Abstract
Two millimeter long secondary root tips of etiolated mung bean (Phaseolus aureus) plants were given 4 minute consecutive treatments of darkness, red light, far red light, and acetylcholine during darkness. We studied the effects of these treatments on exogenous (H+) changes, ATP utilization, O2 uptake, P1 levels, and ATPase activity. Red light and acetylcholine increased the level of P1, O2 uptake, and exogenous H+, but decreased ATP concentrations. Darkness and far red light caused the amount of ATP to increase and decreased the O2 uptake and P1 level. O2 uptake of both excised root tips and isolated mitochondria was promoted by acetylcholine levels of the same order of magnitude that promoted the other photomimetic phenomena. ADP-O ratios indicated that acetylcholine did not cause an appreciable decrease in ATP synthesis. The total ATPase activity remained constant throughout all treatments. Ouabain caused no adhesion to negatively charged glass in the dark, while the inhibitors valinomycin, atractyloside, digitoxin, gramicidin, and oligomycin caused immediate adhesion. All of the inhibitors prevented release from the glass. In red light ouabain increased adhesion, whereas the other inhibitors caused caused immediate and complete adhesion.
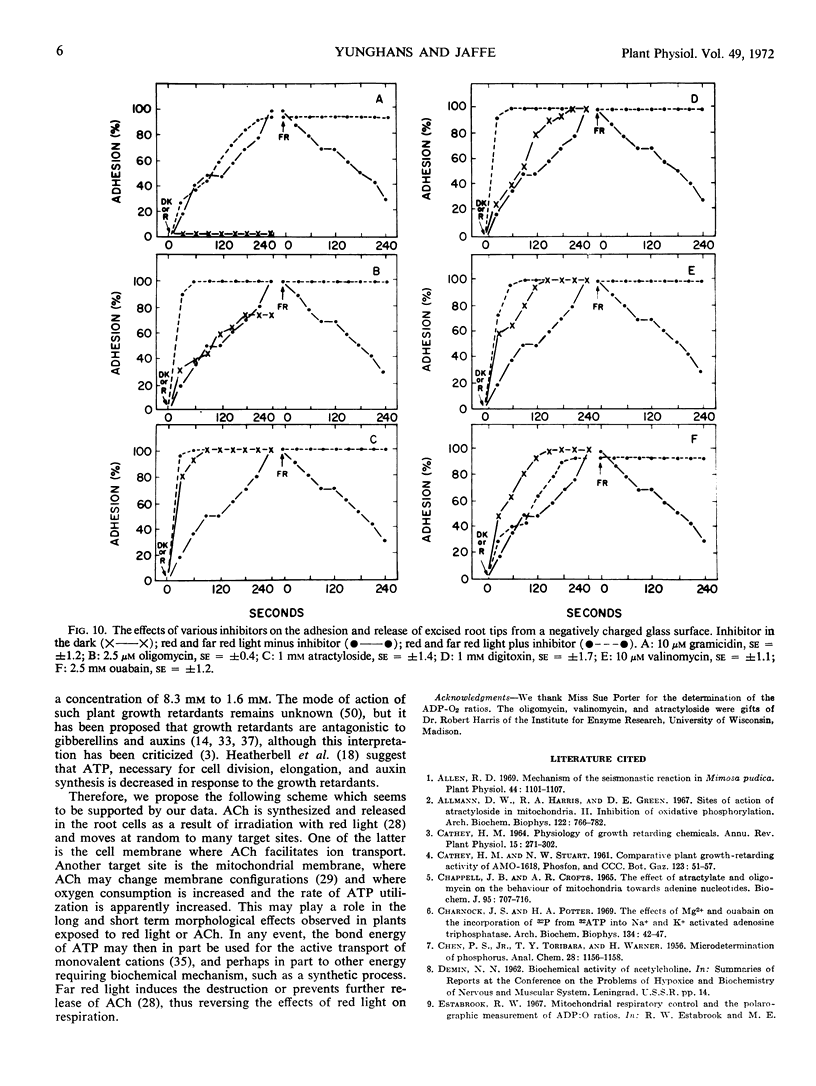
These data seem to imply that one of the functions of the phytochrome-mediated response to red light in roots, regulated by acetylcholine, is to cause the rapid utilization of ATP pools; far red light appears to inhibit this utilization.
