Abstract
Ginseng, the root of Panax ginseng, is one of the oldest herbal medicines. It has a variety of physiological and pharmacological effects. Recently, we isolated a subset of glycolipoproteins that we designated gintonin, and demonstrated that it induced transient change in intracellular calcium concentration ([Ca2+]i) in cells via G-protein-coupled receptor signaling pathway(s). The previous method for gintonin isolation included multiple steps using methanol, butanol, and other organic solvents. In the present study, we developed a much simple method for the preparation of gintonin from ginseng root using 80% ethanol extraction. The extracted fraction was designated edible gintonin. This method produced a high yield of gintonin (0.20%). The chemical characteristics of gintonin such as molecular weight and the composition of the extract product were almost identical as the gintonin prepared using the previous extraction regimen involving various organic solvents. We also examined the physiological effects of edible gintonin on endogenous Ca2+-activated Cl- channel activity of Xenopus oocytes. The 50% effective dose was 1.03±0.3 μg/mL. Finally, since gintonin preparation through ethanol extraction is easily reproducible, gintonin could be commercially applied for ginseng-derived functional health food and/or drug following the confirmations of in vitro and in vivo physiological and pharmacological effects of gintonin.
Keywords: Panax ginseng, Ginseng, Gintonin, Ethanol extraction, Edible gintonin
INTRODUCTION
Ginseng, the root of Panax ginseng Meyer, is a popular herbal medicine consumed as a functional health food globally throughout the world. The oldest Chinese herbal medicine book, Sheng-nong Ben-cao Jing, states that ginseng’s beneficial effects include replenishment of vital energy, mood elevation, and longevity, and recent studies have revealed that it exhibits diverse physiological and pharmacological effects on nervous and non-nervous systems [1]. However, knowledge of the molecular basis of the multiple pharmacological effects of ginseng is rudimentary.
Previously, we demonstrated that a crude ginseng total saponin (cGTS) fraction activated the endogenous Ca2+- activated Cl- channel (CaCC) in Xenopus oocytes via a Gαq/11-phospholipase Cβ3-IP3-Ca2+ pathway and that repeated treatment with the cGTS fraction caused rapid desensitization of the CaCC via GRK2 and β-arrestin I [2-4]. Recently, we isolated a novel glycolipoprotein fraction from the cGTS and named it gintonin [5]. Gintonin contains proteins with a high abundance of hydrophobic and acidic amino acids, and glucose is the main carbohydrate component. It also contains several fatty acids in ester form [5]. Interestingly, gintonin, but not ginseng saponin (also called ginsenoside), induced transient Ca2+ mobilization in mouse Ehrlich ascites tumor cells and activated CaCC in Xenopus oocytes at very low concentration via the same pathway as the cGTS fraction [5]. We demonstrated that active agents that caused CaCC activation in Xenopus oocytes and transiently elevated the intracellular calcium concentration ([Ca2+]i) in mammalian cells are not ginsenosides but gintonin [5]. In addition, we found that the ginseng stem and leaf also contain gintonin [6]. Thus, we provided the evidence that ginseng contains novel ingredients and that one novel ingredient, gintonin, could also be the main agent for Ca2+-mediated various cellular events.
However, the previous methods for crude gintonin preparation included multiple steps using various organic solvents such as methanol, butanol, and other harmful organic solvents [5]. In the present study, we developed a simple method for crude gintonin preparation from ginseng root using ethanol. Here, we report that this procedure could be produce edible gintonin with high yield. The present data indicate report shows the possibility that the edible gintonin could also be useful in functional health food/drug development.
