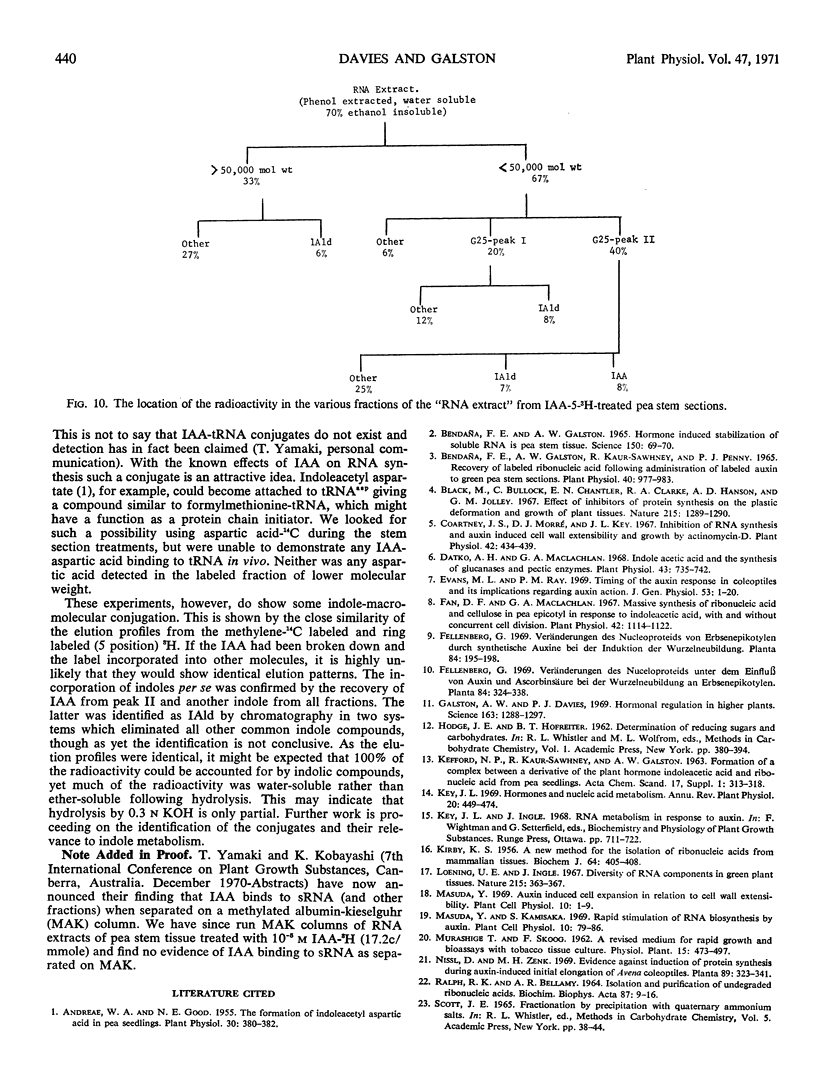
Abstract
Pea (Pisum sativum var. Alaska) and bean (Phaseolus vulgaris var. Red Kidney) stem sections treated with indoleacetic acid-1-14C, indoleacetic acid-2-14C, and indoleacetic acid-5-3H were homogenized, extracted with phenol, and the water-soluble, ethanol-insoluble material subjected to further fractionation. Following an 18-hour incubation period in indoleacetic acid-1-14C, most of the label was found as nonindole-14C in high molecular weight polysaccharide, as phenol extraction is specific for both RNA and polysaccharides. With indoleacetic acid-2-14C and -5-3H, and to a lesser extent with indoleacetic acid-1-14C, radioactive indoles were obtained by hydrolysis from a heterogeneous fraction between about 500 and 30,000 molecular weight, possibly polysaccharide in nature. Indoleacetic acid accounted for 8% and indole aldehyde accounted for 21% of the total radioactivity in the extract.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreae W. A., Good N. E. The Formation of Indoleacetylaspartic Acid in Pea Seedlings. Plant Physiol. 1955 Jul;30(4):380–382. doi: 10.1104/pp.30.4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendaña F. E., Galston A. W. Hormone-induced stabilization of soluble RNA in pea-stem tissue. Science. 1965 Oct 1;150(3692):69–70. doi: 10.1126/science.150.3692.69. [DOI] [PubMed] [Google Scholar]
- Bendaña F. E., Galston A. W., Kaur-Sawhney R., Penny P. J. Recovery of labeled ribonucleic acid following administration of labeled auxin to green pea stem sections. Plant Physiol. 1965 Nov;40(6):977–983. doi: 10.1104/pp.40.6.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coartney J. S., Morre D. J., Key J. L. Inhibition of RNA synthesis and auxin-induced cell wall extensibility and growth by Actinomycin D. Plant Physiol. 1967 Mar;42(3):434–439. doi: 10.1104/pp.42.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datko A. H., Maclachlan G. A. Indoleacetic Acid and the synthesis of glucanases and pectic enzymes. Plant Physiol. 1968 May;43(5):735–742. doi: 10.1104/pp.43.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. L., Ray P. M. Timing of the auxin response in coleoptiles and its implications regarding auxin action. J Gen Physiol. 1969 Jan;53(1):1–20. doi: 10.1085/jgp.53.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galston A. W., Davies P. J. Hormonal regulation in higher plants. Science. 1969 Mar 21;163(3873):1288–1297. doi: 10.1126/science.163.3873.1288. [DOI] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of ribonucleic acids from mammalian tissues. Biochem J. 1956 Nov;64(3):405–408. doi: 10.1042/bj0640405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loening U. E., Ingle J. Diversity of RNA components in green plant tissues. Nature. 1967 Jul 22;215(5099):363–367. doi: 10.1038/215363a0. [DOI] [PubMed] [Google Scholar]
- RALPH R. K., BELLAMY A. R. ISOLATION AND PURIFICATION OF UNDEGRADED RIBONUCLEIC ACIDS. Biochim Biophys Acta. 1964 May 18;87:9–16. doi: 10.1016/0926-6550(64)90041-6. [DOI] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Trewavas A. J. Effect of IAA on RNA and protein synthesis. Effects of 3-indolylacetic acid on the metabolism of ribonucleic acid and protein in etiolated subapical sections of Pisum sativum. Arch Biochem Biophys. 1968 Feb;123(2):324–335. doi: 10.1016/0003-9861(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Winter A., Thimann K. V. Bound indoleacetic Acid in Avena coleoptiles. Plant Physiol. 1966 Feb;41(2):335–342. doi: 10.1104/pp.41.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]


