Abstract
Concurrent O2 evolution, O2 uptake, and CO2 uptake by illuminated maize (Zea mays) leaves were measured using 13CO2 and 18O2. Considerable O2 uptake occurred during active photosynthesis. At CO2 compensation, O2 uptake increased. Associated with this increase was a decrease in O2 release such that a stoichiometric exchange of O2 occurred. The rate of O2 exchange at CO2 compensation was directly related to O2 concentration in the atmosphere at least up to 8% (v/v).
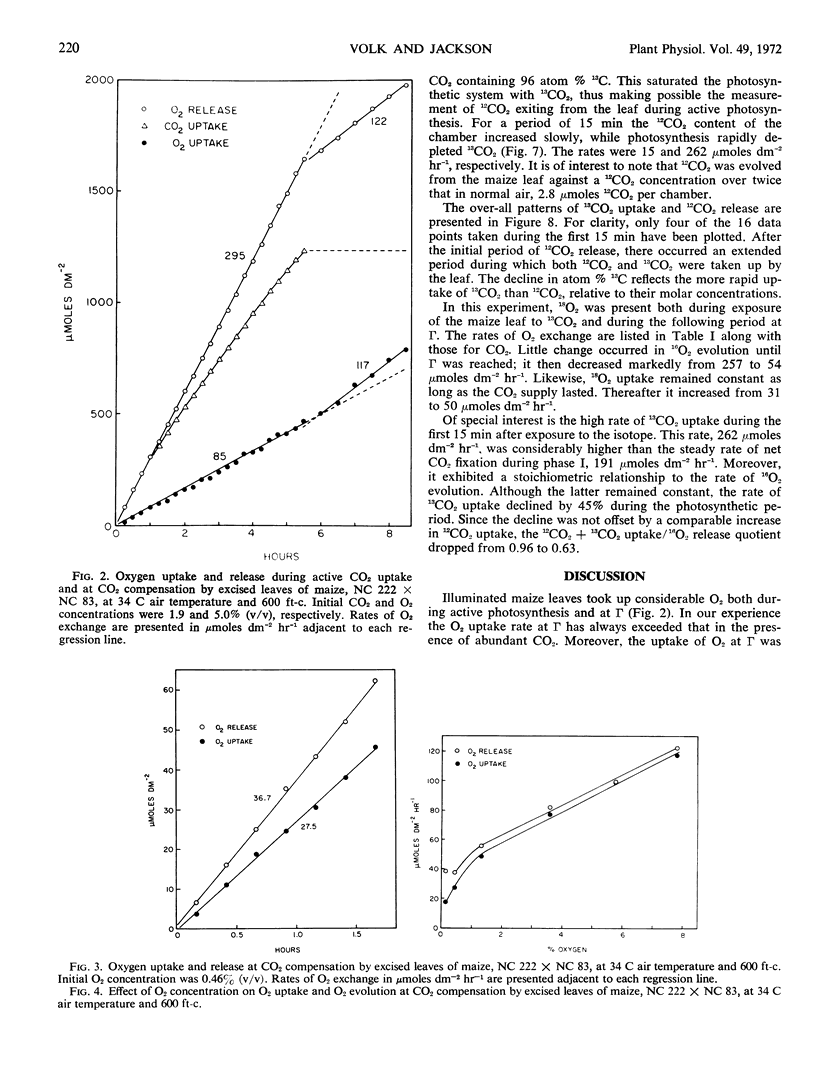
When illuminated maize leaves were exposed to saturating CO2 concentrations containing approximately equal amounts of 12CO2 and 13CO2, the latter was taken up more rapidly, thus depressing the atom% 13C in the atmosphere. Moreover, upon exposure to CO2 containing 96 atom% 13C, there occurred a directly measurable efflux of 12CO2 from the leaves for at least 15 minutes. During this period an equimolar evolution of 16O2 and uptake of 13CO2 was observed. Thereafter, although the rate of 16O2 evolution remained unchanged, the rate of 13CO2 uptake declined markedly, suggesting continual 13C enrichment of the photorespiratory substrate.
