Abstract
Background
The vascular endothelium is the interface between the blood and vascular smooth muscle in arteries. It is easily damaged by oxidative stress. Recent studies show that Asians are more susceptible than Caucasians to impairment of endothelial function. This study examined endothelial function in US-born Caucasians, Asians from Korea, and US-born Asians (almost all Korean decent) and examined the effect of coenzyme Q10 (CoQ10) on endothelial function.
Material/Methods
Twenty Caucasians and 30 Asians participated (<35 years old, males and females). Endothelial function was assessed by the skin blood flow response to local heat using a thermode for 6 minutes at 44°C and by vascular occlusion for 4 minutes followed by release and measurement of skin blood flow for 2 minutes. In the US-born subjects, the experiments were repeated after 2-week administration of CoQ10 or a placebo.
Results
When applying 6 minutes of local heat at 44°C, the skin blood flows were significantly higher in Caucasians than both Asian groups Asians. Likewise after vascular occlusion, the blood flow response was greater in Caucasians compared to Asians. Asians born in Asia had the lowest response of the 3 groups of subjects. Administering CoQ10 for 2 weeks eliminated much of the difference between the groups, whereas there was no difference with a placebo.
Conclusions
These findings suggest that Asians either born in Asia or the US may have lower endothelial function than Caucasians. This may be explained, in part, by genetic variations causing increased oxidative stress from westernized diets in Asians. Co enzyme Q10 administration narrows the difference between the groups.
Keywords: Q10, thrifty gene, endothelial
Background
In previous studies, we and others have shown that the endothelial response seen in Asians newly arrived to the US is lower than that seen in age-matched Caucasians [1–4]. Further, in Native born Koreans, when oxidative markers in the blood were measured, the impaired endothelial response in Koreans, as measured by MDA, was inversely related to endothelial function [3]. When antioxidants were given for 2 weeks, MDA was reduced and the blood flow response to endothelial stress was increased in Koreans compared to Caucasians [3]; however, these studies compared native-born Asians, either in Asia or newly arrived in the United States. In the present investigation, we examined endothelial function in 3 groups of subjects – Caucasians born in the US, People of Asian descent but born in the US, and Asians born in Asia but who were newly arrived to the US to see if this difference in endothelial function in Asians and Caucasians exists in US-born Asians and Asians newly arrived to the US. We gave a 2-week dose of coenzyme q10, an antioxidant, to Asians born in the United States and Caucasians born in the US, to see if this would alter the blood flow response to endothelial stress.
Previous studies have shown that Koreans, Chinese and people from Thailand are more susceptible than Caucasians to free radical damage caused by diet. In Asians newly arrived the United States but born in Asia, at rest and especially 2 hours after a high fat meal, the blood flow response to occlusion and heat diminished in proportion to the increase in blood-borne free radicals. Taking antioxidant vitamins diminished these differences between Asians and Caucasians [3].
A number of studies have been conducted to see if antioxidants might reduce free radicals and hence be protective of blood flow in the myocardium and other organs [5,6]. Because free radicals are strongly associated with cardiovascular disease and diabetes [7–9], natural foods or vitamins might reduce the risk of these pathologies [7–9]. The ability of many different vitamins and additives to reduce free radicals in the blood has been investigated [10–12]. Recently, there has been great interest in coenzyme Q10, an electron acceptor that allows pyruvic acid to enter the mitochondria. It is also the first hydrogen acceptor in oxidative phosphorylation. Pharmaceuticals like Lipitor deplete q10 and can cause atrophy of muscle, as well as muscle cramps and weakness [13]. Q10 is also a potent anti-inflammatory that can reduce inflammation and endothelial damage after a heart attack and may even reduce inflammation from exercise [12]. Some reviews have concluded that it is an ergogenic agent that prevents the loss of muscle strength during exercise and increases endurance by absorbing free radicals in muscle during exercise [12,14]. Coenzyme Q10 supplementation reduces free radicals in the blood as assessed by superoxide dismutase and MDA [5].
One source of free radicals, as cited above, is the ingestion of dietary fat [15–17]. Previous studies in our lab have shown that even the ingestion of a single high fat meal can, in some races, impair blood vessel (endothelial) function [2]. Endothelial function has been measured in previous studies in 2 ways: response to vascular occlusion and response to local heat. The gold standard for assessing endothelial function is the response to vascular occlusion [18], which involves placing an occlusion cuff over the arm at the axilla for 4 minutes and then, after pressure is removed, noting the blood flow response for 2 minutes. Another measure of endothelial function is the skin response to local heat [18–22]. When heat is applied to the skin, there is an increase in blood flow mediated by 2 different mechanisms. Initially, tactile neurons in the skin release Substance P and Calcitonin Gene-Related Peptide when the skin is exposed to local heat [23,24]. This causes an increase in potassium permeability in vascular smooth muscle surrounding the endothelial cells [14,23,25]. Relaxation of vascular smooth muscle then increases skin blood flow, but this response only lasts a few minutes. The sustained response to temperature is mediated by TRPV-4 voltage gated calcium channels in the vascular endothelial cells [26–29]. Above a temperature of 35°C, these cells cause an exponential increase in calcium influx into the endothelial cells from the interstitial space. Calcium activates the enzyme nitric oxide synthase, producing endothelial nitric oxide [30].
