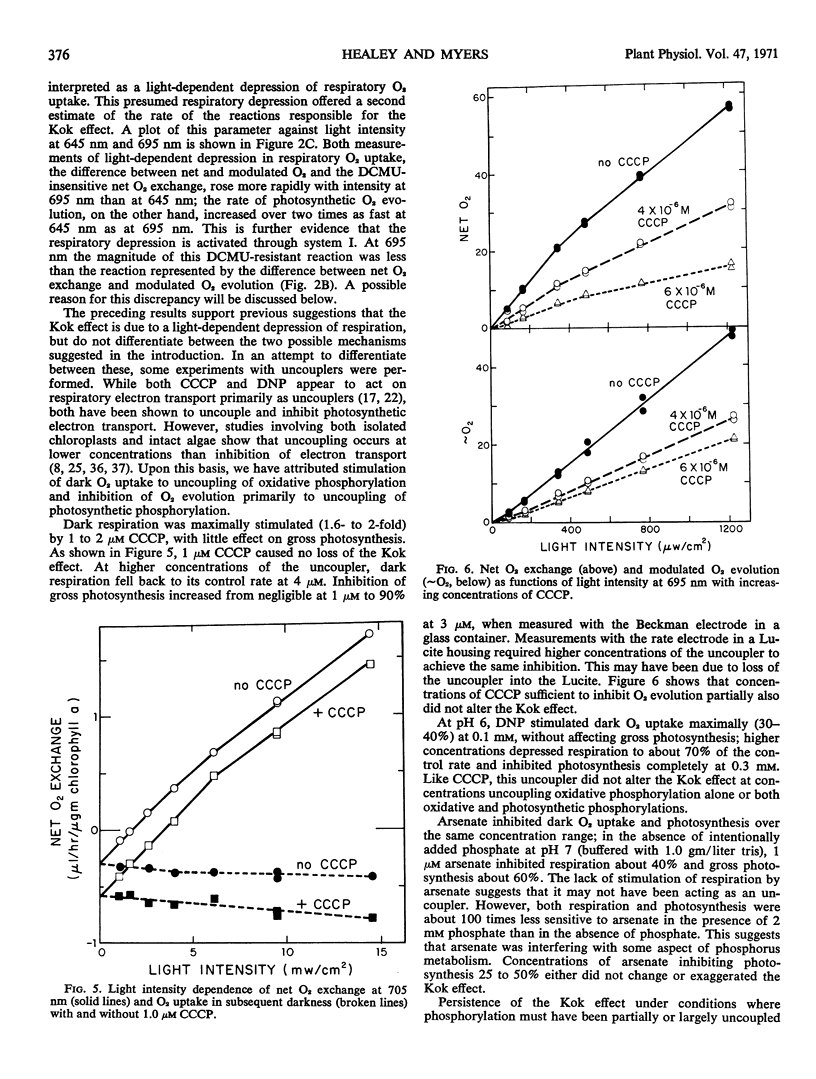
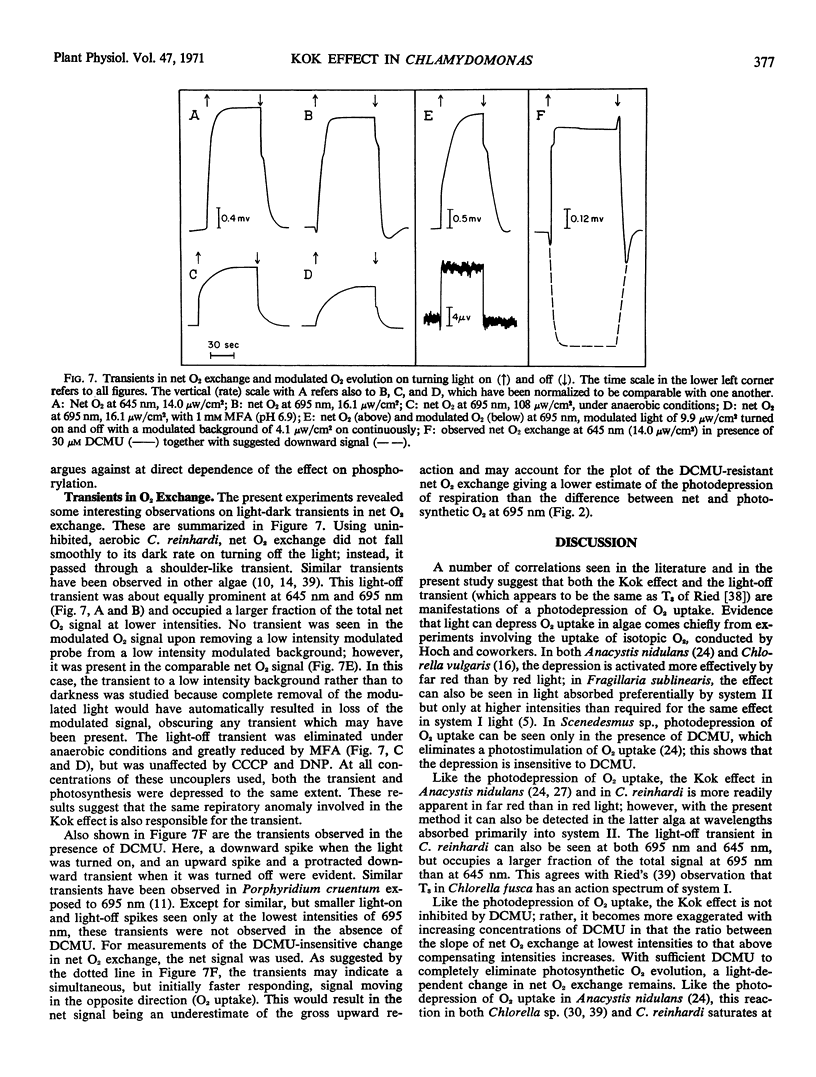
A Haxo-Blinks rate-measuring oxygen electrode together with a modulated light source gave an average current signal (change in net O2 exchange) and a modulated current signal (photosynthetic O2 evolution). Using this apparatus, net O2 exchange and photosynthetic O2 evolution at low intensities have been studied in the green alga, Chlamydomonas reinhardi. At both 645 nm and 695 nm, the curves of net O2 exchange as a function of light intensity were steeper at lowest intensities than about compensation, indicative of the Kok effect. The effect was greater at 695 nm than at 645 nm. The corresponding curves of photosynthetic O2 evolution, on the other hand, showed no Kok effect; here, the slope was lowest at lowest intensity. The absence of the Kok effect in O2 evolution, together with its sensitivity to monofluoroacetic acid, show that it is due to an interaction of photosynthesis and respiration. The effect was exaggerated by 3-(3,4-dichlorophenyl)-1,1-dimethylurea. In the presence of concentrations of this inhibitor sufficient to inhibit O2 evolution completely, a light-induced change in net O2 exchange remained. This was interpreted as a system I dependent depression of respiratory O2 uptake. The Kok effect remained undiminished in concentrations of carbonyl cyanide m-chlorophenylhydrazone and 2,4-dinitrophenol which partially uncoupled either oxidative phosphorylation alone or both oxidative and photosynthetic phosphorylations. The above results can be explained within a model of the Kok effect in which O2 uptake is depressed by diversion of reductant away from respiratory electron transport and into photosystem I. The same photodepression of O2 uptake also appears to account for a transient in net O2 exchange seen in several algae upon turning off the light.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonaventura C., Myers J. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim Biophys Acta. 1969;189(3):366–383. doi: 10.1016/0005-2728(69)90168-6. [DOI] [PubMed] [Google Scholar]