Full text
PDF
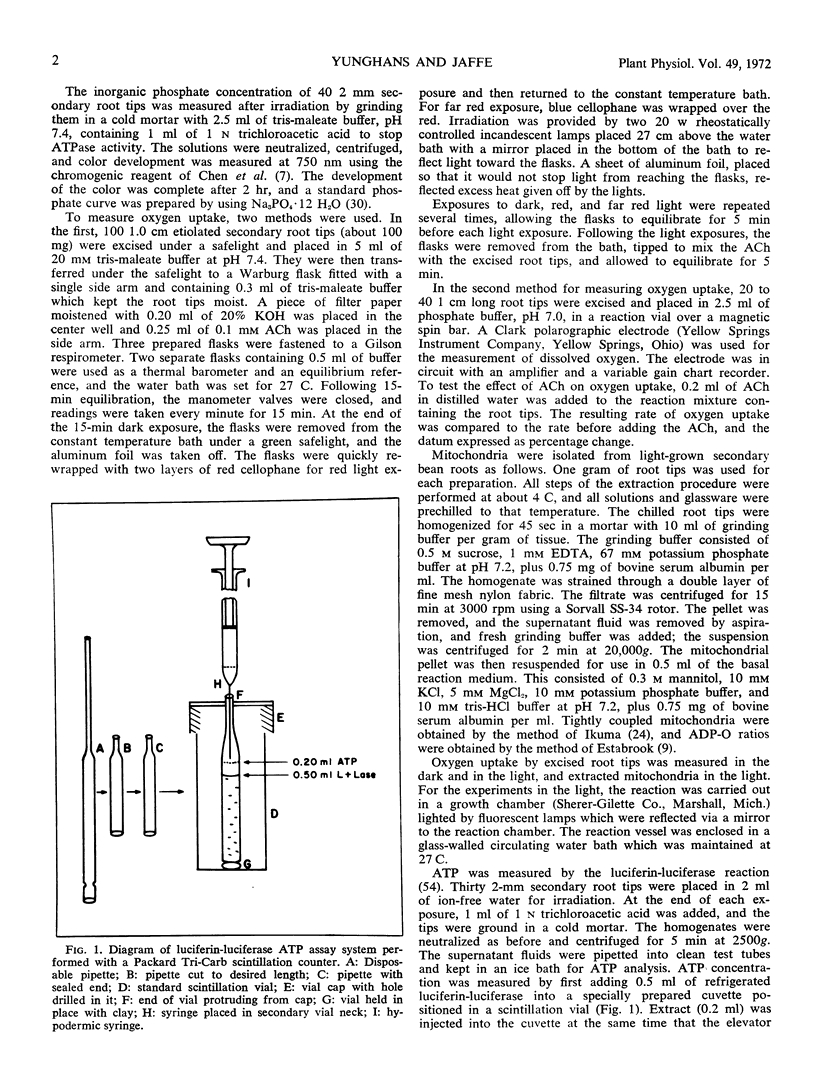
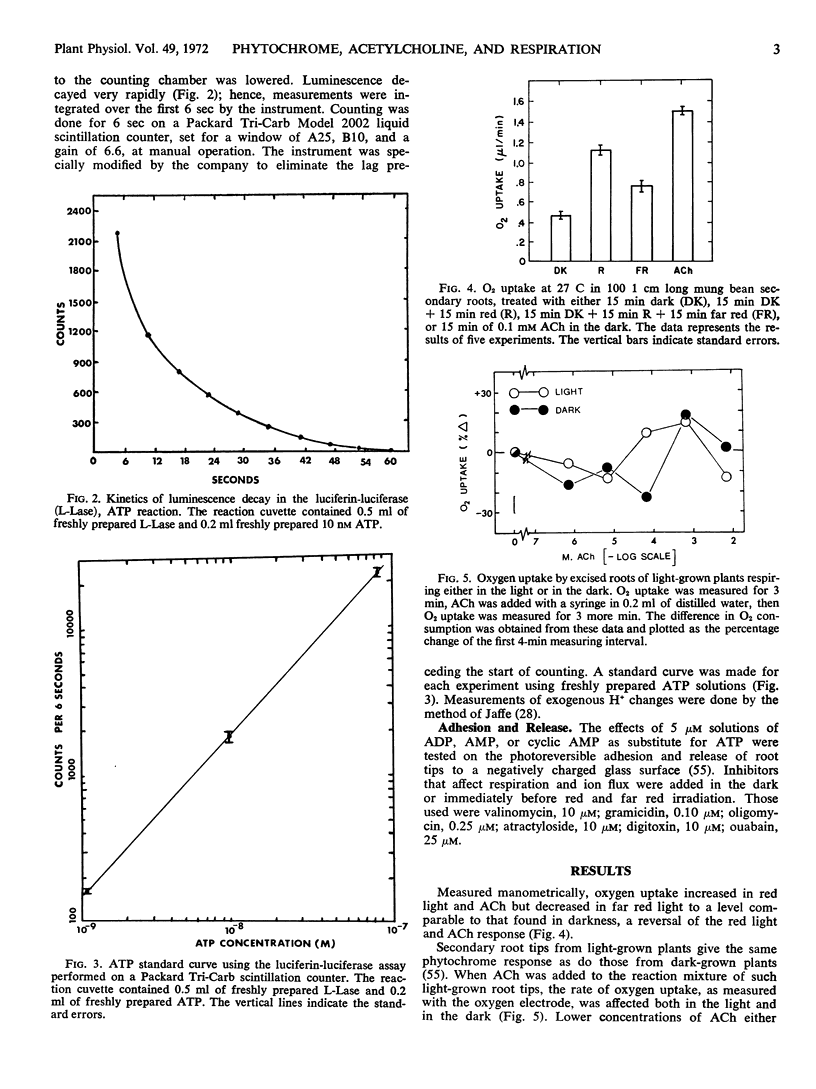
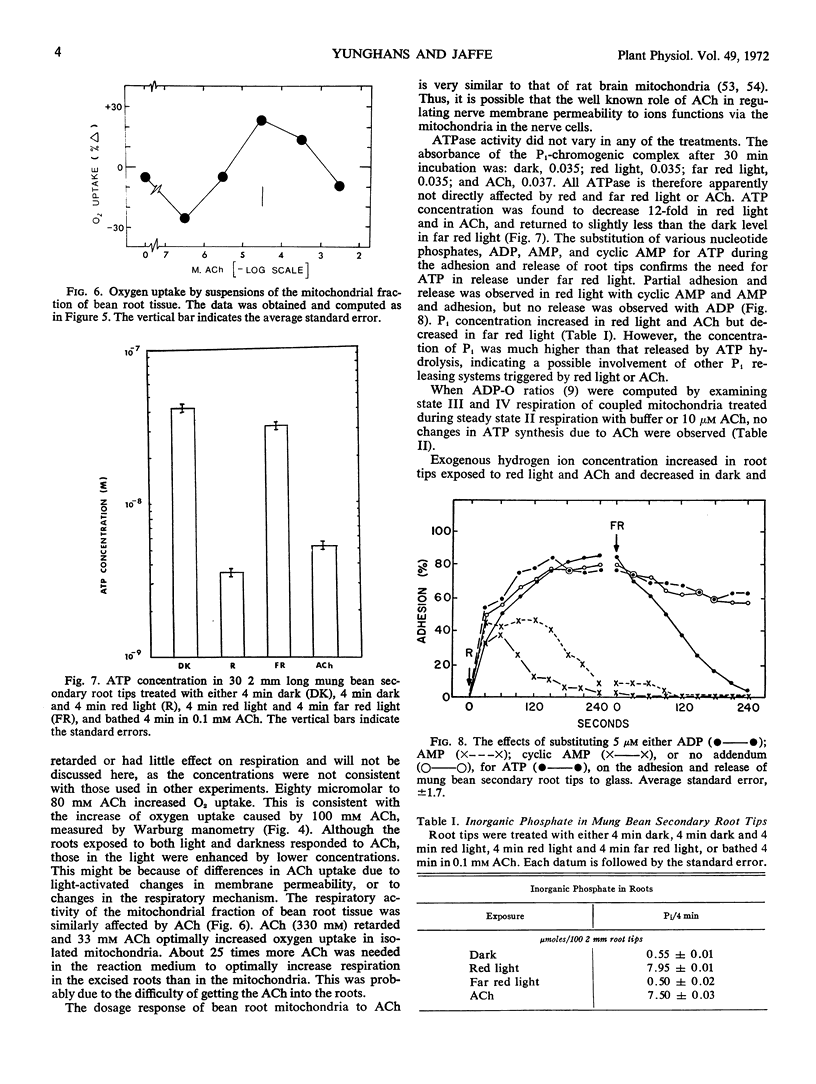
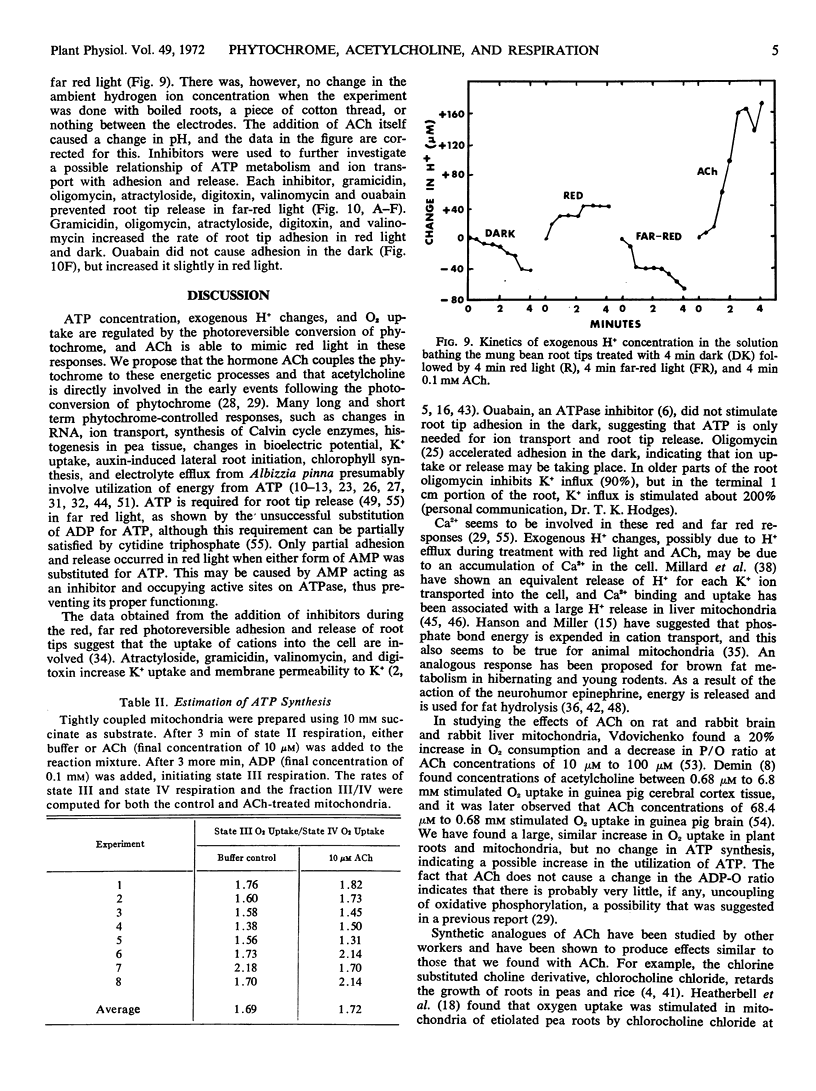

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D. Mechanism of the Seismonastic Reaction in Mimosa pudica. Plant Physiol. 1969 Aug;44(8):1101–1107. doi: 10.1104/pp.44.8.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allmann D. W., Harris R. A., Green D. E. Site of action of atractyloside in mitochondria. II. Inhibition of oxidative phosphorylation. Arch Biochem Biophys. 1967 Dec;122(3):766–782. doi: 10.1016/0003-9861(67)90186-5. [DOI] [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. THE EFFECT OF ATRACTYLATE AND OLIGOMYCIN ON THE BEHAVIOUR OF MITOCHONDRIA TOWARDS ADENINE NUCLEOTIDES. Biochem J. 1965 Jun;95:707–716. doi: 10.1042/bj0950707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnock J. S., Potter H. A. The effect of Mg2+ and ouabin on the incorporation of P32 from gamma-ATP32 into Na plus- and K plus-activated adenosine triphosphatase. Arch Biochem Biophys. 1969 Oct;134(1):42–47. doi: 10.1016/0003-9861(69)90248-3. [DOI] [PubMed] [Google Scholar]
- Fisher J., Hodges T. K. Monovalent ion stimulated adenosine triphosphatase from oat roots. Plant Physiol. 1969 Mar;44(3):385–395. doi: 10.1104/pp.44.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Torrey J. G. The Reversible Inhibition by Red and Far-Red Light of Auxin-Induced Lateral Root Initiation in Isolated Pea Roots. Plant Physiol. 