MATERIALS AND METHODS
Materials
BioLogic DuoFlow chromatography systems were purchased from Bio-Rad (Hercules, CA, USA). Six-year-old Korean red ginseng (Korea Ginseng Corporation, Daejeon, Korea) were purchased from a local ginseng market. The prepacked columns (Superdex 75 10/30/300 GL and HiTrap DEAE FF) and medium (DEAE Sepharose CL-6B) were purchased from GE Healthcare (Uppsala, Sweden). Cellulose membrane with a molecular weight cut of 13,000 and other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Preparation of gintonin fraction from ginseng root
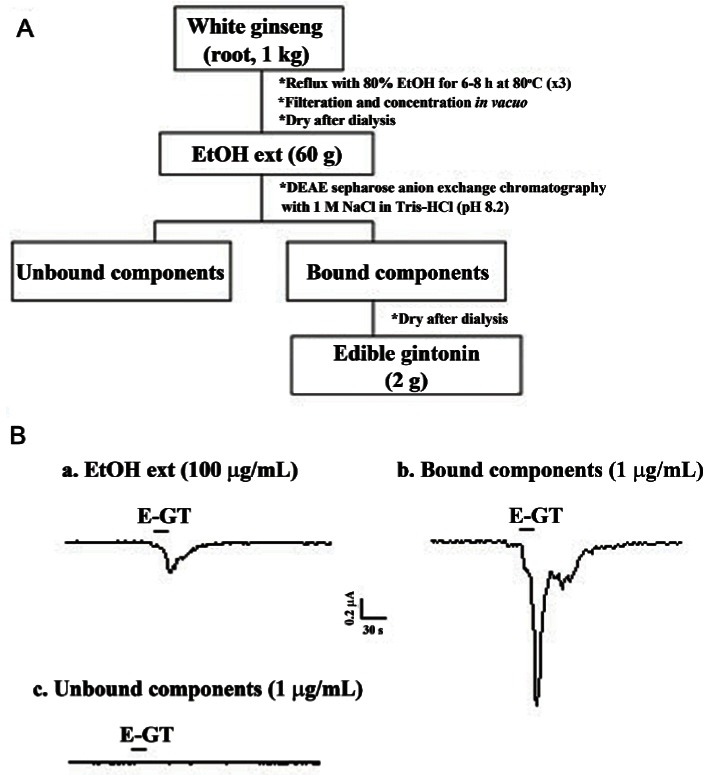
One kilogram of 4-year-old ginseng were ground into small pieces (>3 mm) and refluxed with 80% ethanol (EtOH) three times for 8 h at 80℃ each. The EtOH extracts (60 g) were concentrated and dried after extensive dialysis. The EtOH fraction of Panax ginseng dissolved in phosphate buffered saline (PBS, pH 7.2) was loaded onto a column packed with DEAE sepharose CL-6B (GE Healthcare) and equilibrated with PBS. The unbound materials were eluted with the same buffer and the bound materials were eluted with a linear gradient of 0 to 1 M NaCl in PBS. The eluted fraction was further dialyzed at 4℃ for 8 h with 1,000-fold excess distilled water using a Spectra/Por dialysis membrane (molecular weight cut off 6,000-8,000; Spectrum Laboratories, Rancho Dominguez, CA, USA) to further remove small molecular components such as ginsenosides and other components that might remain in the fraction (2 g) [5]. This fraction was designated edible gintonin (Fig. 1).
Fig. 1. Diagram for an edible gintonin (E-GT) preparation from Panax ginseng root. (A) A complete diagram for crude gintonin preparation from ginseng root. (B) The representative Ca2+-activated Cl- channel (CaCC) current traces are representative of one obtained from each preparation step. Treatment with ethanol extraction (EtOH ext) and the bound component obtained after elution with NaCl in Tris-HCl (pH 8.2) induced endogenous CaCC activation in Xenopus oocytes, whereas unbound component had no effect. The amount of each fraction used to test CaCC activity was 1 or 100 μg/mL. Inward currents were recorded at –80 mV holding potential.
Gel filtration chromatography
Gel filtration chromatography of the gintonin fraction obtained from ginseng root was carried out by a Superdex 75 column (10×300 mm) equilibrated with PBS on BioLogic DuoFlow chromatography systems (Bio-Rad). Fractions were collected with a flow rate of 0.5 mL/min and monitored at 280 nm. Each fraction was tested on endogenous CaCC activations in Xenopus oocytes [5].
Electrophoresis
The crude gintonin from ginseng was subjected to 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) [6]. Edible gintonin (100 μg each) from ginseng root was loaded in each lane. After electrophoresis, gintonin bands were visualized by Coomassie Brilliant Blue R-250 staining [5].