In a previous study, we have shown that both responses are increased in young people ingesting a mixture of antioxidants for 2 weeks. In the present investigation, we examined only the effect of coenzyme q10. It was administered for 2 weeks. Endothelial function was assessed in Asians born in the US, Asians born in Asia (Korea) and Caucasians born in the US. The experiments were repeated before and after Co enzyme Q10 administration in the 2 US born groups.
Material and Methods
Subjects
Sixty subjects participated in the experiments. Twenty subjects were US-born Caucasians. Their data was compared to 2 groups of Asian subjects; 20 subjects whose parents or grandparents were from China and Korea (17 Korea and 3 from China) but who were born in the US and had lived there their whole lives, and 10 subjects who had arrived in the US within the last year from Korea and who were born in Korea. The 20 US-born Asians were physical therapy students: 10 were in the coq10 group and 10 were in the placebo group. Subjects were of similar age, not taking alpha blockers, beta blockers, alpha agonists or antagonists, or any other medication that would affect peripheral blood flow, nor were they taking calcium channel blockers or any pain medications. All subjects were vitamin naïve for at least a month prior to the beginning of this study. No subjects were smokers. All methods and procedures were approved by the Institutional Review Board of Azusa Pacific or Loma Linda University. All subjects signed a statement of informed consent. General characteristics of subjects are shown in Tables 1–3. There were no statistical differences in age, height, weight, or BMI among the 3 groups of subjects (p>0.05).
Table 1.
Demographics of the 20 Caucasian US born subjects.
| Age (years) | Height (cm) | Weight (kg) | BMI | |
|---|---|---|---|---|
| Mean | 23.8 | 171.1 | 69.0 | 23.5 |
| SD | 1.7 | 8.9 | 11.3 | 2.4 |
Table 3.
Demographics of the 10 Asian-born Asian subjects.
| Age (years) | Height (cm) | Weight (kg) | BMI | |
|---|---|---|---|---|
| Mean | 25.1 | 168.6 | 69.2 | 24.5 |
| SD | 4.1 | 9.8 | 16.1 | 3.6 |
Methods
Measurement of skin temperature
Skin temperature was measured with a thermistor (SKT RX 202A) manufactured by BioPac systems (BioPac Inc., Goleta, CA). The thermistor output was sensed by an SKT 100 thermistor amplifier (BioPac Inc., Goleta, CA). The output, which was a voltage between 0 and 10 volts, was then sampled with an analog-to-digital converter at a frequency of a 1000 samples per second with a resolution of 24 bits using a BioPac MP150 analog-to-digital converter. The converted data was then stored on a desktop computer using Acknowledge 4.1 software for later analysis. Data analysis was done over a 5-second period for mean temperature. The temperature was calibrated at the beginning of each day by placing the thermistors in a controlled temperature water bath calibrated against a standard thermometer.
Measurement of skin blood flow
Skin blood flow was measured with a Moor Laser Doppler flow meter (VMS LDF20, Oxford England). The imager uses a red laser beam (632.8 nm) to measure skin blood flow using the Doppler effect. After warming the laser for 15 to 30 minutes prior to use, the laser was applied to the skin through a VP12B fiber optic probe placed above the forearm (Figure 1). The Moor Laser Doppler flow meter measures blood flow through most of the dermal layer of the skin but does penetrate the entire dermal layer. Blood flow is then calculated in a unit called Flux, based on the red cell concentration in red cell velocity with a stated accuracy of ±10%. The tissue thickness sampled is typically 1 mm in depth.
Figure 1.
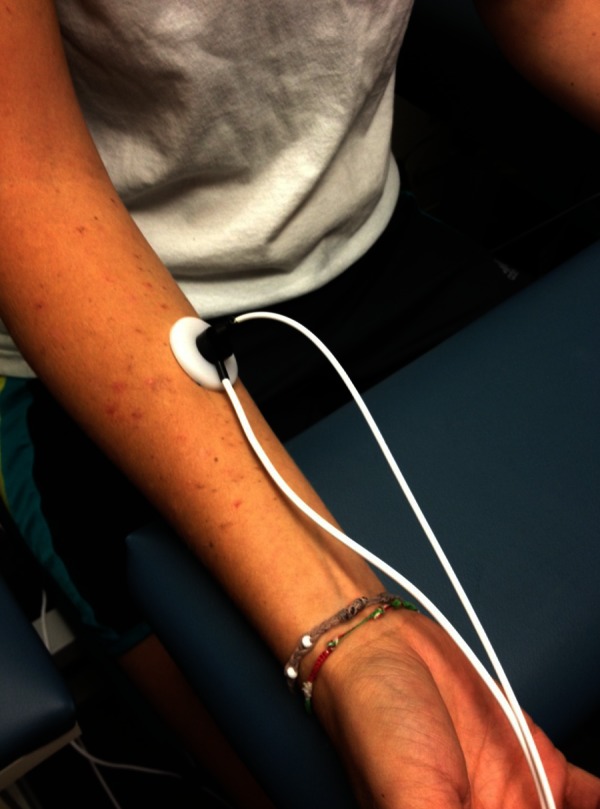
The Moor laser flow meter with the surrounding thermode to heat the skin.
Control of skin temperature
Skin temperature was controlled by a Moor temperature controller (SH02) with an SHO2-SHP1 skin temperature module integrated with the blood flow fiber optic probe (Figure 1). This is a closed loop electric warmer (thermode), where temperature is controlled to within 0.1°C.