1964 Nov;39(6):987–991. doi: 10.1104/pp.39.6.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy A. H. Interaction of Growth-retarding Compounds and Gibberellin on Indoleacetic Acid Oxidase and Peroxidase of Cucumber Seedlings. Plant Physiol. 1963 Nov;38(6):731–737. doi: 10.1104/pp.38.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. B., Miller R. J. Evidence for active phosphate transport in maize mitochondria. Proc Natl Acad Sci U S A. 1967 Aug;58(2):727–734. doi: 10.1073/pnas.58.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks S. B., Borthwick H. A. The function of phytochrome in regulation of plant growth. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2125–2130. doi: 10.1073/pnas.58.5.2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman W. S., Koukkari W. L. Phytochrome Effects in the Nyctinastic Leaf Movements of Albizzia julibrissin and Some Other Legumes. Plant Physiol. 1967 Oct;42(10):1413–1418. doi: 10.1104/pp.42.10.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Evidence for the regulation of phytochrome-mediated processes in bean roots by the neurohumor, acetylcholine. Plant Physiol. 1970 Dec;46(6):768–777. doi: 10.1104/pp.46.6.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J., Galston A. W. Physiological Studies on Pea Tendrils. II. The Role of Light and ATP in Contact Coiling. Plant Physiol. 