Gintonin amino acid composition analysis
Gintonin (30 μg) from ginseng was hydrolyzed in vacuo in 6 N HCl for 24 h at 110℃ for general amino acid analysis. For the analysis of cysteine, gintonin was hydrolyzed in 6 N HCl for 24 h at 110℃ after peroxidation treatment with formic acid:hydrogen peroxide (10:1). For the analysis of tryptophan, the sample was hydrolyzed in 4 M methanesulfonic acid and 4 M KOH was also added. Amino acids converted to phenylisothiocyanate derivates were analyzed by HPLC (Hewlett Packard 1100 series; Hewlett Packard, Palo Alto, CA, USA) with a Waters Nova-Pak C18 column (3.9×300 mm) at the Korea Basic Science Institute (Seoul, Korea). Protein contents were determined by the Bradford method using bovine serum albumin as a standard [5].
Carbohydrate composition
Gintonin from ginseng was hydrolyzed in 2 M trifluoroacetic acid for 4 h at 100℃ for neutral sugar and hydrolyzed in 6 N HCl for 4 h at 100℃ for amino sugar and acid sugar in glass. Carbohydrate compositions of gintonin were analyzed by high performance anion exchange chromatography-pulsed ampherometric detection system (HPAEC-PAD system; Dionex, Sunnyvale, CA, USA) with a CarboPac PA1 column at the Carbohydrate Bioproduct Research Center, Sejong University (Seoul, Korea). The molar ratios of monosaccharides were calculated from peak areas. The carbohydrate contents were also determined by phenol-sulfuric acid method for neutral sugar [7] and the anthrone method for acid sugar [8].
Lipid composition analysis
Gintonin from ginseng was hydrolyzed in 6 N HCl for 4 h at 100℃ or digested by lipoprotein lipase to confirm lipid and hydrophobic moiety. Acid hydrolyzed or digested gintonin was partitioned between distilled water and n-butanol (BuOH). The n-BuOH layer, after concentration, was further partitioned between distilled water and n-hexane. The n-hexane layer was prepared for lipid and hydrophobic moiety analysis by a 6890N GC-MS system (Agilent, Santa Rosa, CA, USA) with a DB5-MS capillary column (30 cm×250 μm×0.25 μm) at the Korea Basic Science Institute (Seoul, Korea) and by gas chromatography using an Agilent 6890N chromatograph equipped with flame ionization detector and a split injection system and fitted with a supelco SPB-1 capillary column (15 m×0.32 mm inside diameter, 0.25 mm thickness) [5].
Oocyte preparation
Xenopus laevis frogs were obtained from Xenopus I (Ann Arbor, MI, USA). Their care and handling were in accordance with the highest standards of Konkuk University guidelines. To isolate oocytes, frogs were operated on under anesthesia with an aerated solution of 3-amino benzoic acid ethyl ester. Oocytes were separated by treatment with collagenase and agitation for 2 h in a Ca2+-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, 2.5 mM sodium pyruvate, 100 units/mL penicillin and 100 μg/mL streptomycin. Stage V-VI oocytes were collected and stored in ND96 (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 50 μg/mL gentamicin [2]. This oocyte-containing solution was maintained at 18℃ with continuous gentle shaking and changed everyday.
Measurements of endogenous Ca2+-activated Cl- channel currents
Two-electrode voltage-clamp recordings were obtained from individual oocytes placed in a small Plexiglas net chamber (0.5 mL), which was continuously superfused with the bathing medium (i.e., ND96). The microelectrodes were filled with 3 M KCl and had a resistance of 0.2-0.7 MΩ. The electrophysiological experiments were performed at room temperature using an OC-725C Oocyte Clamp amplifier (Warner Instrument, Hamden, CT, USA). CaCC was recorded at –80 mV holding potential. Gintonin was applied to oocytes by bath perfusion [2].