Measurement of endothelial function
Endothelial function was measured by arterial occlusion. The blood flow to the arm was occluded for 4 minutes by placing a pneumatic occlusion cuff on the upper arm under the axilla and inflating the cuff for 4 minutes. After the pressure was released, forearm blood flow was measured for 2 minutes to assess the reactivity of the blood vessels to occlusion and anoxia.
Measurement of the response to heat
The response of the skin to heat was measured by applying the heated probe to the skin for 6 minutes. The thermode was set at a temperature of 44°C, warming the skin, and blood flow was then recorded.
Procedures
Subjects were interviewed for inclusion and exclusion criteria. Those subjects that were eligible were enrolled into the study and read and signed a statement of informed consent. Next, subjects rested for 15 minutes while height and weight were taken. Baseline skin blood flow was recorded for 1 minute over the forearm. Then, the thermode was applied upon the arm above the brachioradialis muscle to warm the skin to 44°C. The thermode was left on for 6 minutes. On another day, occlusion was applied by a blood pressure occlusion cuff inflated to 200mmHg for 4 minutes followed by 2 minutes of additional blood flow recording. Skin temperature at this site was measured throughout the experimental period. Each experiment took approximately 10 minutes and was performed on 2 separate days. In the subjects who were born in the United States, the experiments were repeated after subjects had received 300 mg/day of CoQ10 for half the group or a matched appearance placebo for the other half of this group.
Statistical analysis
Data were summarized as means and standard deviations. Baseline characteristics of Caucasians and Asians were compared using ANOVA. A mixed factorial ANOVA was conducted to compare the blood flow response to 4 minutes of vascular occlusion and 6 minutes of local heat. The level of significance was set at p<0.05.
Results
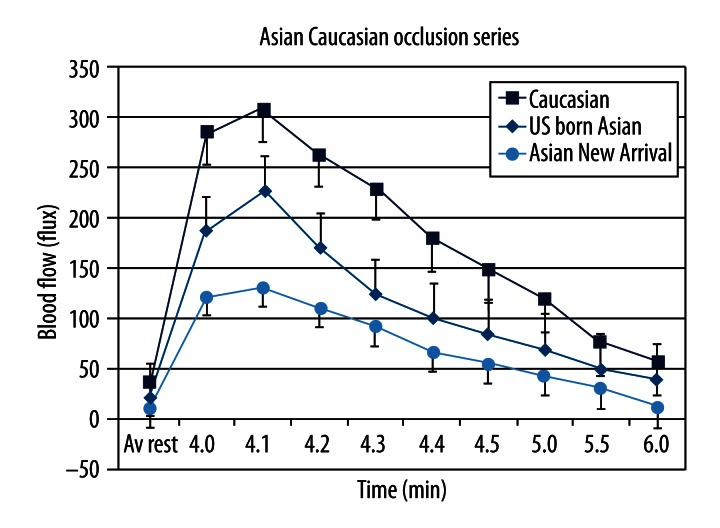
Figure 2 shows the results of the measurement of skin blood flow after 4 minutes of vascular occlusion in the 3 groups of subjects. For all subjects, there was a rapid increase in blood flow after the occlusion cuff was released. Blood flow peaked about 10 seconds after the occlusion was released and then fell exponentially during the 2 minutes after the occlusion time period in which blood flow was measured. The blood flow at rest was significantly higher for the Caucasian group than the 2 Asian groups. The 2 Asian groups were not different from each other (p>0.05). From the end of the occlusion to the 2 minute post occlusion period, skin blood flow was significantly different in the Caucasians vs. the 2 Asian groups and the 2 Asian groups were different form each other (p<0.05). This was better seen in the area under the curve, a measure of total excess blood flow needed to recover from the occlusion: 513±163 cc for the Caucasian group, 418±67 cc for the Asians born in the US, and 156±36 cc for the Asians newly arrived in the US. These values were significantly different from each other (p<0.05).
Figure 2.
Showing the average blood flow in Caucasians, Asians born in the US and Asians from Asia in response to 4 minutes of vascular occlusion. All data is the group mean ± the SD.
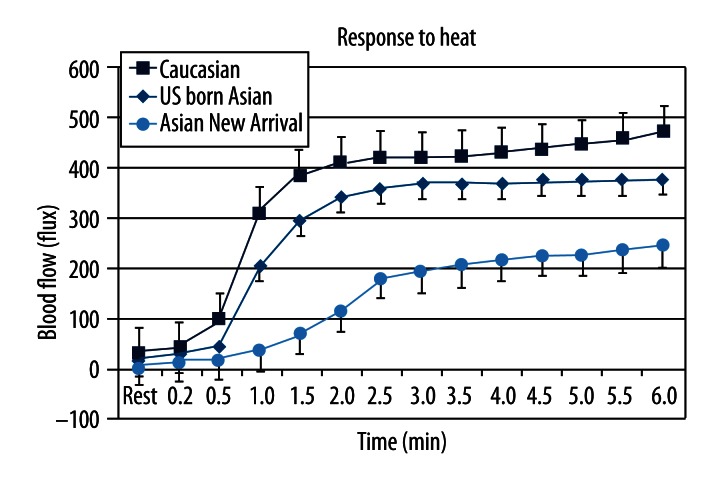
The blood flow response to heat for the 3 groups of subjects is shown in Figure 3. Blood flow rose starting about 30 seconds after heat was applied. For all subjects, blood flow increased steadily for about 2.5 minutes and then more slowly rose thereafter. Caucasian subjects had the greatest increase in blood flow and Asian subjects born in Asia had the least. Blood flow was significantly higher at rest and throughout the 6-minute period in Caucasians compared to Asian newly arrived to the US (p<0.05, ANOVA). The blood flow was significantly higher from 1 to 6 minutes in the Asians born in the US compared to the Asians newly arrived in the US. The blood flow in the Asians born in the US was significantly less than that of US-born Caucasians from 1.5 to 6 minutes (p<0.05, ANOVA).