- Brown A. H., Weis D. Relation Between Respiration and Photosynthesis in the Green Alga, Ankistrodesmus braunii. Plant Physiol. 1959 May;34(3):224–234. doi: 10.1104/pp.34.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Sager R. Oxygen and Light Induced Oxidations of Cytochrome, Flavoprotein, and Pyridine Nucleotide in a Chlamydomonas Mutant. Plant Physiol. 1957 Nov;32(6):548–561. doi: 10.1104/pp.32.6.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. M., Fay P. Special aspects of nitrogen fixation by blue-green algae. Proc R Soc Lond B Biol Sci. 1969 Apr 1;172(1029):357–366. doi: 10.1098/rspb.1969.0026. [DOI] [PubMed] [Google Scholar]
- De Kiewiet D. Y., Hall D. O., Jenner E. L. Effect of carbonylcyanide m-chlorophenylhydrazone on the photochemical reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Sep 27;109(1):284–292. doi: 10.1016/0926-6585(65)90112-3. [DOI] [PubMed] [Google Scholar]
- GOVINDJEE, OWENS O. V., HOCH G. A MASS-SPECTROSCOPIC STUDY OF THE EMERSON ENHANCEMENT EFFECT. Biochim Biophys Acta. 1963 Sep 24;75:281–284. doi: 10.1016/0006-3002(63)90611-5. [DOI] [PubMed] [Google Scholar]
- HEYTLER P. G. uncoupling of oxidative phosphorylation by carbonyl cyanide phenylhydrazones. I. Some characteristics of m-Cl-CCP action on mitochondria and chloroplasts. Biochemistry. 1963 Mar-Apr;2:357–361. doi: 10.1021/bi00902a031. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Healey F. P. The Mechanism of Hydrogen Evolution by Chlamydomonas moewusii. Plant Physiol. 1970 Feb;45(2):153–159. doi: 10.1104/pp.45.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heber U. W., Santarius K. A. Compartmentation and reduction of pyridine nucleotides in relation to photosynthesis. Biochim Biophys Acta. 1965 Nov 29;109(2):390–408. doi: 10.1016/0926-6585(65)90166-4. [DOI] [PubMed] [Google Scholar]
- Herron H. A., Mauzerall D. M. The light saturation curve of photosynthesis. Biochim Biophys Acta. 1970;205(2):312–314. doi: 10.1016/0005-2728(70)90262-8. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Nishimura M., Chance B. Energy and Electron Transfer Systems of Chlamydomonas reinhardi. I. Photosynthetic and Respiratory Cytochrome Systems of the Pale Green Mutant. Plant Physiol. 1969 Apr;44(4):527–534. doi: 10.1104/pp.44.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES L. W., MYERS J. A COMMON LINK BETWEEN PHOTOSYNTHESIS AND RESPIRATION IN A BLUE-GREEN ALGA. Nature. 1963 Aug 17;199:670–672. doi: 10.1038/199670a0. [DOI] [PubMed] [Google Scholar]