Data analysis
To obtain the concentration-response curve in the presence of edible gintonin, the observed peak amplitudes were normalized and plotted and then fitted to the Hill equation below using Origin software (Origin, Northampton, MA, USA): y/ymax = [A]n/([A]n + [EC50]n) where y represents the percent activation at a given concentration of gintonin, ymax represents maximal peak current, EC50 is the concentration of gintonin producing half-maximum effect of the control response to gintonin, A is the concentration of gintonin, and n is the interaction coefficient. All values are presented as mean±SEM. The differences between means of control and gintonin treatment data were analyzed using unpaired Student’s t-test. A value of p<0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Simple method for edible gintonin preparation from ginseng
Gintonin, a novel mixture of glycolipoproteins of ginseng, induces intracellular Ca2+ mobilization via phospholipase C-IP3-Ca2+ pathway in mammalian cells and Xenopus oocytes [5]. Since Ca2+ is a second messenger and is involved in numerous Ca2+-dependent events such as Ca2+-dependent enzymes, ion channels, hormones and neurotransmitter release, and more, gintonin might participate a variety of cellular events. In previous reports, we have shown that ginseng can be extracted with various organic solvents such as methanol and butanol as a first step for gintonin preparation from ginseng root, stem, and leaf [5,6]. For use of gintonin as a functional health food and/or drug, a new method of extraction that does not use harmful organic solvents is necessary. In the present study, we have developed a simple extraction protocol for gintonin preparation using ethanol. As shown in Fig. 1A, we first prepared 80% ethanol extract, directly applied to anion exchange column, eluted bound components by 1 M NaCl, and finally produced gintonin fraction with 0.2% yield. As shown in Fig. 1B, ethanol extract (100 μg/mL) of ginseng slightly induced a inward Cl- current by activating endogenous CaCC in Xenopus oocytes. The unbound components to column had no effect on CaCC activation, whereas the bound component (1 μg/mL) induced a large inward Cl- current by activating endogenous CaCC channels in Xenopus oocytes. These results indicate that the main component for CaCC activation is gintonin that carries charges and that unbound components, which might include ginsenosides and other uncharged or neutral components, had no effects on CaCC activity. Thus, we could prepare gintonin fraction activating CaCC with ethanol and called it edible gintonin fraction.
Determination of molecular weight and gel filtration chromatography of edible gintonin
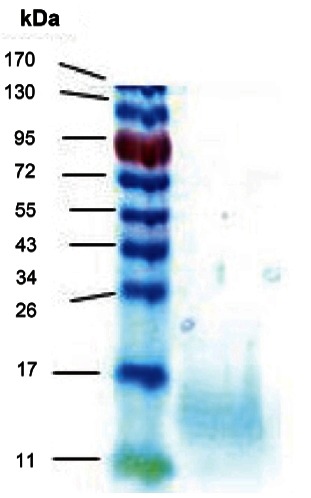
We next estimated the apparent molecular weight of edible gintonin fraction prepared from ginseng. For this, we performed SDS-PAGE using edible gintonin. In SDS-PAGE, the edible gintonin fraction showed a broad but single major band and its apparent molecular weight was about 13 kDa (Fig. 2), revealing that the molecular weight of edible gintonin is the same with the gintonin prepared with other organic solvents [5].
Fig. 2. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) of each gintonin prepared from ginseng root. SDS-PAGE of edible gintonin obtained from anion exchange chromatography as shown in Fig. 1. Coomassie Brilliant Blue staining was used to stain protein moieties of gintonin. Crude gintonin prepared from ginseng root in SDS-PAGE showed that apparent molecular weight of gintonin was about 13 kDa.
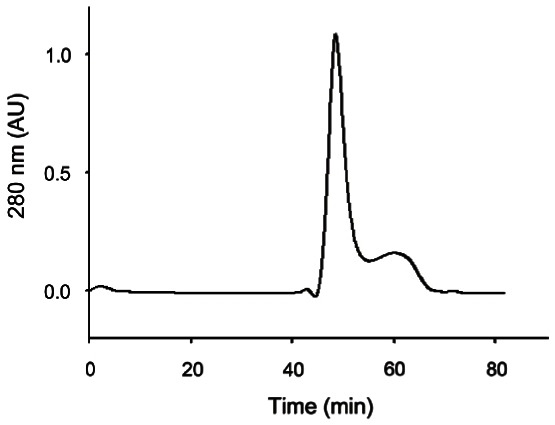
We examined the gel filtration chromatography pattern of the edible gintonin fraction. As shown in Fig. 3, the edible gintonin fraction from ginseng root showed a main peak and a small peak. We found that the main peak, but not the minor peak, of edible gintonin largely exhibited CaCC activation in Xenopus oocytes (data not shown).
Fig. 3. Elution patterns of gintonin prepared from ginseng root by gel chromatography. Gel filtration chromatograms on Superdex 75 column with phosphate buffered saline (pH 7.2) of edible gintonin prepared from ginseng root. Analysis through gel filtration chromatograms showed one main peak and other minor peaks. The main peak was active for the activation of Ca2+-activated Cl- channel.