Figure 3.
Showing the average blood flow in Caucasians, Asians born in the US and Asians from Asia in response to 6 minutes of local heating of the skin. All data is the group mean ± the SD.
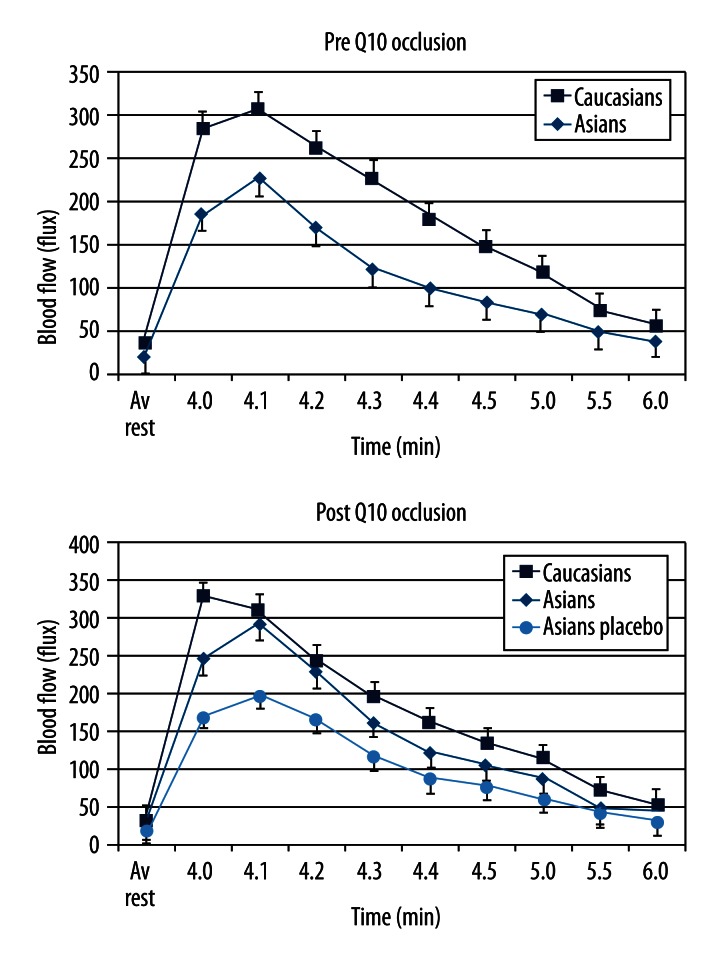
Figures 4 and 5 shows the blood flow in the skin of the Caucasian and US-born Asian groups before and after 2-week administration of Coenzyme q10 or for one group of 10 Asian subjects who received a matched placebo. As shown in Figure 4, the difference in response after occlusion in blood flow between US-born Asians and Caucasians (panel A) was eliminated after 2-week administration of Coq10 panel B (p>0.05, ANOVA). The placebo group showed no change in their response (p>0.05, ANOVA). For the Caucasian group, there was a significant increase in blood flow at 4, 4.1, and 4.2 minutes comparing data on the Caucasians before and after administration of Coenzyme q10 (p<0.05, ANOVA). For the US-born Asians, there was no significant difference in the blood flow response after occlusion compared to Caucasians. Thus, proportionally, there was a greater increase in post-occlusion blood flow in the Asians than in the Caucasians. For the placebo Asian group, there was no difference between pre-and post-placebo values.
Figure 4.
Showing the average blood flow in Caucasians and Asians born in the US in response to 4 minutes of vascular occlusion. All data is the group mean ± the SD. Data is shown before Q10 and after 2 weeks of Q10 administration or placebo administration.
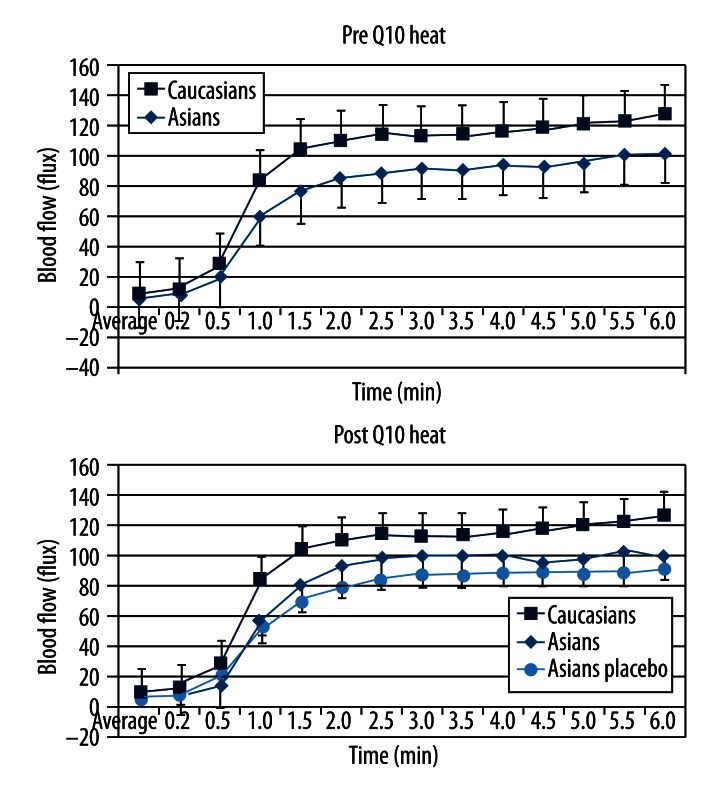
Figure 5.