It is concluded that a finite photorespiratory process occurs in maize and that the CO2 generated thereby is efficiently recycled. Recycling maintains the internal CO2 concentration at a level difficult to detect by most photorespiratory assays.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Coombs J., Wittingham C. P. The mechanism of inhibition of photosynthesis by high partial pressures of oxygen in Chlorella. Proc R Soc Lond B Biol Sci. 1966 Apr 19;164(996):511–520. doi: 10.1098/rspb.1966.0046. [DOI] [PubMed] [Google Scholar]
- Downton W. J., Tregunna E. B. Photorespiration and Glycolate Metabolism: A Re-examination and Correlation of Some Previous Studies. Plant Physiol. 1968 Jun;43(6):923–929. doi: 10.1104/pp.43.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORTI G., JAGENDORF A. T. Photosynthetic phosphorylation in the absence of redox dyes: oxygen and ascorbate effects. Biochim Biophys Acta. 1961 Dec 9;54:322–330. doi: 10.1016/0006-3002(61)90372-9. [DOI] [PubMed] [Google Scholar]
- HOCH G., OWENS O. V., KOK B. Photosynthesis and respiration. Arch Biochem Biophys. 1963 Apr;101:171–180. doi: 10.1016/0003-9861(63)90547-2. [DOI] [PubMed] [Google Scholar]
- Heber U. Conformational changes of chloroplasts induced by illumination of leaves in vivo. Biochim Biophys Acta. 1969 Jun 24;180(2):302–319. doi: 10.1016/0005-2728(69)90116-9. [DOI] [PubMed] [Google Scholar]
- Hew C. S., Krotkov G., Canvin D. T. Determination of the Rate of CO(2) Evolution by Green Leaves in Light. Plant Physiol. 1969 May;44(5):662–670. doi: 10.1104/pp.44.5.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H. S., Hatch M. D. Properties and regulation of leaf nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase and 'malic' enzyme in plants with the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1970 Sep;119(2):273–280. doi: 10.1042/bj1190273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KRALL A. R., BASS E. R. Oxygen dependency of in vivo photophosphorylation. Nature. 1962 Nov 24;196:791–792. doi: 10.1038/196791a0. [DOI] [PubMed] [Google Scholar]
- Krall A. R., Good N. E., Mayne B. C. Cyclic and non-cyclic photophosphorylation in chloroplasts distinguished by use of labeled oxygen. Plant Physiol. 1961 Jan;36(1):44–47. doi: 10.1104/pp.36.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H., BROWN A. H. Studies on reactions of illuminated chloroplasts. III. Simultaneous photoproduction and consumption of oxygen studied with oxygen isotopes. Arch Biochem Biophys. 1952 Jul;38:365–370. doi: 10.1016/0003-9861(52)90042-8. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys. 1951 Dec;34(2):339–351. doi: 10.1016/0003-9861(51)90012-4. [DOI] [PubMed] [Google Scholar]
- MOSS D. N. The limiting carbon dioxide concentration for photosynthesis. Nature. 1962 Feb 10;193:587–587. doi: 10.1038/193587a0. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- Moss D. N. High activity of the glycolic Acid oxidase system in tobacco leaves. Plant Physiol. 1967 Oct;42(10):1463–1464. doi: 10.1104/pp.42.10.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMOTO T., KROGMANN D. W., MAYNE B. Oxygen exchange catalyzed by phosphorylating chloroplasts. J Biol Chem. 1960 Jun;235:1843–1845. [PubMed] [Google Scholar]
- Ogren W. L., Bowes G. Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol. 1971 Mar 31;230(13):159–160. doi: 10.1038/newbio230159a0. [DOI] [PubMed] [Google Scholar]
- Ozbun J. L., Volk R. J., Jackson W. A. Effects of Light and Darkness on Gaseous Exchange of Bean Leaves. Plant Physiol. 1964 Jul;39(4):523–527. doi: 10.1104/pp.39.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABSON R., TOLBERTNE, KEARNEY P. C. Formation of serine and glyceric acid by the glycolate pathway. Arch Biochem Biophys. 1962 Jul;98:154–163. doi: 10.1016/0003-9861(62)90161-3. [DOI] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D. Comparative studies on the activity of carboxylases and other enzymes in relation to the new pathway of photosynthetic carbon dioxide fixation in tropical grasses. Biochem J. 1967 Jun;103(3):660–665. doi: 10.1042/bj1030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack C. R., Hatch M. D., Goodchild D. J. Distribution of enzymes in mesophyll and parenchyma-sheath chloroplasts of maize leaves in relation to the C4-dicarboxylic acid pathway of photosynthesis. Biochem J. 1969 Sep;114(3):489–498. doi: 10.1042/bj1140489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALKER D. A., BROWN J. M. Physiological studies on acid metabolism. 5. Effects of carbon dioxide concentration on phosphoenolpyruvic carboxylase activity. Biochem J. 1957 Sep;67(1):79–83. doi: 10.1042/bj0670079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yemm E. W., Bidwell R. G. Carbon Dioxide Exchanges in Leaves. I. Discrimination Between CO(2) and CO(2) in Photosynthesis. Plant Physiol. 1969 Sep;44(9):1328–1334. doi: 10.1104/pp.44.9.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZELITCH I. The role of glycolic acid oxidase in the respiration of leaves. J Biol Chem. 1958 Dec;233(6):1299–1303. [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Investigation on photorespiration with a sensitive C-assay. Plant Physiol. 1968 Nov;43(11):1829–1837. doi: 10.1104/pp.43.11.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]