Amino acid, carbohydrates, and lipid composition of gintonin prepared from ginseng
The contents of total proteins in crude gintonin by the Bradford method were about 26% for edible gintonin.
Amino acid composition of individual gintonin was summarized in Table 1. In previous reports, we have shown that gintonin contains carbohydrate moieties.
Table 1.
Amino acid composition of edible gintonin
| Amino acid | Result |
|---|---|
|
| |
| Sum of cysteine & cystin | 6.39 |
| Sum of asparagine & asparate | 15.37 |
| Sum of glutamine & glutamate | 11.07 |
| Serine | 4.69 |
| Glycine | 7.88 |
| Histidine | 1.12 |
| Arginine | 3.34 |
| Threonine | 7.19 |
| Alinine | 6.49 |
| Proline | 6.84 |
| Tyrosine | 1.89 |
| Valine | 6.38 |
| Methionine | 1.3 |
| Isoleucine | 3.82 |
| Leucine | 7.18 |
| Phenyalanine | 4.82 |
| Tryptophane | 0.35 |
| Lysine | 3.89 |
| N-isobutylglycine | |
The detail methods for amino acid compositions of edible gintonin are described in Materials and Methods section. Data are presented as percentage.
We next the examined the carbohydrate composition of gintonin using the HPAEC-PAD system (Fig. 4). Table 2 shows that carbohydrate compositions of edible gintonin. Glucose was major component, as shown previously [5]. Finally, the contents of total carbohydrate in crude gintonin were about 23.2%. The above results again confirmed that gintonins are glycoproteins.
Fig. 4. Analysis of carbohydrate components of crude gintonin prepared from ginseng root by high performance anion exchange chromatography-pulsed ampherometric detection (HPAEC-PAD) chromatograms. HPAEC-PAD chromatograms revealed that gintonin to be mainly composed of three different kinds of neutral sugars: glucose, arabinose, galactose, and fucose and one amino sugar, glucosamine. (A) Std stands for standard carbohydrates used; 1. L-fucose, 2. L-rhamnose, 3. D-galactosamine, 4. D-arabinose, 5. D-glucosamine, 6. D-galactose, 7. D-glucose, 8. D-mannose, 9. D-xylose, 10. D-fructose. (B) HPAEC-PAD chromatograms of gintonin.
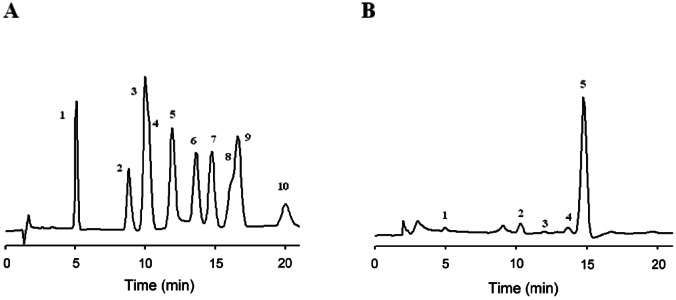
Table 2.
Carbohydrate composition of edible gintonin
| Carbohydrate | Result |
|---|---|
|
| |
| Fuctose | 1.50 |
| Arabinose | 4.32 |
| Glucosamine | 0.87 |
| Galactose | 2.95 |
| Glucose | 90.35 |
Data are presented as percentage. The detailed methods for carbohydrate compositions of edible gintonin are described in Materials and Methods.
We also examined lipid moieties. As shown in Fig. 5 and Table 3, edible gintonin contained fatty acids such as palmitic, stearic, linoleic acid as ester form, and other minor fatty acids. Interestingly, the portion of palmitic acid inntonin was higher than unsaturated fatty acids such as linoleic acid (Fig. 5). Thus, it appears that palmitic acid was the dominant lipid of crudentonin. Next, we estimated the total contents of lipid moiety in gintonin by gas chromatography. The contents of total lipids in crude gintonin were 26%. Again, these results indicated thatntonin prepared using ethanol is composed of glycolipoproteins.