Showing the average blood flow in Caucasians and Asians born in the US in response to 6 minutes of local heat. All data is the group mean ± the SD. Data is shown before Q10 and after 2 weeks of Q10 administration or placebo administration.
The response to heat is shown in Figure 5. Differences between the US born Asian and Caucasian groups were eliminated after administration of Q10. There was no statistical difference between the heat response of the groups (panel B, p>0.05, ANOVA), except for the placebo group, which had no changes comparing data collected before and after vitamin administration and data was still different than the other groups.
Discussion
Asians have developed a genetic mutation in a number of genes that have been termed “thrifty” genes. These genes allow Asians to exist on low-calories diets [31–33]. People with thrifty genes can store fat better than others and are therefore more likely to survive prolonged food shortages [2]. This thrifty genotype, which is composed of many single-nucleotide polymorphisms (SNPs), is a genetic difference regulating lipid metabolism and fat storage, and differs depending on ethnicity [31,33–35], but these same genes have been linked to a sharp increase in the incidence of diabetes and heart disease all across Asia. International studies conducted among different Asian national populations in China, Korea, Philippines, Singapore, and Taiwan have shown increased risk of type 2 diabetes and cardiovascular disease at lower BMI than European populations [36]. According to recent studies, the predicted prevalence of metabolic syndrome is 25% for non-Hispanic Whites compared to 45% of Asians (Korean, Asian Indian, Chinese, Filipino, Japanese, and Vietnamese) and prevalence of diabetes in Asian Americans is 60% higher than in non-Hispanic whites [36,37]. Such ethnic difference could be either due to a naturally lower blood flow in Asians or the influence of the thrifty genotype on endothelial function [31,33,34]. Due to a modern high-fat diet, this genotype may cause endothelial damage. What has been termed a “westernized” diet in Asia led it to have the most rapidly increasing incidence of heart disease and diabetes in the world [38]. The most pronounced effect is on vascular endothelial cells.
The vascular endothelial cell is the interface between the blood and either the surrounding vascular smooth muscle or the movement of substances into and out of the capillaries [24]. Endothelial cells release substances in the arteries that either causes relaxation of the artery (vasodilation) or constriction of smooth muscle [2,10,15,24]. A principal vasodilator is nitric oxide [7,39]. Produced from the reduction of l-arginine to l- citrulline, this fat-soluble substance readily moves to vascular smooth muscle cells and causes muscle relaxation [1,40]. This predominant vasodilator pathway is subject to damage by free radicals [3].
Free radicals are commonly produced and neutralized in the body [41]. Some free radicals are produced and used for cellular communication, and others are produced as a natural product of cellular metabolism [39,42–44].
Older adults have lower levels of antioxidant enzymes [45] and are therefore more susceptible to injury from pro-oxidant challenges [46]. Thus, especially in older individuals, the production of free radicals at rest and during exercise can cause significant damage to tissue, leading to an inflammatory response [41,46,47].
When the free radical concentration reaches a critical level, rather than increasing blood flow, they biodegrade nitric oxide and prostacyclin, a second vasodilator released from vascular endothelial cells, into inactive forms [17,30,48]. In the presence of free radicals such as hydrogen peroxide, nitric oxide is reduced to peroxynitrite (ONOO), a free radical with no influence on circulation[18]. Bioconversion of nitric oxide to peroxynitrite is believed to be one of the mechanisms associated with the reduction in circulation at rest and during stress in older people and people with diabetes, leading to endothelial dysfunction [18].
Previous studies on Japanese in Japan, Japanese Americans, and people in the US from Thailand, Korea, and India, have all shown that even a single high-fat meal can reduce the blood flow response to the skin and muscle in response to occlusion [4,32,33,49,50]. Free radicals have been measured after a single high-fat meal and with a chronic high-fat diet and found to be markedly elevated, especially in Asians. Nappo et al. [51]investigated inflammatory markers after healthy subjects ingested a high-fat meal. They verified significant elevations in serum triglycerides (TG), as well as cytokines such as TNF-α, TL-6, monocyte chemo-attractant protein, and an increase in the expression of adhesion molecules (ICAM-1, VCAM-1), which normally are absent in the endothelium of the vascular wall. In native Koreans, free radicals were higher than in Caucasians [3]. With the use of antioxidants for only 2 weeks, free radicals were reduced after a high-fat meal to levels similar to those found in Caucasians and the blood flow response to occlusion and heat was no different in Koreans than their Caucasian counterparts [49].