- Mann J. E., Myers J. Photosynthetic Enhancement in the Diatom Phaeodactylum tricornutum. Plant Physiol. 1968 Dec;43(12):1991–1995. doi: 10.1104/pp.43.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh H. V., Galmiche J. M., Gibbs M. Effect of Light on the Tricarboxylic Acid Cycle in Scenedesmus. Plant Physiol. 1965 Nov;40(6):1013–1022. doi: 10.1104/pp.40.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J., Graham J. R. Enhancement in Chlorella. Plant Physiol. 1963 Jan;38(1):105–116. doi: 10.1104/pp.38.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann J., Jagendorf A. T. Dinitrophenol as an uncoupler of photosynthetic phosphorylation. Biochem Biophys Res Commun. 1964 Aug 11;16(6):562–567. doi: 10.1016/0006-291x(64)90193-7. [DOI] [PubMed] [Google Scholar]
- Ried A. Interactions between photosynthesis and respiration in chlorella. I. Types of transients of oxygen exchange after short light exposures. Biochim Biophys Acta. 1968 Apr 2;153(3):653–663. doi: 10.1016/0005-2728(68)90192-8. [DOI] [PubMed] [Google Scholar]
- SAGER R., PALADE G. E. Structure and development of the chloroplast in Chlamydomonas. I. The normal green cell. J Biophys Biochem Cytol. 1957 May 25;3(3):463–488. doi: 10.1083/jcb.3.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SETLIK I. Light-dark transients in oxygen exchange of blue-green algae. Biochim Biophys Acta. 1957 May;24(2):436–437. doi: 10.1016/0006-3002(57)90222-6. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Tanner W., Loffler M., Kandler O. Cyclic Photophosphorylation in Vivo and its Relation to Photosynthetic CO(2)-Fixation. Plant Physiol. 1969 Mar;44(3):422–428. doi: 10.1104/pp.44.3.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis D., Brown A. H. Kinetic Relationships Between Photosynthesis and Respiration in the Algal Flagellate, Ochromonas Malhamensis. Plant Physiol. 1959 May;34(3):235–239. doi: 10.1104/pp.34.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]