1966 Sep;41(7):1152–1158. doi: 10.1104/pp.41.7.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe M. J. Phytochrome-mediated bioelectric potentials in mung bean seedlings. Science. 1968 Nov 29;162(3857):1016–1017. doi: 10.1126/science.162.3857.1016. [DOI] [PubMed] [Google Scholar]
- Kuraishi S., Muir R. M. Mode of Action of Growth Retarding Chemicals. Plant Physiol. 1963 Jan;38(1):19–24. doi: 10.1104/pp.38.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARDY H. A., CONNELLY J. L., JOHNSON D. ANTIBIOTIC STUDIES. II. INHIBITION OF PHOSPHORYL TRANSFER IN MITOCHONDRIA BY OLIGOMYCIN AND AUROVERTIN. Biochemistry. 1964 Dec;3:1961–1968. doi: 10.1021/bi00900a030. [DOI] [PubMed] [Google Scholar]
- Lindberg O., de Pierre J., Rylander E., Afzelius B. A. Studies of the mitochondrial energy-transfer system of brown adipose tissue. J Cell Biol. 1967 Jul;34(1):293–310. doi: 10.1083/jcb.34.1.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. Kinetic studies of certain anti-gibberellins. Plant Physiol. 1962 Nov;37(6):759–764. doi: 10.1104/pp.37.6.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard D. L., Wiskich J. T., Robertson R. N. ION UPTAKE BY PLANT MITOCHONDRIA. Proc Natl Acad Sci U S A. 1964 Oct;52(4):996–1004. doi: 10.1073/pnas.52.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachmansohn D. Proteins in excitable membranes: their properties and function in bioelectricity are discussed. Science. 