This, then, agrees with the results of the present investigation in many ways. One major difference is that Asians were compared that were born in the US vs. those that were newly arrived to the US. The Asians newly arrived in the US had more diminished blood flow response to occlusion and heat than US-born Asians. One possibility is that US-born Asians have adapted to the high-fat foods common in a western diet. By being born in the US, the body may develop a type of immunity to the free radicals generated by a westernized diet. This needs to be investigated further.
Another possibility is that their lifestyle is different than that seen in Asians newly arrived to the US. In our study, the US-born Asian subjects were physical therapy students. As such, they exercise more than the general population, especially people newly arrived in the US. Exercise has been shown to boost the immune system and protect the body from free radicals by increasing the concentration of peroxidases and other enzymes that reduce free radicals in the body [52]. This may be the difference seen between the 2 Asian groups.
Of interest is the fact that administration of q10 for just 2 weeks improved the response to occlusion by only a small amount in Caucasians but by a large amount in US-born Asians. A mixture of Q10 and vitamins A and C was used by Yim et al. [3], who showed a similar improvement in native Asians before and after ingesting a high-fat meal. Here, only Q10 was administered but the results were very dramatic. Studies have shown the antioxidant effect of Q10 [53–55]. The present study agrees with these in that administration of Q10 was associated with an increased response to occlusion and a small increase in the response to heat in Caucasians, and to a greater degree in Asians. This is probably due to reduced oxidative stress, but there may be another mechanism associated with metabolic effects on Q10. This needs further investigation.
Conclusions
Asians, either from Asia or born in the United States, have an impaired skin blood flow response to occlusion and heat.
This may be due to a high-fat westernized diet because Asians have a “thrifty” genotype that produces excessive free radicals even from a single high-fat meal.
In the present study, this reduced blood flow response in the skin was seen in Asians born in Asia and to a lesser extent in US-born Asians.
Two-week administration of Coenzyme Q10, a free radical scavenger, increased the skin blood flow response in Asians born in the United States similar to that seen in Caucasians; there was no effect on the skin blood flow response to heat or occlusion to 2-week administration of a placebo.
This study supports the idea that westernized diets are damaging to circulation in Asians but, at least in the short term, this can be neutralized by higher levels of dietary antioxidants.
Table 2.
Demographics of the 20 US-born Asian subjects.
| Age (years) | Height (cm) | Weight (kg) | BMI | |
|---|---|---|---|---|
| Mean | 24.3 | 168.7 | 69.7 | 24.1 |
| SD | 4.3 | 12.3 | 21.3 | 4.1 |
Footnotes
Source of support: Departmental sources
References
- 1.Petrofsky JS, et al. Reduced endothelial function in the skin in Southeast Asians compared to Caucasians. Med Sci Monit. 2012;18(1):CR1–8. doi: 10.12659/MSM.882185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bui C, et al. Acute effect of a single high-fat meal on forearm blood flow, blood pressure and heart rate in healthy male Asians and Caucasians: a pilot study. Southeast Asian J Trop Med Public Health. 2010;41(2):490–500. [PMC free article] [PubMed] [Google Scholar]
- 3.Yim J, et al. Protective effect of anti-oxidants on endothelial function in young Korean-Asians compared to Caucasians. Med Sci Monit. 2012;18(8):467–79. doi: 10.12659/MSM.883266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yim J, et al. Differences in endothelial function between Korean-Asians and Caucasians. Med Sci Monit. 2012;18(6):CR337–43. doi: 10.12659/MSM.882902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee BJ, et al. Effects of coenzyme Q10 supplementation on inflammatory markers (high-sensitivity C-reactive protein, interleukin-6, and homocysteine) in patients with coronary artery disease. Nutrition (Burbank, Los Angeles County, Calif) 2012 doi: 10.1016/j.nut.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Lee YJ, et al. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: a double-blind randomized controlled study. J Med Food. 2011;14(4):386–90. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 7.Yubero-Serrano EM, et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J Gerontol A Biol Sci Med Sci. 2012;67(1):3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez-Mariscal FM, et al. Mediterranean diet supplemented with coenzyme Q10 induces postprandial changes in p53 in response to oxidative DNA damage in elderly subjects. Age (Dordr) 2012;34(2):389–403. doi: 10.1007/s11357-011-9229-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yubero-Serrano EM, et al. Postprandial antioxidant effect of the Mediterranean diet supplemented with coenzyme Q10 in elderly men and women. Age (Dordr) 2011;33(4):579–90. doi: 10.1007/s11357-010-9199-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gokbel H, et al. Effects of coenzyme Q10 supplementation on plasma adiponectin, interleukin-6, and tumor necrosis factor-alpha levels in men. J Med Food. 2010;13(1):216–18. doi: 10.1089/jmf.2008.0310. [DOI] [PubMed] [Google Scholar]