Fig. 5. GC-MS spectral analysis of lipid components of edible gintonin prepared from ginseng root. Acid hydrolyzed gintonin were partitioned between distilled water and n-butanol. The n-butanol layer, after concentration, was further partitioned between distilled water and n-hexane. The n-hexane layer was subjected to gas chromatography-mass spectrometry with a DB5-MS capillary column. Several major peaks were present in hexane fraction of gintonin and were identified. The 20.94 peak was nonanedioic acid that is known to function to defense after infection in plants. The 22.04 peak of palmitic acid (C16:0) and 23.5 peak of linoleic acid (C18:2) were present as dominant fatty acids or their ester forms.
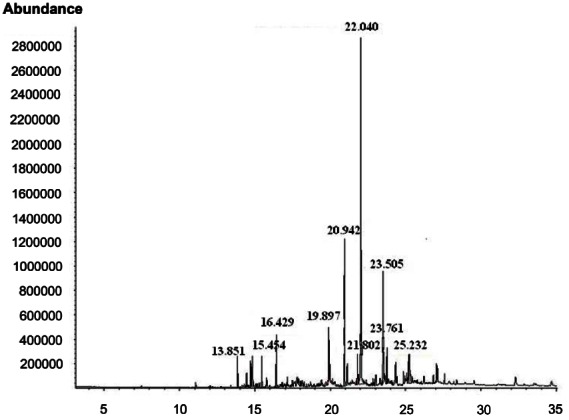
Table 3.
Lipid composition of edible gintonin
| Lipids | Result |
|---|---|
|
| |
| Succinic acid, dibutyl ester | 3.0 |
| Nonanedioic acid, dibutyl ester | 12.0 |
| Palmitic acid or palmitic acid butyl ester form | 41.8 |
| Stearic acid or stearic acid butyl ester form | 6.5 |
| Linoleic acid or linoleic acid butyl or ethyl ester form | 17.6 |
| Others | 7.9 |
Data are presented as percentage. The detail methods for lipid compositions of edible gintonin are described in Materials and Methods.
Effects of edible gintonin prepared from ginseng on endogenous Ca2+-activated Cl- channel in Xenopus oocytes
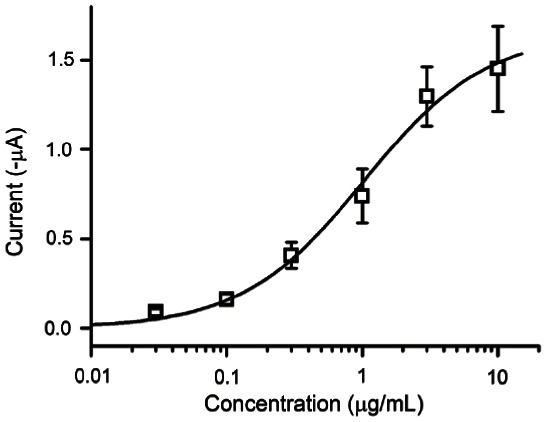
We also examined the effects of edible gintonin for activation of CaCC. As shown in Fig. 6, treatment of edible gintonin induced a large inward Cl- current in a concentration-dependent manner at –70 mV holding potential (Fig. 6). The ED50 was 1.03±0.3 μg/mL. Thus, these results indicate that edible gintonin prepared with ethanol shows same ED50 value in activating CaCC in Xenopus oocytes compared to the previous gintonin prepared with other solvents [5].
Fig. 6. Edible gintonin prepared from ginseng root activates Ca2+-activated Cl- channel in a concentration-dependent manner in Xenopus oocytes. Experimental details are described in Materials and Methods. Data represent mean±SEM (n=7 or 8).
In the present study, we report two major findings. First, we showed that gintonin can be prepared using ethanol. Second, we also used a one-step extraction instead of previous multiple step extractions using methanol and butanol [5,6]. This simplified method for gintonin preparation has at least three merits compared to previous methods. First, the newly developed method uses ethanol, which is permitted for use with functional food and/or drug preparation. Second, this method also requires less organic solvents than the previous methods, making mass production of gintonin conceivable. Third, the procedure for gintonin preparation using ethanol is easily reproducible.