1970 May 29;168(3935):1059–1066. doi: 10.1126/science.168.3935.1059. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Christiansen E. N., Grav H. J. Respiration-linked phosphorylation in mitochondria of guinea-pig brown fat. Biochem Biophys Res Commun. 1968 Aug 13;32(3):492–500. doi: 10.1016/0006-291x(68)90689-x. [DOI] [PubMed] [Google Scholar]
- Pressman B. C., Harris E. J., Jagger W. S., Johnson J. H. Antibiotic-mediated transport of alkali ions across lipid barriers. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1949–1956. doi: 10.1073/pnas.58.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L., Klein W. H. Red, far-red response & chlorophyll synthesis. Plant Physiol. 1961 Nov;36(6):733–735. doi: 10.1104/pp.36.6.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Rossi C., Azzi A., Azzone G. F. Ion transport in liver mitochondria. I. Metabolism-independent Ca++ binding and H+ release. J Biol Chem. 1967 Mar 10;242(5):951–957. [PubMed] [Google Scholar]
- Smith R. E., Horwitz B. A. Brown fat and thermogenesis. Physiol Rev. 1969 Apr;49(2):330–425. doi: 10.1152/physrev.1969.49.2.330. [DOI] [PubMed] [Google Scholar]
- Tanada T. A rapid photoreversible response of barley root tips in the presence of 3-indoleacetic Acid. Proc Natl Acad Sci U S A. 1968 Feb;59(2):376–380. doi: 10.1073/pnas.59.2.376. [DOI] [PMC free article] [PubMed] [Google Scholar]