- 11.Gokbel H, et al. The effects of coenzyme Q10 supplementation on performance during repeated bouts of supramaximal exercise in sedentary men. J Strength Cond Res. 2010;24(1):97–102. doi: 10.1519/JSC.0b013e3181a61a50. [DOI] [PubMed] [Google Scholar]
- 12.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88(4):1243–76. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikka P, et al. Statin intolerance: now a solved problem. J Postgrad Med. 2011;57(4):321–28. doi: 10.4103/0022-3859.90085. [DOI] [PubMed] [Google Scholar]
- 14.Minson CT, Berry LT, Joyner MJ. Nitric oxide and neurally mediated regulation of skin blood flow during local heating. J Appl Physiol. 2001;91(4):1619–26. doi: 10.1152/jappl.2001.91.4.1619. [DOI] [PubMed] [Google Scholar]
- 15.Riccardi G, Rivellese AA. Dietary treatment of the metabolic syndrome – the optimal diet. Br J Nut. 2000;83(Suppl 1):S143–48. doi: 10.1017/s0007114500001082. [DOI] [PubMed] [Google Scholar]
- 16.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clinical nutrition (Edinburgh, Scotland) 2004;23(4):447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Vecchini A, et al. Dietary alpha-linolenic acid reduces COX-2 expression and induces apoptosis of hepatoma cells. J Lipid Res. 2004;45(2):308–16. doi: 10.1194/jlr.M300396-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Farage M, Miller K, Maibach H, editors. Influence of Race, Gender, Age and Diabetes on the Skin Circluation Text Book of Ageing Skin. Springer Berlin; Heidelberg: 2010. [Google Scholar]
- 19.Al-Nakhli HH, et al. The use of thermal infrared imaging to assess the efficacy of a therapeutic exercise program in individuals with diabetes. Diabetes Technol Ther. 2012;14(2):159–67. doi: 10.1089/dia.2011.0187. [DOI] [PubMed] [Google Scholar]
- 20.Petrofsky JS. The effect of type-2-diabetes-related vascular endothelial dysfunction on skin physiology and activities of daily living. J Diabetes Sci Technol. 2011;5(3):657–67. doi: 10.1177/193229681100500319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrofsky J, et al. The interrealtionship between locally applied heat, ageing and skin blood flow on heat transfer into and from the skin. J Med Eng Technol. 2011;35(5):262–74. doi: 10.3109/03091902.2011.580039. [DOI] [PubMed] [Google Scholar]
- 22.Petrofsky J, et al. The ability of the skin to absorb heat; the effect of repeated exposure and age. Med Sci Monit. 2011;17(1):CR1–8. doi: 10.12659/MSM.881315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charkoudian N, Fromy B, Saumet JL. Reflex control of the cutaneous circulation after acute and chronic local capsaicin. J Appl Physiol. 2001;90(5):1860–64. doi: 10.1152/jappl.2001.90.5.1860. [DOI] [PubMed] [Google Scholar]
- 24.Farage MA, Miller KW, Maibach HI, editors. Textbook of Aging Skin 2010. Springer-Verlag; Berlin Heidelberg: [Google Scholar]
- 25.Charkoudian N, et al. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol. 2002;92(2):685–90. doi: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe H, et al. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002;277(49):47044–51. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- 27.Watanabe M, et al. Assessment of mechanical and thermal thresholds of human C nociceptors during increases in skin sympathetic nerve activity. Clin Neurophysiol. 2002;113(9):1485–90. doi: 10.1016/s1388-2457(02)00159-1. [DOI] [PubMed] [Google Scholar]
- 28.Petrofsky J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med Eng Phys. 2008;30(9):1168–76. doi: 10.1016/j.medengphy.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Alderton F, et al. G-protein-coupled receptor stimulation of the p42/p44 mitogen-activated protein kinase pathway is attenuated by lipid phosphate phosphatases 1, 1a, and 2 in human embryonic kidney 293 cells. J Biol Chem. 2001;276(16):13452–60. doi: 10.1074/jbc.M006582200. [DOI] [PubMed] [Google Scholar]
- 30.Wong BJ, Fieger SM. Transient receptor potential vanilloid type-1 (TRPV-1) channels contribute to cutaneous thermal hyperaemia in humans. J Physiol. 2010;588(Pt 21):4317–26. doi: 10.1113/jphysiol.2010.195511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radha V, et al. Role of genetic polymorphism peroxisome proliferator-activated receptor-gamma2 Pro12Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects: Evidence for heterogeneity. Diabetes Care. 2006;29(5):1046–51. doi: 10.2337/diacare.2951046. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi C, et al. Germline mutation of the LKB1/STK11 gene with loss of the normal allele in an aggressive breast cancer of Peutz-Jeghers syndrome. Oncology. 2004;67(5–6):476–79. doi: 10.1159/000082933. [DOI] [PubMed] [Google Scholar]
- 33.Kagawa Y, et al. Single nucleotide polymorphisms of thrifty genes for energy metabolism: evolutionary origins and prospects for intervention to prevent obesity-related diseases. Biochem Biophys Res Commun. 2002;295(2):207–22. doi: 10.1016/s0006-291x(02)00680-0. [DOI] [PubMed] [Google Scholar]