We observed some similarities and differences in the present and previous gintonin. Their molecular weights in SDS-PAGE were almost same (Fig. 4). In amino acid composition, edible gintonin also was shown to contain a high amount of hydrophobic amino acids such as phenyalanine, leucine, isoleucine, alanine, and proline, in agreement with the composition of the previously obtained gintonin (Table 1). In carbohydrate compositions, glucose was predominant in both gintonin preparations obtained using ethanol and other organ solvents (Table 2). Interestingly, palmitic acid (C16:0) was the major lipid component in edible gintonin followed by linoleic acid (C18:2), whereas linoleic acid was dominant one and followed by palmitic acid (Table 3). In addition, we observed the presence of some minor components (Table 3). The discrepancy in lipid content between present and previous gintonin might be derived from batch-to-batch variability and from different sources of ginseng cultivation. Further studies will be needed to establish standardization of gintonin in lipid content.
In previous studies we demonstrated that gintonin induced CaCC activation in Xenopus oocytes through the mobilization of [Ca2+]i [5,6]. When we compared the potency for endogenous CaCC activation in Xenopus oocytes with the previously acquired extract, we found that ethanol gintonin was not much different from that of previous gintonin for the activation of CaCC. These results indicate that ethanol used for gintonin extraction at least did not affect gintonin activity for CaCC activations and also that ethanol could be used for mass production of gintonin for application as a functional health food and/or drug (Fig. 6).
In summary, we have developed a simple method for edible gintonin preparation using ethanol. The composition of edible gintonin and physiological activity of edible gintonin for CaCC activation in Xenopus oocytes through intracellular Ca2+ mobilization was almost same with that of previous ginotonin prepared by other organic solvents. Since elevation of intracellular Ca2+ level as a second messenger plays an important role for variety of cellular functions [9], the present method for edible gintonin could be further applied for functional health food and/or drug development.
Acknowledgments
This paper was supported by Konkuk University in 2010.
References
- 1.Nah SY, Kim DH, Rhim H. Ginsenosides: are any of them candidates for drugs acting on the central nervous system? CNS Drug Rev. 2007;13:381–404. doi: 10.1111/j.1527-3458.2007.00023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi S, Kim HJ, Ko YS, Jeong SW, Kim YI, Simonds WF, Oh JW, Nah SY. G alpha(q/11) coupled to mammalian phospholipase C beta 3-like enzyme mediates the ginsenoside effect on Ca(2+ )-activated Cl(-) current in the Xenopus oocyte. J Biol Chem. 2007;276:48797–48802. doi: 10.1074/jbc.M104346200. [DOI] [PubMed] [Google Scholar]
- 3.Choi S, Rho SH, Jung SY, Kim SC, Park CS, Nah SY. A novel activation of Ca(2+ )-activated Cl(-) channel in Xenopus oocytes by Ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca(2+ ) mobilization. Br J Pharmacol. 2001;132:641–648. doi: 10.1038/sj.bjp.0703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Jeong SM, Lee BH, Noh HS, Kim BK, Kim JI, Rhim H, Kim HC, Kim KM, Nah SY. Prevention of ginsenoside-induced desensitization of Ca(2+ )-activated Cl(-) current by microinjection of inositol hexakisphosphate in Xenopus laevis oocytes: involvement of GRK2 and beta-arrestin I. J Biol Chem. 2004;279:9912–9921. doi: 10.1074/jbc.M310824200. [DOI] [PubMed] [Google Scholar]
- 5.Pyo MK, Choi SH, Hwang SH, Shin TJ, Lee BH, Lee SM, Lim YH, Kim DH, Nah SY. Novel glycolipoproteins from ginseng. J Ginseng Res. 2011;35:92–103. doi: 10.5142/jgr.2011.35.1.092. [DOI] [Google Scholar]
- 6.Pyo MK, Choi SH, Shin TJ, Hwang SH, Lee BH, Kang J, Kim HJ, Lee SH, Nah SY. A simple method for the preparation of crude gintonin from ginseng root, stem, and leaf. J Ginseng Res. 2011;35:209–218. doi: 10.5142/jgr.2011.35.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hounsell EF, Davies MJ, Smith KD. Protein protocol handbook. Humana Press; Totowa: 1997. [Google Scholar]
- 8.Scott TA, Melvin EH. Determination of dextran with anthrone. Anal Chem. 1953;25:1656–1661. doi: 10.1021/ac60083a023. [DOI] [Google Scholar]
- 9.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]