- 34.Nakanishi S, et al. The effect of polymorphism in the intestinal fatty acid-binding protein 2 gene on fat metabolism is associated with gender and obesity amongst non-diabetic Japanese-Americans. Diabetes Obes Metab. 2004;6(1):45–49. doi: 10.1111/j.1463-1326.2004.00313.x. [DOI] [PubMed] [Google Scholar]
- 35.Murphy C, et al. Vascular dysfunction and reduced circulating endothelial progenitor cells in young healthy UK South Asian men. Arterioscler Thromb Vasc Biol. 2007;27(4):936–42. doi: 10.1161/01.ATV.0000258788.11372.d0. [DOI] [PubMed] [Google Scholar]
- 36.Palaniappan LP, et al. Asian Americans have greater prevalence of metabolic syndrome despite lower body mass index. Int J Obes (Lond) 2011;35(3):393–400. doi: 10.1038/ijo.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27(1):66–69. doi: 10.2337/diacare.27.1.66. [DOI] [PubMed] [Google Scholar]
- 38.Cho SY, et al. Hypoplastic myelodysplastic syndrome associated with der(1;7)(q10;p10) presenting as bone marrow failure. Acta Haematol. 2011;126(2):110–13. doi: 10.1159/000328036. [DOI] [PubMed] [Google Scholar]
- 39.Petrofsky J, et al. The interrelationships between electrical stimulation, the environment surrounding the vascular endothelial cells of the skin, and the role of nitric oxide in mediating the blood flow response to electrical stimulation. Med Sci Monit. 2007;13(9):CR391–97. [PubMed] [Google Scholar]
- 40.Petrofsky J, Berk L, Al-Nakhli H. The influence of autonomic dysfunction associated with aging and type 2 diabetes on daily life activities. Exp Diabetes Res. 2012;2012:657103. doi: 10.1155/2012/657103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sacheck JM, Blumberg JB. Role of vitamin E and oxidative stress in exercise. Nutrition (Burbank, Los Angeles County, Calif) 2001;17(10):809–14. doi: 10.1016/s0899-9007(01)00639-6. [DOI] [PubMed] [Google Scholar]
- 42.Durante W, Johnson FK, Johnson RA. Arginase: a critical regulator of nitric oxide synthesis and vascular function. Clin Exp Pharmacol Physiol. 2007;34(9):906–11. doi: 10.1111/j.1440-1681.2007.04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimmeler S, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6(10):964–68. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- 44.Maloney-Hinds C, et al. The role of nitric oxide in skin blood flow increases due to vibration in healthy adults and adults with type 2 diabetes. Diabetes Technol Ther. 2009;11(1):39–43. doi: 10.1089/dia.2008.0011. [DOI] [PubMed] [Google Scholar]
- 45.Gershon D. Current status of age altered enzymes: alternative mechanisms. Mech Ageing Dev. 1979;9(3–4):189–96. doi: 10.1016/0047-6374(79)90098-8. [DOI] [PubMed] [Google Scholar]
- 46.Meydani M, et al. Protective effect of vitamin E on exercise-induced oxidative damage in young and older adults. Am J Physiol. 1993;264(5 Pt 2):R992–98. doi: 10.1152/ajpregu.1993.264.5.R992. [DOI] [PubMed] [Google Scholar]
- 47.Urso ML, Clarkson PM. Oxidative stress, exercise, and antioxidant supplementation. Toxicology. 2003;189(1–2):41–54. doi: 10.1016/s0300-483x(03)00151-3. [DOI] [PubMed] [Google Scholar]
- 48.Petrofsky J, et al. Dry heat, moist heat and body fat: are heating modalities really effective in people who are overweight? J Med Eng Technol. 2009;33(5):361–69. doi: 10.1080/03091900802355508. [DOI] [PubMed] [Google Scholar]
- 49.Petrofsky J, Lee S, Cuneo ML. Gait characteristics in patients with type 2 diabetes; improvement after administration of rosiglitazone. Med Sci Monit. 2005;11(6):PI43–51. [PubMed] [Google Scholar]
- 50.Petrofsky J, et al. Autonomic, endothelial function and the analysis of gait in patients with type 1 and type 2 diabetes. Acta Diabetol. 2005;42(1):7–15. doi: 10.1007/s00592-005-0168-0. [DOI] [PubMed] [Google Scholar]
- 51.Petrofsky JS, et al. Impairment in orthostatic tolerance during heat exposure in individuals with Type I and Type II diabetes. Med Sci Monit. 2005;11(4):CR153–59. [PubMed] [Google Scholar]
- 52.Petrofsky J, Lee S, Cuneo M. Effects of aging and type 2 diabetes on resting and post occlusive hyperemia of the forearm; the impact of rosiglitazone. BMC Endocr Disord. 2005;5(1):4. doi: 10.1186/1472-6823-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mohr D, Bowry VW, Stocker R. Dietary supplementation with coenzyme Q10 results in increased levels of ubiquinol-10 within circulating lipoproteins and increased resistance of human low-density lipoprotein to the initiation of lipid peroxidation. Biochim Biophys Acta. 1992;1126(3):247–54. doi: 10.1016/0005-2760(92)90237-p. [DOI] [PubMed] [Google Scholar]
- 54.Fisher GR, Patterson LH, Gutierrez PL. A comparison of free radical formation by quinone antitumour agents in MCF-7 cells and the role of NAD(P)H (quinone-acceptor) oxidoreductase (DT-diaphorase) Chem Biol Interact. 1993;88(2–3):137–53. doi: 10.1016/0009-2797(93)90088-g. [DOI] [PubMed] [Google Scholar]
- 55.Lee BJ, et al. Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease. Nutrition (Burbank, Los Angeles County, Calif) 2012;28(3):250–55. doi: 10.1016/j.nut.2011.06.004. [DOI] [PubMed] [Google Scholar]